Abstract
A multi-functional liquid chromatography system that performs 1-dimensional, 2-dimensional (strong cation exchange/reverse phase liquid chromatography, or SCX/RPLC) separations and online phosphopeptide enrichment using a single binary nano-flow pump has been developed. With a simple operation of a function selection valve equipped with a SCX column and a TiO2 (titanium dioxide) column, a fully automated selection of three different experiment modes was achieved. Because the current system uses essentially the same solvent flow paths, the same trap column, and the same separation column for reverse-phase separation of 1D, 2D, and online phosphopeptides enrichment experiments, the elution time information obtained from these experiments is in excellent agreement, which facilitates correlating peptide information from different experiments. The final reverse-phase separation of the three experiments is completely decoupled from all of function selection processes; thereby salts or acids from SCX or TiO2 column do not affect the efficiency of the reverse-phase separation.
Introduction
In proteomics, in which complex protein mixtures are analyzed, a liquid chromatography (LC) combined with tandem mass spectrometry (MS/MS) has become a widely used technique1,2. Reverse-phase LC (RPLC) is a preferred mode of separation for LC/MS/MS because of its high separation power and the compatibility of its mobile phase with electrospray ionization sources of mass spectrometers1,2,3,4. The current dominant bottom-up approach to proteomics, which analyzes peptides from proteolytic digestion of proteins, encounters significant under-sampling due to e.g., insufficient efficiency of peptide separation, insufficient speed and sensitivity of mass spectrometric analyses, and other experimental limitations4,5. Various multi-dimensional separation strategies have been developed to improve separation efficiency by increasing peak capacity and thereby increasing the peptide identification rates6,7,8,9. By utilizing two or more separation modes (i.e., ion exchange, reverse phase, hydrophilic interaction, size exclusion, and others) that are preferably orthogonal to each other, multi-dimensional separations provided improved separation efficiencies and increased the number of peptides or proteins identified from proteome samples7,10,11,12,13,14,15,16,17,4,18,19,20,21,22,23. Strong cation exchange-reverse phase (SCX-RP) is widely used and has been extensively investigated in off-line16,19,21,22, on-line biphasic one column4,13 or on-line two column modes11,12,23,24. Another approach to increasing RPLC peak capacity is to utilize long capillary columns up to 100 cm in length operating at ultra–high pressure (~10,000 psi)25. These columns with markedly increased peak capacity provide enhanced separation resolution. With improved chromatographic separation, identification of several thousands of proteins has become possible in LC/MS/MS experiments3,25,26.
An important aspect of proteomics is to probe peptides that are post-translationally modified5. As the modifications of proteins modulate their functions in many vital cellular processes, an efficient and accurate method of analyzing these protein modifications is of great importance27,28. Phosphorylation is an important post-translational modification (PTM) whose analysis is of great research interests in both biology and technical development. However, the stoichiometry of phosphorylation is often very low, resulting in technical difficulties in the detection of phosphopeptides in the presence of abundant non-phosphopeptides28,29. Many attempts have been tried to improve detection of phosphopeptides30,31,32,33,34 by e.g employing online phosphopeptide enrichment steps35,36,37. Automated online and reproducible phosphopeptide enrichment is of particular interest for enabling the sensitive detection of phosphospeptides with reduced sample losses and increased experimental throughput32,37.
Here we describe a simple valve module consisting of three valves: a Z-valve, a function selection valve, and a column valve. When applied to a commercial reverse-phase nanoLC system equipped with an auto-sampler, the valve module turns the LC system into a multi-functional UPLC system that can perform 1D and 2D separations and online phosphopeptide enrichment on the same LC system employing a single binary LC pump. Simple electronic switching of the function selection valve via the LC system was shown to allow fully automated selection of different experiments. LC/MS/MS results showed excellent agreement in peptide elution from the reverse phase column despite the different modes of experiments being employed in the analysis of proteome samples having different levels of complexity.
Experimental
Chemicals and materials
Acetonitrile (ACN), methanol and water were purchased from J. T. Baker (Phillipsburg, NJ, USA). Enolase, β-casein, HPLC grade formic acid, ammonium bicarbonate (NH4HCO3), phosphoric acid (H3PO4) and formic acid (FA) were from Sigma-Aldrich (St. Louis, MO, USA). Sequence-grade modified porcine trypsin was from Promega (Madison, WI, USA). All of the chemicals were of analytical purity grade or HPLC grade and were used as received.
Preparation of proteome samples of various complexities
For the optimization and evaluation of the automated multi-functional UPLC system, protein digests of enolase and β-casein were prepared. Each protein was dissolved in 50 mM ammonium bicarbonate, and trypsin was added at a 1:50 ratio before digesting for 24 h at 37 °C. The digested samples were completely dried and stored at −80 °C. The digests were reconstituted in LC solvent A (0.1% formic acid in water) immediately before the LC/MS/MS experiment.
The rho0 cell without mitochondrial DNA (mtDNA) which was derived from an osteosarcoma cell line (143B) defective of thymidine kinase activity by long-term exposure to ethidium bromide was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 100 mg/mL 5-bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis), 50 μg/mL uridine (Sigma-Aldrich, St. Louis, MO), and 10 % fetal bovine serum (FBS). Southern blot analysis and PCR amplification of mtDNA target sequences confirmed the absence of any residual mtDNA. Using platelets as mtDNA donors, cybrid cells were produced as described previously38. Briefly, the platelet-rich fraction was separated from the blood sample of a patient harboring mtDNA 3243 A>G mutation with informed consent, and 143B rho0 cells were added. Fusion was carried out in the presence of 42 % polyethylene glycol 1500 (Sigma-Aldrich). By limiting dilution of fusion products, cybrid clones with 3243A homoplasmy (wild type) was obtained and analyzed by PCR-Restriction fragment length polymorphism (RFLP). To assure a complete repopulation of mtDNA, the functional assessment of the selected clones was carried out after 2–3 months of successive subcultivation. The harvested cells were suspended with an equal volume of ice-cold phosphate-based saline (PBS) buffer (pH 7.4) and homogenized in a FastPrep apparatus (Bio101, Savant, Carlsbad, CA). The lysate was transferred to a new tube and protein concentration was determined by the BCA assay (Pierce, Rockford, IL).
