Abstract
Links between impulsive compulsive behaviors in treated Parkinson’s disease, behavioral addictions and substance abuse have been postulated, but no direct comparisons have been carried out so far.
We directly compared patients with Parkinson’s disease with and without impulsive compulsive behaviors with illicit drug abusers, pathological gamblers and age-matched healthy controls using the beads task, a test of reflection impulsivity and a working memory task.
We found that all patients with Parkinson’s disease made more impulsive and irrational choices than the control group. Parkinson’s disease patients who had an impulsive compulsive behavior showed similar behavior to illicit substance abusers whereas patients without impulsive compulsive behaviors more closely resembled pathological gamblers. In contrast we found no difference in working memory performance within the Parkinson’s disease groups. However Parkinson’s disease patients without impulsive compulsive behaviors remembered distractors significantly less than all other patients during working memory tests.
We were able to correctly classify 96% of the Parkinson’s disease patients with respect to whether or not they had an impulsive compulsive behavior by analyzing 3 trials of the 80/20 loss condition of the beads task with a negative prediction value of 92.3% and we propose that this task may prove to be a powerful screening tool to detect an impulsive compulsive behavior in Parkinson’s disease. Our results also suggest that intact cortical processing and less distractibility in Parkinson’s disease patients without impulsive compulsive behaviors may protect them from developing behavioral addictions.
Keywords: Impulsive compulsive behavior, Parkinson’s disease, reflection impulsivity, pathological gambling, substance abuse, beads task
Introduction
Although not necessarily maladaptive impulsive decision making is often linked with addiction and has been reported in patients with substance abuse and pathological gambling1, 2. It is also seen in a subgroup of patients with Parkinson’s disease (PD) who develop impulsive compulsive behaviors (ICBs) on dopaminergic medication3. About 14% of PD patients treated with dopamine receptor agonists alone develop ICBs, such as pathological gambling, compulsive sexual behavior, binge eating, excessive shopping and punding4.
It remains unclear why some PD patients are predisposed to ICBs, but risk factors include younger age of disease onset, male gender and a premorbid or family history of substance abuse5. ICBs have also been associated with ‘behavioral addictions’6 sharing clinical withdrawal symptoms of dysphoria, depression and anxiety7, 8 with substance abuse. Functional imaging studies have demonstrated aberrant striatal dopaminergic “reward pathways” and altered function in frontal cortical regions in PD patients with ICBs (PD+ICB) and non-PD patients with addictive behaviors7, 9, 10.
We have used the ‘beads task’11 to compare decision making in PD patients with and without ICBs, pathological gamblers and substance abusers. In addition to the Mini-Mental state examination (MMSE)12 we also included a working memory (WM) task to assess whether impairments in decision making reflected a more generalized cognitive deficit. The beads task assesses how much information participants gather before making a decision that has been referred to as “reflection impulsivity”13, 14. This differs from ‘motor’ impulsivity, the inability to stop an ongoing process and from ‘waiting’ impulsivity, the inability to delay an action15. Early decision on the beads task or ‘jumping to conclusions’ has been also seen in patients with schizophrenia16–18. In a modified version of this task a positive association between impulsivity and problem gambling has been reported19. Reflection impulsivity has been also described in opioid abusers previously14 and has been shown not to correlate with scores on the Barratt impulsiveness scale20.
We predicted that all impulsive patients would jump to conclusions and speculated that PD+ICB patients would show behavior similar to substance abusers and pathological gamblers and make choices which were more impulsive than PD patients without ICBs (PD−ICB). We also speculated that both PD groups would perform worse than matched controls, given recent studies showing that even PD−ICB patients show increased risk taking and temporal discounting21, 22. Negative effects of task irrelevant stimuli (=distractors) on WM performance have been reported23. Given our previous results on WM performance21 we hypothesized that PD+ICB patients would perform significantly worse than controls and PD−ICB patients on the WM task and might have a performance similar to that seen in pathological gamblers and substance abusers. We also speculated that all patients with addictions would remember distractors significantly better than controls and PD−ICB patients.
Methods
All participants provided written informed consent according to the declaration of Helsinki and the study was approved by the UCLH Trust and the University of Lvov ethics committee.
PD and elderly control groups
Twenty seven PD−ICB and 26 PD+ICB patients were recruited from the National Hospital for Neurology and Neurosurgery London. All patients fulfilled the Queen Square Brain Bank criteria for the diagnosis of PD24 and were taking L-dopa. Twenty-one/27 PD−ICB patients were taking a dopamine agonist, whereas only 13/26 PD+ICB patients were still on a dopamine agonist. Eighteen healthy matched elderly volunteers were recruited. Patients who scored under 26/30 points on the MMSE were excluded. All participants were screened for sub-classes of ICBs in a semi-structured interview, using accepted diagnostic criteria for pathological gambling25, compulsive shopping26, compulsive sexual behavior27 and punding28. We also used a self-rated validated questionnaire for impulsive compulsive disorders in PD (QUIP)29.
