Abstract
DNA double-strand breaks (DSBs) in embryonic stem (ES) cells are repaired primarily by homologous recombination (HR). The mechanism by which HR is regulated in these cells, however, remains enigmatic. To gain insight into such regulatory mechanisms, we have asked how protein levels of Rad51, a key component of HR, are controlled in mouse ES cells and mouse embryo fibroblasts (MEFs). The Rad51 protein level is about 15-fold higher in ES cells than in MEFs. The level of Rad51 mRNA, however, is only ∼2-fold higher, indicating that the differences in mRNA levels due to rates of transcription or mRNA stability are not sufficient to account for the large difference in the abundance of Rad51 protein. Comparison of Rad51 half-lives between ES cells and MEFs also did not explain the elevated level of Rad51 protein in the ES cells. A comparative assessment of the Rad51 translation level demonstrated that it is translated with much greater efficacy in ES cells than in MEFs. To determine whether this high level of translation in ES cells is a general phenomenon in these cells or whether it is a characteristic of specific proteins, such as those involved with recombination and cell cycle progression, we compared mechanisms that regulate the level of Pcna in ES cells with those that regulate Rad51. The half-life of Pcna and its rate of synthesis were considerably different from those of Rad51 in ES cells, demonstrating that regulation of Rad51 abundance cannot be generalized to other ES cell proteins and not to proteins involved in DNA replication and cell cycle control. Finally, we show that only a small proportion of the abundant Rad51 protein population is activated under basal conditions in ES cells and recruited to DNA DSBs and/or stalled replication forks.
Keywords: Embryonic stem cell, ES cell, DNA repair, homologous recombination, Rad51, Pcna
Introduction
Embryonic stem (ES) cells are derived from cells of the inner cell mass at the blastocyst stage of embryogenesis and are capable contributing to the development of the organism through multiple rounds of division followed by specifically-timed programmed differentiation. In culture, murine ES cells can be characterized as having a rapid cell cycle, defined by short gap phases with a large proportion of the cell cycle devoted to S-phase (White et al., 2005). This observation implies that a majority of gene products involved in both DNA replication and DNA repair should be active and readily accessible in these cells. Furthermore, the promoters of many of the genes involved in DNA replication contain E2F binding sites, and their expression is coordinately expressed upon entry into S-phase.
The Rad51 protein is a key player in DNA double-strand break (DSB) repair by homologous recombination (HR). The expression level of Rad51 typically varies throughout the cell cycle, with the lowest expression found in the G1 phase, increasing throughout S-phase, and peaking in G2/M (Chen et al., 1997; Flygare et al., 1996; Yamamoto et al., 1996). It is at these late phases of the cell cycle, mid to late S-phase and G2, that sister chromatids are optimally available as templates for homologymediated repair (Saleh-Gohari et al., 2004). In unchallenged cells, Rad51 protein exhibits a predominantly diffuse nuclear staining pattern. However, upon induction of DNA DSBs, Rad51 relocalizes to distinct foci surrounding the sites of DSBs, where it is involved in both the search for homologous sequences to serve as templates for repair as well as the invasion of these templates to facilitate the repair process (Sinha et al., 2008; Sung et al., 2003). The exact mechanism by which Rad51 performs this function is still unresolved.
Proliferating cell nuclear antigen (Pcna) is a homotrimeric ring protein first identified in proliferating cells (Celis et al., 1987) and has previously been utilized as a prognostic indicator of tumor grade (Elias, 1997; Jain et al., 1991; Mangham et al., 1994). Early studies demonstrated that Pcna functions during DNA replication, serving as a processivity factor that tethers DNA polymerases δ and ε to replicating DNA {reviewed in (Kelman, 1997)}. More recent data suggest additional roles for Pcna in mismatch and base-excision repair (Gary et al., 1999; Iyer et al., 2008; Lee et al., 2006; Matsumoto, 2001; Muller-Weeks et al., 1996; Umar et al., 1996), based on protein interactions with components of the repair machinery, as well as roles in both error-free and error-prone lesion bypass {reviewed in (Moldovan et al., 2007)} and apoptosis (He et al., 2009).
We and others have reported that murine ES cells express high levels of Rad51 protein compared with mouse embryo fibroblasts (MEFs), which correlates with the increased propensity of mouse ES cells to repair DNA DSBs by HR (Serrano et al., 2011; Tichy et al., 2010). In the current study, we have assessed the mechanisms by which mouse ES cells maintain abundant Rad51 levels and compared them with those utilized by MEFs. To establish whether our findings are peculiar to Rad51 or whether they can be extended to other ES cell proteins, particularly those involved in cell cycle progression, we have investigated the mechanisms that control the levels of the DNA replication protein Pcna for comparison. Our data prove that the regulation of Rad51 in ES cells differs at several levels from that of Pcna, demonstrating that differential controls exist between cell cycle and HR protein expression, challenging current dogma. Finally, we asked whether the level of Rad51 protein is reflective of a high load of endogenous DNA damage in ES cells and found that although DSBs and/or stalled replication forks are present in unchallenged cells, only a small percentage of total Rad51 localized to these sites.
Results
Characterization of Rad51 and DNA replication gene products in MEFs and ES cells
Several reports demonstrate that many proteins involved in HR, including the Rad51 protein, are expressed at very high levels in ES cells when compared with more differentiated cell types (Serrano et al., 2011; Tichy et al., 2010). This was confirmed in using isogenic MEFs and ES cells (Fig. 1A). Since HR, and consequently Rad51, is active predominantly in the S- and G2-phases of the cell cycle in a majority of cell types, we probed asynchronous MEF and ES cell populations for differences in their cell cycle profiles that might account for the high level of Rad51 in ES cells. To this end, MEFs and ES cells were pulsed for 30 minutes with EdU, a thymidine analog, which is incorporated into DNA during S-phase of the cell cycle. When cells were analyzed for EdU incorporation using bivariate flow cytometry, nearly 90% of the ES cells were in the S- or G2/M-phases of the cell cycle, compared with only about 50% of MEFs under the same conditions (Fig. 1B), consistent with the trend of protein expression seen in Fig. 1A. It should be noted that since 75-80% of passage 3 MEFs incorporate EdU when labeled for 24 hours (Supplementary Fig. 1), the low abundance of Rad51 protein in MEFs cannot be attributed to the presence of quiescent or senescent cells (Trojanek et al., 2003), which typically express low levels of Rad51 protein (Serrano et al., 2011; Tichy et al., 2010). Additionally, the high abundance of Rad51 protein in ES cells is unlikely to be due to contamination of the ES cells with the mitomycin-C treated MEF feeder cells (Supplementary Fig. 2). In fact, ES cells express a higher level of Rad51 in the absence of feeder cells than when harvested along with the feeder layer.
