Abstract
Oxidative stress contributes significantly to brain aging. Animals lacking glutamate transporter type 3 (EAAT3) have a decreased level of glutathione, the major intracellular anti-oxidant, in neurons, and present with early onset of brain aging including brain atrophy and cognitive impairment at 11 months of age. Here, 12-month old male EAAT3 knockout mice received intraperitoneal injection of N-acetylcysteine (NAC) at 150 mg/kg once every day for 4 weeks. NAC is a membrane permeable cysteine precursor that can work as a substrate for glutathione synthesis. EAAT3 knockout mice that received saline injection or did not receive any injection were also included in the study. EAAT3 knockout mice had significantly less freezing behavior than age- and gender-matched wild-type mice in context- and tone-related fear conditioning tests. The knockout mice also had decreased levels of glutathione and increased levels of 4-hydroxy-2-nonenal and proteins containing nitrotyrosine, indicators of oxidative stress, in the cerebral cortex and hippocampus. NAC but not saline injection attenuated these behavioral and biochemical changes in the EAAT3 knockout mice. These results suggest that improvement of anti-oxidative capacity in neurons reverses the existing cognitive impairment in aging brains, implying a potential role of glutathione replacement in cognitive improvement of aging population.
Keywords: cognitive functions, glutamate transporter 3, glutathione, mice, oxidative stress
Introduction
Glutamate transporters, also named excitatory amino acid transporters (EAAT), are plasma membrane proteins that uptake glutamate, the major excitatory neurotransmitter, from extracellular space to intracellular compartments under physiological conditions (Danbolt, 2001). There are five EAATs identified so far (Danbolt, 2001). EAAT3 is the major neuronal EAAT and is distributed widely in the central nervous system, such as cerebrum and hippocampus (Rothstein et al., 1994). In addition to taking up glutamate, EAAT3 also transports cysteine(Chen and Swanson, 2003, Lee et al., 2009), a rate-limiting substrate for the synthesis of glutathione (Dringen et al., 1999). Glutathione is the major intracellular anti-oxidant. It has been shown that uptake of cysteine via EAAT3 is a significant method to provide substrate for glutathione synthesis in neurons (Aoyama et al., 2006). EAAT3 knockout mice have decreased neuronal glutathione concentrations, age-dependent neurodegeneration and cognitive impairment. These animals have obvious aging-like brain changes by 11 months of age (Aoyama et al., 2006). This presentation is consistent with the theory that oxidative stress contributes significantly to brain aging (Floyd and Hensley, 2002, Perluigi et al., 2010).
Decrease of cognitive functions is an important component of brain aging. It has been shown that administration of anti-oxidants slows down aging-related cognitive impairment (Milgram et al., 2005). However, there is very limited information on whether aging-related cognitive impairment is reversible. We hypothesize that improved anti-oxidative capacity in neurons reverses existing cognitive impairment of aging animals. To test this hypothesis, we administrated N-acetylcysteine (NAC), a membrane permeable cysteine precursor, to 12-month old EAAT3 knockout mice. Their cognitive functions and oxidative stress indicators in the brain tissues were evaluated.
Materials and methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23) revised in 1996.
Animals
Twelve-month-old male CD-1 wild-type mice and EAAT3 knockout mice were used in this study. The EAAT3 knockout mice were descendants of mice used in previous studies (Peghini et al., 1997, Li and Zuo, 2011). These mice have a CD-1 mouse gene background. EAAT3 proteins are not expressed in them. These mice also do not have a compensatory increase of EAAT1 or EAAT2 (Peghini et al., 1997, Lee et al., 2010, Li and Zuo, 2011), the other major types of EAATs in the brain. These EAAT3 knockout mice have been backcrossed with wild-type CD-1 mice at least once every 8 generations to prevent genetic drift as recommended from the Banbury Conference (Silva et al., 1997). The wild-type mice produced during the process are used in the study.
