Abstract
Endoglin is an endothelial-specific transforming growth factor beta (TGF-β) co-receptor essential for angiogenesis and vascular remodeling. Endoglin regulates a wide range of cellular processes, including cell adhesion, migration, and proliferation, through TGF-β signaling to canonical Smad and Smad-independent pathways. Despite its overall pro-angiogenic role in the vasculature, the underlying mechanism of endoglin action is poorly characterized. We previously identified β-arrestin2 as a binding partner that causes endoglin internalization from the plasma membrane and inhibits ERK signaling towards endothelial migration. In the present study, we examined the mechanistic role of endoglin and β-arrestin2 in endothelial cell proliferation. We show that endoglin impedes cell growth through sustained inhibition of ERK-induced c-Myc and cyclinD1 expression in a TGF-β-independent manner. The down-regulation of c-Myc and cyclinD1, along with growth-inhibition, are reversed when the endoglin/β-arrestin2 interaction is disrupted. Given that TGF-β-induced Smad signaling potently represses c-Myc in most cell types, our findings here show a novel mechanism by which endoglin augments growth-inhibition by targeting ERK and key downstream mitogenic substrates.
Keywords: Endoglin, β-arrestin2, ERK, c-Myc, TGF-β, Smads
INTRODUCTION
Endoglin is a critical mediator of angiogenesis and vascular morphogenesis. Endoglin is mutated in the human disease, hereditary hemorrhagic telangiectasia (HHT), a condition characterized by vascular dysplasia and arteriovenous malformations (AVMs) [1] and [2]. Up to 25% of patients with HHT develop life-threatening complications such as pulmonary or brain AVMs [3]. Endoglin dysfunction also contributes to preeclampsia, inflammation and tumor-associated angiogenesis [2], [18] and [19].
The overall process of angiogenesis comprises highly regulated series of events, initiated by endothelial degradation of the basement membrane, followed by migration, proliferation, alignment, and capillary tube formation [4]. And while its pro-angiogenic role is clear, how endoglin impacts each of these events at the cellular and molecular level are largely undefined.
The general scheme of TGF-β signaling in endothelial cells describes two distinct types of serine/threonine kinase receptors, the activin receptor-like kinase 1 (ALK1) and ALK5, which target downstream nuclear shuttling proteins, Smad 1/5/8 and Smad 2/3, respectively [5] and [6]. ALK5 is ubiquitously expressed, which upon binding TGF-β, activates Smad 2/3 to inhibit endothelial cell proliferation and migration. ALK1 responds to either TGF-β or bone morphogenetic protein-9 (BMP-9) to activate Smad 1/5/8, culminating in pro-angiogenic transcriptional responses including proliferation and migration [7]. Endoglin modulates the critical balance of these two divergent Smad pathways, in part, by mediating heteromeric receptor complex formation [3] and [7]. It is also becoming evident that endoglin engages several Smad-independent mechanisms to alter endothelial cell behavior via scaffolding/trafficking adaptor molecules [8]. How endoglin mediates both Smad and non-Smad signaling components is a subject of intense investigation.
The analysis of endoglin structure reveals a large extracellular ligand-binding domain, followed by a single transmembrane segment, and a short cytoplasmic tail [9]. While lacking a kinase domain, this cytoplasmic segment functions as important phosphorylation [17] and binding sites for multiple scaffolding/trafficking adaptors including β-arrestin2 [12], Zyxin [10], Zyxin related protein-1 (ZRP-1) [11], and GIPC [13]. In the case of β-arrestin2, binding to endoglin causes internalization of this complex into endocytic vesicles, resulting in transient suppression of ERK1/2 activation and cell migration [12]. But given that ERK modulates the functions of multiple substrates present in the cytoplasm and nucleus, here we examined whether endoglin and β-arrestin2 impacts ERK-induced mitogenic signaling during cellular proliferation.
MATERIALS AND METHODS
Tissue culture and reagents
MEEC and HMEC-1 endothelial cells were cultured as described previously [12]. Ectopic expression in Eng-/- MEECs was achieved by nucleofection with Amaxa (SF kit). HMEC-1s were nucleofected with pLKO.1 puro vector encoding either scramble control or shRNA to human endoglin sequence (purchased from Sigma). The pDisplay expression vectors encoding endoglin wild type (WT) and T650A were described previously [12]. TGFβ-1 was obtained from R&D Systems. Protease and phosphatase inhibitor cocktails (Sigma) were used in the biochemical analyses. MTT was purchased from Sigma.