Figure 1. Characterization of Rad51 and other E2F target gene and protein expression in ES cells and MEFs.

A. Western Blot of Rad51 in asynchronous MEFs and ES cells. Oct4 served as a marker of undifferentiated ES cells and β-actin was used as a loading control. B. Analysis of the cell cycle distribution in asynchronous early-passage MEFs or ES cells. Cells were pulse labeled with 5-ethynyl-2′-deoxyuridine (EdU), a thymidine analog, for 30 minutes before processing and analysis by bivariate flow cytometry. The abscissa represents DNA content by staining with 405-Blue, and the ordinate represents labeling by EdU and Alexa fluorescence. C. Western blots for indicated proteins involved in DNA replication in MEFs and ES cells. Dhfr - dihydrofolate reductase, Ts - thymidylate synthase. D and E. RT-PCR using cDNA generated from asynchronous MEFs and ES cells. Amplification was performed using primers for the genes indicated that are involved in D. DNA replication; or, E. for Rad51.
We hypothesized that maximal expression of Rad51 in ES cells may be coordinated with the entry of cells into S-phase, similar to the behavior of other proteins involved in DNA replication in other cell types (Bracken et al., 2004; Fujii-Yamamoto et al., 2005). To test this proposition, we characterized the expression levels of several proteins that regulate the initiation and progression of DNA replication by western blotting. As shown in Fig. 1C, Cdc6, Cyclin A, Cyclin E, Dihydrofolate Reductase (Dhfr), and Thymidylate Synthase (Ts) all express more robustly in asynchronous ES cells than in MEFs. The promoters of a majority of genes encoding proteins that control the entry of cells into S-phase contain E2F binding sites. Since the Rad51 promoter also contains these elements (Bracken et al., 2004; Ishida et al., 2001; Lee et al., 1995), we postulated that the high abundance of Rad51 protein in ES cells may be regulated in the same manner. Fig. 1D demonstrates a general trend of increased transcription in ES cells of genes containing E2F binding sites, consistent with a previous report (Fujii-Yamamoto et al., 2005). When Rad51 transcripts were compared between MEFs and ES cells, the ES cells expressed a higher level of Rad51 message (Fig. 1E), suggesting that Rad51 may be regulated at the transcriptional level.
The abundance of Rad51 protein is controlled at multiple levels in ES cells
To better understand how the high levels of Rad51 protein in ES cells are controlled and maintained, we first assessed the extent to which Rad51 protein is overexpressed in ES cells. There is approximately 15-fold more Rad51 in ES cells than in MEFs (Fig. 2A). In contrast, quantitative PCR revealed that Rad51 mRNA is only about 2-fold higher in ES cells (Fig. 2B). This finding is consistent with the cell cycle data from Fig. 1 and indicates that differences in transcription levels between the cell types may contribute, but is insufficient, to explain the Rad51 protein elevation in ES cells.
Figure 2. Mechanisms for maintaining elevated levels of Rad51 in ES cells.
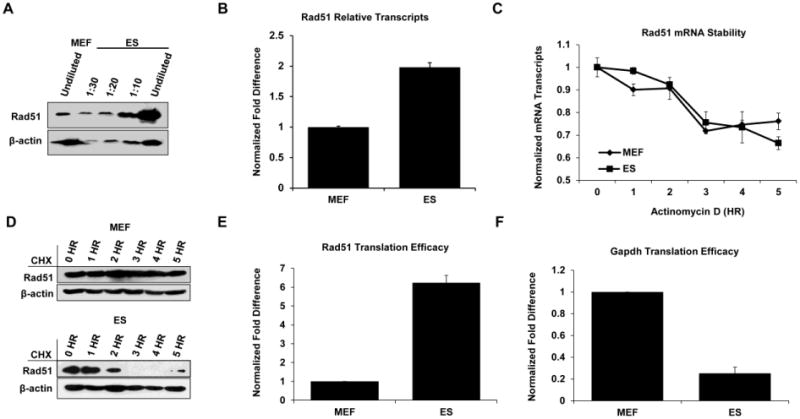
A. Whole cell lysates from MEFs or ES cells were diluted as indicated with loading buffer or left undiluted prior to completing Western blots for Rad51. β-actin served as a loading control. B. Analysis of Rad51 transcript levels by qPCR in MEF and ES cells expressed as the ratio of Rad51/Gapdh and normalized to MEFs. Error bars represent the S.E.M. from at least two independent experiments performed in triplicate. C. MEFs and ES cells were treated with actinomycin D to inhibit mRNA synthesis and were harvested at the indicated times for qPCR analysis. The data are displayed as the ratio of Rad51/Gapdh at each time point with data from each cell type normalized to its respective untreated sample. Error bars represent the S.E.M. from two independent experiments performed in triplicate. D. MEFs (upper panel) or ES cells (lower panel) were treated with cycloheximide to inhibit new protein synthesis before harvesting at the indicated time points for Western blot analysis using antibodies to Rad51 or β-actin. Note: 10-times more lysate was used in the MEF lanes due to the low expression of Rad51 in these cells. E and F. Comparison of relative rates of translation between MEFs and ES cells for Rad51 or Gapdh. Cells were grown in methionine-free medium prior to labeling with 35S methionine for one hour. Cells were lysed, and cell lysates were immunoprecipitated with antibody before SDS-PAGE. Gels were silver stained to determine protein content prior to autoradiography. A minimum of three experiments were used for analysis. Error bars represent the S.E.M.
To determine whether differences in the stability of Rad51 mRNA might contribute to the high abundance of Rad51 in ES cells, MEFs and ES cells were treated with actinomycin D at doses that inhibit transcription mediated by all RNA polymerases (Sobell, 1985). The kinetics of Rad51 mRNA diminution were assessed over a five-hour time course and showed no significant differences in Rad51 mRNA decay between the cell types (Fig. 2C). A five-hour end point was chosen as it corresponds to about one half of the cell cycle time of ES cells grown under standard conditions in our laboratory (Supplementary Figure 3). The stability of the Rad51 protein in ES cells was also assessed and compared with that of MEFs following inhibition of translation by cycloheximide (Schneider-Poetsch et al., 2010). Unexpectedly, the level of Rad51 in MEFs remained constant over the time course of treatment while the Rad51 half-life in ES cells is very short, with no Rad51 observable by 3 hours (Fig. 2D). The short half-life of Rad51 in ES cells clearly eliminates the stability of the protein as a factor contributing to the high levels of this protein in these cells.
