Abstract
We have developed a local anesthetic-eluting suture system which would combine the function and ubiquity of the suture for surgical repair with the controlled release properties of a biodegradable polymeric matrix. Drug-free and drug-loaded poly(lactic-co-glycolic acid) (PLGA) sutures were fabricated by electrospinning, with or without the local anesthetic bupivacaine. The tensile strength of the electrospun sutures decreased as drug content increased, but strains remained relatively similar across all groups. Sutures released their entire drug payload over the course of 12 days and maintained approximately 12% of their initial tensile strength after 14 days of incubation in vitro. In a rat skin wound model, local analgesia was achieved 1 day after surgery and lasted approximately 1 week in 90% of treated animals (n = 10, p < 0.05), and all wounds were able to heal normally without the need for further reinforcement. The sutures caused tissue reaction in vivo that was comparable to that seen with a commercially available suture composed of PLGA. Such sutures may enhance perioperative analgesia and mitigate the need for standard postoperative opioid analgesics.
Keywords: suture, bupivacaine, local anesthetic, poly(lactic-co-glycolic acid), prolonged release
1. Introduction
A fundamental component of nearly any surgical procedure is the utilization of sutures for a wide range of applications including approximating tissues, connecting hollow structures, and the ligation of blood vessels. Although sutures have changed markedly in regards to the materials and manufacturing techniques used since their introduction over four thousand years ago [1], little has been done to enhance the therapeutic value of the suture itself. Recently, there has been interest in using sutures as platforms for releasing drugs for local effect. The rationale is that controlled release systems [2, 3] can create high local drug concentrations without excessive systemic levels (i.e. improvement in the therapeutic index) and/or prolongation of the duration of effect. Unlike other drug delivery systems, since sutures are already utilized in almost all surgical procedures, the use of drug-eluting sutures would not require the surgeon to place an object in the surgical bed that would otherwise not be there, which could create a nidus for infection, introduce undesirable bulk in a tight place, or place materials in a wound that might interfere with healing or other processes. In the past, sutures have been coated with triclosan [4–6], doxycycline in a fibrinogen coating [7], chlorhexidine in polyelectrolye films [8], and antiseptics with fatty acids [9] to achieve antibacterial effect, or a polymer containing the immunosuppressive drug tacrolimus in a bioabsorbable polymer to prevent neointimal hyperplasia [10], and tetracycline [11] and cefotaxime sodium [12] have been released from electrospun sutures.
Here, we have developed electrospun sutures to provide local anesthesia at the site of incision. This approach utilizes a core component of almost every operation as an opportunity to deliver an analgesic agent to reduce postoperative discomfort and the need for supplemental systemic drugs such as opioid narcotics, which may impair patient function, and to possibly obviate the need for other procedures (such as local or regional anesthetic nerve blocks). One challenge in producing such a system using conventional amino-amide local anesthetics is that these drugs are of relatively low potency, which makes high drug loadings necessary. This necessity is compounded by the fact that local anesthesia requires relatively high tissue drug concentrations (compared to those required by biologicals, for example). Such drug loading may affect suture mechanical properties adversely and accelerate suture degradation excessively.
The sutures used in this study are composed of poly(lactic-co-glycolic) acid (PLGA) fibers bundled into a single-stranded construct with varying concentrations of the local anesthetic bupivacaine hydrochloride. PLGA is a biodegradable polymer with good biocompatibility that is approved for use in humans by the Food and Drug Administration (FDA) and is one of the polymers used in commercially available sutures, and bupivacaine hydrochloride is a commonly used amino-amide local anesthetic. The drug-containing sutures were produced by electrospinning, a technique in which a charge is applied to a polymer-drug mixture that has been dissolved in a volatile organic solvent, and this charge causes the solution to be ejected as a thin stream to a grounded collector upon which polymer microfibers are deposited [13]. Using this approach, fibers can be made from almost any polymer without the need for the high operating temperatures which could degrade heat-labile drugs. The flexibility in matrix material selection allows for a wide variety of therapeutics to be incorporated, including: antibiotics [11, 12, 14], anti-cancer drugs [15], proteins [16], and DNA [17]. Additionally, through modifications to the basic electrospinning technique, constructs with different modes of drug loading (coated, embedded, etc.) can be created. Therefore, electrospinning has the advantage – compared to the commonly used fabrication method of hot-melt extrusion – of allowing for a wider variety drug-eluting sutures with different polymer-drug combinations.
