Abstract
Spinal cord injury (SCI) causes profound bone loss due to muscle paralysis resulting in the inability to walk. Sclerostin, a Wnt signaling pathway antagonist produced by osteocytes, is a potent inhibitor of bone formation. Short-term studies in rodent models have demonstrated increased sclerostin in response to mechanical unloading that is reversed with reloading. Although sclerostin inhibition has been proposed as a potential therapy for bone loss, it is not known if sclerostin levels vary with duration of SCI in humans. We analyzed circulating sclerostin in 155 men with varying degrees of SCI who were 1 year or more post-injury. We report that sclerostin levels are greatest in subjects with short-term SCI (≤ 5 years post-injury) and decrease significantly over the first 5 years post-injury. There was no association between sclerostin and injury duration in subjects with long-term SCI (> 5 years post-injury). In subjects with long-term SCI, sclerostin levels were positively associated with lower extremity bone density and bone mineral content. These data suggest that sclerostin levels in SCI are initially increased after SCI in response to mechanical unloading. This response is time-limited and as bone loss progresses, circulating sclerostin is lowest in subjects with severe osteoporosis. These findings support a dual role for sclerostin after SCI: a therapeutic target in acute SCI, and a biomarker of osteoporosis severity in chronic SCI.
Keywords: Osteoporosis, Sclerostin, Spinal Cord Injury, Rehabilitation Medicine
1. INTRODUCTION
Osteocytes, the cells responsible for mechano-transduction in bone, represent the first cellular response to mechanical unloading [1]. Sclerostin is a secreted Wnt signaling antagonist produced primarily by osteocytes. Sclerostin selectively inhibits Wnt/β-catenin, suppressing the activity of osteoblasts as well as the viability of osteoblasts and osteocytes [2]. Mechanical unloading causes up-regulation of sclerostin leading to reduced Wnt/β-catenin signaling and suppression of bone formation [3]. In humans, mechanical unloading of bone occurs in diseases that cause muscle paralysis, including spinal cord injury (SCI). SCI results in profound bone loss that occurs in 2 phases: 1) rapid, acute bone loss that plateaus between 12 and 36 months post-injury and 2) chronic, ongoing bone loss that is more gradual in nature [4, 5]. Based on reports in the rodent literature [3, 6], it is thought that rapid bone loss in the acute phase of SCI is due to increased osteocyte production of sclerostin in response to the abrupt loss of mechanical loading.
Given that sclerostin is a negative regulator of bone formation, clinical trials are underway to test the efficacy of anti-sclerostin antibodies to treat post-menopausal osteoporosis [7]. This emerging anabolic medication may be a powerful, mechanism-based therapy to prevent or treat SCI-induced osteoporosis. However, prior to applying this knowledge to the SCI population, it is imperative to confirm in human studies the role of sclerostin in SCI-induced osteoporosis. We recently assessed the relationship between circulating sclerostin and bone density in 39 subjects with chronic SCI (more than 2 years post-injury) and 10 subjects with no SCI. We found that sclerostin levels were reduced, not elevated, in chronic SCI [8]. These findings are in contrast to the acute sclerostin-mediated bone loss demonstrated in animal models of mechanical unloading where high sclerostin levels suppress bone formation. Our findings suggest that reduced sclerostin levels may be reflective of the severity of bone loss that occurs in chronic SCI. We hypothesize that, similar to the findings observed in animal models, unloading following acute SCI leads to elevated sclerostin levels, which inhibit bone formation by suppressing osteoblastic differentiation and/or function [2, 9]. If inhibition of bone formation proceeds without reintroduction of mechanical loading, extreme bone loss occurs. Severe osteoporosis follows, with fewer bone cells producing sclerostin, which ultimately results in lower than normal sclerostin levels.
Our novel finding of decreased sclerostin levels in chronic SCI in subjects with extremely low bone density strongly suggests that the therapeutic window for targeting sclerostin may be limited in disuse osteoporosis. Elevations in sclerostin have never been demonstrated immediately following SCI in human studies. The optimal time frame for targeting sclerostin in disuse osteoporosis, either with medication or with physical therapy, is currently unknown. To that end, the aim of the current study was to better define the kinetics of the sclerostin response to mechanical unloading; determine the association between sclerostin and bone after SCI; and determine associations with sclerostin and other clinical factors.