The differences in Rad51 protein stability and that of its RNA between MEFs and ES cells cannot explain the elevated Rad51 protein level seen in ES cells. Furthermore, the rate of transcription may only account for about 20% of the 15-fold protein elevation seen in these cells. Consequently, translation efficiencies of the Rad51 protein were compared between the cell types. To this end, cells were grown for one hour in medium containing 35S labeled methionine and cysteine, lysed, and subjected to immunoprecipitation with either IgG control antibody or Rad51 antibody prior to separation by SDS-PAGE and autoradiography. Incorporation of 35S methionine/cysteine into Rad51 was about 6-fold higher in the ES cells than in MEFs (Fig. 2E). These data suggest that the high abundance of Rad51 protein in ES cells compared with MEFs is likely to be a consequence of higher rates of both transcription and translation. To ask whether the greater efficiency of Rad51 translation in ES cells compared with MEFs is generally characteristic of translation in ES cells, the same approach was used to investigate the translation rate of glyceraldehyde 3-phosphate dehydrogenase (Gapdh). In contrast to Rad51, the translation of Gapdh was four times more efficient in MEFs than ES cells (Fig. 2F).
Expression patterns of Rad51 and Pcna in ES cells are similar
To determine whether ES cells regulate Rad51 in the same fashion as other proteins involved in DNA replication or cell cycle progression, we compared the expression of Rad51 with that of Pcna in ES cells. Both Rad51 and Pcna proteins were greatly elevated in ES cells compared with MEFs under basal conditions (Fig. 1A and Fig. 3A). Using a series of lysate dilutions, we determined that the Pcna protein was about five-fold more abundant in ES cells compared with MEFs (Fig. 3B). Since the gene encoding Pcna contains E2F binding sites within its promoter, we expected to see elevated levels Pcna transcription levels in the ES cells similar to that observed with Rad51. However, qPCR analysis of Pcna mRNA in MEFs and ES cells showed only a 1.25 fold elevation in the ES cells (Fig. 3C), demonstrating that Rad51 and Pcna utilize different mechanisms for regulating their respective protein levels.
Figure 3. Analysis of Pcna and Rad51 expression and regulation in ES cells.

A. Western blots for Pcna using MEF and ES whole cell lysates. Undifferentiated cells were identified using Oct4 as a marker. B. ES whole cell lysate was diluted as indicated or left undiluted and compared to undiluted MEF lysate for the level of Pcna expression. C. QPCR analysis of Pcna expression in MEFs and ES cells. Data are displayed as the ratio of Pcna/Gapdh normalized to MEFs. D. ES cells were treated with actinomycin D and harvested at the indicated time points for qPCR analysis using primers for Rad51 or Pcna. Data are presented as relative mRNA units. E. A comparison of the stabilities of Pcna and Rad51 proteins in ES cells after treatment with cycloheximide at the indicated time points by Western blotting. F. Relative rates of translation of Rad51 and Pcna were measured by 35S methionine incorporation over a one hour period in ES cells. Error bars represent the S.E.M.
Pcna is a stable protein in ES cells and is translated with high efficacy
Since differences in the transcript levels are not sufficient to account for the elevated abundance of Pcna levels found in ES cells, compared with MEFs, mRNA stability was examined by treatment of the cells with actinomycin D. Based on this approach, there were no significant differences Pcna and Rad51 mRNA levels (Fig. 3D). Unlike Rad51 protein, however, the Pcna protein level remained stable in ES cells during the five-hour cycloheximide treatment (Fig. 3E), consistent with observations in other cell types (Bravo et al., 1985; Yu et al., 2009). The translation efficacy of Pcna in ES cells is also very high, displaying a rate about 7 times higher than that of Rad51 (Fig. 3F). This difference cannot be attributed to differences in the number of sulfurcontaining amino acids between the two proteins, as they differ by only one residue. In this respect, these data demonstrate that ES cells utilize different mechanisms to regulate the levels of proteins involved primarily in DNA replication versus those with major roles in DNA DSB repair.
ES cells are primed to repair DNA DSBs and/or restart stalled replication forks
While the elevated expression of Pcna protein in ES cells is consistent with its role in DNA replication and the large proportion of the cell cycle devoted to S-phase in these cells, the requirement, if any, for elevated Rad51 protein is less clear. To determine if high Rad51 protein is in response to a large endogenous load of DNA damage in ES cells, we examined the relative levels of activated Rad51 protein in unperturbed ES cells and in MEFs as a control cell type. Phosphorylation of Rad51 on threonine 309 by Chk1 is crucial to promote Rad51 participation in HR (Sorensen et al., 2005). As an additional control, cells were treated with very low doses of several agents that can induce DNA DSBs, including ionizing radiation (IR), etoposide (ETO), and hydroxyurea (HU). Both MEFs and ES cells displayed very low levels of phosphorylated Rad51 protein under basal conditions (Fig. 4A). Phosphorylated Rad51 levels increased in both cell types, in response to DNA damage, regardless of treatment type. There was no significant change, however, in the total levels of Rad51. Under the same conditions, both cell types activated Chk1 (phospho-ser317). After induction of DNA damage, total Chk1 protein levels increased in both cell types, but remained low in unchallenged cells. These data suggest that only a small population of total cellular Rad51 protein participates in DSB repair in unchallenged ES cells
Figure 4. Measurement of Rad51 activation in ES cells.
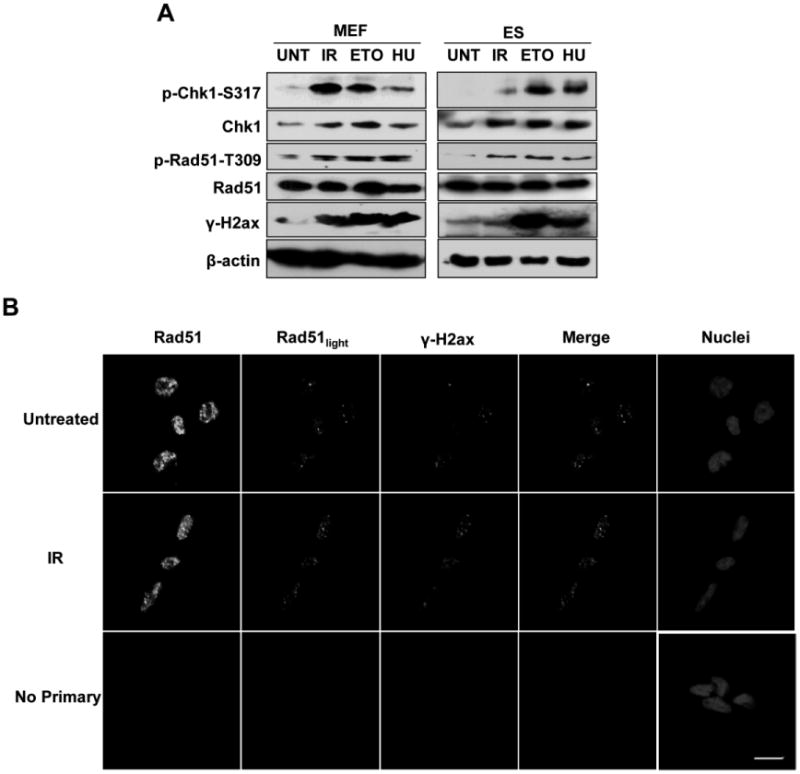
A. ES cells were harvested untreated (UNT) or treated with 2 Gy ionizing radiation (IR), etoposide (ETO), or hydroxyurea (HU) to induce DNA DSBs. Western blots were conducted using antibodies to the indicated proteins. MEFs are included as a control, using 10-times more lysate than for ES cells. B. ES cells were fixed untreated or one hour after treatment with 2 Gy IR and stained with antibodies against Rad51 and γ-H2AX for confocal immunofluorescent analysis. Nuclei were stained with DRAQ5. Scale bar represents 10 μm. Cells in the Rad51light images are the same as those in the Rad51 plane, except laser output was lowered prior to imaging.