2. Methods
2.1. Materials
Poly(lactic-co-glycolic acid) (PLGA, 90:10 glycolide:L-lactide, inherent viscosity: 1.71 dL/g in HFIP at 25°C) was purchased from Purac Biomaterials Inc. (Lincolnshire, IL). 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), bupivacaine HCl, rhodamine 6G, acetonitrile, and sodium phosphate were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Phosphate buffered saline (PBS) was purchased from Invitrogen Corp. (Carlsbad, CA). All materials were used as received unless stated otherwise.
2.2. Suture Fabrication
Electrospun sutures were fabricated using PLGA as the synthetic biodegradable drug-eluting platform. PLGA was dissolved in HFIP at a 10 wt% concentration until a clear and homogenous solution was obtained. The polymer solution was delivered through a stainless steel capillary via Teflon tubing at a rate of 3 mL/hr, and this flow rate was maintained with a syringe pump (Harvard Apparatus, Holliston, MA). A high voltage power supply (Gamma High Voltage Research, Ormond Beach, FL) created a 25 kV potential between the capillary tip and a grounded stainless steel disc 30 cm away. The disc featured a diameter of 280 mm, a thickness of 5 mm, and a sharpened edge with a half-angle of 25° that was spun at a predetermined speed. The applied voltage potential caused the polymer solutions to form a steady jet that underwent whipping instability and solvent evaporation to form microfibers that were then deposited onto the edge of the grounded disc [18]. Fibers were collected for 2, 4 and 7 minutes to create fiber bundles equivalent to sutures sized 6-0 (~0.08 mm diameter), 4-0 (~0.175 mm), and 2-0 (~0.32 mm), respectively. The resulting fiber bundles were removed from the disc manually and allowed to sit at room temperature for 72 hours to let any residual solvent evaporate. Electrospun drug-loaded and dye-loaded sutures were prepared in the same way, but with the addition of bupivacaine HCl or rhodamine 6G dye at concentrations of 5%, 10%, 15%, and 22% by weight to the 10 wt% PLGA solution. Suture diameters were confirmed by taking still images of the sutures using an optical microscope and with a known scale and measuring the diameters using ImageJ (National Institutes of Health, Bethesda, MA).
2.3. Mechanical Testing
Electrospun and Vicryl® sutures were cut into 10 cm segments in preparation for testing. Five samples of each suture diameter for drug-free, drug-loaded, and dye-loaded sutures were tested on an Instron Model 5943 Single Column Testing System (Instron Engineering Corporation, Norwood, MA) with a load cell of 50 N. A cross-head speed of 10 mm/min was used for all tests, and all samples were tested until failure. Post-immersion suture samples from the in vitro release study were tested using the same parameters. Average force and strain at failure, as well as their standard deviations, were obtained.
2.4. Scanning Electron Microscopy
Fiber morphologies of the electrospun sutures were examined using scanning electron microscopy (SEM; Model 5600LV, JEOL Ltd., Japan). Samples were prepared by either spinning fibers directly onto aluminum foil that had been placed onto the edge of the spinning disc or by cutting sutures that had been immersed in PBS into 1 cm long segments. Samples were sputter-coated with gold-palladium alloy to boost their electrical conductivity to facilitate high resolution imaging. An accelerating voltage of 5 kV was used for all images.
2.5. Thermogravimetric Analysis
Residual solvent content in electrospun sutures was determined using thermogravimetric analysis (TGA; Pyris 1 TGA, Perkin Elmer, Waltham, MA). 10 cm segments of electrospun drug-free and drug-loaded sutures were analyzed, and 10 cm segments of Vicryl® suture served as controls. Samples were brought up to 375 C at 10° C/min and held at this temperature for 5 minutes before being cooled back down to 30° C at 20 C/min.
2.6. In vitro Release Study
5 cm suture segments were placed in separate wells of a 24-well plate and submerged in 1 mL of phosphate buffered saline (PBS). The plates were incubated at 37 °C in 5% CO2. After 4, 8 and 12 hours, and then every other day for 21 days, PBS was removed for analysis and replaced by fresh solution. Separate suture segments of each diameter treated in a similar manner were collected every 7 days for 21 total days and stored at −20°C until ready for tensile testing and SEM imaging as described above, at which time they were brought back to room temperature.
2.7. High Performance Liquid Chromatography
Bupivacaine HCl concentrations in collected PBS samples were determined by high-performance liquid chromatography (HPLC; HP/Agilent 1100, Agilent, Santa Clara, CA). 20 μL of each sample was injected into a 4.6 × 250 mm Atlantis dC18 5 μm column. The column was eluted with an aqueous solution of 70:30 acetonitrile:NaH2PO4/H3PO4 (0.01 M, pH = 2.1) at 1.5 mL/min, and bupivacaine was detected by UV absorbance at λ = 210 nm. Standards composed of known concentrations of bupivacaine HCl in PBS were made on the day of analysis.