2. METHODS
2.1 Subjects
Subjects were recruited from veterans who receive care at our VA facility, by advertisement in SCI consumer magazines, and by direct mail to 1) persons who previously received medical care at our acute rehabilitation facility, 2) New England subscribers of New Mobility Magazine, and 3) members of the National Spinal Cord Injury Association. Subjects were eligible if they were 22 years of age or older, 1 year or more after injury, and had no other neuromuscular conditions (i.e., multiple sclerosis, previous stroke, past polio). 196 subjects with SCI were enrolled between August 2009 and January 2011. Because only 29 women enrolled and sclerostin levels vary by gender [10], only male participants were included in this study. Similarly, 12 men taking bisphosphonates were excluded because the effect of bisphosphonate use on circulating sclerostin is unknown. The final cohort consisted of 155 men with SCI.
2.2 Motor Score
Motor level and completeness of injury were confirmed by physical exam by a trained rater. Injury completeness was reported according to the American Spinal Injury Association Impairment Scale (AIS) as previously described [11]. Participants were classified as AIS A or B (motor complete, no motor function below the neurological level of injury); AIS C (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level are not strong enough to overcome gravity); or AIS D (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level strong enough to overcome gravity). Injury severity was then classified in 2 categories: motor complete SCI (AIS A/B) or motor incomplete SCI (AIS C or D).
2.3 Assessment of Bone Mineral Density by Dual X-ray Absorptiometry (DXA) Scanning
Bone mineral density (BMD) was determined by Dual X-ray Absorptiometry (DXA) scan using a 5th generation GE iDXA densitometer. Total body scans were performed to determine leg bone mineral content (BMC). Fractures are most common at the knee after SCI. Therefore, scans were also performed at both SCI-specific (distal femur, proximal tibia) and standard skeletal sites (hip, radius) as previously described (Morse et al. 2009; Morse et al. 2011). Unless there was a previous fracture or instrumentation, the non-dominant lower extremity was scanned. For the distal femur, the proximal edge of the region of interest (ROI) was set at 20% of the femur length (measured from the lateral femoral condyle), and the distal edge was set at the visible intersection between the patella and the femur, excluding the patella from the ROI. For the proximal tibia, the proximal edge was set at the most proximal point of contact between the tibia and fibular head sites, avoiding regions of overlap between the fibula and the tibia. Scans were performed in triplicate at the distal femur and proximal tibia. Customized research software supplied by General Electric was used to determine knee BMD. For subjects age 50 or older, T-score was used to classify hip bone density according to the World Health Organization (WHO) definitions of normal (T-score ≥−1), osteopenia (T-score <−1 and >−2.5) and osteoporosis (T-score ≤−2.5). For subjects under the age of 50, Z-score was used to classify hip bone density as normal (Z-score >−2) or as lower than expected for age and sex (Z-score ≤−2). As a standard procedure, a quality assurance block supplied by the manufacturer was measured at least every 2 days to confirm accuracy of the densitometer.
2.4 Biochemical Analyses
Plasma samples were drawn into an EDTA tube and immediately delivered to the core blood research laboratory at our facility. The samples were centrifuged for 15 min at 2600 rpm (1459 × g) at 4°C and stored at −80°C until batch analysis. All biochemical analyses were performed at the Clinical & Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children’s Hospital in Boston, a state-of-the-art reference laboratory that specializes in micro-analysis. Sclerostin was quantified by ELISA assay (Alpco Diagnostics, Salem, NH) with a detection limit of 8.9 pmol/L. Assays were performed in duplicate and any duplicate with >10% CV was repeated. The inter-assay variation is 5.6% at 105.0 pmol/L and 6.7% at 52.1 pmol/L. 25 OH Vitamin D (25OHD) was quantified by enzyme immunoassay (Immunodiagnostic Systems Inc, Fountain Hills, AZ) with a detection limit of 2.0 ng/ml. Osteocalcin was measured as an indicator of bone formation by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) with a detection limit of 0.50 ng/mL. C-telopeptide was measured as an indicator of bone resorption by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) with a detection limit of 0.07 ng/mL.