Peptide samples were prepared by modified filter-aided sample preparation (FASP)39. 250 μg of proteins was reduced in 100 μL of SDT buffer (4 % SDS and 0.1 M DTT in 0.1 M Tris-HCl, pH 7.6) for 1 h at 37 °C and then boiled for 10 min. After sonication for 10 min, the sample was centrifuged for 5 min at 14,000 g. The proteins in SDT buffer were mixed with 200 μL of 8 M urea (in 0.1M Tris-HCl, pH 8.5) in a membrane filter (Microcon device, YM-30, Millipore, Massachusetts, USA). The membrane filter was centrifuged at 14,000 g at 20 °C for 40–60 min. The concentrate was diluted with 200 μL of 8 M urea and centrifuged to remove the SDS. Subsequently, 100 μL of 50 mM iodoacetamide in 8 M urea were added to the concentrate for alkylation for 25 min at 37 °C. The resulting solution was diluted with 200 μL of 8 M urea and concentrated again. The concentrate was washed twice with 100 μL of 50 mM NH4HCO3 at 14,000 g for 40–60 min each. The resultant protein concentrate in the filter was subjected to proteolytic digestion using 5 μg of trypsin (1:50 of enzyme to protein ratio) for 1 min before overnight digestion without shaking at 37 °C. After the first digestion, trypsin (in 1:200 of enzyme to protein ratio) was added for 6h of digestion. The resultant tryptic peptides after digestion were eluted from the filter by centrifugation for 20–30 min at 14,000 g, and the filter was rinsed with 60 μL of 50 mM NH4HCO3 and the flow-through was mixed with the first eluate. The combined flow-through was vacuum-dried using a SpeedVac concentrator (Thermo, San Jose, CA) and the dried peptides were stored at −70 °C for LC/MS/MS analysis.
Preparation of capillary RPLC, SPE, SCX and TiO2 columns
Sol-gel method was used to form frits inside of fused silica capillaries as described before40. Briefly, one end of a fused silica capillary (Polymicro Technologies, Phoenix, AZ, USA) was put in the sol-gel solution of 5:1 KASIL No.1/formamide (KASIL-2.5:1 SiO2/K2O) and approximately 1-cm of the capillary was filled with the sol-gel solution by capillary action. A frit was formed after baking the capillary at 100 °C for 10 min. RPLC capillary columns (85 cm × 75 μm) were prepared by packing a fit-ended fused-silica capillary with C18-bonded porous particles (3 μm diameter, 300 Å pore size, Jupiter, Phenomenex, Torrance, CA, USA) using the slurry packing technique as previously described41,42,43. SPE column (3 cm × 150 μm) was similarly prepared using the same packing material. Both ends of the SPE column were blocked by the frits. After packing, the columns were sonicated for 5 min at 12,000 psi and the pressure was gradually released overnight to prevent dispersion of the packed C18 materials. For the preparation of SCX column (15 cm × 150 μm), the 5 μm Partisphere SCX resins (Whatman, Clifton, NJ, USA) were used to pack a frit-ended capillary using the same method. The TiO2 column was prepared by packing a frit-ended fused silica capillary (10 cm × 150 μm) with 10 μm Titania resins (titanium dioxide, GL Sciences, Tokyo, Japan).
RPLC separation in various LC experiments
A 60 min or 180 min linear gradient of 2%–60% or 2%–50% solvent B was produced from a nanoACQUITY binary pump (Waters, Milford, MA, USA) at the flow rate of 400 nL/min or 200 nL/min, respectively. The solvent A and B were 0.1 % formic acid in water and 0.1 % formic acid in acetonitrile, respectively. The operation temperature of the RP analytical column was set at 50 °C using a semi-flexible column heater44.
Operation of valves for various LC experiments
Figure 1 shows the schematic diagram of the multi-functional valve module, which encompassed by the red dashed box, for the multi-functional UPLC system. The module is consisted of a Z-valve (Z), a function selection valve (F) and a column valve (C). The valve module is connected to the original sample injection valve (S) of the auto-sampler unit of the NanoACQUITY UPLC system.
Figure 1.
Schematic representation of the fully automated multi-functional UPLC system. S, Z, F, and C denote sample injection valve, the Z-valve, the function selection valve, and the column valve, respectively. The valve’s numeric labels (i.e in F1, F2 and F3) indicate their positions. The current system utilizes a single binary nanoLC pump. The red dashed box includes the valves that were additionally attached to the nanoACQUITY UPLC system.