2.8. In vivo Studies
Animals were cared for in compliance with protocols approved by the Massachusetts Institute of Technology Committee on Animal Care, which conformed to IASP guidelines. Adult male Sprague-Dawley rats weighing 350–400 grams were obtained from Charles River Laboratories, Inc. (Wilmington, MA) and were housed in groups of two in a 6 AM to 6 PM light-dark cycle. Under isoflurane-oxygen anesthesia, a 1.5 cm long full-thickness incision was made on the left dorsal flank, followed by wound closure with either dye-loaded or drug-loaded sutures by standard suturing techniques (single layer, subcuticular). The similarity of rhodamine 6G’s size, pKa, and log P values [19, 20] with those of many of the weakly-basic small molecules that comprise the vast majority of common local anesthetics make the dye a useful representative tracer of the diffusion of such molecules into tissue surrounding the suture after wound closure. The spread of dye around the wound was visually inspected and documented over 14 days under both visible and cobalt blue light. Fluorescent images of dye distribution in rat dermis were taken using a standard single-lens reflex camera with a #16 orange lens filter (Tiffen, Hauppauge, NY).
Animals implanted with bupivacaine-loaded sutures were subjected to pinch testing in order to determine the effectiveness of eluted drug in inducing analgesia. The pinch test used in this study is a modification of established methods that have been used in many published works [21, 22], and our model was validated by pilot experiments in which naïve rats and rats injected with 0.2 mL of 0.25% bupivacaine HCl subcutaneously were subjected to pinch stimuli. These experiments showed that a response was elicited 83.3% of the time in naïve rats, and that rats injected with the bupivacaine HCl solution did not respond to pinch for up to 1 hour. All testing was done by a single, blinded investigator to minimize variations in technique and bias in observation. Testing consisted of using a curved blunt non-serrated forceps (0.8 mm tip width) to apply a brief pinch in six different locations within a 1 cm radius around the entire incision and recording the animal’s response to each pinch. Care was taken to ensure that pinches were applied with near constant force each time and that as little skin was pinched as possible. A normal response to the pinch stimulus was defined as a withdrawal reaction or a reflexive contraction of the musculature at the point of stimulus, and a negative response was defined as no flinch, muscle contraction, or vocalization. Responses were scored as the percentage of pinches where the animal responded to stimulus. Animals were tested 1, 4 and 8 hours post-surgery, then every day for the next 14 days. Animals were euthanized at the end of the study by carbon dioxide inhalation, and the skin tissue from the site of the incision was harvested and processed for histological analysis by hematoxylin and eosin staining.
2.9. Statistical Analysis
Data from tensile testing, in vitro drug release, and pinch testing assessments are presented as means ± one standard deviation. Differences between groups for tensile testing and in vitro drug release experiments were assessed with one-way ANOVA with a Tukey post-test analysis. Statistical comparisons of analgesia scores were made using the Mann-Whitney U test. All analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA), and p < 0.05 was considered statistically significant.
3. Results
3.1. Suture fabrication and characterization
Pilot studies varying disc spin speeds and the concentrations of polymer identified which combination provided the best degree of microfiber alignment and morphology to impart optimal tensile strength to the sutures. Microfibers spun at 1200 rpm were more aligned than those spun at lower speeds (Fig. 1), and significant fiber loss occurred at higher speeds due to turbulence between the Taylor cone and the disc’s edge. A series of polymer solution concentrations used for electrospinning was tested and a concentration of 10% w/w was found to consistently create fibers without irregularities that would have adversely affected suture tensile strength, such as polymer beading (irregular accumulation of polymer along individual fibers) or fiber necking (localized decrease in fiber cross-sectional area). Imaging of the sutures by SEM showed that individual microfibers in each suture were approximately 0.8–1.0 μm in diameter, and the electrospun sutures exhibited a generally uniform cylindrical form not unlike that of commercially available sutures at both the macro and micro scales (Fig. 2). Furthermore, the sizes of the sutures created (ie. 2-0, 4-0, 6-0) were determined by electrospinning time, allowing for the reproducible fabrication of sutures of different sizes (Fig. 3).
Figure 1.

Microfibers spun at a disc speed of 600 rpm (A) were less well aligned than those spun at 1200 rpm (B).
Figure 2.
Representative photographs and SEMs of an electrospun PLGA suture (A) and of a Vicryl® braided suture (B).
Figure 3.

Sutures of various specific sizes could be produced at all drug loadings by controlling the duration of electrospinning. Data are means ± SD; n = 5. Symbols in key are percent drug loadings with bupivacaine. The y-axis on the left indicates the corresponding suture size nomenclature.