2.5 Variable Definition
Information regarding SCI, medical history, medication use, smoking history, and fracture history were obtained by questionnaire at the time of DXA scan. Participants were weighed and supine length measured for the calculation of body mass index (BMI). In subjects with severe joint contractures, length was self-reported (n=14). Usual mobility mode (more than 50% of the time) was considered in the following 2 categories: wheelchair use (motorized wheelchair or hand-propelled wheelchair) or no wheelchair use (walk with aid (crutch, cane, or similar aid) or walk without assistance). Smokers were defined as smoking 20 or more packs of cigarettes or using 12 ounces of tobacco or more in a lifetime, or smoking 1 or more cigarettes per day for at least 1 year. Current smokers reported cigarette use within 1 month of testing. Vitamin D levels were considered as a continuous variable and dichotomously (normal ≥ 20ng/ml and deficient < 20 ng/ml). For fracture history, information was collected on timing (before SCI, at time of SCI, or after SCI), location, and cause of fracture. All fractures that occurred after SCI were considered in the analysis.
2.6 Statistical Analysis
All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). Since the distribution of sclerostin was skewed, natural log-transformation was used to normalize the distribution of the outcome and stabilize the variance. General linear models (PROC GLM) were applied to assess associations between selected covariates and sclerostin. Tukey correction was used to adjust for multiple comparisons when appropriate. Independent samples t-test and chi-square tests were applied to test for differences in subject characteristics based on injury duration.
3. RESULTS
3.1 Subject Characteristics
Subject characteristics are presented in Table 1 based on injury duration in the following 2 groups: short-term SCI (≤5 years post-injury) and long-term SCI (>5 years post-injury). All participants were male and the majority was white. Ages ranged from 25.3–87.6 years, with a mean of 55.7±14.0 years. Injury duration ranged from 1.6–60.8 years, with a mean of 19.4±13.1 years. 96 subjects used a wheelchair and 59 walked independently or with an assistive device. Bone density could not be determined in 4 subjects at the distal femur, 5 subjects at the proximal tibia, 8 subjects at the hip, and 2 subjects at the radius due to knee/hip replacement, fixation rods, heterotrophic ossification, spasms, or contractures preventing proper scan positioning. There were no differences in age, race, wheelchair use, smoking history, vitamin D levels or levels of markers of bone turnover (p=0.21–0.87) between the short-term and long-term SCI groups. A greater percentage of subjects with long-term SCI had motor complete injury than the short-term SCI group (51% vs 22%, p=0.02). Subjects with short-term SCI had significantly greater BMD and BMC at all sublesional skeletal sites (p=0.004–0.03) and higher BMI (p=0.01) than subjects with long-term SCI. There was no difference in BMD at the radius between the two groups (p=0.24). Subjects in the long-term SCI group were more likely to have osteoporosis at the hip (p=0.05) or a history of post-SCI fracture (p=0.0002) than the subjects in the short-term SCI group.
Table 1.