Patients were tested only “on” medication with the beads test to minimize “off” dysphoria and anxiety30, which was however not specifically measured.
For the working memory task, patients were tested both off and on medication, in a counterbalanced order. Patients who were tested “off medication” did not take their anti-Parkinson medication, for at least 12 hours and performed the task between 8.00am and 9.00am. They were then retested “on medication” the following day. Those patients who were tested “on medication” first performed this task usually in mid-morning when their motor symptoms were well controlled. They were re-visited on the following day prior to their medication for the second test. Controls were tested in the same way but did not take any anti-Parkinson medication. All PD patients had an excellent L-dopa response assessed by UPDRS Part III scores during “off” and “on” state. L-dopa equivalent units (LEU-Table 1) were calculated as described previously28(See supplementary material).
Table 1.
Demographic characteristics
| CO-O | CO-Y | PD+ICB | PD−ICB | Addicts | Gamblers | t value, χ2 and F-value | p-value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Participants(no.) | 18 | 18 | 26 | 27 | 13 | 23 | ||
|
| ||||||||
| Age (yrs) | 58.9±12.7 | 33.2±5.5 | 58.7±10.0 | 65.3±5.3 | 32.0±7.1 | 38.0±9.3 | F=58.8 | <0.001* |
|
| ||||||||
| Gender (male) | 15 | 18 | 22 | 24 | 12 | 23 | χ2=6.8 | 0.25 |
|
| ||||||||
| Average draws across all trials | 1.9±0.1 | 2.0±0.1 | 0.15±0.1 | 1.2±0.1 | 0.07±0.1 | 0.9±0.1 | χ2=191 | <0.001* |
|
| ||||||||
| At PD onset | 47.7±9.5 | 55.3±7.4 | t=3.28 | 0.002* | ||||
|
| ||||||||
| PD Disease duration (yrs) | 11.0±4.1 | 10.0±6.5 | t=0.52 | 0.48 | ||||
|
| ||||||||
| Education (yrs) | 13.6±3.2 | 13.9±2.2 | 13.1±2.8 | 14.8±2.5 | 12.0±1.9 | 14.5±2.0 | F=3.1 | 0.011* |
|
| ||||||||
| ICB current | 20 | |||||||
| ICB (>3–12months) | 6 | |||||||
| Gambling (yrs) | 12.1±7.4 | |||||||
| Gambling stopped (months) | 1.8±2.7 | |||||||
| Drug abuse (yrs) | 12.0±5.1 | |||||||
| Replacement therapy (yrs) | 1.4±1.3 | |||||||
|
| ||||||||
| LEU dose(mg/day) | 934.2±407 | 740.1±369 | t=1.8 | 0.072 | ||||
|
| ||||||||
| PD patients currently using DA (N) | 13/26 | 21/27 | χ2=5.1 | 0.024* | ||||
|
| ||||||||
| UPDRS on | 16.2±10.6 | 21.1±9.0 | t=1.7 | 0.09 | ||||
| UPDRS off | 31.0±11.3 | 32.1±10.6 | t=0.5 | 0.6 | ||||
| Improvement in UPRDS (%) | 47.7 | 34.2 | ||||||
|
| ||||||||
| Hypersexuality | 12 | |||||||
| PG | 13 | 3 | 23 | |||||
| Casino games | - | 15 | ||||||
| Sport betting | 2 | 12 | ||||||
| Stock markets | - | 2 | ||||||
| Slot machines | 8 | 3 | ||||||
| Bingo | 4 | - | ||||||
| Scratch cards | 2 | - | ||||||
| Punding | 7 | |||||||
| Shopping | 5 | |||||||
| Substance abuse | 3(past) | 13 | 1 (past) | |||||
| i.v. opiods | 12 | |||||||
| i.v. heroin | 4 | |||||||
| cannabis | 3 | |||||||
| cocaine | 1 | |||||||
| morphine | 1 | |||||||
UPDRS = Unified Parkinson’s Disease Rating Scale; LEU = L-dopa equivalent units; DA = dopamine agonists. All values are mean ±SD. Significant differences are labeled with “*”. Controls (CO-O, elderly controls; CO-Y, young controls), PD patients with (PD+ICB) and without (PD−ICB) impulsive compulsive behaviors, addicts (=illicit substance abusers) and pathological gamblers.
