Abstract
As a general strategy to selectively target antibody activity in vivo, a molecular architecture was designed to render binding activity dependent upon proteases in disease tissues. A protease-activated antibody (pro-antibody) targeting vascular cell adhesion molecule 1 (VCAM-1), a marker of atherosclerotic plaques, was constructed by tethering a binding site-masking peptide to the antibody via a matrix metalloprotease (MMP) susceptible linker. Pro-antibody activation in vitro by MMP-1 yielded a 200-fold increase in binding affinity and restored anti-VCAM-1 binding in tissue sections from ApoE(−/−) mice ex vivo. The pro-antibody was efficiently activated by native proteases in aorta tissue extracts from ApoE(−/−), but not from normal mice, and accumulated in aortic plaques in vivo with enhanced selectivity when compared to the unmodified antibody. Pro-antibody accumulation in aortic plaques was MMP-dependant, and significantly inhibited by a broad-spectrum MMP inhibitor. These results demonstrate that the activity of disease-associated proteases can be exploited to site-specifically target antibody activity in vivo.
Keywords: pro-antibody, protease, targeting, atherosclerosis, vascular cell adhesion protein 1
Introduction
Therapeutic antibodies are among the fastest growing classes of drugs [1, 2]. Although antibody therapeutics are generally well tolerated, they can give rise to undesirable side-effects that result from target binding in normal tissues [2, 3]. Even though such on-target antibody toxicities typically occur in small fraction of patients, they can have substantial detrimental impacts including costly failures during development [2, 4–6], post-marketing regulatory actions [3], and reduced patient compliance [7]. On-target toxicities have been reported for approved antibodies targeting epidermal growth factor receptor (skin toxicity [8]), vascular endothelial growth factor (kidney and gastrointestinal toxicity, [9]), tumor necrosis factor (infections [10]), and HER-2 (cardiotoxicity [11]). Given these problems, several distinct strategies have been proposed to enhance the selectivity of antibody therapeutics for diseased tissues. For example, bi-specific antibodies recognizing two distinct tumor-associated antigens have been demonstrated to increase the selectivity of targeting Erb2+/Erb3+ tumors [12]. Antibody fusions with vascular permeability enhancing agents such as IL-2 have been shown to enhance tumor uptake by several-fold [13]. The therapeutic index of Trastuzumab was improved roughly ten-fold in xenograft tumor models through co-administration of a neuropilin-1-binding peptide (iRGD), that enhanced antibody delivery into extravascular tumor tissue [14]. Despite these successes, general methods to restrict antibody binding activity to sites of disease, while sparing normal tissues, could serve to minimize on-target toxicities.
Proteolytic enzymes are known to be highly specific to, and locally activated in diverse pathologies including oncological [15], autoimmune [16], cardiovascular [17], and neurodegenerative diseases [18]. Consequently, disease-site active proteases have been exploited to locally activate cytotoxic prodrugs [19] and molecular probes for imaging tumors, arthritic joints, and atherosclerotic plaques [16, 20, 21]. In cardiovascular disease, a leading cause of death in developed countries, proteases are selectively activated in pathological atherosclerotic plaques. In particular, members of the matrix metalloprotease (MMP) family (e.g., MMP-1, 3, 8, 14) have been shown to be activated [22] and could influence plaque stability through their ability to degrade type-1 collagen in the fibrous cap [23]. Antibodies and peptide ligands specific for vascular cellular adhesion molecule-1 (VCAM-1, CD106), a marker highly expressed on plaque endothelial and smooth muscle cells [24], have proven useful for detecting plaques in vivo [25]. Therefore, we sought to exploit the elevated MMP activity in plaques to enhance the in vivo selectivity of an anti-VCAM-1 antibody. Thus, we designed a protease-activated antibody (pro-antibody) to target VCAM-1 in plaques that exhibit MMP activity. Although prodrugs face challenges with regard to their rates of local activation, we reasoned that since the serum half-life of an antibody is typically orders of magnitude greater than that of small molecule prodrugs and imaging probes, a pro-antibody might provide a more effective means to detect and respond to protease activities in vivo. To investigate the potential utility of pro-antibodies in enhancing in vivo tissue targeting selectivity, we compared the selectivity of an anti-VCAM-1 pro-antibody for targeting aortic plaques over normal tissues to that of the unmodified antibody in the widely used ApoE(−/−) mouse model [26] of atherosclerosis. ApoE (−/−) mice exhibit reduced clearance of cholesterol and triglycerides, and when fed with a high fat diet, develop atherosclerotic plaques over a period of 6–9 months that mimic many of the features of human atherosclerosis [26]. Our results demonstrate that antibody activity can be selectively targeted to pathological sites where proteases are activated, while sparing normal tissues that do not exhibit elevated protease activity.
Material and Methods
Reagents, strains, and cell lines
All experiments were performed with E. coli strain MC1061 (F-araD139 (ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL (StrR) hsdR2 (rK − mK+ mcrA mcrB1) [27] grown at 37 °C with vigorous shaking (250 rpm) in either LB medium (10 g tryptone, 5 g yeast extract, and 10 g/L NaCl) supplemented with chloramphenicol (Cm) at 34 μg/mL, or low salt LB medium (10 g tryptone, 5 g yeast extract, 5 g NaCl per liter) supplemented with 50 μg/mL Zeocin. FreeStyle 293-F (Invitrogen) cells and HEK 293 cells were grown in FreeStyle medium and DMEM with 10% FBS, respectively, supplemented with penicillin (25 units/mL), and streptomycin (12.5μg/mL). Matrix metalloproteinase-1 (MMP-1, BIOMOL Intl.), oligonucleotides (Operon Biotechnologies, Huntsville), restriction enzymes (New England Biolabs), lipofectamine (Invitrogen), JetPEI (Genesee Scientific), protein A-agarose resin (Sigma-Aldrich), VCAM-1 (Mouse VCAM-1/Fc Chimera, R&D Systems) peroxidase-conjugated goat anti-mouse (Jackson ImmunoResearch,), SIGMAFAST OPD (Sigma-Aldrich), DAB (3,3 Diamino Benzidine Tetrahydrochloride, 5mg tablets, MP Biomedicals), Safeguard (Fisher), Vectashield Mounting medium (Vector labs, H-1200), DPX mounting medium (Sigma) and Methyl green (Aldrich) were used without modification. Experiments were performed with the following sterile-filtered buffers: HBS-CZP buffer (10 mM HEPES, 150 mM NaCl, 2mM CaCl2, 10 μM ZnCl2, 0.005% tween 20, pH 7.4), coating buffer (65 μM Na2CO3, 135 μM NaH2CO3) blocking buffer (PBS, 5% (w/v) BSA), dilution buffer (PBS, 0.05% (v/v) Tween 20, 0.5% (w/v) BSA), wash buffer (PBS, 0.05% (v/v) Tween 20) and TBS (20mM Tris, pH 7.4, 140 mM NaCl).