Subject characteristics
| Variable | Short-term SCI (≤ 5 years) [N=18] | Long-term SCI (>5 years) [N=137] | p | Total [N=155] |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Age (years) [Mean ± SD] | 57.7 ± 18.1 | 55.4 ± 13.4 | 0.52 | 55.7 ± 14.0 |
| Age (years) [Range] | 25.3–85.2 | 27.1–87.6 | 25.3–87.6 | |
| White % | 14 (77.8%) | 122 (89.1%) | 0.30 | 136 (87.7%) |
|
| ||||
| Injury severity | 0.02 | |||
| •Motor complete SCI | 4 (22.2%) | 70 (51.1%) | 74 (47.7%) | |
| •Motor incomplete SCI | 14 (77.8%) | 67 (48.9%) | 81 (52.3%) | |
|
| ||||
| Wheelchair use | 0.55 | |||
| •Wheelchair | 10 (55.6%) | 86 (62.8%) | 96 (61.9%) | |
| •No wheelchair | 8 (44.4%) | 51 (37.2%) | 59 (38.1%) | |
|
| ||||
| BMI (kg/m2) [Mean ± SD] | 30.7 ± 6.2 | 27.2 ± 5.6 | 0.01 | 27.6 ± 5.8 |
| •Normal (< 25) | 3 (16.7%) | 51 (37.2%) | 54 (34.8%) | |
| •Overweight/Obese (≥25) | 15 (83.3%) | 86 (62.8%) | 101 (65.2%) | |
|
| ||||
| Smoking history | 0.72 | |||
| •Current smoker | 2 (11.1%) | 24 (17.5%) | 26 (16.8%) | |
| •Former smoker | 9 (50.0%) | 57 (41.6%) | 66 (42.6%) | |
| •Never smoker | 7 (38.9%) | 56 (40.9%) | 63 (40.7%) | |
|
| ||||
| Bone Mineral Density (BMD) (g/cm2) | ||||
| •Distal femur | 0.890 ± 0.158a | 0.751 ± 0.226c | 0.02 | 0.766 ± 0.224 |
| •Proximal tibia | 0.969 ± 0.210a | 0.787 ± 0.287d | 0.02 | 0.807 ± 0.285 |
| •Femoral neck | 0.940 ± 0.150 | 0.830 ± 0.208e | 0.03 | 0.843 ± 0.205 |
| •Total hip | 0.996 ± 0.195 | 0.838 ± 0.234e | 0.01 | 0.858 ± 0.235 |
| •Radius | 1.011 ± 0.102 | 0.979 ± 0.109c | 0.24 | 0.982 ± 0.108 |
| Leg Bone Mineral Content (BMC) (g) | 1117.0 ± 242.7b | 910.5 ± 276.2 | 0.004 | 933.5 ± 279.7 |
|
| ||||
| Hip bone density classification | 0.05 | |||
| •Normal | 10 (55.6%) | 38 (27.7%) | 48 (31.0%) | |
| •Osteopenia | 5 (27.8%) | 25 (18.2%) | 30 (19.4%) | |
| •Osteoporosis/BMD lower than expected for age/gender | 3 (16.7%) | 66 (48.2%) | 69 (44.5%) | |
| •Hip BMD not available | 0 (0.0%) | 8 (5.8%) | 8 (5.2%) | |
|
| ||||
| Post SCI fracture history | 0.002 | |||
| •Fracture | 0 (0.0%) | 51 (37.2%) | 51 (32.9%) | |
| •No fracture | 18 (100.0%) | 86 (62.8%) | 104 (67.1%) | |
|
| ||||
| 25 OH Vitamin D (ng/mL) | 0.55 | |||
| [Mean ± SD] | 22.3 ± 5.9 | 23.3 ± 10.2 | 23.2 ± 9.8 | |
| •Normal (≥20 ng/mL) | 11 (66.1%) | 81 (59.1% | 92 (59.4%) | |
| •Deficient (< 20 ng/mL) | 7 (38.9%) | 56 (40.9%) | 63 (40.7%) | |
|
| ||||
| Markers of bone turnover (ng/ml) [Mean±SD] | ||||
| •CTX | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.21 | 0.4 ± 0.2 |
| •Osteocalcin | 17.3 ± 5.3 | 20.3 ± 9.6 | 0.85 | 20.0 ± 9.3 |
=16,
=17,
=135,
=134,
=129
3.2 Effect of Age on Sclerostin
In our previous study we found that sclerostin significantly increased with age in 39 subjects with chronic SCI (Morse et al 2011). This finding has also been reported in the general population (Modder et al. 2011). In this current study, we confirmed in a larger group of subjects with long-term SCI that sclerostin increased significantly with age (p=<0.0001). In contrast, there was no association between sclerostin and age (p=0.11) in subjects with short-term SCI, likely reflecting elevations in sclerostin due to mechanical unloading that dramatically alter basal sclerostin levels. Therefore, we adjusted for age only in analyses with long-term SCI.