Pathological gamblers, substance abusers and matched controls
These participants were tested once usually mid-morning. Twenty-three patients with pathological gambling, according to DSM-IV criteria25 were recruited from the National Problem Gambling Clinic, UK. Most gamblers only stopped gambling following financial ruin recently. All were help-seeking and awaiting treatment. None had a current history of substance abuse. Thirteen patients with a recent history of illicit substance abuse, meeting DSM-IV criteria for substance dependence25 were also tested. Patients were recruited from the Replacement Therapy Unit of Lviv and were receiving buprenorphine. None fulfilled DSM-IV criteria for dementia. Twelve out of 13 patients had a long standing history of intravenous opioid abuse (see Table 1).
All pathological gamblers and 12/13 of the substance abusers were males. Results were compared with 18 age matched male controls.
Beads task
The beads task11 was performed on a laptop computer, usually in the participant’s home or in a quiet room to minimize distractions. Participants were required to guess from which of two cups colored beads were being drawn. The cups differed in the proportion of blue and green beads they contained. For example, one of the cups may have contained 80% blue beads and 20% green beads, whereas the other cup may have contained 80% green beads and 20% blue beads. Participants were first shown a bead draw, which was either blue or green. They could then draw another bead, or guess that the bead was being drawn from the predominantly green or blue cup. This was repeated until they chose to guess one of the cups. We were interested in the number of beads drawn before the participant guessed a cup and whether the urn choice represented a rational (e.g. if more blue beads were drawn the participant guessed blue) or irrational (i.e. the cup color guessed was not most probably correct, given the beads drawn) choice. This is referred to as opposite color choice.
Participants completed 4 blocks of 3 trials each. Two blocks contained an 80/20 ratio of beads and 2 blocks a 60/40 ratio of beads in each cup. (See supplementary material).
Working memory task
PD patients were tested prior and after their usual anti-Parkinson medication in a counterbalanced sequence to account for order effects. Twenty four trials of a WM task were completed on a laptop computer (Fig. S1). Participants were asked to memorize either 2 or 3 geometric figures which were shown for 3 seconds, followed by a delay of 2 seconds. During the delay, distractor images were shown. Then another geometric figure was presented and participants were asked whether this figure was within the set that they had to remember before. In half of the trials 2 geometric figures and in the other half 3 had to be remembered. Distractors could be positive, neutral or negative images taken from the validated International Affective Picture System31.
At the end participants were shown 24 distractor images and were asked whether they thought they had seen the images before. Half of the distractors were shown during the WM task. (See supplementary material).
Results
Demographic and clinical features
Demographic variables (Table 1) were analysed using ANOVA, t-test or χ2 tests where appropriate. There were no differences between the control groups and the matched patient groups on any demographic variables. Significantly more PD−ICB (21/27) than PD+ICB (13/26) patients were taking a dopamine agonist (p=0.024), which is in line with accepted clinical guidelines of managing an ICB in PD. Consistent with the literature4 PD+ICB patients showed a trend to be younger than PD−ICB patients and had a significantly younger disease onset relative to PD−ICB patients (t52=3.28, p=0.002). (See supplementary material).
Beads task
We examined the number of draws each participant made in the different conditions (Fig. 1a). (For total numbers draws per group see Table 1). We found significant effects of group (Wald χ2=191.0, p<0.001), beads ratio (Wald χ2=167.9, p<0.001) and a significant beads ratio by loss condition interaction (Wald χ2=9.4, p=0.002). There was no difference between the 2 control groups on number of draws (Wald χ2=1.0, p>0.3). There was also no correlation between age and number of draws in the control groups (r=−0.15, p>0.37). We then combined the 2 control groups and performed pairwise comparisons between the PD+ICB group and the other groups to examine whether or not the PD+ICB group would perform similar to the other groups (Table 2).
Figure 1.
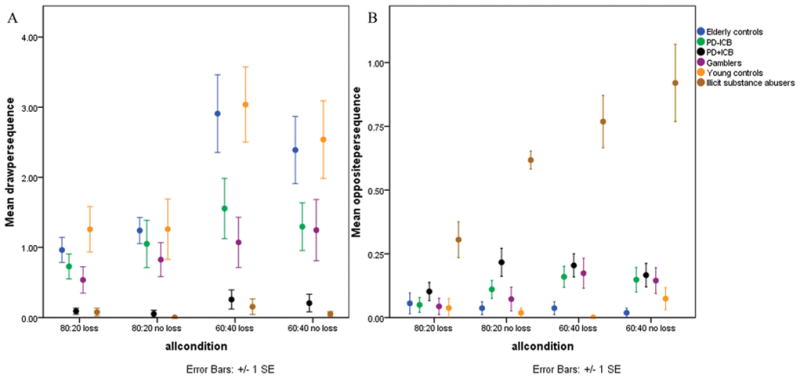
Figure 1a. (left) Average number of draws per condition by group. 1b: (right) Number of times participants chose the opposite color.
