Abstract
Purpose of Study
Prenatal exposure to alcohol often results in disruption to discrete cognitive and behavioral domains, including executive function (EF) and adaptive functioning. In the current study, the relation between these two domains was examined in children with histories of heavy prenatal alcohol exposure, non-exposed children with a diagnosis of attention-deficit/hyperactivity disorder (ADHD), and typically developing controls.
Methods
As part of a multisite study, three groups of children (8-18y, M = 12.10) were tested: children with histories of heavy prenatal alcohol exposure (ALC, N=142), non-exposed children with ADHD (ADHD, N=82), and typically developing controls (CON, N=133) who did not have ADHD or a history of prenatal alcohol exposure. Children completed subtests of the Delis-Kaplan Executive Function System (D-KEFS) and their primary caregivers completed the Vineland Adaptive Behavior Scales-II (VABS). Data were analyzed using regression analyses.
Results
Analyses showed that EF measures were predictive of adaptive abilities and significant interactions between D-KEFS measures and group were present. For the ADHD group, the relation between adaptive abilities and EF was more general, with three of the four EF measures showing a significant relation with adaptive score. In contrast, for the ALC group, this relation was specific to the nonverbal EF measures. In the CON group, performance on EF tasks did not predict adaptive scores over the influence of age.
Conclusion
These results support prior research in ADHD suggesting that EF deficits are predictive of poorer adaptive behavior and extend this finding to include children with heavy prenatal exposure to alcohol. However, the relation between EF and adaptive ability differed by group, suggesting unique patterns of abilities in these children. These results provide enhanced understanding of adaptive deficits in these populations, as well as demonstrate the ecological validity of laboratory measures of executive function.
Keywords: Fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), ADHD, adaptive function, executive functioning, multi-site study, neurobehavioral profile
Introduction
The effects of heavy prenatal alcohol exposure include neuropsychological and behavioral deficits. Such deficits occur on a continuum and are collectively known as fetal alcohol spectrum disorders (FASD; Bertrand et al., 2004). Some, but not the majority of children with heavy prenatal alcohol exposure display characteristic physical features necessary for a diagnosis of fetal alcohol syndrome (FAS; Hoyme et al., 2005, Stratton et al., 1996, Bertrand et al., 2004); the diagnosis of FAS is based on the presence of key facial features (smooth philtrum, thin vermillion border, and small palpebral fissures) along with documented growth and central nervous system deficiencies (Jones and Smith, 1973, Bertrand et al., 2004, Hoyme et al., 2005). Although recent estimates suggest FAS may occur in 2 to 7 cases per 1,000 births in the U.S., cases of FASD are more prevalent, occurring in approximately 9 in 1,000 live births (May et al., 2009, Sampson et al., 1997).
Alcohol-exposed children with and without the characteristic facial features associated with FAS demonstrate qualitatively similar deficits on neuropsychological and behavioral measures (Mattson et al., 1998). These impairments include difficulties with verbal and non-verbal learning (Kaemingk et al., 2003, Aragon et al., 2008), language (McGee et al., 2009), visual-spatial functioning (Chiodo et al., 2009), and attention (Schonfeld et al., 2001). The neurobehavioral implications of heavy prenatal alcohol exposure are pervasive, affecting the individual’s behavioral abilities throughout the lifespan (Fryer et al., 2007, Coles et al., 1991, Thomas et al., 1998, Crocker et al., 2009, Streissguth et al., 2004, Streissguth et al., 1996). Of particular concern for individuals with FASD are adaptive abilities, which encompass the ability to monitor and adjust behavior in changing environments (Sparrow et al., 1984). In FASD, adaptive deficits begin early and functioning fails to improve with age, particularly on measures of socialization and communication (Thomas et al., 1998, Crocker et al., 2009, Whaley et al., 2001, Carr et al., 2010, Steinhausen et al., 1993). Such adaptive impairments may be related to elevated rates of secondary disabilities in FASD, including maladaptive behavioral outcomes, academic failure, and increased delinquency (Streissguth et al., 1994, Streissguth et al., 1990, Streissguth et al., 2004, Howell et al., 2006, Fast et al., 1999).
Executive functions (EF), or higher-order cognitive processes involved in behavioral modification (Welsh and Pennington, 1988), are also significantly impaired in FASD, particularly on tasks of decision-making, concept formation, and set-shifting (Vaurio et al., 2008, McGee et al., 2008b, Schonfeld et al., 2006, Schonfeld et al., 2001). Previous literature has suggested that EF may contribute to adaptive functioning through self-regulation of emotional and social processes (Lezak et al., 2004, Diekhof et al., 2009, Roberts, 2006, Schoenbaum et al., 2009). Determining whether diminished EF contributes to adaptive dysfunction in FASD is of particular importance, as recent literature has focused on the need for clinically relevant interventions for children with FASD (Bertrand, 2009). Establishing how EF abilities contribute to adaptive deficits could aid in the development of future interventions for FASD and provide a measurement for the effectiveness of current intervention programs.
Although no study to date has examined the relation between EF and adaptive behavior in FASD, previous literature has examined the relation between EF and social functioning (Schonfeld et al., 2006, McGee et al., 2008a). Findings indicated that parent reports of EF in children with FASD relate to self-reports of social problem solving (McGee et al., 2008a) and parent and teacher assesments of social skills (Schonfeld et al., 2006). Thus, it appears that EF abilities contribute to social adaption in this population; however, given that adaptive behavior is comprised, in part, of social and communication abilities, further investigations are needed to determine whether overall adaptive deficits in FASD are related to executive dysfunction. The relation between standardized EF measures and adaptive behavior has been examined in other clinical populations, including non-exposed children with attention deficit/hyperactivity disorder (ADHD; Wahlstedt et al., 2008, Thorell and Wahlstedt, 2006, Clark et al., 2002). Given the similar adaptive behavior (Stein et al., 1995, Thorell and Wahlstedt, 2006, Greene et al., 1996, Roizen et al., 1994) and executive function impairments (Toplak et al., 2009, Semrud-Clikeman et al., 2008, Semrud-Clikeman et al., 2010, Muir-Broaddus et al., 2002, Stavro et al., 2007, Miller and Hinshaw, 2010) demonstrated by children with FASD and non-exposed children with ADHD, determining the contribution of specific EF domains to adaptive deficits may promote more effective interventions for both clinical groups.