3.3 Effect of Injury Duration on Sclerostin
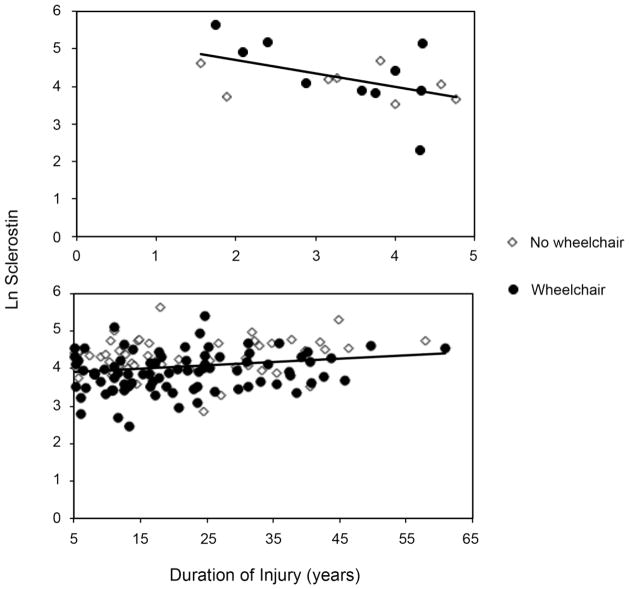
We examined sclerostin levels as a function of injury duration in both short-term and long-term SCI. In the short-term SCI group only, there was a significant negative association between ln sclerostin (natural log transformed sclerostin) and injury duration (Figure 1 top, p=0.04). Those injured less than 3 years (n=6, ln sclerostin 4.69±0.71 corresponding to 108.89 pmol/L) had greater sclerostin levels than those injured 3–5 years (n=12, ln sclerostin 3.98±0.70 corresponding to 53.73 pmol/L, p=0.06). In long-term SCI, after adjusting for age, there was no association between sclerostin and injury duration (Figure 1 bottom, p=0.79).
Figure 1. Sclerostin Levels Based on Duration of Injury.
Sclerostin levels are initially high after SCI and drop significantly with time after injury in the short-term SCI group only (less than or equal to 5 years, top panel, β ± SE= −0.36±0.16, p=0.04). No association was seen between sclerostin and injury duration in long-term SCI (more than 5 years, bottom panel, age-adjusted β ± SE= 0.001±0.004, p=0.79). Sclerostin was plotted using natural log-transformed data.
3.4 Effect of Injury Duration on the Association between Sclerostin and Bone
In the short-term group, there was no association between sclerostin and bone density or bone mineral content at any site tested (p=0.17–0.41). After adjusting for age, there was a significant, positive association between tibia BMD and sclerostin in the long-term SCI group (Figure 2, R2=0.19, β=0.002 ± 0.001, p=0.0004). The results were similar at all sublesional skeletal sites and leg BMC (p=<0.0001–0.01) but not the radius (p=0.15). When considering this association based on wheelchair use in long-term SCI, tibial BMD was significantly associated with sclerostin in the wheelchair users (R2=0.09, β=0.002±0.0007, p=0.03) and was of borderline significance in the non-wheelchair users (R2=0.07, β=0.001±0.0007, p=0.097).
Figure 2. Association between Sclerostin and Bone Density in Long-Term SCI.
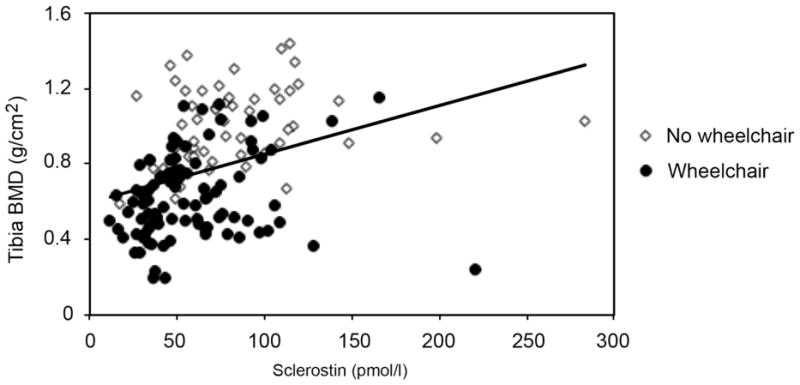
Greater sclerostin levels are positively associated with greater proximal tibia bone mineral density in subjects with long-term SCI (more than 5 years, age-adjusted R2=0.19, β ± SE= 0.002± 0.001, p=0.0004).