The Z-valve (Z) is a six-port and two-channel switching valve (C72MX-4696XD, VICI, Houston, Texas, USA), in which two opposing ports are connected by a fused silica capillary (50 μm id × 360 μm od × 8 cm length). Coupled with a tee, as shown in Figure 1, this valve directed solvent flow from the nano-pump to either the F-valve (Z1 position) or the C-valve (Z2 position). The position of the Z-valve is controlled by asserting (or grounding) digital I/O pins (pin 5 for the Z1 position and pin 6 for the Z2 position) of the micro-electric valve actuator (EH, VICI, Houston, Texas, USA). The function selection valve (F-valve) is a nine-port and four-channel selector valve (C75MFX-4694, VICI, Houston, Texas, USA), on which one SCX and one TiO2 capillary columns are connecting two of the nine-ports of the valve, respectively, as shown in Figure 1. The solvent and sample flow directly to the C-valve, or through the SCX column, or through the TiO2 column before reaching the C-valve by switching the valve to F1, F2, or F3 position, respectively. The position of the F-valve was set by asserting a digital I/O pin (pin 3, step signal pin) for the desired numbers of time. For example, if F1 were set to the home position, positions F2 and F3 would be reached by asserting pin 3 twice and five times, respectively. The column valve (C-valve) is a six-port and three-channel switching valve (C72MX-4696D, VICI, Houston, Texas, USA), on which an SPE and an analytical column were installed. The dead volume between the two columns is the volume of one channel of approximately 40 nL.
The positions of the Z-, F- and C-valves were controlled by asserting the digital I/O pins of the micro-electric valve actuators. The assertion was time-controlled by an electronic switch of the nanoACQUITY system, allowing the valve positionings and the timings of switching during the LC experiments to be controlled by the MassLynx data system of the nanoACQUITY system. A signal relay (ES1E-S-DC5V, Panasonic Electric Works Co., Osaka, Japan) was placed between the electronic switch and the controller of the micro-electric valve actuator to control the valves’ positions via one switch of the commercial LC system.
2D SCX/RPLC/MS/MS analysis
Different percentage salt solutions of 500 mM ammonium acetate (AA) solution in sol A were stored in the auto-sampler of the nanoACQUITY system and 10 μL salt solution aliquots from the auto-sampler were used as elution buffers for the elution of bound peptides from the SCX column. Different salt solutions were used for different samples depending on their peptide complexity. For example, four salt solutions (10 %, 40 %, 90 % 500 mM AA in sol A, and 80 % 500 mM AA in 20 % ACN and sol A) were used for tryptic enolase peptides and seventeen salt solutions (2 %, 4 %, 6 %, 8 %, 10 %, 15 %, 20 %, 25 %, 30 %, 35 %, 40 %, 50 %, 60 %, 80 %, and 100 % 500 mM AA in sol A, 95 % 500 mM AA in 5 % ACN and sol A and 80 % 500 mM AA in 20 % ACN and sol A) were used for tryptic peptides of the whole cell lysates of wild type cybrid. The flow rate during the step salt elution was 1 μL/min. The eluted peptides were loaded onto the SPE column and desalted with 15 μL sol A before their RPLC separation in each salt step.
Online phosphopeptides enrichment
To enrich phosphorylated peptides, the F-valve was set to position F3 and the peptide samples flowed through the TiO2 column on the valve. The flow rate for the injection into the TiO2 column was 0.5 μL/min for 30 min. The bound phosphopeptides were eluted with 10 μL of elution buffer (250 mM H3PO4). Detail experimental conditions and the valves’ positions are given in the supplementary table 3.
Mass spectrometry and data analysis
A 7-tesla Fourier transform ion cyclotron resonance mass spectrometer (FTICR, LTQ-FT, Thermo Electron, San Jose, CA, USA) was used to collect mass spectra. MS precursor ion scans (m/z 500 – 2,000) were acquired in profile mode with an AGC target value of 1.0 × 106, a mass resolution of 1.0 × 105 and a maximum ion accumulation time of 1,000 ms. The mass spectrometer was operated in data-dependent tandem MS mode; the three most abundant ions detected in the precursor MS scan were dynamically selected for MS/MS experiments that simultaneously incorporated a dynamic exclusion option (exclusion mass width low: 1.10 Th; exclusion mass width high: 2.10 Th; exclusion list size: 120; exclusion duration: 30 s) to prevent reacquisition of the MS/MS spectra of the same peptides. Collision-induced dissociations of the precursor ions were performed in an ion trap (LTQ) with collisional energy and isolation width set to 35% and 3 Th, respectively.
The tandem mass spectrometric data from the LC/MS/MS experiments were processed by the integrated post-experiment monoisotopic mass refinement (iPE-MMR) method45. Briefly, DeconMSn46 was used to generate MS/MS data (DTA files) whose precursor masses were further corrected and refined through a modified version of PE-MMR47. The resultant mass-refined DTA files were subjected to systematic correction using DtaRefinery48,49. For tryptic yeast peptides, the MS/MS data after the iPE-MMR processes were searched against a composite target-decoy database, containing human database (IPI ver.3.86, ftp://ftp.ebi.ac.uk/pub/databases/IPI/current/ipi.HUMAN.fasta) and its reversed complements using SEQUEST Sorcerer (Version 27, Revision 12). The searches were performed allowing for semi-tryptic peptides, and the maximum number of internal cleavage sites was set to 3. The mass tolerance was set to 10 ppm for precursor ions and 1 Da for fragment ions. The oxidation of methionine (15.994 920 Da) was used as a variable modification. The FP rate of the peptide assignment was estimated through a composite target/decoy database search. The values of Xcorr and the ΔCn threshold for the 1% FP rate were used to obtain the peptide IDs.