The current study aimed to examine how specific verbal and non-verbal laboratory measures of EF relate to parent-reports of adaptive behavior in children with histories of heavy prenatal alcohol exposure, non-exposed children with ADHD, and non-exposed children without ADHD. It was hypothesized that children with prenatal alcohol exposure would have poorer adaptive and executive functioning than non-exposed children with and without ADHD, and that non-exposed children with ADHD would show greater adaptive and EF impairments than non-exposed children without ADHD. Given previously documented contribution of verbal ability to adaptive behavior in non-exposed children with ADHD (Clark et al., 2002) and greater non-verbal impairments in children with prenatal alcohol exposure (Schonfeld et al., 2001, Vaurio et al., 2008), the current study hypothesized that verbal EF tasks would contribute more to adaptive functioning in the non-exposed ADHD group, whereas non-verbal EF ability would be associated with adaptive ratings in children with prenatal alcohol exposure. Lastly, it was posited that EF abilities would account for a greater amount of explained variance in adaptive scores in the clinical groups compared to the control group.
Materials and Methods
General Methods
Children (N = 357) between the ages 8-18 years (M = 12.10, SD = 2.44) were recruited for an ongoing multisite research study conducted by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which have been described elsewhere (Mattson et al., 2010). Children included in this study comprised 3 groups: those with heavy prenatal exposure to alcohol (the ALC group), non-exposed children with a diagnosis of ADHD (the ADHD group), and typically developing children without histories of prenatal alcohol exposure or ADHD (the CON group).
CIFASD neurobehavioral testing took place at multiple testing centers internationally. However, only data collected in United States testing centers were considered in this analysis to decrease potential impact of cultural and social demands on adaptive behavior. Children in the ALC group were recruited at all testing sites through various methods (for details see Mattson et al., 2010). Children diagnosed with ADHD were recruited for this study from the Center for Behavioral Teratology at San Diego State University, The Fetal Alcohol and Drug Exposure Clinic at Emory University, Center on Alcoholism, Substance Abuse and Addictions at the University of New Mexico, seven different communities throughout North Dakota, South Dakota, and Montana (Northern Plains), and Fetal Alcohol and Related Disorders Clinic at the University of California, Los Angeles (Mattson et al., 2010). Control children were recruited through various modalities from individual sites for on-going research or specifically for CIFASD.
A standardized neuropsychological battery was administered in a single day to each child by a trained examiner, who was blind to the participant’s diagnostic group. Children were assessed on a range of cognitive domains, including general intellectual functioning, attention, memory, and executive functioning. Parent interviews and questionnaires were administered to primary caregivers. Caregivers completed the clinician-assisted National Institute of Mental Health Diagnostic Interview Schedule for Children (DISC; Shaffer et al., 2000) to determine ADHD diagnosis, along with any comorbid psychopathology. Informed assent and consent were obtained from all subjects and their parents prior to testing. Subject incentive was provided to both parents and children. The Institutional Review Board (IRB) at San Diego State University and other CIFASD sites approved this study.
Subjects
The ALC (n = 142) group comprised children with confirmed histories of heavy prenatal alcohol exposure. In the ALC group, 38 (26.8%) children met criteria for FAS and 60 (59.9%) met diagnostic criteria for ADHD based on the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV; American Psychiatric Association, 2000). For all CIFASD sites, children included in the ALC group were recruited retrospectively and had known histories of heavy prenatal alcohol exposure, defined as in utero exposure to an average of 14 drinks per week or more than 4 alcoholic drinks at least once per week on average throughout the pregnancy. Prenatal exposure to alcohol was confirmed through medical history, birth records, social services records, and maternal report and questionnaires, when available. FAS diagnoses were determined via a comprehensive clinical exam by a member of the CIFASD Dysmorphology Core, using a standardized assessment of physical, craniofacial and growth anomalies. Children in the ALC group were categorized as having FAS if they met the following criteria: structural abnormality (i.e., two or more of the following facial features: short palpebral fissure length, smooth philtrum, thin vermillion border) and either growth deficiency or microcephaly. Additional detail on the CIFASD Dysmorphology Core diagnostic criteria can be found elsewhere (Mattson et al., 2010, Jones et al., 2010, Jones et al., 2006).
The ADHD group (n = 82) consisted of non-exposed children who met full DSM-IV diagnostic criteria for ADHD per the DISC. The CON group (n = 133) consisted of typically developing children who did not meet diagnostic criteria for ADHD, and had histories of little to no prenatal exposure to alcohol, as described above. Children were excluded from the CON group if they demonstrated clinical or subclinical symptoms of ADHD, as defined by the DISC. At all testing locations, children in the ADHD and the CON groups were screened for prenatal alcohol exposure and were only included if exposure levels were less than minimal exposure, defined as one drink per week on average and never more than 2 drinks on a single occasion throughout gestation. Exclusion criteria for all groups were: history of significant head injury or loss of consciousness > 30 minutes, non-fluent English speaker, inability to participate due to psychiatric or physical disability, or had been adopted from abroad after the age of 5 years old or less than 2 years before assessment.
Measures
Vineland Adaptive Behavior Scales-II (VABS)
The Vineland Adaptive Behavior Scales-II Parent/Caregiver Rating Form (Sparrow et al., 2005) is a standardized parent questionnaire assessing adaptive behavior. Behavioral questions are scored as 0, 1, or 2, with 0 representing a response of never, 1 representing sometimes or partially, and 2 representing usually. The measure provides a standard adaptive composite score (population M = 100, SD = 15), which is derived from standardized domain scores: communication, socialization, and daily living skills. The VABS standard adaptive score is standardized and normed for age. Computerized scoring software was used to derive standardized adaptive scores. Information regarding VABS standardization, validity and reliability can be found in the Survey Forms Manual (Sparrow et al., 2005).