Pro-antibody construction, expression and purification
The rat anti-mouse VCAM-1 monoclonal antibody was produced using hybridoma cell line MK271 and purified with an anti-rat IgG resin [28]. A bacterial display peptide library with fifteen randomized amino acids fused to the scaffold’s surface exposed N-terminus [29] was screened for library members that bind anti-VCAM-1 IgG. Magnetic cell separation was used to enrich anti-VCAM-1 binders (2 μM IgG) and then binders were further screened for binding to IgG (200 nM) using FACS (fluorescence activated cell sorting), in the presence of unlabeled, pooled mouse IgG (2 μM). The secondary biotinylated detection reagent was changed in each round (i.e., Streptavidin-Phycoerythrin (PE), Neutravidin-PE, anti-biotin IgG-PE) to disfavor peptides that bind to the secondary reagent.
To construct VCAM-1 pro-antibody expression plasmids, a scFv encoding gene was amplified by PCR, including sequence coding a GGSGGSGGS linker and MMP-1 substrate (VLVPMAMMAS) [28] on the scFv’s N-terminus, while introducing a NcoI restriction site at the 3′ end of the PCR product. PCR products were reamplified to add a GSGGSGG linker and anti-VCAM-1 IgG binding peptide (DGWSCVPTVLRPCA), while introducing an EcoRI site at the 5′ end of the product. A pro-antibody with a non-binding mask that binds streptavidin (WVCHPMWEVMCLR) was similarly constructed. The resulting PCR products were digested with EcoRI and NcoI, ligated into a similarly digested pFUSE-mIgG2A-Fc2 (Invivogen), and transferred to electrocompetent E. coli. For antibody/pro-antibody production, HEK293 cells or FreeStyle 293-F were transfected with scFv-Fc fusion vectors. To produce a stable cell line HEK293 cells were transfected with JetPEI (20 μg plasmid DNA / 1 x 107 cells). On day 2, the medium was replaced with FreeStyle medium for expression and selection. To produce transiently transfected cells 293-F cells were transfected using Lipofectamine (375 μg plasmid DNA / 2.5 x 108 cells) and grown in FreeStyle medium. Antibody titers were measured using ELISA. ScFv-Fc was purified using protein A agarose (Pierce) following the manufacturer’s protocol, concentrated using Amicon spin columns (10 kDa cutoff), and dialyzed in HBS-CZP buffer for 16 hrs.
Characterization of pro-antibody activation by ELISA and surface plasmon resonance
To assess antibody masking and protease mediated activation using ELISA the extracellular domain of VCAM-1 (R&D systems) was incubated in a 96-well plate (Nunc Immunosorb) for 12–20 hrs at 4°C (1 ng/μL, 100 μL/well). Plates were blocked for 2 hrs at 37°C and antibody/pro-antibody samples were incubated either with buffer only (HBS-CZP), or with 30 nM MMP-1 for 2 hrs at 37°C. Wells were washed three times with wash buffer and incubated with the indicated dilution of antibody or pro-antibody for 2 hrs at 25°C. Wells were washed three times and incubated with peroxidase-conjugated goat-anti-mouse Fc IgG (Jackson Immunoresearch, 0.4 nM) for 1–2 hrs at room temperature. Wells were washed three times and 200 μL of Sigma Fast OPD solution was added to each well. Antibody binding was quantified by measurement of A450 nm using a Tecan Safire spectrophotometer. Background signal (wells without antigen pre-incubation) was subtracted from the gross signal.
To confirm pro-antibody activation, binding was measured before and after treatment with MMP-1 using SPR (surface plasmon resonance, BIAcore 3000, GE Healthcare). The VCAM-1 ED was coupled to CM5 sensor chips activated with a 1:1 (v/v) mixture of 0.4 M EDC (N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide) and 0.1 M NHS (N-hydroxysuccinimide) for 7 min at a flow rate of 5 μL/min using HBS-EP running buffer. After coupling, excess activated carboxyl groups were blocked using 1 M ethanolamine (pH 8.5) injected at 5μL/min for 7 min. The VCAM-1 ED was diluted in 10 mM sodium acetate buffer (pH 4.5) and immobilized (2800 RU). Another flow-cell surface was activated and deactivated to serve as a reference. Binding measurements were made using HBS-CZP as the running buffer. Samples were exposed to 30 nM MMP-1 for 4 hrs at 37 °C, and both protease-treated and untreated pro-antibody were injected for 10 min at 25 μL/min. Responses from the reference surface were subtracted.
Measurement of tissue specific protease activity 2and ex vivo pro-antibody binding
ApoE(−/−) mice were euthanized and tissue was collected immediately following anesthesia. Samples were frozen in liquid nitrogen until used for the assay. Frozen tissue samples were homogenized in homogenizing buffer (HBS-CZP, 0.1% triton X-100 (w/v), protease inhibitor cocktail). The inhibitor cocktail (Sigma) selected did not contain MMP inhibitors or metal chelators. Homogenized samples were centrifuged at 21k rcf at 4 °C for 10 min. Supernatant was collected and frozen in nitrogen. For the assay, 5-fold dilutions of the homogenate were incubated with indicated concentrations of the antibodies for 2 hrs at 37 °C. Samples were diluted and ELISA was performed as described previously.
Purified antibody was labeled using Alexa-546 labeling kit (Invitrogen) and was tested for binding activity. For confocal microscopy imaging, anti-VCAM-1 (100 nM) antibody was used to stain explanted tissues. Samples were blocked for 1 hour with TBS supplemented with 4 % (v/v) FBS, then incubated with the antibody at 4 °C for 16 hrs, washed, and mounted with DAPI-containing mounting solution (Vector labs). For semi-quantitative analysis, samples were treated the same as above using 50 nM antibody.