Results and Discussion
Reproducible retention of peptides from different experiments with the multi-functional UPLC system
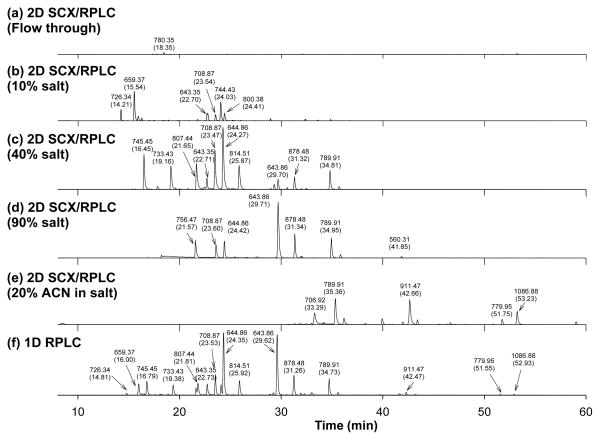
Table 1 lists the positions of the sample injection valve (S-valve), the Z-valve, the function selection valve (F-valve) and the column valve (C-valve) for the different steps of various experiments. The use of a tee in conjunction with the Z-valve allowed the solvent from the binary nano-flow pump to flow either to the F-valve (i.e in the Z1 position) and the C-valve or directly to the C-valve (i.e in the Z2 position), bypassing the F-valve. The positions of the Z-valve and the C-valve (Z2 and C2) remained unchanged for the reverse-phase separation in all the 1D, 2D, and online phosphopeptide enrichment experiments (Table 1). In the Z2 and C2 positions, the solvent took a flow path from the pump to the Z-valve to the C-valve (Figure 1). Regardless of the operation modes of 1D, 2D, or phosphopeptide enrichment, the flow path, flow rate, and solvent gradient conditions of reverse phase separation remained the same, enabling reproducible peptide retentions across all three experiments. For tryptic enolase peptides, the result of the 1D RPLC experiment is compared with those of 2D SCX/RPLC experiment in Figure 2. For 2D SCX/RPLC separation, the tryptic enolase peptides were injected and loaded into the F-valve to pass to the SCX column (i.e., F2 position). After RPLC/MS analysis (Figure 2a) of the flow-through, the SCX fractionation of the first salt step was performed by injecting 10 μL 10 % 500 mM ammonium acetate from the auto-sampler. The eluted peptides from the first salt step were trapped in the SPE, and excess salts were removed by flowing 15 μL of solvent A through the SPE column before RPLC separation of the trapped peptides. The SCX fractionation was repeated with increasing percentages of 500 mM ammonium acetate to 40% and 90% and the remaining peptides were eluted by the 20% ACN in 500 mM ammonium acetate in the last salt step. The five chromatograms of the 2D SCX/RPLC experiments (Figure 2a–2e) show different peptides of tryptic enolase being analyzed at each of SCX fractions. The RP elution times of peptides during 2D SCX/RPLC experiments were in good agreements with those observed during the 1D RPLC experiment. As describe above, the flow path from the nano-flow pump to the RP column, the column flow rate, and the solvent gradient of RP separation were essentially the same during both experiments in the current system, resulting in excellent agreement in elution times. This is a desirable feature, particularly when the 2D SCX/RPLC proteomic data are mapped to identify 1D RPLC proteomic data. Importantly the RP separation in these experiments is completely decoupled from the 1D and 2D function selection processes. As a result of this, the salt elution of peptides from SCX column had no adverse effects on the reverse-phase separation because the salts were effectively removed during the trapping in the SPE column.
Table 1.
Valve positions and LC conditions for the different steps of various experiments
| Experiment Type | Experimental Steps | Valve Position | Other conditions | |||
|---|---|---|---|---|---|---|
| Sa | Zb | Fc | Cd | |||
| 1-D RPLC | Sample Injection/Desalting | S1 | Z1 | F1 | C1 | Sol A, 3 μL/min, 5 min |
| RP Separation | S1 | Z2 | F1 | C2 | Sol A/B, 0.4 μL/min or 0.2 μL/min | |
| 2-D SCX/RPLC | Sample Injection/0% Salt | S1 | Z1 | F2 | C1 | Sol A, 1 μL/min, 15 min |
| RP Separation | S1 | Z2 | F1 | C2 | Sol A/B, 0.4 μL/min or 0.2 μL/min | |
| SCX Step Elution | S1 | Z1 | F2 | C1 | 10 μL of X% salt, Sol A, 1 μL/min, 15 min | |
| Online Phospho-peptides Enrichment | Enriching phosphopeptides | S1 | Z1 | F3 | C1 | Sol A, 0.5 μL/min, 30 min |
| Removal non-phosphopeptides from SPE | S1 | Z1 | F1 | C1 | Sol B, 1 μL/min, 10 min | |
| Elution of phosphopeptides | S1 | Z1 | F3 | C1 | 10 μL of 250 mM H3PO4 | |
| RP Separation | S1 | Z2 | F1 | C2 | Sol A/B, 0.4 μL/min | |
| Common Steps | Sample Loading | S2 | Z2 | F1 | C2 | Sol A, 0.4 μL/min |
| Washing | S1 | Z1 | F1 | C1 | Sol A/B, 1 μL/min | |
| SPE/Column Equilibration | S1 | Z2 | F1 | C2 | Sol A, 0.4 μL/min | |
S denotes for the sample injection valve.
Z denotes for the Z-valve.
F denotes for the function selection valve.
C denotes for the column valve.
Figure 2.
Comparison of the base peak chromatograms of (a–e) 2D SCX/RPLC/MS/MS experiments and (f) 1D RPLC/MS/MS experiment using tryptic enolase peptides. The masses of base peaks are indicated and their elution times are given in parentheses.