3.5 Clinical Factors Associated with Sclerostin Levels
We examined the association between sclerostin and various clinical factors known to be relevant to bone health in the general population. We found no significant association with BMI, smoking history (current, former, or never), history of post-SCI fracture (ever or in the previous 2 years), or markers of bone turnover (p=0.34–0.97) in the short-term or long-term group. Adjusting for injury duration, subjects in the short-term group who were vitamin D deficient had significantly lower sclerostin levels compared to those who were not vitamin D deficient (p=0.02, 42.78 pmol/ml vs 91.32 pmol/ml, Table 2).
Table 2.
Association between sclerostin and vitamin D status, adjusted for injury duration, in short-term SCI (≤ 5 years)
| R2 | β ± SE | eβ | p | |
|---|---|---|---|---|
|
| ||||
| Injury duration (years) | 0.49 | −0.33±0.14 | 0.72 | 0.03 |
|
|
|
|||
| ln sclerostin LS means±SE | e(ln sclerostin) | p | ||
|
|
|
|||
| Vitamin D status | ||||
| •Normal (≥ 20 ng/mL) | 4.51±0.18 | 91.32 | 0.02 | |
| •Deficient (<20 ng/mL) | 3.76±0.22 | 42.78 | ||
In the long-term SCI group, adjusting for age, sclerostin levels were significantly lower in wheelchair users compared with those who walk (p=0.01), in subjects with motor complete SCI compared with motor incomplete SCI (p=0.01), and in subjects with a diagnosis of osteoporosis at the hip compared with those with normal bone density (p=<0.0001). In multivariate regression models adjusting for age and including tibia BMD (Table 3 Model A) or osteoporosis diagnosis (Table 3 Model B), the relationship between sclerostin and wheelchair use or injury severity (motor complete vs incomplete) was no longer significant (p=0.20–0.78).
Table 3.
Association between sclerostin and bone density or osteoporosis diagnosis, adjusted for age, in long-term SCI (≥5 years)
| Model A (R2=0.35)
| ||||
|---|---|---|---|---|
| N | β ± SE | eβ | p | |
|
| ||||
| Age (years) | 134 | 0.01±0.003 | 1.01 | <0.0001 |
|
|
|
|||
| Tibia BMD (g/cm2) | 0.70±0.14 | 2.01 | <0.0001 | |
|
| ||||
|
Model B (R2=0.40)
| ||||
| N | β ± SE | eβ | p | |
|
| ||||
| Age (years) | 129 | 0.01±0.003 | 1.01 | <0.0001 |
|
|
|
|||
| Mean ln sclerostin ± SE | e (ln sclerostin) | p | ||
|
|
|
|||
| Osteoporosis diagnosis | ||||
| •Normal | 4.32±0.07 | 75.05 | ||
| •Osteopenia | 4.19±0.09 | 66.28 | 0.51 | |
| •Osteoporosis/BMD lower than expected for age/gender | 3.83±0.06 | 45.85 | <0.0001 | |
4. DISCUSSION
In this study we examined bone and circulating sclerostin levels in 155 men with varying degrees of SCI who were 1 year or more post-injury. We report that sclerostin levels are greatest in subjects with short-term SCI (≤5 years) and decrease significantly over the first 5 years post-injury. We found no association between sclerostin and injury duration in subjects with long-term SCI (>5 years). In long-term SCI, sclerostin levels are positively associated with lower extremity bone density and bone mineral content. These associations are stronger in subjects who use a wheelchair compared to those who do not.
This is, to our knowledge, the first study to report elevated sclerostin in response to paralysis in short-term SCI. Our findings are consistent with a previous report of elevated sclerostin in institutionalized women with mobility impairments following stroke [12]. However, we report much greater sclerostin levels in men with short-term SCI (108.89 pmol/L) than those reported in women after stroke (42.9 pmol/l) or in postmenopausal women (51.04 pmol/l) [13]. Men have higher sclerostin levels than women [10], and this may account for some of the difference. Another factor may be the degree of mechanical unloading that occurs after SCI compared to stroke or postmenopausal osteoporosis. To be included in the stroke study, women could not walk without assistance. No information was provided on the number of women with stroke who ambulated regularly with assistance. However, 14 of the 40 women with stroke performed passive standing with a standing device. Therefore, some may have performed weight-bearing activities regularly despite their mobility impairments. Similarly, post-menopausal osteoporosis is not necessarily associated with the inability to walk.