N-acetylcysteine administration
NAC (Sigma, St Louis, MO) was dissolved in 0.9% saline and administrated intraperitoneally at 150 mg/kg once every day for 4 consecutive weeks to the EAAT3 knockout mice. Two additional groups of EAAT3 knockout mice received either no injection or daily injection of 0.9% saline. The wild-type mice did not receive any injection.
Fear conditioning
Twenty-seven days after the start of NAC or saline injection, mice were subjected to fear conditioning. Fear conditioning is a sensitive and non-effort-dependent test of learning and memory. As we described previously (Lin et al., 2011), each animal was placed in a test chamber (SD Instruments, San Diego, CA) wiped with 70% alcohol and subjected to 3 tone-foot shock pairings (tone: 2000 Hz, 85 db, 30 s; foot shock: 1 mA, 2 s) with an inter-pairing interval 1 min in a relatively bright room. The shocks were delivered to the animal through the stainless steel bars and the tone was delivered by a speaker installed in the cover of the test chamber. The animal was removed from this test chamber 1 min after the completion of the conditioning training. The animal was placed back to the same chamber 24 h later for 5 min in the absence of tone and shock and was video-taped. Freezing behavioral events were counted in a 6-s interval. A percentage of freezing events for each animal was calculated by using the following formula: 100 × n/t where n is the number of freezing events for the animal and t is the total possible events. Two hours later, the animal was put in a test chamber that had different context, shape and smell from the first test chamber (this second chamber was wiped with 1% acetic acid) in a relatively dark room. After adaptation for 2 min in this new chamber, the auditory stimulus was turned on for 3 cycles, each cycle for 30 s followed by a 1-min inter-cycle interval (4.5 min in total). The freezing behavioral events within the 4.5 min were counted in a 6-s interval. These tests detect hippocampus-dependent (context-related) and hippocampus-independent (tone-related) learning and memory functions (Kim and Fanselow, 1992).
Measurement of glutathione, glutathione disulfide (GSSG), nitrotyrosine-containing proteins and 4-hydroxy-2-nonenal (HNE)
The next day after fear conditioning test, mice were deeply anesthetized with isoflurane and transcardially perfused with 0.9% saline. Brains were removed. Hippocampi and cerebral cortices were dissected out immediately on ice. The brain tissues were homogenized in ice-cold 0.1 M potassium phosphate buffer (pH 6.8) containing 1 mM EDTA. Homogenates were centrifuged at 13,000 g for 20 min at 4°C. Protein concentration of the supernatant was measured by Bradford protein assay. Glutathione Assay kit (Cayman Chemical, Ann Arbor, MI) was used to measure the levels of glutathione and GSSG in the samples according to the manufacture’s protocols. OxiSelect Nitrotyrosine enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs Inc., San Diego, CA) and the OxiSelect HNE-His Adduct ELISA kit (Cell Biolabs Inc.) were used to assess the contents of proteins containing nitrotyrosine and HNE, respectively, in the samples according to the manufacturer’ instructions. All assays were performed using samples obtained from CD-1 wild-type mice and EAAT3 knockout mice treated in parallel.
Statistical analysis
Results are presented as means ± SEM. (n≥4). They were analyzed by one way analysis of variance followed by the Student-Newman-Keuls test after confirmation of normal distribution of the data or by t-test as appropriate. A P < 0.05 was accepted as significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA).
Results
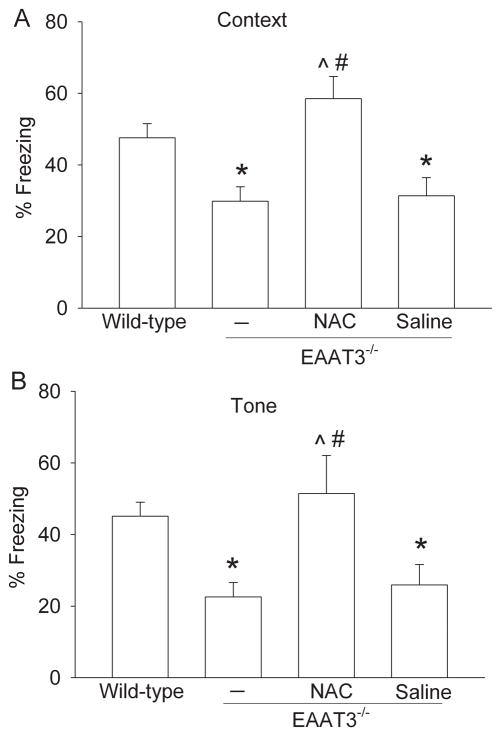
A previous study showed that 11-month old EAAT3 knockout mice performed poorly in Morris Water maze (Aoyama et al., 2006). Here, the 12-month old EAAT3 knockout mice had significantly less freezing behavior in both context- and tone-related fear conditioning tests than wild-type mice. This reduced freezing behavior was abolished by NAC injection but was not affected by saline injection (Fig. 1). These results suggest that EAAT3 knockout mice have hippocampus-dependent and hippocampus-independent cognitive impairment. NAC reverses this impairment.