Biochemical analyses
ERK activation was assessed by immunoblotting with phosphor-specific and total ERK antibodies (Cell Signaling). Endoglin expression in cell lysate was detected by immunoblotting with P4A4 antibody (University of Iowa Hybridoma). c-Myc and cyclin D1 were purchased from Cell Signaling, while β-actin was obtained from Sigma. Prior to TGF-β dose-response and time course studies, MEECs were serum starved for 4-5 h in MCDB-131 media supplemented with L-glutamine. Cells lysates were normalized for equal protein loading through Bio-rad assay using lysis buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 10 mM NaF, 1 mM EDTA, supplemented with protease and phosphatase inhibitor cocktails).
MTT assay
MEECs plated at 100,000 cells were grown overnight in 6 well plates. Cells were treated with MTT (Thiazolyl Blue Tetrazolium Bromide) in serum free media (MCDB-131 supplemented with L-glutamine) for 2 h at various time points (12, 24, 48 h). Cells were briefly washed with PBS, then the MTT metabolic product (formazan) was extracted in DMSO and the optical density was read at 570 nm in a microplate reader.
RESULTS
While TGF-β is a potent inhibitor of proliferation in most cell types, the precise role of endoglin in in endothelial proliferation is unclear. A number of in vitro and in vivo studies have shown that endoglin and ALK1 enhance proliferation through TGF-β-induced Smad1/5/8 signaling [2] and [5], while others have reported the opposite results [14] and [15]. To better understand endoglin function in this cellular context, we sought to determine whether endoglin engages non-Smad mechanisms that contribute to cell proliferation.
In particular, the potential role for endoglin in regulating ERK-mediated mitogenic signaling was tested. It was previously found that endoglin impairs the rapid and transient activation of ERK (5-10 min) in response to low dose concentrations of TGF-β [12]. To further define endoglin function in ERK signaling, we first performed a TGF-β dose-response to compare ERK phosphorylation in endoglin knockout (Eng-/-) and control (Eng+/+) mouse embryonic endothelial cells (MEECs). Eng+/+ cells displayed a significantly lower basal ERK activity, with little to no response to increasing TGF-β concentrations, relative to Eng-/- cells (Fig.1A; 0 to 200pM TGF-β). Next, to determine whether endoglin exerts a long-term suppression of ERK activation, a time course experiment was performed. Irrespective of TGF-β treatment over the course of 12 h, there were significantly higher levels of ERK activation in Eng-/- MEECs (Fig. 1B).
Fig. 1.
Endoglin constitutively inhibits ERK activation. (A) Eng+/+ and Eng-/- MEECs were serums starved for 4 h prior to TGF-β dose-response at the indicated concentrations for 15 min. Phospho-specific (upper panel) and total ERK (lower panel) immunoblotting are shown. (B) A time-course experiment involved Eng+/+ and Eng-/- MEECs being treated with 20 pM TGF-β for the indicated time points.
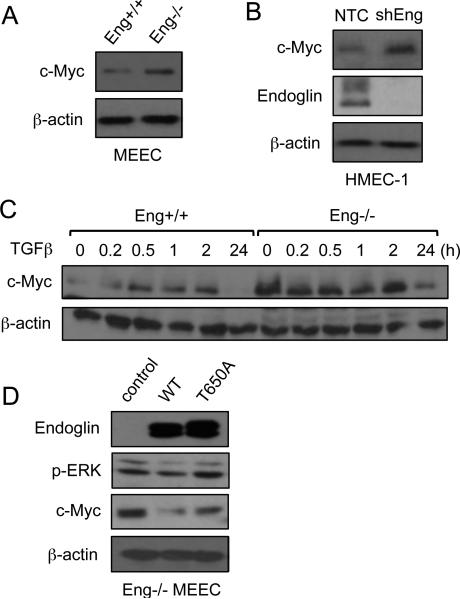
As this sustained ERK activation in Eng-/- cells could potentially contribute to cellular proliferation, we assessed c-Myc expression as a downstream mitogenic target of ERK signaling. As expected, the results showed greater c-Myc expression in Eng-/- than Eng+/+ MEECs (Fig.2A). To further demonstrate this was an endoglin-specific effect, we silenced endoglin expression in human microvascular endothelial cells (HMEC-1) and observed an elevated level of c-Myc upon endoglin knockdown (Fig.2B). Next, we examined how endoglin affects c-Myc expression over a 24 h period in the presence of TGF-β. Consistent with the ERK activation, Eng-/- MEECs displayed relatively higher levels of c-Myc expression than Eng+/+ throughout the 24 h TGF-β treatment (Fig. 2C). Taken together, our data suggested that endoglin constitutively down-regulates c-Myc.