Imaging of anti-VCAM-1 antibody localization after administration to ApoE(−/−) mice
Atherosclerotic plaques in transgenic mice homozygous for the Apoetm1Unc mutation (ApoE-null mice, The Jackson Laboratory) were induced by keeping the mice on high fat diet [30, 31] for 6 to 8 months. Healthy aortas were obtained from age-matched C57BL/6 wild-type mice fed on a normal diet or normal BALB/c mice. The mice were housed and all procedures performed according to standards of the UCSB Institutional Animal Care and Use Committee. To assess the in vivo selectivity of the anti-VCAM-1 antibody or pro-antibody for plaques, mice were injected intravenously with FITC-conjugated anti-VCAM-1 at 4 mg/kg, 80–150 μL per injection, via the retro-orbital route under isoflurane inhalation (isoflurane 2 % –3 % (vol/vol); 2 L/min O2). After circulation for 22 hrs, blood was cleared from anesthetized mice (under Avertin, 30 mg/mL) by perfusing with high glucose DMEM media through the left ventricle. Tissues including aorta were excised for cellular extract preparation or flash-frozen in liquid nitrogen and embedded in OCT blocks. Fresh frozen OCT-embedded tissues were serially cross-sectioned (7 μm thickness) and immediately fixed with acetone. Samples were then blocked with Tris buffered saline (TBS) supplemented with 4% (v/v) FBS for one hour at room temperature, and then incubated with anti-FITC conjugated to peroxidase (GeneTex) diluted 1:300 in TBS supplemented with 0.4 % (v/v) FBS for 16 hrs at 4 °C. Following washing, sections were incubated with DAB (3,3-diamino benzidine tetrahydrochloride, 5 mg tablets, MP Biomedicals) for 2–10 min., and terminated in water. Samples were stained with Methyl green (1 % (w/v), Sigma) for 4 min. Samples were dehydrated by washes with ethanol and SopV (Safeguard, Fisher) and lastly covered with DPX mounting solution (Sigma) and cover slips.
Explanted tissue sections were imaged using an Olympus Fluoview 500 microscope equipped with a 20x objective lens. An Olympus IX 70 with Q-imaging camera was used for the semi-quantitative analysis of ex-vivo staining (Fig. 3b) with 60x magnification. For each section, an image was acquired with blue filtration (DAPI staining), to confirm comparable cell number. An Olympus BX60 with MacroFire camera was used for imaging of immunohistochemical staining.
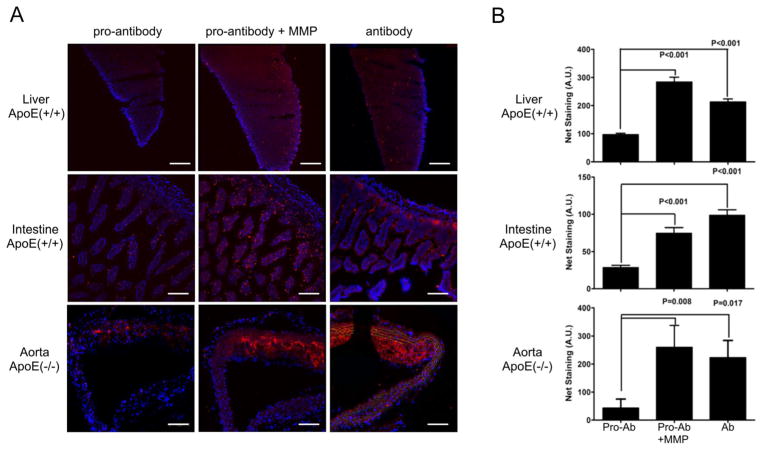
Figure 3.
Analysis of anti-VCAM-1 antibody and pro-antibody binding to explanted tissue sections using fluorescence microscopy. (A) Tissue sections from ApoE(+/+) (liver, intestine) and ApoE(−/−) (aortas) mice were stained with Alexa546-conjugated anti-VCAM-1 antibody or pro-antibody (100 nM), before and after treatment with MMP-1. (Red - Alexa546;– Blue). Original magnification ×20, Scale bars, 100 μm.. (B) Explanted tissues were stained with Alexa546-conjugated anti-VCAM-1 antibody or pro-antibody (50 nM). Bars represent average and standard error of the integrated fluorescence signals from 4–6 fields for each tissue. Significance was confirmed using both Student’s t-test (shown), and one-way ANOVA analysis with Bonferroni’s correction. However, the staining signal difference in the aorta between the pro-antibody and antibody samples did not reach statistical significance as determined using Bonferroni’s multiple comparison test.
Measurement of protease-mediated pro-antibody binding in aortas
Explanted aorta from ApoE(+/+) and ApoE(−/−) mice were perfused with 40 mL DMEM, and cut en-face to expose plaques. Explants were incubated with FITC labeled anti-VCAM-1 pro-antibody (200 nM) or anti-VCAM FITC (200 nM; eBioscience) with or without 33 μM Galardin (~1000 x highest known MMP IC50; ) in HZP buffer (minus Tween20) a slow rotary shaker (80 rpm) for 3hrs in the dark. Samples were washed (3X) with PBS at 37 °C with rotation (75 rpm) for 5 min, partitioned, and embedded in OCT for sectioning and immunohistochemical analysis.
Statistical methods
P-values indicate the confidence of statistically significant differences between samples calculated using two-tailed Student’s t-test, Bonferroni’s multiple comparison test, or Mann-Whitney test where indicated.