Table 2.
Pair-wise comparisons between groups for number of draws (above) and for opposite color choices (below)
| Group (χ2, p-value) | PD−ICB | Illicit substance abusers | Gamblers | Controls |
|---|---|---|---|---|
|
| ||||
| PD+ICB | ||||
| Draws | 27.1, p < 0.001 | 0.38, p = 0.53 | 13.9, p < 0.001 | 75.1, p < 0.001 |
| Opposite color | 4.0, p = 0.044 | 12.2, p < 0.001 | 3.6, p = 0.055 | 30.3, p < 0.001 |
|
| ||||
| PD−ICB | ||||
| Draws | 13.4, p < 0.001 | 0.45, p = 0.8 | 65.1, p < 0.001 | |
| Opposite color | 29.4, p < 0.001 | 0.001, p > 0.97 | 15.0, p < 0.001 | |
|
| ||||
| Addicts | ||||
| Draws | 8.3, p=0.004 | 34.8, p < 0.001 | ||
| Opposite color | 24.0, p < 0.001 | 60.8, p < 0.001 | ||
|
| ||||
| Gamblers | ||||
| Draws | 34.0, p < 0.001 | |||
| Opposite color | 13.9, p < 0.001 | |||
All p-values shown are uncorrected. Values less than 0.0125 (highlighted in bold) for the PD+ICB group are significant. All p-values in this and subsequent tables are for main effect of group.
We found that PD+ICBs were drawing significantly fewer than PD−ICBs (Wald χ2=27.1, p<0.001), pathological gamblers (Wald χ2=13.9, p<0.001) and controls (Wald χ2=75.1, p<0.001). For completeness we also report comparisons between the other groups (Table 2). All pair-wise comparisons showed main effects of beads ratio. Only the PD−ICB vs. control pair-wise comparison additionally showed an interaction between group and beads ratio (Wald χ2=8.0, p=0.005).
Opposite color choice
Next we examined the number of times participants made an irrational choice, summed across all conditions (Fig. 1b). We found a main effect of group (Wald χ2=72.1, p<0.001) and examined effects pair-wise, between groups. Again there was no difference between the two control groups (Wald χ2=0.07, p=0.8), so they were combined (Table 2). Pair-wise comparisons showed that substance abusers chose the opposite color significantly more often than all other groups (all p values<0.001). Further all patients chose the opposite color significantly more often than controls (p<0.001). There was no difference between PD+ICB and PD−ICB patients or pathological gamblers.
Classification of PD+ICBs on the basis of drawing behavior
We used the drawing behavior of individual participants in the 80/20 loss condition to try to predict group membership between the PD+ICB and PD−ICB groups. We used an unblended, supervised classification technique, which required labeled data and found that we correctly classified 25 out of 26 (>96%) PD+ICB patients. We also correctly classified 44% of PD−ICB patients as not having an ICB, giving a positive predictive value of 62.5% and a negative predictive value of 92.3%.
Working memory task
Detailed results are reported in supplemental material. For the WM performance pairwise comparison showed all groups performed better than substance abusers (Table 3), but there were no differences between all the other groups (Fig. 2a). For remembering distractors in the WM task we found a main effect of group (Wald χ2=59.7, p<0.001) and pairwise comparisons showed that PD+ICB patients (Wald χ2=7.2, p=0.007) and pathological gamblers (Wald χ2=15.4, p<0.001) remembered distractors significantly better than PD−ICB patients (Table 3, Fig. 2b).
Table 3.