Fig. 1. Performance in the fear conditioning test.
Twelve-month-old male EAAT3 knockout mice received N-acetylcysteine (NAC) or saline injection for 4 weeks. Age-matched male wild-type and EAAT3 knockout mice that did not receive any injection were also included in the study. Results are mean ± SEM. (n = 12 - 13). * P < 0.05 compared with the wild-type mice. ^ P < 0.05 compared with EAAT3 knockout mice that did not receive any injection. # P < 0.05 compared with EAAT3 knockout mice that received saline injection.
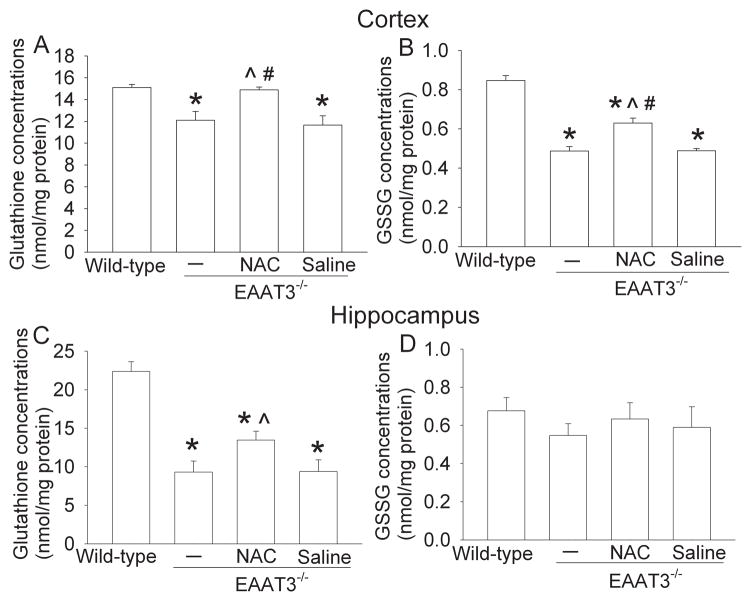
The EAAT3 knockout mice had significant less glutathione in their cerebral cortex and hippocampus than the wild-type mice. NAC, but not saline, attenuated this reduction. EAAT3 knockout mice also had reduced GSSG in the cerebral cortex. This reduction was attenuated by NAC but was not affected by saline (Fig. 2).
Fig. 2. Levels of glutathione and glutathione disulfide (GSSG) in the cerebral cortex and hippocampus.
Twelve-month-old male EAAT3 knockout mice received N-acetylcysteine (NAC) or saline injection for 4 weeks. Age-matched male wild-type and EAAT3 knockout mice that did not receive any injection were also included in the study. Panels A and B present data from the cerebral cortex. Panels C and D are data from the hippocampus. Results are mean ± SEM. (n = 10 – 13). * P < 0.05 compared with the wild-type mice. ^ P < 0.05 compared with EAAT3 knockout mice that did not receive any injection. # P < 0.05 compared with EAAT3 knockout mice that received saline injection.