We identified distinct clinical factors associated with sclerostin in short-term SCI compared with long-term SCI. In this study sclerostin increases with age for subjects with long-term SCI as previously reported [8, 10]. However, we found no significant association between age and sclerostin in those subjects with short-term SCI (5 years or less). Our findings are limited by a small sample size, but suggest that mechanical unloading dramatically alters sclerostin levels, and that this elevation is sustained for as long as 5 years after injury. In short- term SCI, sclerostin is negatively associated with injury duration. After adjusting for injury duration, Vitamin D deficiency is associated with lower sclerostin levels. The physiological significance of this observation remains to be clarified. The role of sclerostin in Wnt signaling is well described in the literature, but less is known about the complex regulation of sclerostin expression. Mechanical unloading stimulates sclerostin expression. The addition of 1,25-dihydroxyvitamin D3 in combination with bone morphogenetic protein (BMP)-4 to human primary osteoblasts significantly stimulated sclerostin expression in culture [14]. More recently, a positive association between sclerostin and 25-hydroxy-cholecalciferol was reported in dialysis patients [15]. Taken together, these reports and our observation suggest that vitamin D is required for osteocyte expression of sclerostin. It is not known if the magnitude of sclerostin-mediated bone loss is less in people with SCI who are vitamin D deficient compared with those with normal vitamin D levels.
When considering long-term SCI, we found no association between BMI or post-SCI fracture history. There is limited information on sclerostin levels in response to a fracture or during the fracture healing process. BMI was previously reported to be associated with sclerostin levels in a large study of post-menopausal women with normal bone density, vitamin D levels 50 ng/ml or greater, and no history of fracture in the prior 2 years [16]. We did identify several factors associated with sclerostin in long-term SCI. In addition to age, we found the following factors were significantly associated with sclerostin: wheelchair use (yes/no), injury completeness (motor complete vs. incomplete), a diagnosis of osteoporosis based on T- or Z-score at the hip, lower extremity bone density (at all skeletal sites tested), and lower extremity bone mineral content. Bone density at the radius was not associated with sclerostin. The radius may be more reflective of age-related bone loss than SCI-induced bone loss, but more work is needed to confirm this. In separate multivariate models (adjusting for age and injury completeness or wheelchair use) lower extremity bone density, leg bone mineral content, and osteoporosis diagnosis based on hip BMD all remain positively associated with sclerostin levels. Fracture risk prediction based on bone density is poorly defined in SCI. Therefore, a diagnosis of osteoporosis currently has little clinical utility in this population. In this study, a diagnosis of osteoporosis at the hip was more strongly correlated with sclerostin levels in long-term SCI than bone density or bone mineral content at any skeletal site tested. Further work is needed to identify the skeletal site, whether SCI-specific (distal femur, proximal tibia) or traditional (femoral neck, total hip), that has the greatest predictive value for osteoporotic fractures in SCI. It is possible that circulating sclerostin, when used in conjunction with osteoporosis diagnosis, sublesional bone density, or leg bone mineral content, may enhance fracture risk prediction after SCI.
Our findings suggest that long-term, motor-complete SCI and subsequent wheelchair use leads to low bone density, ultimately resulting in low circulating sclerostin levels. Therefore, sclerostin may be a good biomarker of bone density, and, perhaps, fracture risk in SCI. These findings validate our previously reported conceptual model [8] that complete paralysis in short-term SCI results in elevated sclerostin leading to inhibition of bone formation, and that this response is modulated by the ability to walk (mechanically load bones). These concepts need to be confirmed in a larger study and to be assessed in the context of a longitudinal study. The elevation in sclerostin in short-term SCI may be predictive of the amount of resulting bone loss, and ultimately fracture risk. In long-term SCI, on the other hand, sclerostin may be a candidate circulating biomarker of bone health and fracture risk.