Drug-loaded sutures composed of PLGA (90 glycolide:10 L-lactide) were produced with varying proportions of the local anesthetic bupivacaine, and were subjected to mechanical testing as produced or after incubation in PBS at 37 °C for one week. Tensile testing revealed that the suture’s strength decreased as the concentration of drug incorporated into the polymer increased. However, failure strains, or the normalized measure of the suture’s deformation at failure, showed no relation to either drug concentration or suture size. Stress-strain curves of electrospun sutures showed these same trends, with a decrease in stress at failure with increasing drug loading (Fig. S1). In most cases, both tensile failure force and strain decreased with incubation in PBS (Figs. 4 & 5), in some cases significantly (e.g. for 5% and 10% loaded 2-0 sutures, p < 0.0001). However, in four of the five drug concentrations investigated to make 6-0 drug-loaded sutures, the decrease in tensile strength was not statistically significant (e.g. p = 0.162 for 0% loading). Beyond 7–10 days, the drug-loaded sutures were degraded to the point where mechanical testing was extremely difficult, as the constructs would disintegrate upon manipulation, and thus could not be tested. Those that could be tested, however, retained approximately 12% of their original tensile strength after 14 days of immersion. Drug-free sutures maintained their physical integrity approximately one week longer than drug-loaded sutures.
Figure 4.
Tensile failure forces of electrospun sutures (A) at day 0 across all sizes and concentrations; before and after 7 days of immersion in PBS for (B) 6-0 sutures (C) 4-0 sutures and (D) 2-0 sutures. Numbers in the keys are the drug loadings in wt% of the various sutures. Data are means ± SD; n = 4; * = p < 0.05.
Figure 5.
Strains of electrospun sutures (A) at day 0 across all sizes and concentrations; before and after 7 days of immersion in PBS for (B) 6-0 sutures (C) 4-0 sutures and (D) 2-0 sutures. Numbers in the keys are the drug loadings in wt% of the various sutures. Data are means ± SD; n = 4; * = p < 0.05.
Thermogravimetric analysis of electrospun sutures of various sizes and drug loadings revealed an average loss of mass of 9.5 ± 5.3% (p < 0.001) that began at around 55 C, which is close to the boiling point of HFIP (58.2 C), suggesting that residual solvent was present in those sutures. Vicryl® sutures displayed no mass loss occurring until approximately 200 C, which is approximately the melting temperature of 90:10 PLGA (Fig. S2).
3.2. In vitro release study
Release of bupivacaine from suture segments into PBS in 24-well plates was measured by HPLC. Total drug release was dependent on both the diameter of the suture and the initial drug loading (Fig. 6). In general, absolute drug release over time increased with thicker sutures and sutures with larger percent loadings, while normalized release over time increased as suture size decreased (Fig. S3). The release profiles of all groups were similar, with a short period (1–2 days) of relatively slow and linear release, a subsequent relatively rapid increase in release rate, and a final plateau. At all drug concentrations and sizes, the rate of drug release was greatest approximately 3 to 7 days after incubation in PBS, and bupivacaine could not be detected in samples collected after 12 days. Comparison of total drug released at the end of the study with the amount of drug initially incorporated in the sutures revealed that the total amount released from the sutures ranged from approximately 80% – 97%, with 22% 2-0 sutures being the closest to achieving 100% total cumulative release.
Figure 6.
(A–D) Release of bupivacaine HCl from electrospun sutures of varying sizes and drug loadings: (A) 5% loading, (B) 10% loading, (C) 15% loading, and (D) 22% loading. (E) Comparison of release normalized to suture mass for all drug loadings from 6-0 sutures. Numbers in the keys are the suture sizes (A–D) or drug loadings in wt% of the various sutures (E). Please note that the y-axes are scaled differently in some plots. Data are means ± SD; n ≥ 8; * = p < 0.05.
Immediately after fabrication, SEM images of both drug-free and drug-loaded sutures showed slight differences in morphology: drug-loaded microfibers appeared to be less aligned than their drug-free counterparts, and there were a greater number of free particles on the surfaces of the drug-loaded fibers. After 14 days of immersion and incubation in PBS, the differences in morphology between drug-free and drug-loaded sutures were more apparent (Fig. 7). The drug-loaded microfibers appeared to have degraded into flattened ribbons and were very porous, while drug-free microfibers still retained their initial cylindrical form, albeit with widespread fiber fragmentation.
Figure 7.