Pair-wise comparisons for WM performance(above) and for recalling the distractors (below)
| Group (χ2, p-value) | PD−ICB | Illicit substance abusers | Gamblers | Controls Old | Controls Young |
|---|---|---|---|---|---|
|
| |||||
| PD+ICB | |||||
| WM | 2.05, p = 0.15 | 7.2, p= 0.007 | 0.3, p > 0.58 | 4.3, p=0.038 | 0.74, p=0.38 |
| Distractor | 22.8, p < 0.001 | 0.6, p > 0.4 | 12.2, p<0.001 | 5.1, p = 0.023 | 0.002, p>0.9 |
|
| |||||
| PD−ICB | |||||
| WM | 17.0, p< 0.001 | 0.44, p > 0.5 | 0.86, p > 0.35 | 0.1, p > 0.74 | |
| Distractor | 10.1, p=0.001 | 59.8, p=0.001 | 2.4, p = 0.1 | 15.8, p<0.001 | |
|
| |||||
| Addicts | |||||
| WM | 9.3, p = 0.002 | 18.8, p< 0.001 | 10.1, p=0.001 | ||
| Distractor | 13.8, p < 0.001 | 1.6, p = 0.2 | 0.46, p=0.5 | ||
|
| |||||
| Gamblers | |||||
| WM | 1.9, p = 0.16 | 0.78, p > 0.7 | |||
| Distractor | 24.3, p=0.001 | 8.2, p=0.004 | |||
|
| |||||
| Control Old | |||||
| WM | 1.1, p > 0.28 | ||||
| Distractor | 3.7, p=0.055 | ||||
All p-values shown are uncorrected. Values less than 0.0125 are significant.
Figure 2.
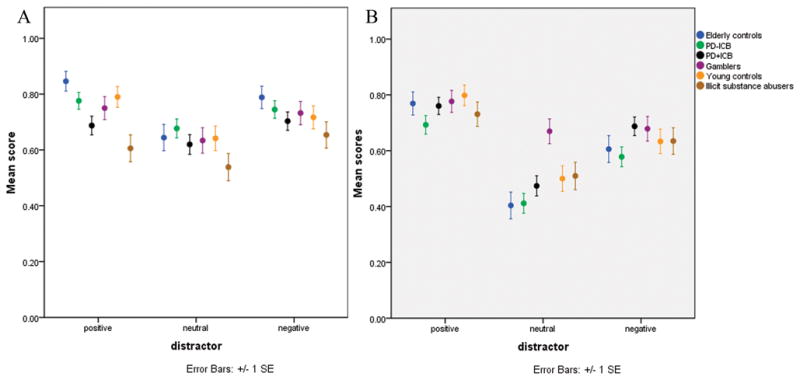
Figure 2a. (left) WM performance. 2b: (right) recalling distractors (positive, neutral, negative).
QUIP questionnaires
Consistent with previous studies29, 32 we found a high sensitivity to detect an ICB (96.1%) for both the patient and caregiver rated QUIP. 40.7% of PD−ICB patients, who did not meet the diagnostic criteria for having an ICB, had at least 1 ICB symptom either self-rated or by their caregiver, consistent with a previous study32. There was no correlation of the QUIP and drawing behavior (see supplementary material).
Discussion
We have examined ‘reflection impulsivity’ using the beads task, an information gathering paradigm in which participants controlled the amount of information they gathered before making a decision11. We compared PD patients with and without ICBs, pathological gamblers and substance abusers and found evidence for impairment even in treated PD patients without clinically apparent ICBs. Across groups we found an effect of the beads ratios, such that participants drew more when the beads ratios were closer to chance (60/40) than when the ratio was greater between the cups (80/20). Further, the loss condition interacted with the beads ratio condition, such that subjects drew relatively more in the higher loss conditions.
Despite all groups showing behavior adaptive to the specific condition, the PD+ICB group drew significantly fewer beads than controls, PD−ICBs and pathological gamblers before making a decision. Significantly less PD+ICB than PD−ICB patients were taking a dopamine agonist and yet they still gathered less information. The fact that the PD+ICB group drew fewer beads than pathological gamblers is intriguing, given that half of the PD+ICB patients had clinically defined pathological gambling. Slot machines, scratch cards and bingo were the most commonly played gambles in PD, pathological gamblers preferred skilled games, such as spread betting and electronic casino games (see Table 1)33–35, which may be of relevance in the interpretation of the results.
Direct comparison between groups on the beads task suggests greater similarities between PD+ICB patients and substance abusers, compared to the pathological gamblers or PD−ICB patients. Positron emission tomography studies have shown sensitization of the ventral striatum in PD+ICB patients36, 37 and also in patients with substance abuse10, 38. Furthermore ‘reflection impulsivity’ does not recover even after prolonged abstinence in substance abusers14. This is consistent with the fact that dopamine agonists have often been withdrawn for a long period in the PD+ICB group leading to alleviation of impulsive symptoms, and yet they still make impulsive choices in the beads task. PD+ICB patients also become irritable when their addictive behavior is restricted28, 39, reminiscent of withdrawal symptoms in drug abusers.