Online 2DLC experiments for the effective analysis of complex proteome samples
The multi-functional LC system was used to analyze the tryptic peptides of the whole cell lysate of wild type cybrid to demonstrate its application in the analysis of complex proteomes. Figure 3 shows chromatograms obtained by analyzing 1 μg and 10 μg of tryptic peptides by the 1D RPLC (Figure 3r) and 2D SCX/RPLC (Figure 3a–3q), respectively. A total of 38,358 non-redundant peptides were identified in the 2D SCX/RPLC experiments while the 1D RPLC experiment resulted in 8,890 peptides. The peak capacities50,51,52 of RPLC separation in both 1D RPLC and 2D SCX/RPLC remained essentially the same as the RP separation was completely decoupled from the SCX separation in 2D SCX/RPLC as described above (data not shown). Approximately 70 % of the total of 38,378 peptides from the 2D SCX/RPLC experiments were observed in one or two SCX fractions (supplementary Figure 1), demonstrating the SCX as an effective online fractionation to couple with subsequent RP separation.
Figure 3.
Base peak chromatograms from (a–q) 2D SCX/RPLC/MS/MS experiments and (r) 1D RPLC/MS/MS experiment using tryptic whole cell peptides. The salt contents for the SCX elution were as follows: 2% salt fraction (a), 4% salt fraction (b), 6% salt fraction (c), 8% salt fraction (d), 10% salt fraction (e), 15% salt fraction (f), 20% salt fraction (g), 25% salt fraction (h), 30% salt fraction (i), 35% salt fraction (j), 40% salt fraction (k), 50% salt fraction (l), 60% salt fraction (m), 80% salt fraction (n), 100% salt fraction (o), 95% salt and 5% ACN fraction (p), 80% salt and 20% ACN fraction (q).
Correlation of 1D RPLC and 2D SCX/RPLC data
As described above, the elution times of peptides in both 1D and 2DLC experiments using this multi-functional UPLC system were very similar (if not same). The estimated difference in the elution time between 1D and 2D for common peptides was calculated to be −0.01 ± 1.40 min (mean ± standard deviation, supplementary Figure 2). The similarity in retention times facilitates utilizing the peptide identification obtained from the 2D SCX/RPLC/MS/MS experiment in identifying peptide features of the 1D RPLC/MS/MS data (Figure 4a). A total of 64,905 unique mass classes53 (i.e peptide features) were measured on the MS data of 1D RPLC/MS/MS experiment. Due to the inherent under-sampling, only 8,890 of the UMCs resulted in positive peptide identification after data analysis described in the Experimental section. When peptide IDs from the 2D SCX/RPLC/MS/MS data, as shown in Figure 4b, were matched to the unassigned UMCs with mass and elution time tolerances of ±10 ppm and 5 min, respectively, 12,549 peptide IDs were additionally assigned to unidentified UMCs (Figure 4c). The increased peptide identifications of 1D RPLC/MS/MS should provide more peptides to be quantified by intensity based label-free quantitation54 in which MS intensities of common peptides among two or more 1DLC/MS/MS data are compared.
Figure 4.
(a) Virtual 2D display of identified UMCs from 1D RPLC/MS/MS experiment. 8,890 of 64,905 UMCs were identified in 1D RPLC/MS/MS experiment. (b) Virtual 2D display of a total of 33,358 identified peptides from 2D SCX/RPLC/MS/MS experiment. (c) Virtual 2D display of the 8,890 originally identified UMCs (in blue) with additionally identified UMCs by 2D SCX/RPLC/MS/MS (in pink). 12,549 peptide identifications were additionally assigned to the unidentified UMCs.
Online phosphopeptides enrichment
The F-valve was set to the position F3 for phosphopeptide enrichment and the peptide sample was then loaded onto the TiO2 column after passing through the Z-valve. The flow-through, most likely non-phosphopeptides, was loaded onto the SPE column of the C-valve. After switching the Z- and C-valves to the Z2 and C2 positions, respectively, the RP separation experiment was performed on the flow-through peptides. The Z- and C-valves were then switched back to Z1 and C1 positions, while the F-valve remained in the F3 position. The bound phosphopeptides were subsequently eluted with the injection of 10 μL of elution buffer (250 mM H3PO4) from the autosampler; the eluent from the TiO2 column was trapped on the SPE column and subjected to desalting. Detailed experimental steps and the valve positions are listed in Supplementary Table 3.
Online phosphopeptide enrichment on the multi-functional UPLC system was demonstrated with a simple phosphopeptide model sample. Figure 5a shows the base peak chromatogram of tryptic β-casein peptides without online phosphopeptide enrichment. Figures 5b and 5c show the base peak chromatograms of the bound peptides and the flow through peptides, respectively, in the online phosphopeptide enrichment mode. As shown in Figure 5b, a monophosphorylated peptide (FQS(p)EEQQQTEDELQDK) of β-casein was observed as a base peak in the chromatogram along with other minor peaks, three of which were identified as DIG(p)SESTEDQAMEDIK and TVDME(p)STEVFTK, YKVPQLEIVPN(p)SAEER (supplementary figure 3) from a-S1 casein and a-S2 casein.
Figure 5.
(a) Base peak chromatogram of tryptic β-casein peptides with no enrichment, (b) base peak chromatogram of tryptic β-casein peptides after online phosphopeptide enrichment using the TiO2 column, and (c) the base peak chromatogram of the flow through portion of tryptic β-casein peptides from the TiO2 column.
Conclusions
A fully automated multi-functional UPLC system was developed for advanced proteomic analyses. By utilizing specially designed valves such as the Z-valve and function selection valve in a valve module, the LC system enables the 1D-RPLC, 2D-SCX/RPLC, and online phosphopeptide enrichment experiments with improved reproducibility in RPLC peptide elution among the experiments. Despite the addition of extra valves, the current system was fully automated and could operate in the different modes via simple electronic valve switching. In order to achieve higher throughputs, we are currently expanding the single RPLC column system to a dual RPLC column system. The feasibility of incorporating additional modes of operation, such as online fast digestion55 and online 3D-phosphopeptide separation, is also presently being evaluated.