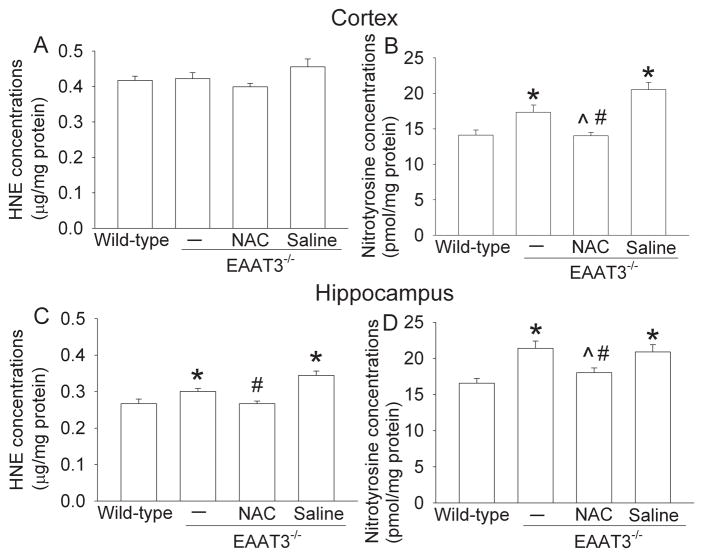
There was a significant increase of proteins containing nitrotyrosine in the cerebral cortex and hippocampus of EAAT3 knockout mice. NAC, but not saline, abolished this increase. EAAT3 knockout mice also had an increase of HNE in their hippocampi. NAC injection, but not saline application, inhibited this increase (Fig. 3). These results suggest that EAAT3 knockout mice have increased oxidative stress in their brain tissues.
Fig. 3. Expression of proteins containing nitrotyrosine and 4-hydroxy-2-nonenal (HNE).
Twelve-month-old male EAAT3 knockout mice received N-acetylcysteine (NAC) or saline injection for 4 weeks. Age-matched male wild-type and EAAT3 knockout mice that did not receive any injection were also included in the study. Panels A and B present data from the cerebral cortex. Panels C and D are data from the hippocampus. Results are mean ± SEM. (n = 12 – 13). * P < 0.05 compared with the wild-type mice. ^ P < 0.05 compared with EAAT3 knockout mice that did not receive any injection. # P < 0.05 compared with EAAT3 knockout mice that received saline injection.
Discussion
Synthesis of glutathione requires cysteine (Dringen et al., 1999, Dringen, 2000). Most brain cells acquire cysteine via hetero-exchange of intracellular glutamate with extracellular cystine that is then converted into cysteine inside the cells (Dringen et al., 1999, Dringen, 2000, Wu et al., 2004). Mature neurons lack the proteins that perform the hetero-exchange (Sato et al., 2002). It has been shown that cysteine transport via EAAT3 is a major mechanism to supply neurons with cysteine (Chen and Swanson, 2003, Aoyama et al., 2006). Consistent with this role, EAAT3 has a higher affinity for cysteine than other EAATs (Zerangue and Kavanaugh, 1996, Lee et al., 2009). EAAT3 is also mainly expressed in the neurons (Rothstein et al., 1994, Danbolt, 2001). EAAT3 knockout mice have a decreased neuronal glutathione level (Aoyama et al., 2006) . In line with these previous findings, our results showed that EAAT3 knockout mice had decreased glutathione levels in the cerebral cortex and hippocampus. NAC, a membrane permeable cysteine precursor, attenuated this decrease, suggesting that the decreased glutathione level is due to intraneuronal deficit of cysteine. Similar pattern of changes also occurred to GSSG, the oxidized form of glutathione, suggesting the overall reduction of glutathione storage.
Associated with the reduced glutathione levels in the EAAT3 knockout mice, our study showed a significant increase of HNE and proteins containing nitrotyrosine in the brain tissues of these mice. These results are similar to our and others’ previous findings (Aoyama et al., 2006, Li and Zuo, 2011). Since HNE and nitrotyrosine are oxidative stress indicators for lipids and proteins, respectively, these results suggest that the EAAT3 knockout mice have an increased oxidative stress in the brain tissues. Our study also showed that NAC reduced this increased oxidative stress, suggesting that the lack of intracellular cysteine and the subsequent reduction of glutathione in the neurons are the cause of this increased oxidative stress in the brain tissues of EAAT3 knockout mice.