To determine whether Rad51 protein localizes to DNA DSBs in ES cells, immunofluorescence experiments were performed in the absence or presence of DNA damage (Fig. 4B). Under basal conditions, γ-H2AX foci, suggestive of DNA DSBs, were present in the cells at a low level, and are likely the result of DSBs induced by endogenous processes. Although Rad51 protein was predominantly distributed diffusely throughout the nucleus, several discrete foci were detectable under low intensity laser excitation. A majority of the Rad51 foci loosely co-localized with γ-H2AX, suggesting that HR was taking place at these sites. When ES cells were challenged with IR, the abundance of both Rad51 and γ-H2AX increased, as did their co-localization. These data suggest that ES cells do not carry a large burden of endogenous DSBs, but appear to consistently maintain an excess of Rad51 protein which can rapidly respond to DSBs as they arise.
In addition to repair of DSBs arising from collapsed replication forks, another role for Rad51 is the restart of stalled replication forks prior to their collapse, which is independent of HR activity (Petermann et al., 2010). Furthermore, replication fork arrest can also induce H2AX phosphorylation (Gagou et al., 2010), which may explain the endogenous γ-H2AX foci observed in ES cells in the absence of challenge. To identify the contribution of stalled replication forks that may induce Rad51 foci formation in ES cells, we have first investigated the expression level of XRCC3, a protein that can collaborate with Rad51 in response to DNA DSBs, and is required for replication fork restart (Petermann et al., 2010). We found higher expression of XRCC3 protein in the ES cells than in MEFs, similar to trends observed with Rad51 (Supplementary Fig. 4A). We then investigated the amount of chromatin-bound Rad51 prior to and after treatment of the cells with thymidine (Supplemental Fig. 4B), which can promote the slowing and eventual stalling of replication forks without inducing DNA DSBs (Lundin et al., 2002) by causing imbalance of nucleotide pools, and found a small proportion of Rad51 bound to chromatin in the absence of challenge. The proportion of chromatin-bound Rad51 increased following thymidine treatment. Thus, the possibility that Rad51 localizes to stalled replication forks in unchallenged ES cells to promote their restart cannot be eliminated.
Discussion
Rad51 plays a critical role in HR, which is the predominant pathway of DSB repair in mouse (Serrano et al., 2011; Tichy et al., 2010) and human (Adams et al., 2010) ES cells. This is in contrast to somatic cells where NHEJ predominates. Recently, Serrano et al. (Serrano et al., 2011) have shown that HR-mediated repair in murine ES cells is not confined to the S-phase and G2, but occurs throughout the cell cycle. This finding contrasts with the conventional dogma for somatic cells which argues that HR occurs predominantly in late S- and G2-phases of the mammalian cell cycle when more homologous templates in the form of sister chromatids are available for recombination. To better understand how HR repair is controlled in ES cells, we investigated how the expression of Rad51 is regulated in mouse ES cells as a preliminary step to determining how HR may be controlled in these cells. The principal finding was that the abundance of Rad51 protein in ES cells is controlled by a combination of selectively elevated transcription and translation when compared with isogenic MEFs, which were used as the cell type representing differentiated somatic cells.
Since the Rad51 promoter contains binding sites for the E2F transcription factor, which is primarily active during entry of cells into S-phase (Bracken et al., 2004; Dyson, 1998; Shirodkar et al., 1992), one might expect that the proportion of the cell cycle spent in S-phase might account in part for the difference in Rad51 transcript levels between ES cells and MEFs. Indeed, the proportion of ES cells in S-phase is about twice that of MEFs, which is similar to the difference in Rad51 mRNA levels between the two cell types. While the two-fold difference in transcript level is significant between MEFs and ES cells, it is not sufficient to explain the 15-fold difference in protein level between the two cell types. Additionally, we did not observe any significant differences of Rad51 protein expression in ES cells throughout the cell cycle (Supplementary Fig. 3), confirming that the observed elevation is not merely the product of E2F-dependent control. When these data are integrated with the observed increased level of Rad51 translation in the ES cells, however, the difference in protein levels between MEFs and ES cells can be reconciled. This observation correlates well with several studies, which suggest that the abundance of a majority of cellular proteins is controlled at the level of translation, and is less dependent on respective protein and mRNA stabilities using NIH 3T3 cells (Schwanhausser et al., 2011) or ES cells (Lu et al., 2009).
There are very few additional studies using ES cells that have systematically measured rates of translation for comparative purposes with differentiated cell types. The most common rationale for studying translation in ES or ES-like cells is to better identify markers characteristic of the least differentiated cells, with the aim of enhancing their separation from unwanted differentiated cells. The typical protocol utilizes a stepwise approach that is coined translation state array analysis (TSAA). The strategy includes sorting for known markers of undifferentiated and differentiated cells after immunostaining, obtaining microarray data from the differentially sorted populations, and identifying polysome-associated mRNAs from those datasets, particularly those proteins which would be found at the cell surface (Kolle et al., 2009). In this manner, novel candidates could be identified that are differentially expressed at the protein level between undifferentiated and differentiating ES cells. Recently, using this approach while asking a different question, Sampath et al. discovered major differences in the translation of multiple transcripts between undifferentiated ES cells and ES cells that were induced to differentiate for 5 days into embryoid bodies (EBs) (Sampath et al., 2008). Surprisingly, the majority of proteins profiled were translated at significantly higher levels in the differentiated EBs than in ES cells, which associated with increased in mTOR activity. The elevated mTOR activity resulted in increased phosphorylation of 4E-BP1, an inhibitory protein that binds the translation initiation factor eIF4E, which in turn promoted the release of eIF4E and activated higher levels of translation. The results of this study imply that ES cells typically translate proteins with lower efficiency than differentiated cell types, at least at the time point chosen.