Results
Construction and characterization of an anti-VCAM-1 pro-antibody
To construct an antibody whose binding activity is activated by site specific proteolysis (i.e., pro-antibody), we reasoned that peptides that bind specifically to the antibody’s antigen-binding site would effectively block or mask the antibody-antigen interaction if tethered via the N-terminus adjacent to the antibody binding-site (Fig. 1). Thus, binding site-masking peptide ligands were identified by screening a large bacterial display peptide library [32] using flow cytometry for binders to an anti-mouse VCAM-1 IgG. To favor high specificity for the anti-VCAM-1 antibody binding site, screening was performed in the presence of excess pooled serum IgG (2 μM)[33]. Six cycles of sorting and re-growth enriched a population of cells displaying peptides specific for anti-VCAM-1 IgG. Three of the identified peptides (DGWSCVPTVLRPCAH; RLDGWTCVPTVEAAC; TCVPTRYGSCITDAR) exhibited a consensus of S/TCVPTVxxxC, and their apparent affinities for anti-VCAM-1 IgG were measured. The peptide with the highest apparent (bivalent) affinity (DGWSCVPTVLRPCA; KDAPP = 80 nM) for anti-VCAM-1 IgG was chosen to construct a pro-antibody. The identified peptide mask was then tethered to the N-terminus of the antibody using a linker containing a peptide substrate (VLVPMA↓MMAS) that is efficiently hydrolyzed by collagenases including MMP-1, 8, and -14 [34]. Since this substrate, like other reported peptide substrates for MMPs, is not selective for one single MMP species, hereafter we refer to it as an MMP-cleavable substrate. For pro-antibody and antibody production, single-chain variable fragments (scFv) with and without the masking peptide were directly fused to the N-terminus of a mouse constant domain (Fc). Stable, antibody/pro-antibody secreting HEK293 cell lines were generated, and constructs were purified using protein A affinity chromatography (Fig. S1, Supplementary information).
Figure 1.
Protease-activated antibody (pro-antibody) design and construction. A pro-antibody is constructed by tethering a peptide (mask) that blocks the antibody’s binding site to the antibody N-terminus via a flexible linker containing a protease substrate. A scFv-Fc fusion was used for simplicity. Masking attenuates binding activity until selective substrate cleavage releases the mask and fully restores antibody-binding activity.
Pro-antibody binding affinity is enhanced by proteolysis
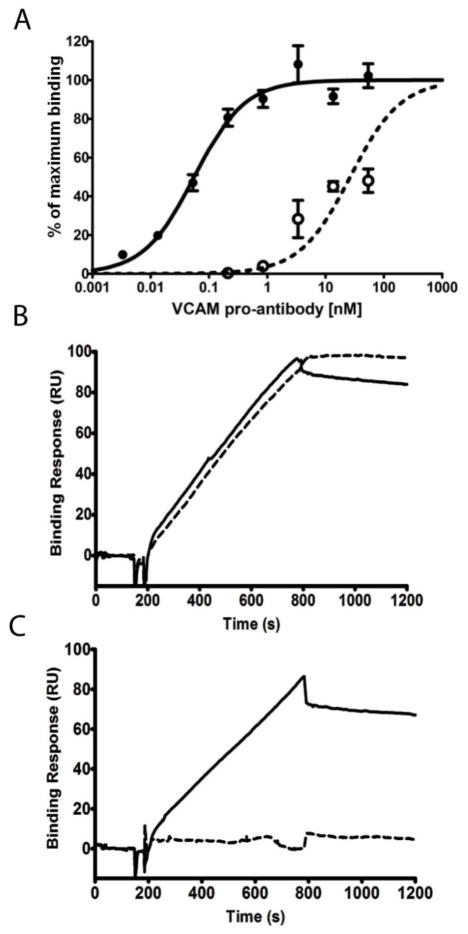
To determine whether a peptide mask tethered to the antibody N-terminus attenuated antibody binding affinity, binding was measured before and after proteolysis. The pro-antibody exhibited a roughly 200-fold increase in apparent binding affinity after exposure to MMP-1, when measured by ELISA (Fig. 2A). Since the unbound mask could potentially contribute to a reduced apparent binding affinity of the pro-antibody through steric blocking of the combining site or closely spaced antigen binding sites [**], we constructed a pro-antibody wherein the masking peptide was replaced with a non-binding peptide. In contrast to the masking peptide, the non-binding mask reduced affinity by less than two-fold and was unaffected by MMP-1 treatment (Fig. S2A). Similarly, surface plasmon resonance measurements revealed that the anti-VCAM-1 pro-antibody exhibited protease dependent VCAM-1 binding, while the unmodified antibody was unaffected by MMP-1 treatment (Fig. 2B, C). The pro-antibody association rate was substantially increased by proteolysis, while the dissociation rate was unchanged, indicating that the masking effect is mediated primarily by a reduction in the association rate constant.
Figure 2.
Pro-antibody binding affinity is enhanced by proteolysis. (A) Binding activity of the anti-VCAM-1 pro-antibody before (○) and after (●) proteolysis, as measured by ELISA. Association and dissociation of (B) the anti-VCAM-1 antibody and (C) pro-antibody before (--) and after (-) proteolysis as measured by SPR.
Given the very slow association rate exhibited by the masked pro-antibody, pro-antibody association with VCAM-1 was measured over prolonged durations to simulate in vivo experimental conditions. Antibody and pro-antibody binding activities were measured at intervals over 24 hrs by ELISA. At low antibody concentrations (5 nM) that approximate normal tissue extravascular therapeutic IgG concentrations [35], the unmodified anti-VCAM-1 antibody exhibited maximum binding after eight hours while the pro-antibody did not exhibit detectable binding (Fig. S2B, Supplementary information). At higher concentrations (25 nM), the level of unmodified antibody binding reached equilibrium within roughly ten minutes, while the pro-antibody exhibited slow association, and an attenuated binding after four hours. As expected, pre-treatment of the pro-antibody with MMP-1 restored the normal association rate, and increased the maximum signal at equilibrium (Fig. S2C, Supplementary information). Thus, at concentrations comparable to those in normal tissues after IV administration, pro-antibody association was substantially reduced when compared to the unmodified antibody.
Plaque specific protease activity restores antibody binding activity
To determine whether pro-antibody binding to VCAM-1 was attenuated in normal tissues without MMP activity, tissue sections from ApoE(−/−) or ApoE(+/+) mice were incubated with Alexa546-conjugated antibodies or pro-antibodies, that were either pre-activated or not activated with MMP-1 (Fig. 3). The unmodified anti-VCAM-1 antibody exhibited staining in aortic plaques, and weak to moderate staining in the liver and intestine (Fig. 3). In contrast, pro-antibody staining occurred only in aortic plaques. The pro-antibody exhibited a significant reduction of staining of normal tissue (p < 0.001 for liver & intestine) and aortic plaque tissue sections (p = 0.008) Fig. 3B). However, when the same pro-antibody was pre-activated with MMP-1, staining in both normal tissues and plaques was comparable to that of the unmodified antibody. Similar results were observed in the spleen and lung tissues (Fig. S3, Supplementary information). Thus, the pro-antibody but not the antibody exhibited reduced binding in normal tissues, and pro-antibody binding could be restored by pre-activation with MMP-1.