Analysis of the QUIP revealed that 41% of PD−ICB patients had at least 1 symptom of an ICB, either self-rated or rated by their caregiver consistent with previous studies32. Using the beads tasks we classified 56% of PD−ICB patients as having tendencies towards impulsivity, suggesting that this task may be a more sensitive screening tool to detect hidden impulsive traits. Consistent with this, there was no difference in the behavioral pattern between PD−ICB patients and pathological gamblers. This is particularly interesting since none of the gamblers had received any treatment for their impulsivity and none of the PD−ICB patients had clinically defined ICBs. We also found that PD−ICB patients drew significantly less than matched controls.
Several studies have demonstrated increased impulsivity and changes on behavioral tasks in PD−ICB patients after starting dopaminergic medication40–42 in contrast to treatment naive PD patients who perform similarly to controls43. Whether impulsivity arises as a result of increased impulsive drive, decreased inhibitory control or a combination of both is still unclear. However, the results in the PD−ICB group could reflect an underlying increased impulsivity driven by excessive dopamine levels in the ventral striatum. In PD there is much less dopamine loss in the ventral than the dorsal striatum44. Therefore, treatment with dopaminergic medication to increase dopamine levels in the dorsal striatum may lead to excessive levels in the ventral striatum. This may result in a tendency in all treated patients to increased impulsivity, which however does not manifest as clinically significant impulsiveness due to intact inhibitory corticostriatal pathways. Hypoactivation of the orbitofrontal cortex is seen in pathological gamblers, illicit substance abusers45, 46 and in treated PD+ICB patients, but not in PD−ICB patients8. The ventromedial plus the orbitofrontal part of the prefrontal cortex is important for impulse control8, 47, 48 and is associated with ‘jumping to conclusions’ on the beads task49. Thus, intact inhibitory control driven by these cortical areas might prevent PD−ICB patients from clinical impulsivity8.
Jumping to conclusions can also occur in psychosis18. Consistent with this, previous work has shown that PD+ICB participants score highly on measures of schizotypy, a personality trait related to psychosis50. Delusional thinking, defined as a belief based on incorrect inference25, has been reported in PD+ICB patients35, 51 and has been positively correlated with fewer draws on the beads task in delusional patients with and without schizophrenia17. Both PD groups also guessed the opposite color more often than controls and anecdotally some stated that they “anticipated” that the opposite color was more likely and therefore chose the less likely cup. In fact there was no group difference between PD patients and pathological gamblers. However substance abusers chose the opposite color significantly more often than the other groups.
There are important differences between risk taking behaviour, temporal discounting and the beads task. Previous studies have found no21 or restricted52 group differences in risk taking between PD+ICB and PD−ICB patients. In contrast, results on the beads task in the two PD groups were highly significant. The standard temporal discounting task3 is more closely related to self-report questionnaires than metric tasks, and measures sensitivity to rewards delayed by weeks or months. In contrast, drawing more beads only delayed possible rewards by seconds. Not drawing often leads to not winning, or losing in the loss blocks which contrasts with waiting for a larger reward, as occurs with temporal discounting.
Since memory plays an important role in reward learning53, we examined whether the results on the beads task could have been confounded by poor WM. In this WM task we examined the role of distractibility during the delay intervals. There was no correlation between the beads task and WM capacity, which suggests that early decisions relating to the beads were not driven by poor cognitive capacity. We also found that substance abusers had a significantly worse WM capacity than the other groups. This is consistent with previous studies demonstrating poorer attention in substance abusers when required to ignore salient stimuli during WM tasks54. However this finding has to be interpreted with caution since the substance abusers were taking opioid replacement therapy which is known to interfere with WM function55.
Many patients with ICBs conceal their behavior due to shame or denial56. By analyzing data from the 80/20 loss condition we were able to correctly identify ICB patients with a sensitivity of 96%. The beads task might therefore provide a simple screening tool to detect patients at greater risk of ICBs or confirm a clinically suspected but concealed ICB. These results also suggest that a significant proportion of PD−ICB patients is at risk of developing impulsive behavior and thus over time may develop ICBs57. Poor performance on this task suggests that these patients should be monitored frequently by their treating physician and the results taken into consideration when deciding on the use of dopamine agonist treatment. This study is free from the limitations of an indirect study design58 and contains a large number of different groups. Further our results also might have clinical implications, since they imply that PD+ICB patients should be treated like substance abusers rather than patients with behavioral addictions. Additional studies comparing PD−ICB patients on and off dopamine agonists will be necessary to explore the role of dopaminergic medication in cognitive impulsivity.
Supplementary Material
Acknowledgments
The authors wish to thank the patients and families who participated in the study. This research was supported in part by the Intramural Research Program of the NIH, NIMH.