Delis-Kaplan Executive Function System (D-KEFS)
The D-KEFS is a set of performance-based measures used to assess executive function abilities in individuals between 8-89 years of age (Delis et al., 2001). Tasks used in the study included Verbal Fluency-Switching (VF), Design Fluency-Switching (DF), Trail Making Test-Switching (TMT), and Color-Word Interference-Inhibition/Switching (CWI). Only subtests with a switching condition were used for this study, as these measures are considered to be the most sensitive to impairments in higher order cognitive flexibility (multi-tasking) and set-shifting abilities, which have been demonstrated in FASD (Mattson et al., 2006). Additionally, specific tasks allowed for assessment of both non-verbal (DF and TMT) and verbal (VF and CWI) EF.
The D-KEFS does not provide a standardized composite score and thus, subtests were analyzed separately. Dependent variables for TMT and CWI are based on completion times, those for DF are based on the total number of correct designs completed within 60 seconds, and those for VF are based on the total number of correct category switches in 60 seconds. All raw scores were transformed into standard scores (population M = 100, SD = 15) prior to analysis using a computerized software program; higher standard scores for all variables reflect stronger performance (Delis et al., 2001). Normative data is stratified across sex, ethnicity/race, age, and education level for each of the D-KEFS subtests. Subtests of the D-KEFS are shown to have strong internal and test-retest reliability, and high construct validity for decision-making, inhibition, multitasking, concept formation, abstract thought and planning abilities (Delis et al., 2001).
Data Analysis
Demographic data were analyzed using Pearson Chi-Square (sex, race, ethnicity, and handedness) and standard Analysis of Variance (ANOVA) (age, and FSIQ). Significant group differences on ANOVA were followed up using pair-wise comparisons [Tukey Honestly Significant Difference (Tukey HSD) test]. Alpha per test rate was set at p < .05 for all primary analyses.
Group differences on D-KEFS tasks (VF, DF, TMT, CWI) and adaptive scores were analyzed using univariate ANOVAs. Group (ALC, ADHD, CON) served as the independent variable and D-KEFS switching measures (i.e., Verbal Fluency, Design Fluency, Trail Making Test, Color-Word Interference) and VABS adaptive score served as separate dependent variable. Significant main effects were followed up with pairwise comparisons (Tukey HSD).
In order to determine whether specific D-KEFS tasks were predictive of adaptive scores, four hierarchical stepwise regression analyses were conducted, with the EF scores analyzed separately. For each analysis, adaptive score was entered as the dependent variable and the EF score of interest and Group were entered as predictors. Planned contrast codes (displayed in Table 3) were created for the Group (ALC, ADHD, CON) variables. Since the relation between age and adaptive behavior has been previously shown to differ in children with prenatal alcohol exposure compared to non-exposed children with and without ADHD (Crocker et al., 2009, Thomas et al., 1998, Whaley et al., 2001), age was included in the model as a covariate. On step one of each regression analysis, Age, Group and the D-KEFS variable of interest (e.g., VF) were entered into the model. On step two, to test whether the relation between each D-KEFS tasks and adaptive scores differed by group, interaction terms between Group and D-KEFS variables were added to the model. Follow-up regression analyses were conducted within each group to examine the relative magnitude between EF and adaptive scores with Age and D-KEFS measures entered as predictor variables. Using Bonferroni adjustment, an Alpha per test rate of p = .017, respectively, was used for all follow-up tests. Additional regression analyses were also conducted to examine differences within the ALC group.
Table 3.
Hierarchical Multiple Regression results for children with heavy prenatal alcohol exposure (ALC), children with attention-deficit/hyperactivity disorder (ADHD), and typically developing controls (CON). Planned contrast codes used for each analysis are included in parentheses.
| Verbal Fluency | Design Fluency | Trail Making Test | Color-Word Interference |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Step 1: Group and EF task |
F(4, 300) = 57.28 p < 0.001, R2 = 0.433 |
F(4, 299) = 55.41 p < 0.001, R2 = 0.426 |
F(4, 296) = 55.46 p < 0.001, R2 = 0.428 |
F(4, 284) = 52.96, p < 0.001, R2 = 0.427 |
||||
|
|
||||||||
| b | p | b | p | b | p | b | p | |
|
|
||||||||
| ALC(0) vs. CON(1) | 23.62 | < 0.001 | 24.32 | < 0.001 | 23.87 | < 0.001 | 23.99 | < 0.001 |
| ALC(0) vs. ADHD(1) | 6.53 | 0.006 | 6.75 | 0.005 | 7.04 | 0.003 | 5.47 | 0.029 |
| ADHD(0) vs. CON(1) | 17.09 | < 0.001 | 17.58 | < 0.001 | 16.84 | < 0.001 | 18.52 | < 0.001 |
| AGE | −0.86 | 0.019 | −1.03 | 0.005 | −0.72 | 0.053 | −0.81 | 0.040 |
| D-KEFS Task | 1.10 | < 0.001 | 1.11 | < 0.001 | 0.99 | < 0.001 | 1.15 | < 0.001 |
|
| ||||||||
| Step 2: Two-way interactions |
F(6, 298) = 40.02, p < 0.001, R2 = 0.446 |
F(6, 297) =37.44, p < 0.001, R2 = 0.431 |
F(6, 294) = 36.75, p < 0.001, R2 = 0.429 |
F(6, 282) = 35.70, p < 0.001, R2 = 0.432 |
||||
| Δ R2 |
F(6, 298) = 3.55, p = 0.030, R2 = 0.013 |
F(6, 297) = 1.29, p = 0.277, R2 = 0.005 |
F(6, 294) = 0.04 p = .964, R2 = 0.001 |
F(6, 282) = 1.09, p = 0.338, R2 = 0.004 |
||||
|
|
||||||||
| b | p | b | p | b | p | b | p | |
|
|
||||||||
| D-KEFS TASK X ALC vs. CON | −0.36 | 0.570 | −1.13 | 0.117 | −0.01 | 0.997 | 0.12 | 0.863 |
| D-KEFS TASK X ALC vs. ADHD | 1.62 | 0.026 | −0.85 | 0.288 | −0.14 | 0.800 | 1.06 | 0.148 |
| D-KEFS TASK X ADHD vs. CON | −1.98 | 0.011 | −0.28 | 0.713 | −0.14 | 0.842 | −0.93 | 0.273 |
SPSS statistical software package version 19.0 was used for statistical analyses (SPSS, 2010).