To investigate whether MMP activity is sufficiently elevated in aortic plaques to activate pro-antibodies, activation was measured in homogenates prepared from aortas and normal tissues from both ApoE(−/−) and healthy ApoE(+/+) mice (Fig. 4). Pro-antibody binding activity resulting from exposure to tissue homogenates was measured quantitatively by ELISA. Importantly, the pro-antibody was significantly activated (p = 0.0012) by aortic homogenates prepared from ApoE(−/−) mice, but not those from healthy ApoE(+/+) mice (Fig. 4B). For comparison, identically prepared homogenates from the intestine did not significantly activate the anti-VCAM-1 pro-antibody. Kidney homogenates partially activated the pro-antibody at a level well below that induced by aorta homogenates. Thus, under these assay conditions, proteases present in normal tissues did not appreciably activate the pro-antibody. The VCAM-1 binding activity of the unmodified antibody was not affected by incubation with any tissue extracts indicating that other proteases present in these samples do not modulate VCAM-1 binding activity (Fig. 4A). These results are in agreement with prior reports that MMP activity is increased in aortic plaques in the ApoE(−/−) mouse model of atherosclerosis [36], and demonstrate that plaque localized proteases can activate the anti-VCAM-1 pro-antibody.
Figure 4.
Aortic plaque homogenates proteolytically activate pro-antibodies. (A) Anti-VCAM-1 Antibody and, (B) pro-antibody binding activity after incubation in assay buffer or with tissue homogenates from ApoE(−/−) or ApoE(+/+) mice, as measured by ELISA. Error bars represent the standard error of the mean of triplicate measurements. Significance was confirmed using both students T-test (shown), and one-way ANOVA analysis with Bonferroni’s multiple comparison test.
A pro-antibody selectivity targets plaques in vivo in an MMP-dependent manner
To determine whether the anti-VCAM-1 pro-antibody selectively targets plaques in vivo in the ApoE(−/−) mouse model of atherosclerosis [26], the pro-antibody and antibody were administered by intravenous injection (4 mg/kg) to mice that were on a high fat diet for approximately 32 weeks. To assess the bio-distribution of the antibody and pro-antibody, one day after administration, tissues were recovered for immunohistochemical analysis. Analysis of frozen aorta tissue sections prepared from mice injected with the antibody or pro-antibody (n = 2) revealed large plaques that stained strongly for the presence of the anti-VCAM-1 antibody and pro-antibody (Fig. 5A, F). Antibody and pro-antibody staining in aortic plaques co-localized with the endothelial cell marker CD31 and, to a lesser extent, with macrophage cell marker CD11b (Fig. S3, Supplementary information). The unmodified anti-VCAM-1 antibody reproducibly localized at low to moderate levels in the intestine, kidney, lung and pancreas (Fig. 5B–E). In contrast, the pro-antibody was not detected in any of these tissues (Fig. 5G–J) despite the strong staining observed in plaques (Fig. 5A, F). Additionally, the pro-antibody did not localize to the aorta or other tissues of normal mice after IV administration (Fig. 5K–O). Thus, the anti-VCAM-1 pro-antibody, but not the unmodified antibody, selectively localized to aortic plaques that exhibit both VCAM-1 expression and MMP activity.
Figure 5.
Immunohistochemical localization of anti-VCAM-1 antibody and pro-antibody accumulation in vivo in ApoE(−/−) and ApoE(+/+) mice. Anti-VCAM-1 antibody or pro-antibodies were administered to ApoE (− /− (A–J) or ApoE(+/+) (K–O) mice, and the indicated tissues were recovered after 24 hrs and analyzed for the presence of the antibody (A–E) or pro-antibody (F–O) by immunoperoxidase staining (indicated by brown color). Representative images (n = 4) of immunoperoxidase staining of tissues from mice injected with anti-VCAM-1 pro-antibody showed positive signal only in plaques, unlike tissues from anti-VCAM-1 antibody-treated mice. Original magnification: A,F,K: X10, B–E, G–J, L–O: ×20. Scale bars, 100 μm.
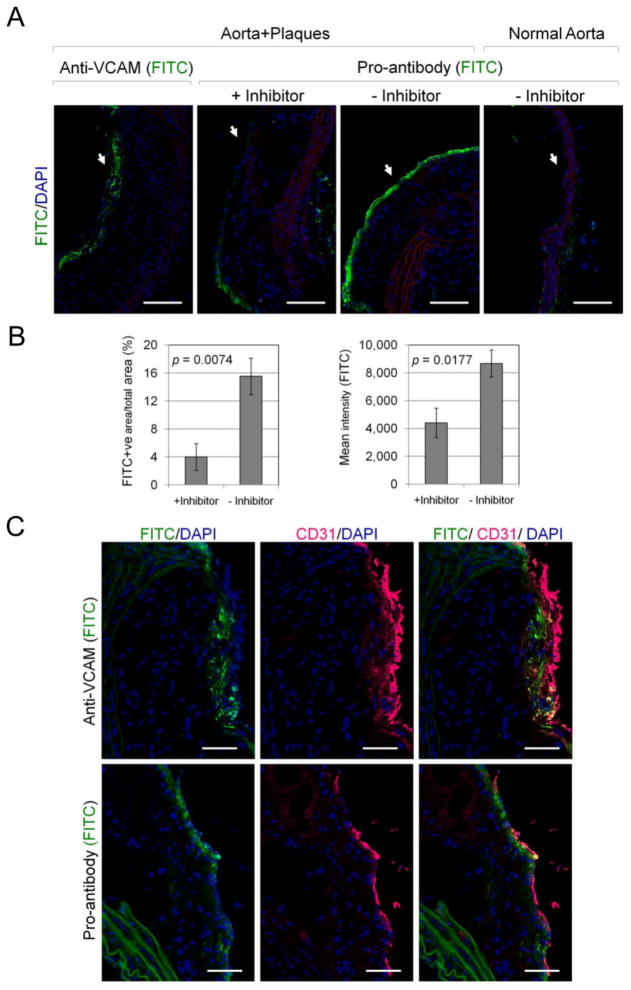
To determine whether pro-antibody accumulation in aortic plaques is mediated by proteolysis, as opposed to protease-independent binding, aorta explants were incubated with the pro-antibody in the presence and absence of the MMP inhibitor Galardin [37] (Fig. 6). In the absence of MMP inhibitor, the pro-antibody robustly stained aortic plaques from ApoE(− /−) mice but not aorta from normal mice. However, in the presence of the MMP inhibitor, pro-antibody accumulation in aortic plaques was decreased by four-fold (FITC-positive image area; p = 0.0074) and two-fold with regard to FITC fluorescence intensity (p = 0.018, n=3) (Fig. 6B). Antibody and pro-antibody staining in aortic plaques co-localized with endothelial cell marker CD31 (Fig. 6C), indicating that both molecules target activated endothelial cells. Thus, the accumulation of anti-VCAM-1 pro-antibody in aortic plaques was significantly enhanced by MMP activity present in plaques.