Fig. 2.
Endoglin down-regulation of c-Myc requires β-arrestin2 interaction. (A) Comparison of c-Myc expression in Eng+/+ and Eng-/- MEECs. (B) Comparison of c-Myc in HMEC-1 in non-targeting control (NTC) versus endoglin knockdown (shEng) after 48 h. (C) c-Myc expression of Eng+/+ and Eng-/- MEECs in response to TGF-β treatment over 24 h period. (D) Comparison of ERK activation and c-Myc expression in Eng-/- MEECs expressing no endoglin (control), WT endoglin, or endoglin point mutant unable to bind β-arrestin2 (T650A).
We next tested whether endoglin requires β-arrestin2 to inhibit c-Myc expression. To do so, endoglin rescue experiments were performed where endoglin expression was restored in Eng-/- MEECs with either endoglin wild type (WT) or point mutant unable to bind β-arrestin2 (T650A). As was observed in Eng+/+ cells, endoglin WT markedly reduced ERK activation relative to the control vector and T650A mutant expressing Eng-/- MEECs (Fig. 2D; second panel), suggesting that endoglin-dependent ERK inhibition is β-arrestin2-dependent. Further, ERK activity was closely correlated with c-Myc expression wherein the control and T650A expressing Eng-/- MEECs had enhanced expression relative to WT (Fig. 2D; third panel).
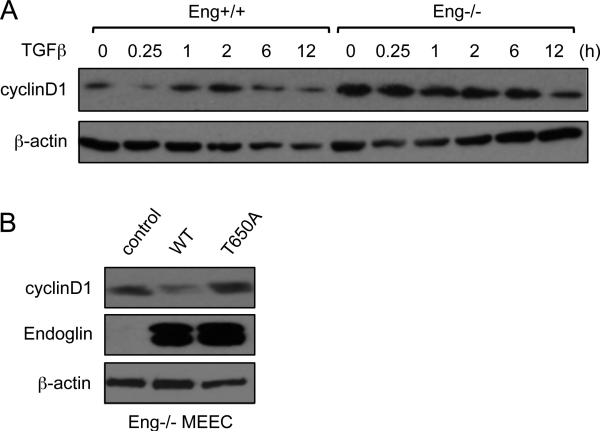
Another downstream target of ERK signaling is cyclin D1, an oncogene that, like c-Myc, promotes proliferation and cell cycle progression. We examined whether cyclin D1 is also associated with endoglin regulation of ERK and c-Myc during endothelial proliferation. As Fig. 3A demonstrates, there was reduced basal cyclin D1 levels in Eng+/+ cells than the knockout, while surprisingly, TGF-β treatment had little impact over the course of 12 h. To test whether β-arrestin2 mediates this effect, WT or T650A expression was restored in Eng-/- cells. Similar to ERK activity and c-Myc expression, WT endoglin markedly reduced cyclin D1 whereas it was unchanged in T650A expressing Eng-/- cells (Fig. 3B), further indicating the importance of endoglin/β-arrestin2 interaction in down-regulating ERK downstream targets.
Fig. 3.
Endoglin inhibits cyclin D1 expression. (A) Comparison of cyclin D1 expression in Eng+/+ and Eng-/- MEECs upon TGF-β treatment for the indicated time points (0-12 h). (B) Comparison of cyclin D1 in Eng-/- MEECs expressing no endoglin (control), WT endoglin, or endoglin mutant T650A.