Results
Demographic Data
Groups did not differ on handedness [χ2 (df = 4) = 6.33, p = .176], race [χ2 (df = 12) = 18.76, p = .094], ethnicity [χ2 (df = 4) = 3.87, p = .423], or age [F (2, 331) = 6.120, p = 0.114]. However, groups did differ on sex [χ2 (df = 2) = 9.75, p = .008], and, as expected, on Full Scale IQ [FSIQ; F (2, 331) = 92.37, p < 0.001]. For FSIQ, pairwise comparisons indicated that the ALC group had lower scores than the ADHD group (p < 0.001) and both had lower scores than the CON group (p < 0.001). For sex, the ADHD group had significantly more males than the ALC (p = 0.005) and CON (p = 0.004) groups, which did not differ from each other (p = 0.889). The male to female ratio in the ADHD group is thought to be representative of sex differences estimated in the ADHD population (Graetz et al., 2001, Merikangas et al., 2010, Cantwell, 1996). Demographic information is presented in Table 1.
Table 1.
Demographic data for children in the alcohol-exposed (ALC), attention-deficit/hyperactivity disorder (ADHD), and control (CON) groups.
| ALC (N = 142) |
ADHD (N = 82) |
CON (N = 133) |
|
|---|---|---|---|
|
| |||
| Site [N (%)] | |||
| Atlanta | 25 (17.6) | 17 (20.7) | 19 (14.3) |
| Los Angeles | 27 (19.0) | 2 (2.4) | 18 (13.5) |
| Plains States | 22 (15.9) | 11 (13.4) | 20 (15.0) |
| New Mexico | 12 (8.5) | 11 (13.4) | 18 (13.5) |
| San Diego | 56 (39.4) | 41 (50.0) | 58 (43.6) |
|
| |||
| Handedness [N (% Right)] | 120 (84.5) | 73 (89.0) | 124 (93.2) |
|
| |||
| FAS [N (%)] | 38 (26.8) | 0 (0) | 0 (0) |
|
| |||
| ADHD Diagnosis | 85 (59.9) | 82 (100) | 0 (0) |
| [N (% Positive)] | |||
| Inattentive | 23 | 36 | |
| Hyperactive/Impulsive | 23 | 24 | |
| Combined | 39 | 21 | |
|
| |||
| Sex [N (% Males)] * | 77 (54.2) | 60 (73.2) | 71 (53.4) |
|
| |||
| Race [N (%White)] | 78 (54.9) | 56 (68.3) | 89 (66.9) |
|
| |||
| Ethnicity [N (% Hispanic)] | 22 (15.5) | 19 (23.2) | 24 (18.0) |
|
| |||
| Age [M (SD)] | 12.25 (2.28) | 11.59 (2.55) | 12.25 (2.51) |
|
| |||
| FSIQ [M (SD)] * | 84.25 (17.35) | 93.05 (18.28) | 110.55 (11.87) |
p < .05
Group Differences in Executive Function and Adaptive Behavior
Average executive function and adaptive scores for the three groups are presented in Table 2. There was a significant main effect of Group on all four D-KEFS measures; post hoc comparisons indicated that the ALC and ADHD groups differed from the CON group (ALC, ADHD < CON), but did not differ significantly from each other (p > .10). There was also a significant main effect of Group on adaptive scores and post hoc comparisons indicated that all groups differed significantly from one another (ALC < ADHD < CON).
Table 2.
Mean (Standard Deviation) D-KEFS and VABS adaptive scores for children in the alcohol-exposed (ALC), attention-deficit/hyperactivity disorder (ADHD), and control (CON) groups.
| ALC GROUP N = 142 |
ADHD GROUP N = 82 |
CON GROUP N = 133 |
Group Main Effect |
Pairwise Comparisons (Tukey HSD) |
|
|---|---|---|---|---|---|
|
Verbal
Fluency |
8.25 (3.41) |
9.27 (2.83) |
11. 68 (2.90) |
F (2, 282) = 40.30, p < .001 |
ALC, ADHD < CON |
|
Design
Fluency |
8.47 (2.50) |
8.97 (3.08) |
11.21 (2.98) |
F (2, 282) = 28.84, p < .001 |
ALC, ADHD < CON |
|
Trail Making
Test |
7.25 (3.86) |
7.98 (4.57) |
10.71 (2.57) |
F (2, 282) = 28.64, p < .001 |
ALC, ADHD < CON |
|
Color Word
Interference |
8.52 (3.68) |
9.53 (3.08) |
10.97 (2.38) |
F (2, 822) = 18.17, p < .001 |
ALC, ADHD < CON |
|
Adaptive
Composite |
84.78 (17.27) |
92.22 (16.94) |
111.44 (13.81) |
F (2, 282) = 83.58, p < .001 |
ALC < ADHD < CON |
Relation between Executive Function and Adaptive Behavior
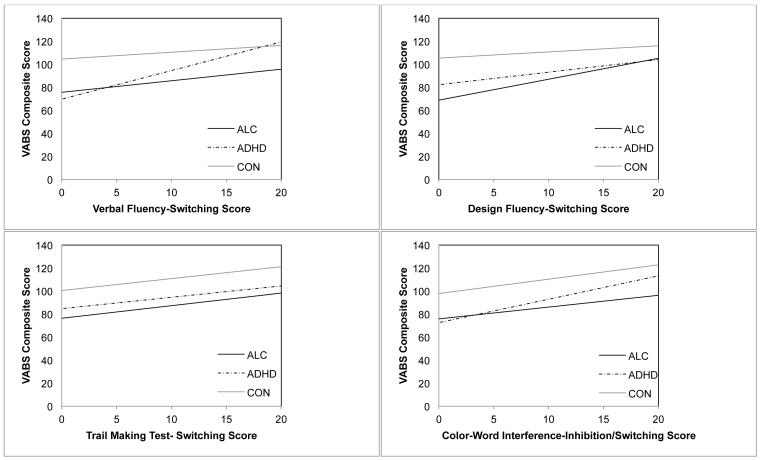
Separate hierarchical multiple regression analyses were conducted to evaluate the effect of Age, Group (ALC, ADHD, CON), and D-KEFS measure (VF, DF, TMT, CWI) on adaptive scores. Regression results are summarized in Table 3 and graphs illustrating the relation between each D-KEFS task and adaptive scores are presented in Figure 1.