Figure 6.
Pro-antibody binding to aorta explants in the presence and absence of MMP inhibitor. (A) Cross-section of aortas comparing the bound FITC-labeled monoclonal VCAM-1 antibody and pro-antibody within luminal endothelial surface of plaque tissue (arrow; green). Original magnification ×20. Scale bars, 100 μm. (B) Quantitative analysis of pro-antibody binding to plaques in the presence and absence of MMP inhibitor expressed as FITC-positive area / total selected area (Mean % ± SE; from n=3 mice/group), and mean fluorescence intensity (Mean ± SE; from n=3 mice/group). (C) Localization of endothelial cell marker (anti-CD31, magenta) and anti-VCAM-1 antibody or pro-antibody-FITC (green) in aorta sections. Original magnification ×40. Scale bars, 50 μm. Significance was confirmed using Student’s t-test (shown) and a Mann-Whitney test.
Discussion
Here, we demonstrated that an MMP-activated anti-VCAM-1 antibody, or pro-antibody, more selectively targets aortic plaques in ApoE(−/−) mice where MMPs are active when compared to a conventional antibody. And importantly, the pro-antibody did not accumulate to the same levels as the conventional antibody in healthy tissues at 24 hrs. Pro-antibody accumulation in aortic plaques without accumulation in healthy tissues is consistent with a localization mechanism wherein i) circulating pro-antibodies biodistribute into tissues in a manner similar to that observed for conventional antibodies, ii) locally distributed pro-antibody is activated by MMP cleavage, and iii) the activated species associates with antigen. Anti-VCAM-1 antibodies and peptides have been used previously to target imaging agents to atherosclerotic plaques [38–40] and other pathologies [41]. However, since VCAM-1 expression is up-regulated in both early and late plaques, VCAM-1 expression does not provide a measure of cardiovascular risk [42, 43]. In contrast, the local activation of MMPs capable of degrading the collagen fibrous cap [44], has been proposed to play a role in plaque destabilization [45, 46]. In agreement with prior reports, our results indicate that MMP inhibitor sensitive proteases are activated in large advanced plaques in ApoE(−/−) mice. Thus, an MMP-activated VCAM-1 pro-antibody might be useful to differentiate plaques with low or high MMP activity. More generally, since the pro-antibody exhibited reduced accumulation in normal tissues, similarly constructed pro-antibodies could be useful for the development of in vivo molecular imaging and diagnostic agents.
Here, recombinant tethering of a binding-site masking peptide adjacent to the antibody binding-site of the pro-antibody provided a means to achieve high local mask concentration to drive the equilibrium pro-antibody state towards a masked state (Fig. 1). For a highly flexible linker with a time-averaged length of 1–2 nm, the local effective concentration can be estimated to be greater than 1 mM [47]. Consequently, even masks with modest affinity (~1 μM) for the antibody binding-site should be capable of masking the antibody’s binding activity. The reduced apparent binding affinity of the pro-antibody could be potentially be impacted by steric hinderance and/or by shielding antigen binding sites adjacent to the antigen-antibody complex as has been described for PEGylated antibodies [48]. However, a pro-antibody wherein the masking peptide did not bind to the antibody binding site exhibited only slight reductions in apparent affinity (<2-fold) while the masked antibody exhibit more than 200-fold affinity reduction. This result indicates that the primary mechanism for affinity attenuation is through binding of the masking peptide to the antibody. In the present study, non-optimized peptide masks identified by bacterial display peptide library screening were capable of inhibiting the activity of the antibody by roughly 200-fold in vitro. However, in vivo targeting applications could benefit from an improved dynamic range between the affinity of the masked and unmasked pro-antibody. Previous studies indicate that the masking effect and dynamic range can be improved by modulating linker length and mask affinity. Covalent tethering of a masking ligand (KD of 500 nM) to a catalytically inactive enzyme via a flexible linker increased the apparent equilibrium dissociation constant of the protein towards it’s ligand from 0.2 nM (without masking) to 6 μM (masked) - a 30,000-fold change in the apparent affinity [47]. Similarly, a tethered receptor ligand system consisting of tumor necrosis factor alpha (TNFα) joined to a TNF receptor fragment attenuated the activity of the fusion protein by 1000-fold [49]. These results suggest that intramolecular inhibitors of modest affinity are sufficient to attenuate intermolecular binding. From the reported study of an enzyme-tethered inhibitor [47], one can estimate that a mask affinity of 1 nM – 1 μM will maintain a balance between efficient masking, and mask dissociation upon cleavage. Given these observations, affinity maturation of masking peptides using display technologies can be applied to further attenuate pro-antibody binding activity.