There are limitations to the current study that must be considered. We report on few subjects 5 years post-injury or less. A larger study is needed in short-term SCI to better define the kinetics of the sclerostin response immediately after SCI. This is a cross-sectional study of men. Longitudinal studies that include women are also needed. Limited information exists regarding clinical factors that may modulate the sclerostin response to mechanical unloading, such as parathyroid hormone levels, spasticity or comorbidities including Diabetes Mellitus. The general consensus in the literature is that PTH levels are lower in acute SCI (17) due to hypercalciuria and higher in chronic SCI (18). It is possible that variations in PTH levels may modulate the sclerostin response to acute unloading. In chronic SCI, PTH levels may also account for some of the variation in the relationship between bone density and circulating sclerostin levels. These relationships have not been studied previously. Despite these limitations, we offer evidence that sclerostin mediates bone loss after short-term SCI. The optimal time frame for targeting sclerostin in SCI-induced osteoporosis, either with medication or with physical therapy, is currently unknown. Our studies, although still preliminary, indicate that the first 5 years post-injury may be the most beneficial time frame to use anti-sclerostin antibodies to treat SCI-induced bone loss. Our results also suggest that physical therapy programs that reintroduce mechanical loading soon after SCI may effectively reduce or block sclerostin-mediated bone loss. These findings have important implications for rehabilitation following SCI.
Highlights.
We analyzed sclerostin in 155 men with SCI who were 1 year or more after injury
Sclerostin levels are initially increased in SCI due to mechanical unloading
In long term SCI sclerostin levels are reduced due to severe osteoporosis
Sclerostin may have dual roles after SCI: therapeutic target and bone biomarker
Acknowledgments
We thank Sam Davis, clinical research coordinator and technician, VA Boston Healthcare System, for assisting with bone density scans; Rachel Burns and Heather Colburn, research assistants, VA Boston Healthcare System, for collection of anthropometric data; and C.W. Wolff, research coordinator, Spaulding Rehabilitation Hospital, for editorial assistance.
This study received support from: the National Institute of Child Health and Human Development [R21HD057030 and R21HD057030-02S1], the National Institute of Arthritis and Musculoskeletal and Skin Diseases [1R01AR059270-02], the Office of Research and Development, Rehabilitation Research and Development [Merit Review Grant B6618R], and the Massachusetts Veterans Epidemiology Research and Information Center, Cooperative Studies Program, Department of Veterans Affairs.
Footnotes
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–75. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278:24113–7. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- 3.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 4.Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36:822–5. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- 5.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–92. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 7.Lewiecki EM. Sclerostin monoclonal antibody therapy with AMG 785: a potential treatment for osteoporosis. Expert Opin Biol Ther. 2011;11:117–27. doi: 10.1517/14712598.2011.540565. [DOI] [PubMed] [Google Scholar]
- 8.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic SCI. J Bone Miner Res. 2011 doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, III, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–9. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirshblum SC, Memmo P, Kim N, Campagnolo D, Millis S. Comparison of the revised 2000 American Spinal Injury Association classification standards with the 1996 guidelines. Am J Phys Med Rehabil. 2002;81:502–5. doi: 10.1097/00002060-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–53. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 13.Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95:1991–7. doi: 10.1210/jc.2009-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherland MK, Geoghegan JC, Yu C, Winkler DG, Latham JA. Unique regulation of SOST, the sclerosteosis gene, by BMPs and steroid hormones in human osteoblasts. Bone. 2004;35:448–54. doi: 10.1016/j.bone.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, Diarra D, Patsch JM, Kainberger F, Bohle B, Haas M. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant. 2012;27:226–30. doi: 10.1093/ndt/gfr270. [DOI] [PubMed] [Google Scholar]
- 16.Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH. Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res. 2011;26:2812–22. doi: 10.1002/jbmr.479. [DOI] [PubMed] [Google Scholar]
- 17.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1998;77(1):28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bauman WA, Zhang RL, Morrison N, Spungen AM. Acute suppression of bone turnover with calcium infusion in persons with spinal cord injury. The journal of spinal cord medicine. 2009;32(4):398–403. doi: 10.1080/10790268.2009.11754393. [DOI] [PMC free article] [PubMed] [Google Scholar]