Figure 1.
Relation between D-KEFS executive function measures and the VABS adaptive score for children with heavy prenatal alcohol exposure (ALC), children with attention-deficit/hyperactivity disorder (ADHD), and typically developing controls (CON).
Verbal Fluency
On step one of the regression analysis, Age, Group, and VF accounted for a significant amount of the variance in adaptive scores. As shown in the univariate analyses described previously, groups differed significantly on adaptive scores (ALC < ADHD < CON). Age was significantly negatively associated with adaptive scores across groups. Scores on VF were significantly positively associated with adaptive scores across groups. On step two, the addition of the two-way interaction term between VF and Group accounted for a significant increase in explained variance in adaptive scores. The VF X ALC vs. ADHD and VF X ADHD vs. CON interactions were significant, indicating that the relation between VF and adaptive scores is different in the ADHD group compared to the ALC and the CON groups.
Regression analyses were rerun to explore the relation between VF and adaptive scores within each group (results are displayed in Table 4). Age and VF were entered as predictor variables on adaptive score. Using an adjusted alpha per test rate of .017, the analyses revealed that VF was only significantly related to VABS adaptive score in the ADHD group. Age was significantly negatively associated with adaptive scores in the CON group, but not in the other two groups. Thus, VF, but not Age, explains variance in adaptive scores in the ADHD group, although this pattern was not true in the ALC or CON groups.
Table 4.
Regression results showing the relation between individual D-KEFS executive function measures, age, and VABS adaptive scores for children in the alcohol-exposed (ALC), attention-deficit/hyperactivity disorder (ADHD), and control (CON) groups.
| ALC GROUP N = 142 |
ADHD GROUP N = 82 |
CON GROUP N = 133 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | b | p | R2 | b | p | R2 | b | p | |
| VF | 0.235 | 0.89 | .050 | 0.455 | 2.48 | < .001 | 0.297 | 0.48 | .261 |
| Age | −0.85 | .235 | −0.01 | .984 | −1.52 | .003 | |||
| DF | 0.318 | 1.80 | .002 | 0.213 | 1.13 | .084 | 0.320 | 0.77 | .065 |
| Age | −1.11 | .103 | 0.29 | .719 | −1.68 | .001 | |||
| TMT | 0.272 | 1.03 | .011 | 0.309 | 1.11 | .015 | 0.339 | 1.03 | .030 |
| Age | −0.80 | .258 | 1.02 | .221 | −1.56 | .002 | |||
| CWI | 0.267 | 0.81 | .078 | 0.390 | 2.10 | .002 | 0.314 | 0.86 | .109 |
| Age | −1.22 | .114 | 0.55 | .507 | −1.33 | .010 | |||
Design Fluency
On step one of the regression, Age, Group, and DF accounted for a significant amount of the variance in adaptive scores. Group was significantly associated with adaptive score (ALC < ADHD < CON). Age was significantly and negatively associated with adaptive score across groups. Finally, scores on DF were significantly positively associated with adaptive score across groups. Explained variance in adaptive scores did not significantly increase with the addition of the two-way interaction terms on step two. Follow-up analyses revealed that DF was significantly and positively related to adaptive scores in the ALC group, but not the ADHD or the CON group. Age was significantly negatively associated with adaptive scores in the CON group, but not in the other two groups. Thus, DF, but not Age, explains variance in adaptive scores in the ALC group, but not in the ADHD or the CON groups.
Trail Making Test
On step one of the regression, Age, Group, and TMT accounted for a significant amount of the variance in adaptive scores. Group was significantly associated with adaptive scores (ALC < ADHD < CON). Age was not significantly associated with adaptive scores across groups. Finally, scores on TMT were significantly positively associated with adaptive scores across groups. Explained variance in adaptive scores did not significantly increase with the addition of the two-way interaction terms on step two.Follow-up regression analyses revealed that TMT was significantly related to adaptive scores in the ALC and the ADHD groups, but not in the CON group. Age was significantly negatively associated with adaptive scores in the CON group, but not in the other two groups. Thus, TMT explains variance in adaptive scores in the ALC and the ADHD groups, but not in the CON group.
Color-Word Interference
On step one of the regression, Age, Group, and CWI accounted for a significant amount of the variance in adaptive scores. Group was significantly associated with adaptive score (ALC < ADHD < CON). Age was also significantly negatively associated with adaptive score across groups. Finally, scores on CWI were significantly positively associated with adaptive score. Explained variance in adaptive scores did not significantly increase with the addition of the two-way interaction terms on step two. Follow-up regression analyses revealed that CWI was significantly related to adaptive score in the ADHD group, but not the other two groups. Age was significantly negatively associated with adaptive scores in the CON group, but not in the ADHD group. Thus, CWI explains variance in adaptive scores in the ADHD group, but not in the ALC or the CON groups.
Additional Covariates
To further investigate the relation between EF tasks and adaptive score, other variables were entered as model covariates. Although IQ differed between groups, it was not considered an appropriate covariate. Prior studies have addressed potential confounds when IQ is covaried, including population representativeness and non-linearity between IQ and other neuropsychological domains (Dennis et al., 2009). However, given the documented sex differences on adaptive behavior in FASD (Schonfeld et al., 2006), Sex was considered as a covariate. Generally, analyses yielded similar results as above. When entered on step one, Group, D-KEFS tasks, Age, and Sex accounted for a significant amount of explained variance in adaptive score (F = 42.63-46.16, p < .001). Group was significantly associated with adaptive score (ALC < ADHD < CON), across Sex. Across, levels of Group and Sex, Age was significantly negatively associated with adaptive scores in the VF (b = −0.85, p = .021), DF (b = −1.02, p = .006), and CWI (b = −0.79, p = .043) analyses, but was not significantly associated with adaptive scores in the TMT analysis (b = −0.71, p = .059). VF (b = 1.07, p < .001), DF (b = 1.06, p = .001), TMT (b = .96, p < .001), and CWI (b = 1.12, p < .001) were significantly positively associated with adaptive scores. For all analyses, Sex was not significantly associated with adaptive score, across groups (b < 2.148, p > .237). The addition of the interaction terms on step two did not significantly increase amount of explained variance in adaptive scores. Thus, including Sex as a model covariate did not significantly alter prior results.