Oxidative stress has been considered as a major mechanism for aging process (Floyd and Hensley, 2002, Perluigi et al., 2010). There are at least two lines of evidence to indicate the role of oxidative stress in brain aging: aging brain has increased markers for oxidative stress and antioxidants attenuate the cognitive impairment associated with aging (Floyd and Hensley, 2002, Head et al., 2008). Our results showed that increase of neuronal anti-oxidative ability improved the cognitive functions of EAAT3 knockout mice to the levels of age-matched wild-type mice. These results suggest the potential of reversing existing cognitive impairment in aging animals by anti-oxidants, adding evidence for the role of oxidative stress in aging-related brain functional changes.
EAAT3 is expressed widely in brain tissues (Danbolt 2001, Rothstein et al. 1994). Consistent with its distribution pattern, our results showed that the EAAT3 knockout mice had disturbed redox status in both cerebral cortex and hippocampus as well as impaired hippocampus-dependent and hippocampus-independent learning and memory.
We observed that EAAT3 knockout mice had a significant increase of proteins containing nitrotyrosine in the hippocampus and cerebral cortex and an increase of HNE in the hippocampus. However, our results did not show an increased HNE level in the cerebral cortex of these animals. The reasons for this no detectable change in the cerebral cortex are not known. A dilution effect from other brain cells, such as glial cells, may have contributed to it. This dilution effect may have greatly underestimated the biochemical changes occurred in the neurons.
The learning and memory impairment of the EAAT3 knockout mice can be due to impaired glutamate uptake. Glutamate neurotransmission has been known to be involved in learning and memory (McEntee and Crook, 1993). However, NAC reversed the cognitive impairment in the EAAT3 knockout mice, suggesting that reduced glutamate uptake in these mice may not be a major mechanism for their learning and memory impairment.
In summary, we have shown that 12-mouth old male EAAT3 knockout mice have significant cognitive impairment, reduced glutathione and increased oxidative stress in the brain. These aging-like brain changes are attenuated by NAC, suggesting the potential of reversing existing cognitive impairment of aging brains by increasing neuronal glutathione levels.
Research highlights.
EAAT3 knockout mice have hippocampus-dependent and independent cognitive impairment
EAAT3 knockout decreases glutathione levels and increases oxidative stress in many brain regions
N-acetylcysteine reverses existing cognitive and biochemical impairment in EAAT3 knockout mice
Acknowledgments
Funding: This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, Virginia.
Abbreviations
- EAAT
excitatory amino acid transporter or glutamate transporter
- ELISA
enzyme-linked immunosorbent assay
- GSSG
glutathione disulfide
- HNE
4-hydroxy-2-nonenal
- NAC
N-acetylcysteine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cystein uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Head E, Rofina J, Zicker S. Oxidative stress, aging, and central nervous system disease in the canine model of human brain aging. Vet Clin North Am Small Animal Practice. 2008;38:167–178. doi: 10.1016/j.cvsm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg. 2009;109:1506–1510. doi: 10.1213/ANE.0b013e3181b6709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neuroscience. 2010;171:788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Feng C, Cao M, Zuo Z. Volatile anesthetics may not induce significant toxicity to human neuron-like cells. Anesth Analg. 2011;112:1194–1198. doi: 10.1213/ANE.0b013e3181fdf69d. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 1993;111:391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Giorgi A, Schinina ME, Coccia R, Cini C, Bellia F, Cambria MT, Cornelius C, Butterfield DA, Calabrese V. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res. 2010;88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Simpson EM, Takahashi JS, Lipp H-P, Nakanishi S, Wehner JM, Giese KP, Tully T, Abel T, Chapman PF, Fox K, Grant S, Itohara S, Lathe R, Mayford M, McNamara JO, Morris RJ, Picciotto M, Roder J, Shin HP, Slesinger PA, Storm DR, Stryker MP, Tonegawa S, Wang Y, Wolfer DP. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]