Supplementary Material
Supplementary Table 1. Detailed conditions for the 1D RPLC analysis.
Supplementary Table 2. Detailed conditions for the 2D SCX/RPLC analysis.
Supplementary Table 3. Detailed conditions for the phosphopeptides enrichment.
Supplementary Figure 1. Frequency distribution of all identified peptides in different SCX fractions of Figure 3.
Supplementary Figure 2. Distribution of elution time differences for common peptides between 1D RPLC/MS/MS and 2D SCX/RPLC/MS/MS.
Supplementary Figure 3. MS/MS spectra of the enriched phosphopeptides.
Acknowledgments
This study was supported in part by grants of A111218–11-CP02 (to SL) from the National Project for Personalized Genomic Medicine, Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea, and NIH grant RR018522/GM103493–10 (to RDS). SL also acknowledges the Priority Research Centers Program (NRF20100020209), the Proteogenomic Research Program through the National Research Foundation of Korea (NRF) and the Converging Research Center Program (Grant 2011K000897) funded by the Ministry of Education, Science and Technology. We thank K. W. Roh for the technical assistance with instrumentation.
Footnotes
Supporting Information Available: This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Fournier ML, Gilmore JM, Martin-Brown SA, Washburn MP. Multidimensional separations-based shotgun proteomics. Chem Rev. 2007;107(8):3654–86. doi: 10.1021/cr068279a. [DOI] [PubMed] [Google Scholar]
- 2.Motoyama A, Yates JR., 3rd Multidimensional LC separations in shotgun proteomics. Anal Chem. 2008;80(19):7187–93. doi: 10.1021/ac8013669. [DOI] [PubMed] [Google Scholar]
- 3.Motoyama A, Venable JD, Ruse CI, Yates JR., 3rd Automated ultra-high-pressure multidimensional protein identification technology (UHP-MudPIT) for improved peptide identification of proteomic samples. Anal Chem. 2006;78(14):5109–18. doi: 10.1021/ac060354u. [DOI] [PubMed] [Google Scholar]
- 4.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17(7):676–82. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 5.Mann M, Kelleher NL. Precision proteomics: the case for high resolution and high mass accuracy. Proc Natl Acad Sci U S A. 2008;105(47):18132–8. doi: 10.1073/pnas.0800788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Y, Zhang R, Moore RJ, Kim J, Metz TO, Hixson KK, Zhao R, Livesay EA, Udseth HR, Smith RD. Automated 20 kpsi RPLC-MS and MS/MS with chromatographic peak capacities of 1000–1500 and capabilities in proteomics and metabolomics. Anal Chem. 2005;77(10):3090–100. doi: 10.1021/ac0483062. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Lin D, Yates JR., 3rd Multidimensional separations for protein/peptide analysis in the post-genomic era. Biotechniques. 2002;32(4):898, 900, 902. doi: 10.2144/02324pt01. passim. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov AR, Zang L, Karger BL. Low-attomole electrospray ionization MS and MS/MS analysis of protein tryptic digests using 20-microm-i.d polystyrene-divinylbenzene monolithic capillary columns. Anal Chem. 2003;75(20):5306–16. doi: 10.1021/ac030163g. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Tolic N, Zhao R, Pasa-Tolic L, Li L, Berger SJ, Harkewicz R, Anderson GA, Belov ME, Smith RD. High-throughput proteomics using high-efficiency multiple-capillary liquid chromatography with on-line high-performance ESI FTICR mass spectrometry. Anal Chem. 2001;73(13):3011–21. doi: 10.1021/ac001393n. [DOI] [PubMed] [Google Scholar]
- 10.Opiteck GJ, Jorgenson JW. Two-dimensional SEC/RPLC coupled to mass spectrometry for the analysis of peptides. Anal Chem. 1997;69(13):2283–91. doi: 10.1021/ac961156d. [DOI] [PubMed] [Google Scholar]
- 11.Nagele E, Vollmer M, Horth P. Two-dimensional nano-liquid chromatography-mass spectrometry system for applications in proteomics. J Chromatogr A. 2003;1009(1–2):197–205. doi: 10.1016/s0021-9673(03)01034-3. [DOI] [PubMed] [Google Scholar]
- 12.Davis MT, Beierle J, Bures ET, McGinley MD, Mort J, Robinson JH, Spahr CS, Yu W, Luethy R, Patterson SD. Automated LC-LC-MS-MS platform using binary ion-exchange and gradient reversed-phase chromatography for improved proteomic analyses. J Chromatogr B Biomed Sci Appl. 2001;752(2):281–91. doi: 10.1016/s0378-4347(00)00547-8. [DOI] [PubMed] [Google Scholar]
- 13.Wolters DA, Washburn MP, Yates JR., 3rd An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73(23):5683–90. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 14.Vollmer M, Nagele E, Horth P. Differential proteome analysis: two-dimensional nano-LC/MS of E. coli proteome grown on different carbon sources. J Biomol Tech. 2003;14(2):128–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using RP-RP-HPLC system with different pH in first and second separation dimensions. J Sep Sci. 2005;28(14):1694–703. doi: 10.1002/jssc.200500116. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Jacobs JM, Camp DG, 2nd, Fang R, Moore RJ, Smith RD, Xiao W, Davis RW, Tompkins RG. Ultra-high-efficiency strong cation exchange LC/RPLC/MS/MS for high dynamic range characterization of the human plasma proteome. Anal Chem. 2004;76(4):1134–44. doi: 10.1021/ac034869m. [DOI] [PubMed] [Google Scholar]