Another study by Ingolia et al. utilized a ribosomal profiling approach to compare undifferentiated mouse ES cells and ES cells induced to differentiate and harvested at different times after induction (Ingolia et al., 2011). During the early stages of differentiation, their findings were similar to those reported by Sampath et al., 2008. At later time points of differentiation into EBs, however, translation of ribosomal proteins was less efficient compared with undifferentiated ES cells, even though expression of mRNAs encoding those proteins remained elevated in the EBs. Additionally, these authors showed that 5′ UTR translation decreased by 25% in the differentiating EBs compared with the ES cells, demonstrating that the translational control program changes dramatically during the differentiation process (Ingolia et al., 2011). When the translation of Rad51 and Pcna was examined in ES cells before and after LIF withdrawal, there was a slight downregulation in the production of both of these proteins in the differentiating ES cells, which the authors attribute to a downregulation in the abundance of mRNA transcripts (Ingolia et al., 2011).
In our study, we have observed that both Rad51 and Pcna are translated at higher efficiencies in ES cells than in MEFs using a radiolabeled amino acid incorporation approach. This finding is not necessarily in conflict with previous reports, since we used terminally differentiated MEFs rather than EBs, and in MEFs, Gapdh was translated at a higher rate than in ES cells. Our data imply that there is a select set of proteins that ES cells preferentially translate with high efficiency, the majority of which are likely cell cycle regulatory proteins and proteins involved in DNA repair pathways.
The question remains as to why Rad51 is expressed at such high levels in the ES cells. Both Rad51 and Chk1 are phosphorylated in ES cells under basal conditions, but the level of activated proteins represents only a small fraction of the protein population (Fig. 3). To validate these findings and gain better insight into the role of elevated Rad51 in ES cells, we probed for focus formation in the absence of and following treatment with agents that damage DNA. Several Rad51 foci co-localized loosely with γ-H2AX, consistent with other reports (Banath et al., 2009; Serrano et al., 2011), suggesting that the Rad51 foci are associated with sites of DNA DSBs, stalled replication forks, or with a particular chromatin architecture that induces the phosphorylation of H2AX. In many cell types, some proteins involved in DNA damage signaling and repair are expressed at a high level but remain in an inactive state until they are triggered to respond to DNA damage (Chaturvedi et al., 1999; Chen et al., 2004). Others, such as p53, are induced to high levels by either an increase in their production and/or a decrease in their degradation, but only in response to DNA damage (Kubbutat et al., 1997). The former case may be applicable to Rad51, particularly in ES cells, since only a portion of the total Rad51 population responds to both endogenous and exogenous DNA damage by forming foci that co-localize with γ-H2AX. Additionally, there are no increases in Rad51 protein production after induction of DNA damage, suggesting that the high level of Rad51 protein present in ES cells is available for deployment to sites of DNA DSBs as they arise. There is also a lack of Rad51 protein production in MEFs after treatment with DNA damaging agents that induce DSBs, at least within the time frame investigated, indicating that the level of Rad51 protein present in a cell determines the capacity of that cell to undergo HR.
The rapid cycling times of murine ES cells and the fact that many of these cells are in S-phase at any given time may account for the localization of Rad51 foci to DNA DSBs under basal conditions, perhaps as a consequence of replication fork slowing/stalling and/or collapse. We cannot eliminate either of these possibilities as contributing to the level of Rad51 and γ-H2AX colocalization, since we do see increases in chromatin-associated Rad51 after chronic treatment of ES cells with high doses of thymidine, which would create slowed/stalled replication forks but not induce DNA DSBs (Supplemental Fig. 4B). Treatment with HU also produces unbalanced nucleotide pools, promoting replication fork slowing/stalling, however, induction of DSBs have also been reported after this treatment (Petermann et al., 2010). While our treatment of cells with HU was acute and at a low concentration, we believe, given the rapid cell cycles of ES cells, that our treatment was sufficient to induce DNA DSBs in these cells (Fig. 4B). An important point to note is that stalled/collapsed replication forks should only take place in S-phase of the cell cycle. While a large proportion of asynchronous ES cells are in S-phase, there are still approximately 25% of the cells in other phases of the cell cycle. Serrano et al. has shown that DSBs and HR occur throughout the murine ES cell cycle, proving that Rad51 is not only responding to stalled/collapsed replication forks to promote their restart (Serrano et al., 2011).
Endogenous DNA DSBs may also arise as a result of ROS production derived from oxidative phosphorylation. This latter possibility is less likely since in ES cells, like in many cancer cells, glycolysis is the predominant energy production pathway and oxidative phosphorylation is reduced (Kondoh et al., 2007). Furthermore, ES cells express antioxidant proteins at much higher levels than do differentiated cells, which may ensure that levels of free radicals are kept to a minimum (Saretzki et al., 2004; Saretzki et al., 2008).
There are several reports that demonstrate roles for Rad51 in addition to its participation in classical HR-mediated DSB repair. For example, in S. cerevisiae that lack telomerase or certain telomere binding proteins, the telomeres can be maintained in rare survivors by alternative recombination events dependent on either Rad50 (type II) or Rad51(type I) (Grandin et al., 2003; Le et al., 1999; Lin et al., 2009). In mammalian cells, an association between Rad51 and telomeres at the late S and G2 phases of the cell cycle has been described, and has been proposed to aid in the formation of the tloop (Verdun et al., 2006). Additionally, a recent report has shown that knockdown of Rad51 protein with shRNA in p53 deficient MEFs or in MEFs with a conditional loss of BRCA2, which is responsible for loading Rad51 onto DNA, resulted in significant erosion of telomeres compared with wildtype cells (Badie et al., 2010). The tight regulation of Rad51 and consequent HR at telomeres, however, is essential for the prevention of chromosomal aberrations stemming from telomere sister chromatid exchanges that are attributed to inappropriate recombination (Gauthier et al., 2011). While it is possible that the Rad51 foci in unchallenged ES cells are associated with telomeres, the fact that many of these Rad51 foci also colocalize with γ-H2AX would suggest that the telomeres are damaged, since γ-H2AX is also used as a marker of uncapped or damaged telomeres (Hao et al., 2004; Nakamura et al., 2009).
In addition to its roles in telomere maintenance and replication, Rad51 can physically associate with mitochondrial DNA (mtDNA), and in conjunction with other Rad51 family members Rad51C and XRCC3, is critical for maintaining proper mtDNA copy number (Sage et al., 2010). However, ES cells have few mitochondria per cell (Saretzki et al., 2008), and all of the detectable Rad51 observed by immunofluorescence is localized in the nucleus, suggesting that the predominant role of the elevated Rad51 protein expression in these cells is to participate in DNA DSB repair and/or other potential nuclear functions.