Disclosure
| Stock Ownership in medically- related fields |
Consultancies | Advisory Boards |
Partnerships | Honoraria | Grants | Intellectual Property Rights |
Expert Testimony |
Employment | Contracts | Royalties | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | none | none | none | none | none | none | none | none | none | none | none | none |
| SOS | none | none | none | none | Britannia Pharmaceuticals | none | none | none | none | none | none | none |
| YS | none | none | none | none | none | none | none | none | none | none | none | none |
| SS | none | none | none | none | none | none | none | none | none | none | none | none |
| YM | none | none | none | none | none | none | none | none | none | none | none | none |
| TF | none | none | none | none | Abbott, St Jude Medical, Data Monitoring Committee for Oxford Biomedical | Parkinson’s UK, Cure Parkinson’s Trust and European Union FP7 | none | none | none | none | none | none |
| RM | none | none | none | none | none | none | none | none | none | none | none | none |
| IAO | none | none | none | none | none | none | none | none | none | none | none | none |
| LF | none | none | none | none | none | none | none | none | none | none | none | none |
| KMD | none | none | none | none | none | none | none | none | none | none | none | none |
| YF | none | none | none | none | none | none | none | none | none | none | none | none |
| MS | none | none | none | none | none | none | none | none | none | none | none | none |
| HBD | none | none | none | none | none | none | none | none | none | none | none | none |
| EJ | none | none | none | none | none | none | none | none | none | none | none | none |
| AJL | none | Genus | none | none | Novartis, Teva, Meda, Boehringer Ingelheim, GSK, Ipsen, Lundbeck, Allergan, Orion | PSP Association, Weston Trust – The Reta Lila Howard Foundation | none | none | none | none | none | none |
| BBA | none | none | none | none | none | Wellcome, and the Intramural research program of the NIH | none | none | none | none | none | none |
Footnotes
All authors report no conflict of interest in the content of this paper.
Author Roles:
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique;
AD: 1, 2, 3A
SOS: 1A, 1B, 2C, 3B
YS: 1A, 1B, 2C, 3B
SS: 1A, 1B, 3B
YM: 1A, 1B, 2B
TF: 1B, 2A, 2C, 3B
RM: 1A, 1B, 3B
IAO: 1B, 2C, 3B
LF: 1B, 2C, 3B
KMD: 1B, 2C, 3B
YF: 1B, 2C, 3B
MS: 1A, 1B, 3B
HBD: 1A, 1B, 3B
EJ: 1A, 1B, 2C, 3B
AJL: 1A, 1B, 2C, 3B
BBA: 1A, 1B, 2, 3B
References
- 1.Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121(3):543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalczuk R, Bowden-Jones H, Verdejo-Garcia A, Clark L. Impulsivity and cognitive distortions in pathological gamblers attending the UK National Problem Gambling Clinic: a preliminary report. Psychol Med. 2011:1–11. doi: 10.1017/S003329171100095X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207(4):645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub D. Impulse control disorders in Parkinson’s disease: prevalence and possible risk factors. Parkinsonism Relat Disord. 2009;15 (Suppl 3):S110–113. doi: 10.1016/S1353-8020(09)70794-1. [DOI] [PubMed] [Google Scholar]
- 6.Stacy M. Impulse control disorders in Parkinson’s disease. F1000 Med Rep. 2009:1. doi: 10.3410/M1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Eimeren T, Pellecchia G, Cilia R, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75(19):1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61(4):502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011;31(48):17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 14.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60(5):515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Averbeck BB, Evans S, Chouhan V, Bristow E, Shergill SS. Probabilistic learning and inference in schizophrenia. Schizophr Res. 2011;127(1–3):115–122. doi: 10.1016/j.schres.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12(1):46–77. doi: 10.1080/13546800600750597. [DOI] [PubMed] [Google Scholar]
- 18.Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38 ( Pt 2):113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- 19.Mishra S, Lalumiere M, Williams R. Gambling as a form of risk-taking: Individual differences in personality, risk-accepting attitudes, and behavioral preferences for risk. Personality and Individual Differences. 2010;49(6):616–621. [Google Scholar]
- 20.Barratt ES, Patton J, Olsson NG, Zuker G. Impulsivity and paced tapping. J Mot Behav. 1981;13(4):286–300. doi: 10.1080/00222895.1981.10735254. [DOI] [PubMed] [Google Scholar]
- 21.Djamshidian A, Jha A, O’Sullivan SS, et al. Risk and learning in impulsive and nonimpulsive patients with Parkinson’s disease. Mov Disord. 2010;25(13):2203–2210. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milenkova M, Mohammadi B, Kollewe K, et al. Intertemporal choice in Parkinson’s disease. Mov Disord. 2011;26(11):2004–2010. doi: 10.1002/mds.23756. [DOI] [PubMed] [Google Scholar]
- 23.Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20(4):859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text rev. [Google Scholar]