Comparisons Within the Alcohol-Exposed Group
We also sought to examine whether the relation between EF and adaptive behavior differs for children with histories of prenatal alcohol exposure as a function of FAS or ADHD diagnosis. Since number of children in the ALC group with ADHD did not differ for children with FAS (n = 23) or without FAS (n = 14) FAS [χ2 (df = 1) = .16, p = .693], FAS and ADHD diagnosis were collapsed so that the effects of each factor could be examined separately. To do so, initial two-step regression analyses, as described above, were repeated comparing the following groups: (1) ALC with FAS vs. ALC without FAS, and (2) ALC with ADHD vs. ALC without ADHD. Since VF and CWI were not significantly associated with adaptive scores in alcohol-exposed children, only DF and TMT were included in the following analyses.
When comparing alcohol-exposed children with and without FAS, on step one, Age, FAS status, and DF accounted for a significant amount of explained variance in adaptive scores (R2 =.117, p = .004). FAS status was marginally positively associated with adaptive scores (b = 6.54, p = .062). Thus children with FAS have lower adaptive scores compared to alcohol-exposed children without FAS, although the difference did not reach statistical significance. Age was not significantly associated with adaptive scores (p = .134) across groups. Additionally, across groups, DF (b = 1.75, p = .004) significantly predicted adaptive scores. The DV X Group interaction term entered on step two did not significantly increase explained variance in adaptive scores (ΔR < .001, p = .989). Thus, the relation between DF and adaptive scores in the alcohol-exposed subjects was not dependent on the presence of an FAS diagnosis. When examining TMT, TMT, Age, and Group were entered on step one and accounted for a significant amount of explained variance in adaptive scores (R2 =.110, p = .007). FAS diagnosis was marginally positively associated with adaptive scores (b = 6.74, p = .057). Thus children with FAS have lower adaptive scores compared to alcohol-exposed children without FAS, although this difference did not reach statistical significance. Age was not significantly associated with adaptive scores (p = .428) across groups. Additionally, across groups, TMT (b = 1.13, p = .007) significantly predicted adaptive score. The TMT X Group interaction term entered on step two did not significantly increase explained variance in adaptive scores (ΔR < .001, p = .964). Thus, the relation between DF adaptive scores in alcohol-exposed subjects was not dependent on the presence of an FAS diagnosis.
When ALC with ADHD subjects were compared to ALC without ADHD subjects, Age, Group, and DF accounted for a significant amount of explained variance in adaptive scores (R2 = .229, p < .001) when entered on step one. ADHD diagnosis was a significant predictor of adaptive scores (b = 8.42, p < .001). Thus, the ALC with ADHD group had significantly lower adaptive scores compared to the ALC without ADHD group. Across groups, Age was not significantly associated with adaptive scores (p = .425). The DF X Group interaction terms entered on step two did not account for an increase in explained variance of adaptive scores (ΔR = .003, p = .512). Thus, the relation between DF and adaptive scores in alcohol-exposed subjects was not dependent on the presence of an ADHD diagnosis. When examining TMT, TMT, Age, and ADHD group were entered on step one and accounted for a significant amount of explained variance in adaptive score (R = .199, p < .001). ADHD diagnosis was a significant predictor of adaptive scores (b = 8.98, p < .001). Thus, the ALC with ADHD had significantly lower adaptive ratings compared to the ALC without ADHD group. TMT (b = .94, p = .018) was significantly associated with adaptive scores across groups, but Age was not significantly associated with adaptive scores (p = .715), across groups. Step two did not significantly add to the explained variance in adaptive scores (ΔR2 = .008, p = .319). Though ADHD diagnosis was, on its own, associated with adaptive scores, the relations between non-verbal EF tasks (DF, TMT) and adaptive scores in alcohol-exposed subjects was not dependent on the presence of an ADHD diagnosis.
Discussion
The aim of the present study was to examine whether or not performance on verbal and non-verbal executive function tasks predicted adaptive behavior outcomes in children with histories of heavy prenatal alcohol exposure compared to non-exposed children with ADHD and typically developing controls. Contrary to what was expected, the ALC and the ADHD group displayed similar performance on all D-KEFS tasks, although both groups did perform more poorly than the CON group. These results confirm previous findings that children with prenatal alcohol exposure have poorer EF capabilities when compared to typical controls (Vaurio et al., 2008, Mattson et al., 1998, Connor et al., 2000, Burden et al., 2009, Coles et al., 1997, Carmichael Olson et al., 1998) and that EF deficits occur in non-exposed children with ADHD (Wahlstedt et al., 2008, Lambek et al., 2010, Semrud-Clikeman et al., 2010, Semrud-Clikeman et al., 2008, Coolidge et al., 2000, Thorell and Wahlstedt, 2006). However, these results are, in part, different from our earlier report which suggested both similarities and differences in EF between these two groups, since in the current study, the ALC and ADHD groups did not differ significantly on any EF task (Vaurio et al., 2008). The two studies differed on variable selection and sample size, which may have resulted in these differences. As was expected, the ALC and the ADHD groups were rated more poorly than controls on measures of adaptive behavior, with the ALC group demonstrating more severe deficits compared to the ADHD group (Crocker et al., 2009, Thomas et al., 1998, Whaley et al., 2001, Mikami et al., 2007, Sukhodolsky et al., 2005, Stein et al., 1995, Roizen et al., 1994, Greene et al., 1996). Additionally, the majority of EF tasks (VF, TMT, and CWI) were strongly associated with adaptive behavior in the ADHD group, whereas only non-verbal EF tasks (DF, TMT) were significantly associated with adaptive scores in the ALC group. In the CON group, none of the D-KEFS tasks accounted for an increased amount of explained variance in adaptive scores, though standard scores decreased with age.