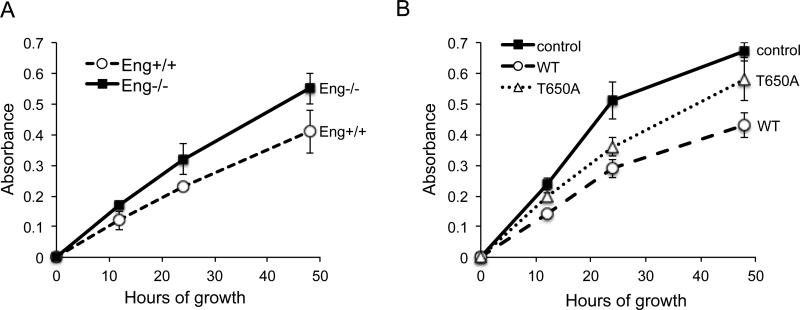
Based on our findings that endoglin inhibits sustained ERK signaling towards c-Myc and cyclin D1, we examined whether this mechanism contributes to the inhibition of cell growth. In agreement with our data on overall c-Myc and cyclin D1 expression, a MTT cell growth assay revealed that Eng-/- cells grew at a rate roughly 30-40% faster than Eng+/+ cells at 48 h time point (Fig. 4A). The rescue expression of WT in Eng-/- cells attenuated cell growth by approximately 55% compared to Eng-/- control (Fig. 4B). In contrast, T650A expression was unable to impede cell growth as effectively as the WT, suggesting that endoglin regulates endothelial cell growth in large part via β-arrestin2-mediated down-regulation of ERK mitogenic signaling.
Fig. 4.
β-arrestin2 mediates endoglin regulation of endothelial cell growth. (A) MTT growth assay of Eng+/+ and Eng-/- MEECs for the indicated time points (0, 12, 24, 48 h). (B) MTT growth assay of Eng-/- MEECs expressing no control, WT or T650A for the indicated time points. Data are representative of four independent experiments. Error bars represent ± SE of triplicates for each time point.
DISCUSSION
The pro-angiogenic role of endoglin in the vasculature is well established. It is highly expressed in rapidly proliferating vascular endothelium, making it more specific and sensitive than many pan-endothelial markers (i.e. CD34 and CD31) [16]. In addition, endoglin expression strongly correlates with tumor progression, survival rate and metastases [2] and [16]. But precisely how endoglin contributes to angiogenesis at the molecular level is not fully understood, and numerous in vitro and in vivo studies have yielded conflicting data. Many earlier studies have reported pro-proliferative and pro-migratory responses elicited by endoglin and ALK1 through Smad1/5/8 signaling [5] and [6], whereas others have shown that endoglin inhibits several key components of angiogenesis, including proliferation and migration [15]. Given that endoglin can engage non-Smad pathways and undergo ectodomain shedding to dampen TGF-β signaling, it is likely that these disparate findings are largely attributable to the different experimental and culturing conditions.
Our present study is an important extension of our previous work that identified β-arrestin2 as a novel endoglin binding partner [12]. We reported that endoglin/β-arrestin2 interaction inhibits transient TGF-β-induced ERK activation, which impedes endothelial migration. Here we examined how endoglin/β-arrestin2 interaction might regulate ERK signaling downstream to c-Myc and cyclin D1, two prominent mitogenic targets essential for proliferation during angiogenesis. Our results clearly indicate a role for endoglin in down-regulating ERK activity and its ability to limit c-Myc and cyclin D1 expression. While there was a transient and rapid ERK activation (5 min) in Eng-/- MEECs in response to TGF-β (data not shown), overall the ability for endoglin to inhibit ERK was largely TGF-β-independent according to our dose-response and time-course data (Fig. 1A and B). However, β-arrestin2 does play a key role in this process, as evidenced by endoglin rescue studies in which the WT but not T650A was able to down-regulate ERK, c-Myc and cyclin D1. These findings are particularly interesting considering that TGF-β-induced Smads are potent transcriptional repressors of c-Myc [22]. Our data strongly suggests that endoglin further limits c-Myc expression, but does so via ERK inhibition in a TGF-β-independent manner. Interestingly, the linker regions of Smad 2 and 3 are known phosphorylation sites for deactivation by ERK [20] and [21]. Hence, there may be a role for endoglin/β-arrestin2 in down-regulating ERK signaling so that the TGF-β-induced Smads can function more effectively as c-Myc repressors. Whether a crosstalk between Smads 2/3 and ERK is necessary to achieve growth inhibition during angiogenesis remains to be investigated.