- 26.McElroy SL, Keck PE, Jr, Pope HG, Jr, Smith JM, Strakowski SM. Compulsive buying: a report of 20 cases. J Clin Psychiatry. 1994;55(6):242–248. [PubMed] [Google Scholar]
- 27.Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- 28.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19(4):397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Hoops S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim SY, Evans AH, Miyasaki JM. Impulse control and related disorders in Parkinson’s disease: review. Ann N Y Acad Sci. 2008;1142:85–107. doi: 10.1196/annals.1444.006. [DOI] [PubMed] [Google Scholar]
- 31.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL, USA: University of Florida; 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 32.Papay K, Mamikonyan E, Siderowf AD, et al. Patient versus informant reporting of ICD symptoms in Parkinson’s disease using the QUIP: validity and variability. Parkinsonism Relat Disord. 2011;17(3):153–155. doi: 10.1016/j.parkreldis.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djamshidian A, Cardoso F, Grosset D, Bowden-Jones H, Lees AJ. Pathological gambling in Parkinson’s disease-a review of the literature. Mov Disord. 2011 doi: 10.1002/mds.23821. [DOI] [PubMed] [Google Scholar]
- 34.Wardle H, Sproston K, Orford J, et al. British Gambling Prevalence Survey. London Stationary office; 2007. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007;22(12):1757–1763. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 36.Evans AH, Pavese N, Lawrence AD, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59(5):852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 37.Steeves TD, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132(Pt 5):1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan GB, Leite-Morris KA, Fan W, Young AJ, Guy MD. Opiate Sensitization Induces FosB/DeltaFosB Expression in Prefrontal Cortical, Striatal and Amygdala Brain Regions. PLoS One. 2011;6(8):e23574. doi: 10.1371/journal.pone.0023574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans AH, Strafella AP, Weintraub D, Stacy M. Impulsive and compulsive behaviors in Parkinson’s disease. Mov Disord. 2009;24(11):1561–1570. doi: 10.1002/mds.22505. [DOI] [PubMed] [Google Scholar]
- 40.Bodi N, Keri S, Nagy H, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132(Pt 9):2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41(11):1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 42.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 43.Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson’s disease. Mov Disord. 2010;25(10):1432–1436. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- 44.Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111 ( Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- 45.Potenza MN, Winters KC. The neurobiology of pathological gambling: translating research findings into clinical advances. J Gambl Stud. 2003;19(1):7–10. doi: 10.1023/a:1021219012324. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 (Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 47.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 48.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123 ( Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 49.Lunt L, Bramham J, Morris R, et al. Prefrontal cortex dysfunction and ‘Jumping to Conclusions’: Bias or deficit? Journal of Neuropsychology. 2011 doi: 10.1111/j.1748-6653.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- 50.Housden CR, O’Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology. 2010;35(11):2155–2164. doi: 10.1038/npp.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolters E, van der Werf YD, van den Heuvel OA. Parkinson’s disease-related disorders in the impulsive-compulsive spectrum. J Neurol. 2008;255 (Suppl 5):48–56. doi: 10.1007/s00415-008-5010-5. [DOI] [PubMed] [Google Scholar]
- 52.Voon V, Gao J, Brezing C, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain. 2011;134(Pt 5):1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 54.Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93(3):270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Rapeli P, Fabritius C, Kalska H, Alho H. Memory function in opioid-dependent patients treated with methadone or buprenorphine along with benzodiazepine: longitudinal change in comparison to healthy individuals. Subst Abuse Treat Prev Policy. 2009;4:6. doi: 10.1186/1747-597X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh A, Kandimala G, Dewey RB, Jr, O’Suilleabhain P. Risk factors for pathologic gambling and other compulsions among Parkinson’s disease patients taking dopamine agonists. J Clin Neurosci. 2007;14(12):1178–1181. doi: 10.1016/j.jocn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Joutsa J, Martikainen K, Vahlberg T, Voon V, Kaasinen V. Impulse control disorders and depression in Finnish patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011 doi: 10.1016/j.parkreldis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Gartlehner G, Moore CG. Direct versus indirect comparisons: a summary of the evidence. Int J Technol Assess Health Care. 2008;24(2):170–177. doi: 10.1017/S0266462308080240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