To examine whether the relation between specific EF tasks and adaptive behavior differed between groups, Group X D-KEFS task interactions were analyzed. For the most part, results were consistent across groups. However, the significant Group X VF interaction suggested that VF was uniquely associated with adaptive scores in the ADHD group relative to the ALC and the CON groups; follow-up analyses revealed that VF significantly predicted adaptive behavior only for the ADHD group. Follow-up tests also indicated that adaptive scores in the ADHD group were associated with performance on both verbal (VF and CWI) tasks and select non-verbal (TMT) EF tasks. These findings support prior studies (Clark et al., 2002) suggesting that when considered together, verbal and EF abilities account for adaptive deficits observed in non-exposed children with ADHD. However, given the relation between TMT (in addition to the verbal tasks) and adaptive behavior, the relation between cognitive set-shifting and adaptive behavior may be more general in this group. Thus, results indicate that as cognitive set-shifting ability increases, adaptive deficits decrease in non-exposed children with ADHD. Additionally, the large amount of variability in adaptive scores explained by VF, TMT, and CWI in the ADHD group may support prior findings that executive dysfunction, considered to be a hallmark deficit of ADHD (Nigg and Casey, 2005, Barkley, 1997, Pennington and Ozonoff, 1996), results in adaptive impairments in non-exposed children with ADHD.
Contrary to findings from the ADHD group, performance on non-verbal EF tasks (DF, TMT) accounted for a large amount of explained variance in adaptive scores in the ALC group. Deficits on non-verbal EF tasks, including TMT and DF have previously been reported following heavy prenatal alcohol exposure (Schonfeld et al., 2001, Vaurio et al., 2008). In particular, non-verbal EF deficits on DF have been shown to be particularly sensitive to prenatal alcohol exposure (Schonfeld et al., 2001). Additionally, deficits in spatial learning and memory are also documented in studies of prenatal alcohol exposure (Uecker and Nadel, 1998, Kaemingk and Halverson, 2000, Aragon et al., 2008), although further research needs to address such impairments. Given the wider range of difficulties typically observed in children with prenatal alcohol exposure relative to children with ADHD, it is therefore possible that a combination of deficits are contributing to impaired adaptive ability in these children. Since EF processes are difficult to disentangle from other higher-order cognitive processes (Fletcher and Henson, 2001), such as spatial working memory, the relation between specific non-verbal EF tasks (DF and TMT) and adaptive ratings observed in the ALC group may indicate the influence of other neuropsychological processes on adaptive behavior. Future studies should evaluate the contribution of different domains of cognitive function, including spatial working memory, to better understand the nature of adaptive difficulties in children with histories of prenatal alcohol exposure.
Subgroup comparisons within the ALC group revealed that even though there was a trend towards FAS children being rated as more impaired on adaptive behavior than non-dysmorphic alcohol-exposed subjects, EF performance predicted adaptive behavior similarly for dysmorphic and non-dysmorphic alcohol-exposed subjects. Similarly, children in the ALC group with ADHD had significantly lower adaptive scores than children in the ALC group without ADHD, but the presence of an ADHD diagnosis did not affect the relation between EF and adaptive behavior. Thus, it appears that while having prenatal alcohol exposure and a diagnosis of ADHD, and to a lesser extent having a diagnosis of FAS, places alcohol-exposed children at increased risk for adaptive dysfunction than history of heavy prenatal alcohol exposure alone, the presence of these diagnoses do not change the relation between nonverbal EF performance and adaptive behavior.
In the CON group, follow-up tests revealed that none of the EF tasks significantly predicted adaptive scores, indicating that age is more predictive of adaptive outcomes than verbal and non-verbal EF ability in this group. However, the significant negative relation between age and adaptive scores in the CON group should not be interpreted as decreasing adaptive abilities with age, but rather that standard scores in this group did not correspond with chronological expectations. There are several interpretations for this negative relation. First, this finding may result from the CON group being self-referred, which may possibly represent a more impaired population. However, the average performance of this group was still within or above normal limits for executive function, adaptive behavior, and IQ scores, suggesting an alternative explanation is necessary. Another possibility is that parents of typically developing adolescents, particularly those who are high functioning, may have higher expectations regarding adaptive abilities than what is typically demonstrated in this age range.
This study provides new information about the relation between non-verbal EF abilities and adaptive behavior in children with prenatal alcohol exposure. Although the relation between EF and adaptive functioning has not been examined with laboratory measures previously in FASD, prior studies have found significant relations between parent rating of social skills and social problem solving and parent ratings of EF (McGee et al., 2008a, Schonfeld et al., 2006). Specifically, the current investigation found that non-verbal EF tasks (DF, TMT) accounted for a large amount of explained variance in adaptive scores in the ALC group. This relation may be due to other factors which impact adaptive ability, including home environment, which was not considered in this study. Home environment may impact adaptive functioning, particularly for children with prenatal exposure to alcohol (Jester et al., 2000), who often reside outside of their biological homes (Fryer et al., 2007), and are more likely to have had unstable and/or adverse home environments (Streissguth et al., 2004). Poor behavioral outcomes have been reported for children with prenatal alcohol exposure when the child is subjected to non-advantageous or stressful home environments (Coggins et al., 2007, Fagerlund et al., 2011). The literature also suggests that children with ADHD also have home environments characterized by increased conflict and disorganization (Pressman et al., 2006) and thus alternative explanations may also exist. Group differences between D-KEFS tasks, adaptive behavior, and the relation between the two further support the notion that children with prenatal alcohol exposure and non-exposed children with ADHD may be differentiated on these domains. Previous studies comparing these groups show that groups perform similarly on set-shifting measures, but differ on measures of verbal and non-verbal fluency (Schonfeld et al., 2001, Vaurio et al., 2008). Additionally, previous findings have shown that, on measures of adaptive behavior, both groups show impairment, but only children with prenatal alcohol exposure fail to show improvement on adaptive functioning with age (Crocker et al., 2009).
Findings from the current study are also consistent with previous studies examining the relation between EF and adaptive ability in children with ADHD (Clark et al., 2002). Previous studies have demonstrated that early EF impairments are predictive of later behavioral problems in non-exposed children with ADHD (Wahlstedt et al., 2008) and that early ADHD diagnosis is associated with poorer adaptive functioning at an older age (Roizen et al., 1994). Additional studies in ADHD have found that childhood EF performance is predictive of adolescent social functioning (Miller and Hinshaw, 2010), which may contribute to poorer adaptive ratings in non-exposed children with ADHD.