SEM images of drug-free fibers drug-loaded fibers at the start of the release study and after 14 days of immersion in phosphate buffered saline. The insets highlight the small pores that appear in the surface of degraded drug-loaded fibers and the lack of such pores on the surface of degraded drug-free fibers.
3.3. In vivo studies
Drug-free electrospun sutures and sutures loaded with fluorescent dye or bupivacaine were used to close a 1.5 cm incision made in the dorsal flank of rats. The sutures had adequate tensile strength to hold tissue together, such that proper healing of the wound could occur despite animal movement and the exposure of the wound site to grooming (Figs. 8A and B). Furthermore, there was no wound disruption or dehiscence. In vivo degradation timeframes were consistent with those found in vitro: whole sutures were noted 7 days after implantation, while only small fragments remained after 14 days.
Figure 8.
Images of full-thickness incision on the left dorsal flank of a rat (A) immediately after closure with drug-eluting suture and (B) the same incision after 14 days. Images illustrating elution of dye (orange) from dye-loaded sutures in vivo after 7 days: (C) entire incision on dorsal flank in vivo and (D) cross-section of same incision and subjacent dermal tissue ex vivo.
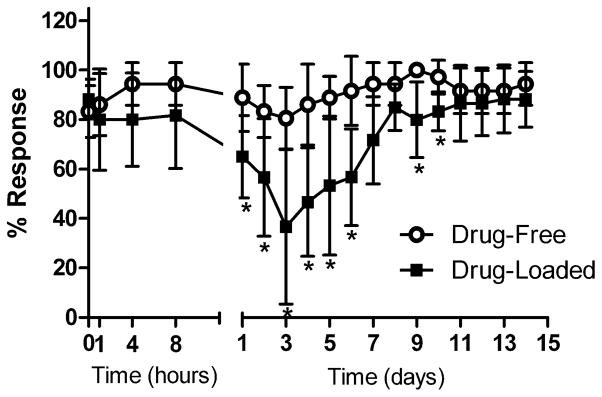
Fluorescent images of dye elution from sutures showed that dye was present along the entire length of the incision and was highly concentrated at both ends of the wound. Cross-sections showed dye penetration through the entire dermal layer in addition to an approximately 2–4 mm radial spread of dye around the incision (Figs. 8C and D). Studies with 22%-loaded 4-0 sutures did not elicit any analgesic effect (Fig. S4), but local analgesia was observed in nine out of ten animals treated with 22%-loaded 2-0 sutures, with onset of analgesia occurring 1 day after surgery (e.g. p = 0.007 on day 3). The greatest degree of analgesia was achieved at approximately 3 days, and was followed by full recovery 7–9 days after initial onset (Fig. 9).
Figure 9.
Pinch responses of animals sutured with drug-free and 22% bupivacaine-loaded size 2-0 electrospun sutures. Data are means ± SD; n ≥ 6; * = p < 0.05.
Histological analysis of epidermal tissue surrounding electrospun sutures with and without drug that had been collected 14 days after implantation revealed signs of a mild inflammatory reaction, with macrophages almost completely surrounding the periphery of the implanted polymer (Figs. 10A and B). Photomicrographs of tissue surrounding a commercial 2-0 Vicryl® control showed an inflammatory reaction that was not significantly different in nature from that produced by the electrospun sutures (Fig. 10C). In all cases, the spread of inflammatory cells did not extend more than 50 μm away from the suture.
Figure 10.

Representative photomicrographs of hematoxylin and eosin stained sections of tissue surrounding (A) drug-free and (B) bupivacaine-loaded electrospun sutures, and (C) Vicryl® sutures. White arrows = inflammatory cells, S = suture fragments. Graphical Abstract
4. Discussion
In this study, we evaluated the potential of electrospun drug-eluting sutures for local anesthesia. We demonstrated that such sutures had acceptable mechanical properties and drug release kinetics, and most importantly, showed that the sutures allowed for tissue penetration of drug and achievement of local therapeutic effect. Further optimization of the release kinetics might reduce or obviate the need for supplemental analgesia in the early healing period, at least as far as pain originating in the immediate vicinity of the sutures is concerned. The sutures degraded in a clinically relevant time frame without compromising wound healing, and the tissue reaction was benign.