Therapeutics that are selectively active at sites of pathology, or pathologically-activated therapeutics [50], represent an attractive strategy to overcome side effects that result from target blockade in normal tissues. This concept has been most extensively applied to develop small molecule prodrugs [51]. For example, the N-methyl-d-aspartate blocker Memantine, approved for the treatment of Alzheimer’s disease, preferentially targets pathologically open ion channels over normal ion channels, and is well tolerated with few side effects [52]. The pathological activation of proteases in tumors has been successfully exploited to develop protease-activated cytotoxic drugs including legumain-activated doxorubicin [53], and legumain-activated auristatin [54]. Legumain-activated auristatin was capable of suppressing tumor growth and metastasis without the toxicity associated with the naked drug. In contrast to small molecule prodrugs that have short half-lives, a pro-antibody would be expected to exhibit an in vivo half-life comparable to an antibody (15–20 days). Although pro-antibody half-life was not measured in this study, this long half-life could provide a greatly extended window for protease-mediated activation, provided that the substrate remains stable in circulation. Consequently, similarly constructed pro-antibodies might have utility for drugging targets present at high levels in normal tissues, that cannot be safely drugged by conventional antibodies or antibody drug conjugates.
The use of pro-antibodies to increase the selectivity of antibody targeting in vivo could have utility for decreasing the toxicities of antibody therapeutics, particularly those intended to act in well-defined tissue compartments including, for example, plaques, tumors and joint synovium where proteases have been shown to play critical roles in pathology. Anti-VCAM-1 therapeutic antibodies have exhibited efficacy in small animal models of arthritis [55], glioma [56], CDD-induced colitis [57], and atopic dermatitis [58]. Despite the encouraging results of these studies, successful translation of anti-VCAM-1 therapeutic antibodies may require strategies to restrict antibody activity to disease sites since a single dose of anti-VCAM-1 antibody has been shown sufficient to induce leukocytosis in healthy mice within 20 hrs [55]. agreement with this finding, antibody blockade of the VCAM-1 binding integrins has been shown to result in altered leukocyte trafficking and fetal hematopoeisis [59]. The enhanced selectivity of pro-antibodies could also be useful for increasing the selectively of antibody-drug conjugates wherein low level targeting of normal tissues has resulted in dose limiting side effects [60, 61] and fatalities [5]. Similarly, pro-antibodies might provide a means to reduce the potential toxicities of bi-specific and multi-targeted antibodies in development [62], or to drug targets that have been discarded because of their broad tissue distribution. As has been observed for antibodies that target antigens restricted to pathological sites, pro-antibodies might be useful to avoid normal tissue sinks that increase the rate of antibody clearance from circulation [63].
Conclusions
Here, we demonstrated that a protease- activated antibody can selectively target sites of protease activation in vivo, while sparing normal tissues that are targeted by an antibody. Specifically, we showed that an anti-VCAM-1 pro-antibody can be activated by the atherosclerotic plaque associated protease MMP-1, and can selectivity target MMP producing aortic plaques in vivo with reduced binding to normal and other tissues. The enhanced in vivo tissue selectively of pro-antibodies may be a useful to develop diagnostic imaging agents and antibody therapeutics whose activity is restricted to sites of protease activation.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants 5 U54 CA119335-04; PEN 5 U01 HL080718-04, 1 R21 CA159232-01, 1 R01 GM097114-01A1 and a postdoctoral fellowship to O.E. from the California Institute for Regenerative Medicine. We thank Claudia Gottstein for providing the anti-VCAM-1 antibody.
Footnotes
Disclosure Statement: The authors declare competing financial interests. PSD and JMT named co-inventor’s in patent applications relating to this work. PSD is a consultant, SAB member, and stock holder of CytomX Therapeutics, Inc. (South San Francisco).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 3.Giezen TJ, Mantel-Teeuwisse AK, Straus SM, Schellekens H, Leufkens HG, Egberts AC. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA. 2008;300(16):1887–1896. doi: 10.1001/jama.300.16.1887. [DOI] [PubMed] [Google Scholar]
- 4.Li JL, Jubb AM, Harris AL. Targeting DLL4 in tumors shows preclinical activity but potentially significant toxicity. Future Oncol. 6(7):1099–1103. doi: 10.2217/fon.10.62. [DOI] [PubMed] [Google Scholar]
- 5.Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, van Dongen GA. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12(20 Pt 1):6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 6.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 65(4):765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gridelli C, Morabito A, Gebbia V, Mencoboni M, Carrozza F, Vigano MG, Verusio C, Bollina R, Mattioli R, Valerio MR, Valmadre G, Maione P, Rossi A, Cascone T, Morgillo F, Di Maio M, Piccirillo MC, Gallo C, Perrone F, Ciardiello F. Cetuximab and gemcitabine in elderly or adult PS2 patients with advanced non-small-cell lung cancer: The cetuximab in advanced lung cancer (CALC1-E and CALC1-PS2) randomized phase II trials. Lung Cancer. 67(1):86–92. doi: 10.1016/j.lungcan.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9):1425–1433. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 9.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 11.Perez EA. Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clin Breast Cancer. 2008;8(Suppl 3):S114–120. doi: 10.3816/cbc.2008.s.007. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, von Mehren M, Shchaveleva I, Simmons HH, Marks JD, Adams GP. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99(9):1415–1425. doi: 10.1038/sj.bjc.6604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBerthon B, Khawli LA, Alauddin M, Miller GK, Charak BS, Mazumder A, Epstein AL. Enhanced tumor uptake of macromolecules induced by a novel vasoactive interleukin 2 immunoconjugate. Cancer Res. 1991;51(10):2694–2698. [PubMed] [Google Scholar]
- 14.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 16.Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004;50(8):2459–2465. doi: 10.1002/art.20379. [DOI] [PubMed] [Google Scholar]
- 17.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115(17):2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 18.Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9(1):3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson JM, Siller CS, Gill JH. Tumour endoproteases: the cutting edge of cancer drug delivery? Br J Pharmacol. 2008;153(7):1344–1352. doi: 10.1038/sj.bjp.0707657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26(24):4012–4021. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105(23):2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 22.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69(3):625–635. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92(6):1565–1569. [PubMed] [Google Scholar]