While the results of MTT assay revealed only a moderate difference in cell growth between Eng+/+ and Eng-/- MEECs (Fig. 4A; ~30 % at 48 h), a more pronounced growth-inhibition was observed upon comparing Eng-/- control cells with those transiently expressing endoglin WT (Fig. 4B; ~ 55%). The fact that the expression of T650A yielded an intermediate growth-inhibition between the control and WT in the knockout cells suggests that the endoglin/β-arrestin2 interaction is not the sole mediator of this process. Indeed, as mentioned earlier, endoglin interacts with multiple other adaptor proteins including Zyxin, ZRP-1 and GIPC. Whether these binding partners also contribute to growth inhibition through regulation of ERK signaling, other MAPKs and/or Smads will need to be determined.
In summary, we have here defined a new mechanism by which endoglin regulates endothelial cell growth. By interacting with β-arrestin2, endoglin restricts a sustained ERK response to reduce c-Myc and cyclin D1 expression during endothelial cell proliferation. These findings support a non-Smad function for endoglin and β-arrestin2 during angiogenesis and vascular remodeling.
- Endoglin inhibits ERK activation in endothelial cells
- Endoglin is a regulator of c-Myc and cyclin D1 expression
- beta-arrestin2 interaction with endoglin is required for ERK/c-Myc repression
- Endoglin impedes cellular proliferation by targeting ERK-induced mitogenic signaling
Acknowledgments
The research was funded by NIH grant to N.Y.L. (R00HL 103791) and startup funds from the Division of Pharmacology and Davis Heart and Lung Research Institute (The Ohio State University).
ABBREVIATIONS
- Eng
endoglin
- MEEC
mouse embryonic endothelial cell
- HMEC-1
human microvascular endothelial cell-1
- HHT
hereditary hemorrhagic telangiectasia
- ALK
activin receptor-like receptor kinase
- TGF-β
transforming growth factor beta
- BMP
bone morphogenetic protein
- AVM
arteriovenous malformation
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43(2):97–110. doi: 10.1136/jmg.2005.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-β family in vascular morphogenesis and disease. Trends in Cell Biology. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). American Journal of Medical Genetics. 2000;91(1):66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells, Molecules, and Diseases. 2007;39(2):212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur H, Dijke PT. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. The EMBO Journal. 2004;23:4018–28. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebrin F, Deckers M, Bertolino P, Dijke P. TGF-beta receptor function in the endothelium. Cardiovascular Research. 2004;65(3):599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Bernabeu C, Conley BA. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem. 2007;102:1375–88. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and Cytoplasmic Domains of Endoglin Interact with the Transforming Growth Factor- Receptors I and II. J Biol Chem. 2002;277(32):29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 10.Conley BA, Koleva R, Smith JD, Kacer D, Zhang D, Bernabéu C, Vary CP. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J Biol Chem. 2004;279(26):27440–9. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- 11.Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CP, Bernabéu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem. 2004;279(31):32858–68. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- 12.Lee NY, Blobe GC. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J Biol Chem. 2007;282(29):21507–17. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- 13.Lee NY, Ray B, How T, Blobe GC. Endoglin Promotes Transforming Growth Factor β-mediated Smad 1/5/8 Signaling and Inhibits Endothelial Cell Migration through Its Association with GIPC. J Biol Chem. 2008;283(47):32527–33. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SO, Lee YJ. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–42. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pece-Barbara N, et al. Endoglin null endothelial cells proliferate faster and are more responsive to transforming growth factor beta 1 with higher affinity receptors and an activated ALK1 pathway. J Biol Chem. 2005;280(30):27800–8. doi: 10.1074/jbc.M503471200. [DOI] [PubMed] [Google Scholar]
- 16.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): A marker of tumor vasculature and potential target for therapy. Clinical Cancer Research. 2008;14:1931–37. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 17.Koleva RI, Conley BA, Romero D, Riley KS, Marto JA, Lux A, Vary CP. Endoglin structure and function determinants of endoglin phosphorylation by transforming growth factor-β receptors. J Biol Chem. 2006;19(281):25110–23. doi: 10.1074/jbc.M601288200. [DOI] [PubMed] [Google Scholar]
- 18.Fonsatti E, et al. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22(42):6557–63. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- 19.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature Medicine. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 20.Funaba M, Zimmerman CM, Mathews LS. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem. 2002;277(44):41361–8. doi: 10.1074/jbc.M204597200. [DOI] [PubMed] [Google Scholar]
- 21.Massague J. Integration of Smad and MAPK pathways: a link and linker revisited. Genes & Development. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 22.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes & Development. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]