The drug release profiles seen in this study were governed by a combination of diffusion and polymer matrix erosion. Initial drug release was diffusion-based and slow, except in small sutures with higher loading where an initial burst release was seen, perhaps due to the presence of drug on or near the surface of the microfibers. Over the next few days, drug release accelerated as the polymer matrix began to degrade through hydrolysis, and the time-frame of this accelerated release correlated with that of the loss of mechanical strength and physical integrity. Release profiles also corresponded well with drug loading as accelerated release occurred earlier as loading increased, indicating a relationship between suture degradation rate and drug content. The porous morphology of the degraded drug-loaded sutures suggests that the pores may have once held pockets of bupivacaine crystals and/or aggregates that have since been released from the matrix into the media. These pores would allow for a greater surface area for water to hydrolytically break down PLGA, thereby causing the observed effect of drug-loaded sutures degrading at a faster rate than their drug-free counterparts. It should be noted, however, that the observed accelerated degradation of our 90:10 PLGA electrospun sutures both in vitro and in vivo is unusual, as previous works have documented that the degradation of this particular polymer blend is on the order of weeks or months, rather than days [23, 24]. The presence of residual solvent, although relatively low, may have contributed to this effect, as its high volatility could lead to the formation of additional pores that would then lead to accelerated drug release. These observations point to the possibility of using trace quantities of plasticizers or other molecules as a method of modulating drug release from sutures.
Despite having some physicochemical similarities to bupivacaine, rhodamine 6G clearly did not behave exactly like bupivacaine in these sutures (Figs. S5 & S6): mechanical tests of sutures with loadings of dye equivalent to that of drug showed that dye sutures on average were approximately 2.0 times stronger than drug-loaded sutures, and in vitro release studies showed that rhodamine 6G eluted at a slower rate than bupivacaine (total encapsulated dye had not been fully released even after 12 days), and with a nearly zero-order release profile. Additionally, dye-loaded sutures did not degrade as quickly as their bupivacaine-loaded counterparts. The dye might provide a better approximation of the behavior of the hydrophobic free base of bupivacaine. Nonetheless, the dye is useful in demonstrating that these sutures, like many controlled release systems, provide high local drug concentration and that this will occur with more than one drug. It also illustrates illustrates the possibility of adjusting drug-eluting suture properties by simply changing the incorporated drug, e.g. to the free base of bupivacaine.”
The amount of drug incorporated in these sutures was comparable to the maximum bolus dose of bupivacaine for rats of the size used in this study (2 mg/kg, or 0.8 mg), but was released over many days; we did not see any signs of significant systemic toxicity [25]. These findings are consistent with observations in numerous reports that controlled release systems can create high local tissue local anesthetic concentrations with minimal systemic distribution. This has been demonstrated clearly in the case of polymeric microspheres containing 75% (w/w) bupivacaine at a dosage sufficient to cause serious toxicity in rats, but pharmacokinetic studies showed that blood levels were low [26]. Local analgesia was achieved with these sutures, and the neurobehavioral data correlated well with in vitro release kinetics and confirmed the drug-eluting capabilities of our sutures. Maximum analgesia was achieved on day 3, which was also the day on which the rate of drug elution was greatest for 22% loaded 2-0 sutures, and recovery occurred at approximately the same time that drug release was shown to have reached a plateau. These data also correlate well with the clinical reality that the greatest need for postoperative analgesia is for 7–10 days after surgery. Having the option of providing directed pain relief at the operative site without the need for catheters or other regional pain techniques that require catheters or external mechanical devices (pumps, etc.) is a significant advantage. Local anesthetics injected directly into the wound last only as long as the drug is present (a few hours at most), whereas with the sustained release of this suture, the drug is released continuously over the several days when it is most needed. This benefit would likely decrease or even remove altogether the need for adjuvant medications (opioids, nonsteroidal anti-inflammatory drugs, etc.) that can interfere with the normal wound healing process, cognitive function, and the patient’s recovery.
As with any other implanted medical device that is composed of a synthetic material, safety considerations are paramount [27]. The mild inflammatory response seen in the tissue surrounding both drug-free and drug-loaded electrospun sutures was reassuring, particularly as it was comparable to the response seen with Vicryl® and other common suture materials such as polypropylene and silk [28], as well as electrospun PLLA sutures [29]. Although our sutures contained residual HFIP, our data showed no evidence of resulting toxicity in vitro or in vivo. Furthermore, the concentrations of HFIP that would be achieved if all the residual solvent came out at once was far below concentrations known to be toxic to cells in vitro [30]. Others who have used this solvent for electrospun scaffolds showed no ill effects on seeded cells [31] or in vivo [32] despite the lack of post-fabrication solvent removal. Nonetheless, it is difficult to predict local concentrations of residual solvent and their effect in the body; as it is not desirable it will have to be removed or replaced for eventual use in humans. These proof-of-principle prototype sutures may still need optimization, particularly with respect to mechanical properties, even though our electrospun sutures were able to perform their primary task of closing a wound and allowing it to heal properly in an in vivo model without any additional reinforcements. Although they were strong enough to allow for wounds to heal without any observed complications such as dehiscence, they were not as robust as commercially available sutures with the same polymer composition (Vicryl®). For example, we found that 2-0 Vicryl® sutures had an average tensile failure force of 29.77 ± 5.40 N, while the average failure force of 2-0 drug-free electrospun sutures was only 2.39 ± 0.30 N. After 7 days, Vicryl® still retained 96% of its initial strength, while drug-free electrospun sutures only retained 57% of their initial strength. The observed difference between the tensile properties of Vicryl® and our sutures may be due to the fact that Vicryl® has a braided structure composed of 6–8 yarns of melt-extruded PLGA fibers. Work is ongoing to enhance the strength of our sutures by braiding, as well as by using other fabrication modifications to maximize suture drug loading without adversely affecting tensile strength. Since HFIP functions as a plasticizer, residual HFIP could also explain in part the relatively weak tensile strength of these sutures [33]. Further optimization is required in removing residual solvent, as the presence of such residues could have an adverse impact on the mechanical properties, degradation characteristics, and safety of electrospun sutures.