In conclusion, murine ES cells regulate the expression of Rad51 by multiple mechanisms, which are different than those in differentiated cells. Additionally, high Rad51 protein expression in these cells does not change significantly during the cell cycle, nor is it the result of a high level of endogenous DNA double-strand breaks. The most plausible hypothesis to explain these observations is that the excess Rad51 protein produced in ES cells constitutes a mechanism by which the cells can rapidly respond to DNA DSBs or slowed/stalled replication forks in a manner that does not affect the rate of cell proliferation. At the same time this mechanism would help preserve genomic integrity by utilizing high fidelity homologous recombination to repair endogenous DNA lesions resulting from DSBs.
Materials and Methods
Cell Culture and Drug Treatments
129/Sv ES cells and primary isogenic MEFs were cultured as described (Tichy et al., 2010). ES cells were separated from feeder cells using a standard protocol and harvested immediately or plated on 0.2% gelatin coated plates prior to use in experiments. MEFs were used between passages 2 and 3 for all experiments. Cells were never more than 60% confluent at the time of harvest. For protein stability experiments, cells were treated with 25 μg/mL cycloheximide (RPI Corp.) and harvested at the indicated time points. For RNA stability experiments, cells were treated with 10 μg/mL actinomycin D (RPI Corp.) and harvested at indicated times. To induce DNA DSBs, MEFs and ES cells were treated with either 2 Gy IR (from a 137Cs source) and harvested after 1 hour, or treated with either 5 μM etoposide (A.G. Scientific) or 1 mM hydroxyurea (Sigma) for two hours, prior to harvesting.
Western Blotting
Whole cell lysates were prepared from 5×106 cells using RIPA buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% Igepal Ca-630, 1% SDS, 0.5% Sodium deoxycholate), including a protease inhibitor cocktail (RPI Corp.) prior to the addition of 2X protein loading buffer (20% glycerol, 120 mM Tris, pH 6.8, 4% SDS, 0.01% bromophenol blue). Lysates were boiled for 10 minutes and subjected to SDS-PAGE, with minimum of 30 μg protein extract loaded per lane. Proteins were transferred to PVDF membranes and blocked with 3% milk-PBS-T. Antibodies raised against Cdc6 (Invitrogen; DCS180), Chk1 (Santa Cruz; G-4), Chk1 phospho-S317 (Abcam), Cyclin A (Santa Cruz; C-19), Cyclin E (Santa Cruz; E-4), Dihydrofolate reductase (Dhfr; BD Biosciences; 49), Oct4 (Santa Cruz; H-134), Pcna (Santa Cruz; PC10), Rad51 (Santa Cruz; H-92), Rad51 phospho-T309 (Abcam), Thymidylate Synthase (TS; Invitrogen; TS106), β-actin (Sigma), and γ-H2AX (Millipore; JBW301) were added and incubated overnight at 4°C. Blots were subsequently incubated with the appropriate secondary antibodies conjugated with horseradish peroxidase prior to incubating with ECL reagent and exposure to X-ray film.
Cell Proliferation Assays
Cell proliferation was analyzed using the Click-iT EdU Alexa Fluor 647 kit (Invitrogen), according to the manufacturer's instructions with minor modifications. For bivariate analysis by flow cytometry, MEFs and ES cells were incubated with 20 μM EdU for 30 minutes prior to processing. DNA was stained with Cell cycle 405-blue provided with the kit. Data were collected using a BD LSR II (BD Biosciences) with FACSDiVa software. Cells were excited with either a 635 nm (for Alexa Fluor 647) or a 405 nm (for DNA dye) laser. Logarithmic or linear fluorescence was collected for Alexa Fluor 647 or DNA dye, respectively, using either a 660/20 or 450/50 band pass filter. Compensation was not required between the two dyes. Data were plotted as Alexa Fluor 674 intensity versus DNA dye intensity.
Immunofluorescence microscopy
ES cells were seeded on gelatin-coated coverslips in the absence of feeder cells and allowed to grow for 24 hours. Cells were irradiated with 2 Gy IR or left untreated and were fixed 1 hour post-irradiation with 1% paraformaldehyde in PBS for 10 minutes. Cells were processed and stained as described previously (Serrano et al., 2011) using anti-Rad51 (Santa Cruz; H-92) and γ-H2AX (Millipore; JBW301) antibodies. DNA was stained with Draq5 (1/2000 in PBS; Biostatus Ltd.). Images were taken on a LS-510 confocal microscope (Carl Zeiss) with accompanying LSM software.
Semi-quantitative PCR
RNA from 5×105 129/Sv ES cells or MEFs was isolated using Tri-Reagent (Molecular Research Center), according to the manufacturer's instructions. Complimentary DNA was generated by reverse transcription using the Superscript III two step RT-PCR system (Invitrogen), using an oligo-dT primer. Four-hundred nanograms of resultant cDNA was amplified for 28-32 cycles using primers directed against: Cdc6 (F 5′-TGATCGTGTTGGTGTTGGACGAGA-3′; R 5′-GCAAACATCCAGCGCTTTACGGAT-3′), Cyclin A1 (F 5′-TGGACAGGTTTCTCTCCTGCATGT-3′; R 5′-TTCAAGAACGGGTCAGCTTCCAGA-3′), Cyclin A2 (F 5′-TCAGTAAACAGCCTGCCTTCACCA-3′; R 5′-AAGGATCGCCCTCATGCTGGTAGT-3′), Cyclin E (F 5′-TGTCCAAGTGGGCTATGTCAACGA-3′; R 5-TGGGCTTGGTCCAGCAAATCCAAG-3′), Dhfr (F 5′-TCCGCTCAGGAACGAGTTCAAGTA-3′; R 5′-TGCCTCCGACTATCCAAACCATGT-3′), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh-F 5′-CTCCACTCACGGCAAATTCAA-3′;R 5′-GATGACAAGCTTCCCATTCTCG) and Ts (F 5′-TCCTCTGCTCACAACCAAACGAGT-3′; R 5′ TACAACTGACAGAGGGCATGGCAA-3′). PCR products were electrophoresed on agarose gels containing ethidium bromide and visualized under UV light.
Quantitative PCR (qPCR)
QPCR preparation and analysis was performed as described previously (Tichy et al., 2010). For RNA stability studies, target gene expression is displayed as the ratio of target/Gapdh, normalized to MEFs and was carried out in duplicate. Primers used were: Gapdh (F 5′-CTCCACTCACGGCAAATTCAA-3′ and R 5′GATGACAAGCTTCCCATTCTCG-3′), Pcna (F 5′-AAAGAAGAGGAGGCGGTAACCA-3′ and R 5′-GGAGACAGTGGAGTGGCTTTTG-3′), and Rad51 (F 5′-TGATGAGTTTGGTGTCGCAGTG-3′ and R 5′-CGAACATGGCTGCTCCATCTAC-3′).