- 17.Dai J, Shieh CH, Sheng QH, Zhou H, Zeng R. Proteomic analysis with integrated multiple dimensional liquid chromatography/mass spectrometry based on elution of ion exchange column using pH steps. Anal Chem. 2005;77(18):5793–9. doi: 10.1021/ac050251w. [DOI] [PubMed] [Google Scholar]
- 18.Evans CR, Jorgenson JW. Multidimensional LC-LC and LC-CE for high-resolution separations of biological molecules. Anal Bioanal Chem. 2004;378(8):1952–61. doi: 10.1007/s00216-004-2516-2. [DOI] [PubMed] [Google Scholar]
- 19.Masuda J, Maynard DM, Nishimura M, Ueda T, Kowalak JA, Markey SP. Fully automated micro- and nanoscale one- or two-dimensional high-performance liquid chromatography system for liquid chromatography-mass spectrometry compatible with non-volatile salts for ion exchange chromatography. J Chromatogr A. 2005;1063(1–2):57–69. doi: 10.1016/j.chroma.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 20.Nagele E, Vollmer M, Horth P, Vad C. 2D-LC/MS techniques for the identification of proteins in highly complex mixtures. Expert Rev Proteomics. 2004;1(1):37–46. doi: 10.1586/14789450.1.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J Proteome Res. 2003;2(1):43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 22.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17(10):994–9. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 23.Winnik WM. Continuous pH/salt gradient and peptide score for strong cation exchange chromatography in 2D-nano-LC/MS/MS peptide identification for proteomics. Anal Chem. 2005;77(15):4991–8. doi: 10.1021/ac0503714. [DOI] [PubMed] [Google Scholar]
- 24.Nagele E, Vollmer M, Horth P. Improved 2D nano-LC/MS for proteomics applications: a comparative analysis using yeast proteome. J Biomol Tech. 2004;15(2):134–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Min HK, Hyung SW, Shin JW, Nam HS, Ahn SH, Jung HJ, Lee SW. Ultrahigh-pressure dual online solid phase extraction/capillary reverse-phase liquid chromatography/tandem mass spectrometry (DO-SPE/cRPLC/MS/MS): a versatile separation platform for high-throughput and highly sensitive proteomic analyses. Electrophoresis. 2007;28(6):1012–21. doi: 10.1002/elps.200600501. [DOI] [PubMed] [Google Scholar]
- 26.Wang N, Xie C, Young JB, Li L. Off-line two-dimensional liquid chromatography with maximized sample loading to reversed-phase liquid chromatography-electrospray ionization tandem mass spectrometry for shotgun proteome analysis. Anal Chem. 2009;81(3):1049–60. doi: 10.1021/ac802106z. [DOI] [PubMed] [Google Scholar]
- 27.Yan JX, Packer NH, Gooley AA, Williams KL. Protein phosphorylation: technologies for the identification of phosphoamino acids. J Chromatogr A. 1998;808(1–2):23–41. doi: 10.1016/s0021-9673(98)00115-0. [DOI] [PubMed] [Google Scholar]
- 28.Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 2002;20(6):261–8. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 29.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101(33):12130–5. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlosser A, Bodem J, Bossemeyer D, Grummt I, Lehmann WD. Identification of protein phosphorylation sites by combination of elastase digestion, immobilized metal affinity chromatography, and quadrupole-time of flight tandem mass spectrometry. Proteomics. 2002;2(7):911–8. doi: 10.1002/1615-9861(200207)2:7<911::AID-PROT911>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101(2):269–95. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- 32.Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol. 2002;20(3):301–5. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- 33.Dunn JD, Reid GE, Bruening ML. Techniques for phosphopeptide enrichment prior to analysis by mass spectrometry. Mass Spectrom Rev. 2010;29(1):29–54. doi: 10.1002/mas.20219. [DOI] [PubMed] [Google Scholar]
- 34.McLachlin DT, Chait BT. Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr Opin Chem Biol. 2001;5(5):591–602. doi: 10.1016/s1367-5931(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhang Y, Jiang H, Cai Y, Qian X. Phosphopeptide detection using automated online IMAC-capillary LC-ESI-MS/MS. Proteomics. 2006;6(2):404–11. doi: 10.1002/pmic.200500223. [DOI] [PubMed] [Google Scholar]
- 36.Dai J, Wang LS, Wu YB, Sheng QH, Wu JR, Shieh CH, Zeng R. Fully automatic separation and identification of phosphopeptides by continuous pH-gradient anion exchange online coupled with reversed-phase liquid chromatography mass spectrometry. J Proteome Res. 2009;8(1):133–41. doi: 10.1021/pr800381w. [DOI] [PubMed] [Google Scholar]
- 37.Pinkse MW, Mohammed S, Gouw JW, van Breukelen B, Vos HR, Heck AJ. Highly robust, automated, and sensitive online TiO2-based phosphoproteomics applied to study endogenous phosphorylation in Drosophila melanogaster. J Proteome Res. 2008;7(2):687–97. doi: 10.1021/pr700605z. [DOI] [PubMed] [Google Scholar]