In principle, the approach applied here could easily be applied to the delivery of other anesthetics. The concepts demonstrated here are not limited to electrospun sutures, but could apply to the spectrum of suture/filament production methods and should be amenable to modification by a broad range of ways to control drug release. One can envision sutures that release more than one drug, particularly drug combinations that can have marked synergistic effects on the duration of local anesthesia, such as site 1 sodium channel blockers [34, 35], glucocorticoids [36, 37] or both [38, 39]. Such combinations would allow the limited payload of sutures to be much more effective.
5. Conclusions
The results of this study demonstrate the ability to create drug-eluting electrospun PLGA sutures providing sustained release of bupivacaine in vitro and prolonged analgesia at the site of incision in vivo. Tissue reaction was mild, similar to that seen with commercially available and FDA-approved PLGA sutures. Suture mechanical properties were adequate to ensure proper wound healing. The simplicity of the electrospinning process and the ability to fabricate sutures from almost any polymer-drug combination will facilitate the continued development of sutures that enhance perioperative analgesia, thereby mitigating the need for standard postoperative opioid analgesics.
Supplementary Material
Acknowledgments
Funding & Support:
Support for this work was provided in all or part by the CHMC Surgical Foundation (CBW, DSK), the Tisch Family Faculty Development Fund (CBW) and the W. Hardy Hendren Faculty Development Fund (CBW), and NIH R01 GM073626 (DSK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukherjee DP. Sutures. In: Kroschwitz JI, editor. Polymers: Biomaterials and Medical Applications. John Wiley & Sons; New York: 1989. pp. 535–545. [Google Scholar]
- 2.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. 1994 Whitaker Lecture: polymers for drug delivery and tissue engineering. Ann Biomed Eng. 1995;23:101–111. doi: 10.1007/BF02368317. [DOI] [PubMed] [Google Scholar]
- 4.Ming X, Nichols M, Rothenburger S. In vivo antibacterial efficacy of MONOCRYL Plus antibacterial suture (Poliglecaprone 25 with triclosan) Surg Infect (Larchmt) 2007;8:209–214. doi: 10.1089/sur.2006.004. [DOI] [PubMed] [Google Scholar]
- 5.Ming X, Rothenburger S, Nichols MM. In vivo and in vitro antibacterial efficacy of PDS Plus (polidioxanone with triclosan) suture. Surg Infect (Larchmt) 2008;9:451–457. doi: 10.1089/sur.2007.061. [DOI] [PubMed] [Google Scholar]
- 6.Ming X, Rothenburger S, Yang D. In vitro antibacterial efficacy of MONOCRYL plus antibacterial suture (Poliglecaprone 25 with triclosan) Surg Infect (Larchmt) 2007;8:201–208. doi: 10.1089/sur.2006.005. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak B, Rehn M, Andersen L, Agren MS, Heegaard AM, Tengvall P, Aspenberg P. Doxycycline-coated sutures improve mechanical strength of intestinal anastomoses. Int J Colorectal Dis. 2008;23:271–276. doi: 10.1007/s00384-007-0401-0. [DOI] [PubMed] [Google Scholar]
- 8.Harnet JC, Le Guen E, Ball V, Tenenbaum H, Ogier J, Haikel Y, Vodouhe C. Antibacterial protection of suture material by chlorhexidine-functionalized polyelectrolyte multilayer films. J Mater Sci Mater Med. 2009;20:185–193. doi: 10.1007/s10856-008-3559-2. [DOI] [PubMed] [Google Scholar]
- 9.Matl FD, Zlotnyk J, Obermeier A, Friess W, Vogt S, Buchner H, Schnabelrauch H, Stemberger A, Kuhn KD. New anti-infective coatings of surgical sutures based on a combination of antiseptics and fatty acids. J Biomater Sci Polym Ed. 2009;20:1439–1449. doi: 10.1163/092050609X12457418973107. [DOI] [PubMed] [Google Scholar]
- 10.Morizumi S, Suematsu Y, Gon S, Shimizu T. Inhibition of neointimal hyperplasia with a novel tacrolimus-eluting suture. J Am Coll Cardiol. 2011;58:441–442. doi: 10.1016/j.jacc.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 11.He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009;89:80–95. doi: 10.1002/jbm.a.32004. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Huang ZM, Liu XY. Development of braided drug-loaded nanofiber sutures. Nanotechnology. 2010;21:315104. doi: 10.1088/0957-4484/21/31/315104. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16:1151–1170. [Google Scholar]