- 24.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171(3):223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay AC, Choudhury RP. Form to function: current and future roles for atherosclerosis imaging in drug development. Nat Rev Drug Discov. 2008;7(6):517–529. doi: 10.1038/nrd2588. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 27.Casadaban MJ, Cohen SN. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 28.Dienst A, Grunow A, Unruh M, Rabausch B, Nor JE, Fries JW, Gottstein C. Specific occlusion of murine and human tumor vasculature by VCAM-1-targeted recombinant fusion proteins. J Natl Cancer Inst. 2005;97(10):733–747. doi: 10.1093/jnci/dji130. [DOI] [PubMed] [Google Scholar]
- 29.Rice JJ, Daugherty PS. Directed evolution of a biterminal bacterial display scaffold enhances the display of diverse peptides. Protein Eng Des Sel. 2008;21(7):435–442. doi: 10.1093/protein/gzn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Maeda N, Johnson L, Kim S, Hagaman J, Friedman M, Reddick R. Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis. 2007;195(1):75–82. doi: 10.1016/j.atherosclerosis.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daugherty PS. Protein engineering with bacterial display. Curr Opin Struct Biol. 2007;17(4):474–480. doi: 10.1016/j.sbi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Hall SS, Daugherty PS. Quantitative specificity-based display library screening identifies determinants of antibody-epitope binding specificity. Protein Sci. 2009;18(9):1926–1934. doi: 10.1002/pro.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulware KT. PhD. University of California; Santa Barbara: 2007. Cellular libraries of peptide substrates (CLiPS): A high-throughput method for proteolytic enzyme characterization, Chemical Engineering; p. 123. [Google Scholar]
- 35.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60(12):1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng C, Tempel D, van Haperen R, van Damme L, Algur M, Krams R, de Crom R. Activation of MMP8 and MMP13 by angiotensin II correlates to severe intra-plaque hemorrhages and collagen breakdown in atherosclerotic lesions with a vulnerable phenotype. Atherosclerosis. 2009;204(1):26–33. doi: 10.1016/j.atherosclerosis.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Wu W, Yang G, Li J, Ye Q. Tetrahydroisoquinoline based sulfonamide hydroxamates as potent matrix metalloproteinase inhibitors. Bioorg Med Chem Lett. 2004;14(1):47–50. doi: 10.1016/j.bmcl.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116(3):276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 39.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2(10):1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyte LC, Brooks KJ, Nagel S, Akhtar A, Chen R, Mardiguian S, McAteer MA, Anthony DC, Choudhury RP, Buchan AM, Sibson NR. Molecular magnetic resonance imaging of acute vascular cell adhesion molecule-1 expression in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(6):1178–1187. doi: 10.1038/jcbfm.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tekin G, Tekin A, Sipahi I, Kaya A, Sansoy V. Plasma concentration of soluble vascular cell adhesion molecule-1 and oncoming cardiovascular risk in patients with unstable angina pectoris and non-ST-segment elevation myocardial infarction. Am J Cardiol. 2005;96(3):379–381. doi: 10.1016/j.amjcard.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 43.de Lemos JA, Hennekens CH, Ridker PM. Plasma concentration of soluble vascular cell adhesion molecule-1 and subsequent cardiovascular risk. J Am Coll Cardiol. 2000;36(2):423–426. doi: 10.1016/s0735-1097(00)00742-7. [DOI] [PubMed] [Google Scholar]
- 44.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99(19):2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 45.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77(5):863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 46.Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med. 2007;17(8):253–258. doi: 10.1016/j.tcm.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamurthy VM, Semetey V, Bracher PJ, Shen N, Whitesides GM. Dependence of effective molarity on linker length for an intramolecular protein-ligand system. J Am Chem Soc. 2007;129(5):1312–1320. doi: 10.1021/ja066780e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubetzko, Sarkar CA, Pluckthun A. Protein PEGylation Decreases Observed Target Association Rates via a Dual Blocking Mechanism. Mol Pharmacol. 2005;68(5):1439–1454. doi: 10.1124/mol.105.014910. [DOI] [PubMed] [Google Scholar]
- 49.Wuest T, Gerlach E, Banerjee D, Gerspach J, Moosmayer D, Pfizenmaier K. TNF-Selectokine: a novel prodrug generated for tumor targeting and site-specific activation of tumor necrosis factor. Oncogene. 2002;21(27):4257–4265. doi: 10.1038/sj.onc.1205193. [DOI] [PubMed] [Google Scholar]
- 50.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8(10):803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 51.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 52.Thomas SJ, Grossberg GT. Memantine: a review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clinical Interventions in Aging. 2009;4:367–377. doi: 10.2147/cia.s6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu W, Luo Y, Sun C, Liu Y, Kuo P, Varga J, Xiang R, Reisfeld R, Janda KD, Edgington TS, Liu C. Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Cancer Res. 2006;66(2):970–980. doi: 10.1158/0008-5472.CAN-05-2591. [DOI] [PubMed] [Google Scholar]
- 54.Bajjuri KM, Liu Y, Liu C, Sinha SC. The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity. ChemMedChem. 2011;6(1):54–59. doi: 10.1002/cmdc.201000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter RA, Campbell IK, O’Donnel KL, Wicks IP. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol. 2002;128(1):44–51. doi: 10.1046/j.1365-2249.2002.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan Q, Yue W, Shaoshan H. The inhibitory effect of photodynamic therapy and of an anti-VCAM-1 monoclonal antibody on the in vivo growth of C6 glioma xenografts. Braz J Med Biol Res. 2011;44(5):489–490. doi: 10.1590/S0100-879X2011007500052. [DOI] [PubMed] [Google Scholar]
- 57.Soriano A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80(10):1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 58.Chen L, Lin SX, Amin S, Overbergh L, Maggiolino G, Chan LS. VCAM-1 blockade delays disease onset, reduces disease severity and inflammatory cells in an atopic dermatitis model. Immunol Cell Biol. 2010;88(3):334–342. doi: 10.1038/icb.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wehner NG, Shopp G, Oneda S, Clarke J. Embryo/fetal development in cynomolgus monkeys exposed to natalizumab, an alpha4 integrin inhibitor. Birth Defects Res B Dev Reprod Toxicol. 2009;86(2):117–130. doi: 10.1002/bdrb.20190. [DOI] [PubMed] [Google Scholar]
- 60.Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, Girish S, Tibbitts J, Yi JH, Sliwkowski MX, Jacobson F, Lutzker SG, Burris HA. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 28(16):2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 61.Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ, Scher HI. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26(13):2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 62.Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, Fuh G. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323(5921):1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- 63.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, Papenfuss AT, Poon AM, Hopkins W, Smyth FE, MacGregor D, Cher LM, Jungbluth AA, Ritter G, Brechbiel MW, Murphy R, Burgess AW, Hoffman EW, Johns TG, Old LJ. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci U S A. 2007;104(10):4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.