- 38.Chomyn A. Platelet-mediated transformation of human mitochondrial DNA-less cells. Methods Enzymol. 1996;264:334–9. doi: 10.1016/s0076-6879(96)64031-2. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6(5):359–62. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 40.Zhao R, Ding SJ, Shen Y, Camp DG, 2nd, Livesay EA, Udseth H, Smith RD. Automated metal-free multiple-column nanoLC for improved phosphopeptide analysis sensitivity and throughput. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(8–9):663–70. doi: 10.1016/j.jchromb.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y, Zhao R, Berger SJ, Anderson GA, Rodriguez N, Smith RD. High-efficiency nanoscale liquid chromatography coupled on-line with mass spectrometry using nanoelectrospray ionization for proteomics. Anal Chem. 2002;74(16):4235–49. doi: 10.1021/ac0202280. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y, Moore RJ, Zhao R, Blonder J, Auberry DL, Masselon C, Pasa-Tolic L, Hixson KK, Auberry KJ, Smith RD. High-efficiency on-line solid-phase extraction coupling to 15–150-microm-i.d clumn liquid chromatography for proteomic analysis. Anal Chem. 2003;75(14):3596–3605. doi: 10.1021/ac0300690. [DOI] [PubMed] [Google Scholar]
- 43.Shen Y, Tolic N, Masselon C, Pasa-Tolic L, Camp DG, 2nd, Hixson KK, Zhao R, Anderson GA, Smith RD. Ultrasensitive proteomics using high-efficiency on-line micro-SPE-nanoLC-nanoESI MS and MS/MS. Anal Chem. 2004;76(1):144–54. doi: 10.1021/ac030096q. [DOI] [PubMed] [Google Scholar]
- 44.Hyung SW, Kim MS, Mun DG, Lee H, Lee SW. The effect and potential of using a temperature controlled separation column with ultra-high pressure microcapillary liquid chromatography/tandem mass spectrometry on proteomic analysis. Analyst. 2011;136(10):2100–5. doi: 10.1039/c0an00724b. [DOI] [PubMed] [Google Scholar]
- 45.Jung HJ, Purvine SO, Kim H, Petyuk VA, Hyung SW, Monroe ME, Mun DG, Kim KC, Park JM, Kim SJ, Tolic N, Slysz GW, Moore RJ, Zhao R, Adkins JN, Anderson GA, Lee H, Camp DG, 2nd, Yu MH, Smith RD, Lee SW. Integrated post-experiment monoisotopic mass refinement: an integrated approach to accurately assign monoisotopic precursor masses to tandem mass spectrometric data. Anal Chem. 2010;82(20):8510–8. doi: 10.1021/ac101388b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayampurath AM, Jaitly N, Purvine SO, Monroe ME, Auberry KJ, Adkins JN, Smith RD. DeconMSn: a software tool for accurate parent ion monoisotopic mass determination for tandem mass spectra. Bioinformatics. 2008;24(7):1021–3. doi: 10.1093/bioinformatics/btn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin B, Jung HJ, Hyung SW, Kim H, Lee D, Lee C, Yu MH, Lee SW. Postexperiment monoisotopic mass filtering and refinement (PE-MMR) of tandem mass spectrometric data increases accuracy of peptide identification in LC/MS/MS. Mol Cell Proteomics. 2008;7(6):1124–34. doi: 10.1074/mcp.M700419-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Petyuk VA, Jaitly N, Moore RJ, Ding J, Metz TO, Tang K, Monroe ME, Tolmachev AV, Adkins JN, Belov ME, Dabney AR, Qian WJ, Camp DG, 2nd, Smith RD. Elimination of systematic mass measurement errors in liquid chromatography-mass spectrometry based proteomics using regression models and a priori partial knowledge of the sample content. Anal Chem. 2008;80(3):693–706. doi: 10.1021/ac701863d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petyuk VA, Mayampurath AM, Monroe ME, Polpitiya AD, Purvine SO, Anderson GA, Camp DG, 2nd, Smith RD. DtaRefinery, a software tool for elimination of systematic errors from parent ion mass measurements in tandem mass spectra data sets. Mol Cell Proteomics. 2010;9(3):486–96. doi: 10.1074/mcp.M900217-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wren SA. Peak capacity in gradient ultra performance liquid chromatography (UPLC) J Pharm Biomed Anal. 2005;38(2):337–43. doi: 10.1016/j.jpba.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 51.Neue UD. Theory of peak capacity in gradient elution. J Chromatogr A. 2005;1079(1–2):153–61. doi: 10.1016/j.chroma.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Shen Y, Smith RD, Unger KK, Kumar D, Lubda D. Ultrahigh-throughput proteomics using fast RPLC separations with ESI-MS/MS. Anal Chem. 2005;77(20):6692–701. doi: 10.1021/ac050876u. [DOI] [PubMed] [Google Scholar]
- 53.Zimmer JS, Monroe ME, Qian WJ, Smith RD. Advances in proteomics data analysis and display using an accurate mass and time tag approach. Mass Spectrom Rev. 2006;25(3):450–82. doi: 10.1002/mas.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neilson KA, Ali NA, Muralidharan S, Mirzaei M, Mariani M, Assadourian G, Lee A, van Sluyter SC, Haynes PA. Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics. 2011;11(4):535–53. doi: 10.1002/pmic.201000553. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Ferrer D, Petritis K, Robinson EW, Hixson KK, Tian Z, Lee JH, Lee SW, Tolic N, Weitz KK, Belov ME, Smith RD, Pasa-Tolic L. Pressurized pepsin digestion in proteomics: an automatable alternative to trypsin for integrated top-down bottom-up proteomics. Mol Cell Proteomics. 2011;10(2):M110 001479. doi: 10.1074/mcp.M110.001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Detailed conditions for the 1D RPLC analysis.
Supplementary Table 2. Detailed conditions for the 2D SCX/RPLC analysis.
Supplementary Table 3. Detailed conditions for the phosphopeptides enrichment.
Supplementary Figure 1. Frequency distribution of all identified peptides in different SCX fractions of Figure 3.
Supplementary Figure 2. Distribution of elution time differences for common peptides between 1D RPLC/MS/MS and 2D SCX/RPLC/MS/MS.
Supplementary Figure 3. MS/MS spectra of the enriched phosphopeptides.