35S Methionine Incorporation and Immunoprecipitation
For 35S labeling, cells were incubated in DMEM (lacking methionine and cysteine; supplemented with 15% dialyzed fetal bovine serum) for one hour to eliminate unlabeled and unincorporated methionine prior to the addition of 50 μCi/mL Tran35S Label (comprised of ∼ 70% methionine and 15% cysteine; MP Biomedicals). Cells were lysed in one of two buffers for immunoprecipitation. For Rad51 and Pcna, cells were lysed in 150 mM NaCl, 0.05% SDS, 0.05% Sodium deoxycholate, and 50 mM Tris, pH 7.5 containing a protease inhibitor cocktail (RPI Corp.). For immunoprecipitation of Gapdh, cells were lysed in 150 mM NaCl, 0.5% Igepal CA-630, 0.1% Brij 35, 0.1% Sodium deoxycholate, and 10 mM Hepes, pH 7.9 containing protease inhibitors. To shear DNA, lysates were passed through a 26 gauge needle. Debris was removed by centrifugation at 16,000 × g for 1 minute. Lysates were incubated overnight at 4°C with a polyclonal anti-Rad51 antibody (Abcam), monoclonal Pcna antibody (Santa Cruz), polyclonal anti-Gapdh antibody (Abcam), or appropriate normal IgG as a control. Forty microliters of Protein A/G Plus agarose beads (Santa Cruz) was added and lysates were incubated for 2 hours at 4°C. After low-speed centrifugation (850 × g), the supernatant was discarded and the pellet washed 4X with IP wash buffer (160mM NaCl, 0.1% SDS, 0.05% Sodium deoxycholate, 1% Igepal Ca-630, 50 mM Tris, pH 7.5) for Rad51 and Pcna, or with Gapdh lysis buffer for 10 minutes each at 4°C. Beads were boiled for 10 minutes after addition of 2X protein loading buffer and subjected to SDS PAGE using 12% polyacrylamide gels. Silver staining was carried out using a commercially available kit (Thermo) to gauge the amount of immunoprecipitated protein loaded onto the gel. Gels were dried and exposed to a phosphorimager screen. Bands were visualized using the Storm Phosphorimager (Molecular Dynamics/GE Healthcare) and quantitated using Imagequant software.
Supplementary Material
Highlights.
Rad51 protein is highly expressed in undifferentiated mouse ES cells.
Abundance of Rad51 is the result of elevated transcription & translation.
Only a portion of Rad51 localizes to DNA double strand breaks.
Acknowledgments
We thank Drs. Robert Holdcraft and David Myer for assistance with experimental design and technical assistance. This work was supported in part by grants R01 ES012695 and R01 ES12695-4S1 to PJS from the National Institutes of Health and the Center for Environmental Genetics, P30 ES006096, and by NIH grants ES011633 and P30 ES005022 to JAT. EDT was supported by a NIH training grant T32 ES007250.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams BR, Golding SE, Rao RR, Valerie K. Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants . PLoS One. 2010;5:e10001. doi: 10.1371/journal.pone.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, Blasco MA, Jonkers J, Tarsounas M. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol. 2010;17:1461–1469. doi: 10.1038/nsmb.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banath JP, Banuelos CA, Klokov D, MacPhail SM, Lansdorp PM, Olive PL. Explanation for excessive DNA single-strand breaks and endogenous repair foci in pluripotent mouse embryonic stem cells. Exp Cell Res. 2009;315:1505–1520. doi: 10.1016/j.yexcr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Bracken AP, ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985;4:655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis JE, Madsen P, Celis A, Nielsen HV, Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987;220:1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, Scott GF, Li X, Carr SA, Johnson RK, Winkler JD, Zhou BB. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Chen F, Nastasi A, Shen Z, Brenneman M, Crissman H, Chen DJ. Cell cycle-dependent protein expression of mammalian homologs of yeast DNA double-strand break repair genes Rad51 and Rad52. Mutat Res. 1997;384:205–211. doi: 10.1016/s0921-8777(97)00020-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sanchez Y. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair (Amst) 2004;3:1025–1032. doi: 10.1016/j.dnarep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Elias JM. Cell proliferation indexes: a biomarker in solid tumors. Biotech Histochem. 1997;72:78–85. doi: 10.3109/10520299709082216. [DOI] [PubMed] [Google Scholar]
- Flygare J, Benson F, Hellgren D. Expression of the human RAD51 gene during the cell cycle in primary human peripheral blood lymphocytes. Biochim Biophys Acta. 1996;1312:231–236. doi: 10.1016/0167-4889(96)00040-7. [DOI] [PubMed] [Google Scholar]
- Fujii-Yamamoto H, Kim JM, Arai K, Masai H. Cell cycle and developmental regulations of replication factors in mouse embryonic stem cells. J Biol Chem. 2005;280:12976–12987. doi: 10.1074/jbc.M412224200. [DOI] [PubMed] [Google Scholar]
- Gagou ME, Zuazua-Villar P, Meuth M. Enhanced H2AX phosphorylation, DNA replication fork arrest, and cell death in the absence of Chk1. Mol Biol Cell. 2010;21:739–752. doi: 10.1091/mbc.E09-07-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Kim K, Cornelius HL, Park MS, Matsumoto Y. Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. J Biol Chem. 1999;274:4354–4363. doi: 10.1074/jbc.274.7.4354. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Granotier C, Hoffschir F, Etienne O, Ayouaz A, Desmaze C, Mailliet P, Biard DS, Boussin FD. Rad51 and DNA-PKcs are involved in the generation of specific telomere aberrations induced by the quadruplex ligand 360A that impair mitotic cell progression and lead to cell death. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin N, Charbonneau M. The Rad51 pathway of telomerase-independent maintenance of telomeres can amplify TG1-3 sequences in yku and cdc13 mutants of Saccharomyces cerevisiae. Mol Cell Biol. 2003;23:3721–3734. doi: 10.1128/MCB.23.11.3721-3734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Strong MA, Greider CW. Phosphorylation of H2AX at short telomeres in T cells and fibroblasts. J Biol Chem. 2004;279:45148–45154. doi: 10.1074/jbc.M403924200. [DOI] [PubMed] [Google Scholar]
- He X, Wei C, Song T, Yuan J, Zhang Y, Ma Q, Shi W, Zhong H. Proliferating cell nuclear antigen destabilizes c-Abl tyrosine kinase and regulates cell apoptosis in response to DNA damage. Apoptosis. 2009;14:268–275. doi: 10.1007/s10495-009-0313-2. [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–4699. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RR, Pohlhaus TJ, Chen S, Hura GL, Dzantiev L, Beese LS, Modrich P. The MutSalpha-proliferating cell nuclear antigen interaction in human DNA mismatch repair. J Biol Chem. 2008;283:13310–13319. doi: 10.1074/jbc.M800606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Filipe MI, Hall PA, Waseem N, Lane DP, Levison DA. Prognostic value of proliferating cell nuclear antigen in gastric carcinoma. J Clin Pathol. 1991;44:655–659. doi: 10.1136/jcp.44.8.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Pereira E, Maxfield M, Russell B, Goudelock DM, Sanchez Y. Regulation of Chk1 includes chromatin association and 14-3-3 binding following phosphorylation on Ser-345. J Biol Chem. 2003;278:25207–25217. doi: 10.1074/jbc.M300070200. [DOI] [PubMed] [Google Scholar]
- Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- Kolle G, Ho M, Zhou Q, Chy HS, Krishnan K, Cloonan N, Bertoncello I, Laslett AL, Grimmond SM. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells. 2009;27:2446–2456. doi: 10.1002/stem.182. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Le S, Moore JK, Haber JE, Greider CW. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Chiang WH, Chiang SH, Liu YC, Hwang J, Ng SY. Regulation of cyclin D1, DNA topoisomerase I, and proliferating cell nuclear antigen promoters during the cell cycle. Gene Expr. 1995;4:95–109. [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Alani E. Analysis of interactions between mismatch repair initiation factors and the replication processivity factor. PCNA J Mol Biol. 2006;355:175–184. doi: 10.1016/j.jmb.2005.10.059. [DOI] [PubMed] [Google Scholar]
- Lin YH, Chang CC, Wong CW, Teng SC. Recruitment of Rad51 and Rad52 to short telomeres triggers a Mec1-mediated hypersensitivity to double-stranded DNA breaks in senescent budding yeast. PLoS One. 2009;(4):e8224. doi: 10.1371/journal.pone.0008224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Markowetz F, Unwin RD, Leek JT, Airoldi EM, MacArthur BD, Lachmann A, Rozov R, Ma'ayan A, Boyer LA, Troyanskaya OG, Whetton AD, Lemischka IR. Systems-level dynamic analyses of fate change in murine embryonic stem cells. Nature. 2009;462:358–362. doi: 10.1038/nature08575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin C, Erixon K, Arnaudeau C, Schultz N, Jenssen D, Meuth M, Helleday T. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol Cell Biol. 2002;22:5869–5878. doi: 10.1128/MCB.22.16.5869-5878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangham DC, Rowlands DC, Newbold KM, Reynolds GM, Fielding JW, Hallissey MT. Expression of proliferating cell nuclear antigen (PCNA) in gastric carcinoma: no evidence for prognostic value. J Clin Pathol. 1994;47:473–474. doi: 10.1136/jcp.47.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y. Molecular mechanism of PCNA-dependent base excision repair. Prog Nucleic Acid Res Mol Biol. 2001;68:129–138. doi: 10.1016/s0079-6603(01)68095-4. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Muller-Weeks SJ, Caradonna S. Specific association of cyclin-like uracil-DNA glycosylase with the proliferating cell nuclear antigen. Exp Cell Res. 1996;226:346–355. doi: 10.1006/excr.1996.0235. [DOI] [PubMed] [Google Scholar]
- Nakamura AJ, Redon CE, Bonner WM, Sedelnikova OA. Telomeredependent and telomere-independent origins of endogenous DNA damage in tumor cells. Aging (Albany NY) 2009;1:212–218. doi: 10.18632/aging.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyureastalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JM, Gildemeister OS, Knight KL. Discovery of a novel function for human Rad51: maintenance of the mitochondrial genome. J Biol Chem. 2010;285:1898418990. doi: 10.1074/jbc.M109.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh-Gohari N, Helleday T. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 2004;32:3683–3688. doi: 10.1093/nar/gkh703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath P, Pritchard DK, Pabon L, Reinecke H, Schwartz SM, Morris DR, Murry CE. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22:962–971. doi: 10.1634/stemcells.22-6-962. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, von Zglinicki T, Lako M. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Serrano L, Liang L, Chang Y, Deng L, Maulion C, Nguyen S, Tischfield JA. Homologous recombination conserves DNA sequence integrity throughout the cell cycle in embryonic stem cells. Stem Cells Dev. 2011;20:363–374. doi: 10.1089/scd.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Sinha M, Peterson CL. A Rad51 presynaptic filament is sufficient to capture nucleosomal homology during recombinational repair of a DNA double-strand break. Mol Cell. 2008;30:803–810. doi: 10.1016/j.molcel.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell HM. Actinomycin and DNA transcription. Proc Natl Acad Sci U S A. 1985;82:5328–5331. doi: 10.1073/pnas.82.16.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, Dhingra U, Rathjen P, Walker D, Dalton S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21:8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- Sung P, Krejci L, Van Komen S, Sehorn MG. Rad51 recombinase and recombination mediators. J Biol Chem. 2003;278:42729–42732. doi: 10.1074/jbc.R300027200. [DOI] [PubMed] [Google Scholar]
- Taniura H, Glass C, Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol. 1995;131:33–44. doi: 10.1083/jcb.131.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy ED, Pillai R, Deng L, Liang L, Tischfield J, Schwemberger SJ, Babcock GF, Stambrook PJ. Mouse embryonic stem cells, but not somatic cells, predominantly use homologous recombination to repair double-strand DNA breaks. Stem Cells Dev. 2010;19:1699–1711. doi: 10.1089/scd.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanek J, Ho T, Del Valle L, Nowicki M, Wang JY, Lassak A, Peruzzi F, Khalili K, Skorski T, Reiss K. Role of the insulin-like growth factor I/insulin receptor substrate 1 axis in Rad51 trafficking and DNA repair by homologous recombination. Mol Cell Biol. 2003;23:7510–7524. doi: 10.1128/MCB.23.21.7510-7524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar A, Buermeyer AB, Simon JA, Thomas DC, Clark AB, Liskay RM, Kunkel TA. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–720. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- White J, Dalton S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005;1:131–138. doi: 10.1385/SCR:1:2:131. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Taki T, Yagi H, Habu T, Yoshida K, Yoshimura Y, Yamamoto K, Matsushiro A, Nishimune Y, Morita T. Cell cycle-dependent expression of the mouse Rad51 gene in proliferating cells. Mol Gen Genet. 1996;251:1–12. doi: 10.1007/BF02174338. [DOI] [PubMed] [Google Scholar]
- Yu Y, Cai JP, Tu B, Wu L, Zhao Y, Liu X, Li L, McNutt MA, Feng J, He Q, Yang Y, Wang H, Sekiguchi M, Zhu WG. Proliferating cell nuclear antigen is protected from degradation by forming a complex with MutT Homolog2. J Biol Chem. 2009;284:19310–19320. doi: 10.1074/jbc.M109.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