- 14.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Wang CH. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm Res. 2006;23:1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 16.Chew SY, Wen J, Yim EK, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 17.Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release. 2007;120:111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Theron A, Zussman E, Yarin AL. Electrostatic field-assisted alignment of electrospun nanofibers. Nanotechnology. 2001;12:384–390. [Google Scholar]
- 19.Khurana TK, Santiago JG. Effects of carbon dioxide on peak mode isotachophoresis: simultaneous preconcentration and separation. Lab Chip. 2009;9:1377–1384. doi: 10.1039/b815460k. [DOI] [PubMed] [Google Scholar]
- 20.Steele TWJ, Huang CL, Kumar S, Widjaja E, Boey FYC, Loo JSC, Venkatraman SS. High-throughput screening of PLGA thin films utilizing hydrophobic fluorescent dyes for hydrophobic drug compounds. J Pharm Sci. 2011;100:4317–4329. doi: 10.1002/jps.22625. [DOI] [PubMed] [Google Scholar]
- 21.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–1025. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Leem JW, Park ES, Paik KS. Electrophysiological evidence for the antinociceptive effect of transcutaneous electrical stimulation on mechanically evoked responsiveness of dorsal horn neurons in neuropathic rats. Neurosci Lett. 1995;192:197–200. doi: 10.1016/0304-3940(95)11644-c. [DOI] [PubMed] [Google Scholar]
- 23.Cai Q, Bei J, Wang S. Relationship among drug delivery behavior, degradation behavior and morphology of copolylactones derived from glycolide, L-lactide and ε-caprolactone. Polym Adv Technol. 2002;13:105–111. [Google Scholar]
- 24.Chu CC. A comparison of the effect of pH on the biodegradation of two synthetic absorbable sutures. Ann Surg. 1982;195:55–59. doi: 10.1097/00000658-198201001-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohane DS, Sankar WN, Shubina M, Hu D, Rifai N, Berde CB. Sciatic nerve blockade in infant, adolescent, and adult rats: a comparison of ropivacaine with bupivacaine. Anesthesiology. 1998;89:1199–1208. doi: 10.1097/00000542-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Dräger C, Benziger D, Gao F, Berde CB. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–979. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Kohane DS, Langer R. Biocompatibility and drug delivery systems. Chem Sci. 2010;1:441–446. [Google Scholar]
- 28.Yaltirik M, Dedegolu K, Bilgic B, Koray M, Ersev H, Issever H, Dulger O, Soley S. Comparison of four different suture materials in soft tissues of rats. Oral Diseases. 2003;9:284–286. doi: 10.1034/j.1601-0825.2003.00954.x. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Huang ZM. Biocompatibility of braided poly(L-lactic acid) nanofiber wires applied as tissue sutures. Polym Int. 2010;59:92–99. [Google Scholar]
- 30.Nam J, Huang Y, Agarwal S, Lannutti J. Materials selection and residual solvent retention in biodegradable electrospun fibers. J Appl Polym Sci. 2008;107:1547–1554. [Google Scholar]
- 31.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen fibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 32.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 33.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 34.Adams HJ, Blair MR, Jr, Takman BH. The local anesthetic activity of tetrodotoxin alone and in combination with vasoconstrictors and local anesthetics. Anesth Analg. 1976;55:568–573. [PubMed] [Google Scholar]
- 35.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–131. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, Wilder R, Langer R, Berde C. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–1166. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Colombo G, Padera R, Langer R, Kohane DS. Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A. 2005;75:458–464. doi: 10.1002/jbm.a.30443. [DOI] [PubMed] [Google Scholar]
- 38.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NG, Berde CB, Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104:415–421. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 39.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci USA. 2009;106:7125–7130. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.