In sum, these results support the idea that higher-order cognitive and strategic planning deficits may contribute to the adaptive deficits observed in children and adolescents with prenatal alcohol exposure and non-exposed children with ADHD. Additionally, these findings demonstrate that although age is related to adaptive behavior in the clinical groups, EF abilities account for an additional amount of explained variance in adaptive behavior ratings.
Limitations
While these results provide important information about the relation between EF and adaptive behavior in children with prenatal alcohol exposure, there are several limitations that should be considered, including the use of parent questionnaires, which may have increased subjectivity of adaptive ratings. It is possible that parent/caregiver expectations of daily functioning may be different for exposed vs. non-exposed children (Streissguth et al., 2004, McGee et al., 2008a). In addition, this study only included EF tasks with switching components, as previous literature has shown that children with histories of heavy prenatal alcohol exposure demonstrate deficits in complex set-shifting (McGee et al., 2008b). However, EF is a multifaceted construct and other executive abilities might contribute to adaptive functioning in children in these two clinical groups. Finally, IQ was not considered in our analyses, given statistical and theoretical limitations (Dennis et al., 2009) and it is possible that overall ability levels impacted the findings of this study. However, lowered IQ scores are inherent in the effects of heavy prenatal alcohol exposure and these results are generalizable to the larger population of alcohol-exposed.
Despite these limitations, this study has several notable strengths. This is the first study to examine the relation between standardized laboratory measures of EF and adaptive behavior outcomes in children with confirmed histories of prenatal alcohol exposure compared to non-exposed children with ADHD and healthy controls. Additionally, the sample sizes included in this study are large relative to other studies examining the effects of prenatal alcohol exposure. Furthermore, this study incorporates multiple testing centers across the United States, increasing generalizability of findings to a broader range of children who have histories of heavy prenatal alcohol exposure and/or ADHD.
Implications and Future Directions
These findings should be considered a first step towards identifying specific neurocognitive domains that contribute to secondary deficits, including adaptive abilities, in children with histories of heavy prenatal alcohol exposure. Based on these results, interventions that aim to strengthen non-verbal executive function skills (Bertrand, 2009) may be utilized to impact adaptive behavior. These interventions may be particularly important for individuals who have comorbid prenatal alcohol exposure and ADHD. Additionally, studies involving neuroimaging techniques aimed at the identification of specific brain regions associated with adaptive behaviors and complex higher-order processes, such as EF, could strengthen the evidence for a direct link between adaptive functioning deficits and cognitive processes in children with histories of prenatal alcohol exposure.
Acknowledgements
Research described in this paper was supported by NIAAA grant numbers U01 AA014834 (Mattson), U24 AA014811 (Riley), U24 AA014818 (Barnett), and U24 AA014815 (Jones). We would like to acknowledge the efforts in data collection of Kristina Hubbard, Delilah Bolo, and Heather Holden in San Diego; Suzanne Houston, Ariel Starr, and Genevieve Rodriguez in Los Angeles; Sharron Paige-Whitaker in Atlanta; and Alfredo Aragon, Ethan White, and Stephanie Rueda in Albuquerque.
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Aragon AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA. Neuropsychological study of FASD in a sample of American Indian children: Processing simple versus complex information. Alcoholism: Clinical and Experimental Research. 2008;32:2136–2148. doi: 10.1111/j.1530-0277.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bertrand J. Interventions for children with fetal alcohol spectrum disorders (FASDs): Overview of findings for five innovative research projects. Research in Developmental Disabilities. 2009;30:986–1006. doi: 10.1016/j.ridd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O’Connor M, Riley EP, Johnson KA, Cohen DE. National Task Force on FAS/FAE: Guidelines for Referral and Diagnosis. Centers for Disease Control and Prevention; Atlanta, GA: 2004. [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, Robinson LK, Khaole N, Nelson CA, Jacobson JL, Jacobson SW. The effects of fetal alcohol syndrome on response execution and inhibition: An event-related potential study. Alcoholism: Clinical and Experimental Research. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Attention deficit disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:978–987. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcoholism: Clinical and Experimental Research. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcoholism: Clinical and Experimental Research. 2010;34:1022–1032. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Janisse J, Delaney-Black V, Sokol RJ, Hannigan JH. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcoholism: Clinical and Experimental Research. 2009;33:634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. Journal of Child Psychology and Psychiatry. 2002;43:785–796. doi: 10.1111/1469-7610.00084. [DOI] [PubMed] [Google Scholar]
- Coggins TE, Timler GR, Olswang LB. A state of double jeopardy: Impact of prenatal alcohol exposure and adverse environments on the social communicative abilities of school-age children with fetal alcohol spectrum disorder. Language, Speech, and Hearing Services in Schools. 2007;38:117–127. doi: 10.1044/0161-1461(2007/012). [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicology and Teratology. 1991;13:357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21:150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental Neuropsychology. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Coolidge FL, Thede LL, Young SE. Heritability and the comorbidity of attention deficit hyperactivity disorder with behavioral disorders and executive function deficits: A preliminary investigation. Developmental Neuropsychology. 2000;17:273–287. doi: 10.1207/S15326942DN1703_1. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33:2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive Function System. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional interactions guiding adaptive processing of behavioral significance. Human Brain Mapping. 2009;30:3325–3331. doi: 10.1002/hbm.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund A, Autti-Ramo I, Hoyme HE, Mattson SN, Korkman M. Risk factors for behavioural problems in foetal alcohol spectrum disorders. Acta Paediatrica. 2011 doi: 10.1111/j.1651-2227.2011.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast DK, Conry J, Loock CA. Identifying fetal alcohol syndrome among youth in the criminal justice system. Journal of Developmental and Behavioral Pediatrics. 1999;20:370–372. doi: 10.1097/00004703-199910000-00012. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Graetz BW, Sawyer MG, Hazell PL, Arney F, Baghurst P. Validity of DSM-IV ADHD subtypes in a nationally representative sample of Australian children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1410–1417. doi: 10.1097/00004583-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Greene RW, Biederman J, Faraone SV, Ouellette CA, Penn C, Griffin SM. Toward a new psychometric definition of social disability in children with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:571–578. doi: 10.1097/00004583-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: A longitudinal follow-up. Journal of Pediatric Psychology. 2006;31:116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JM, Jacobson SW, Sokol RJ, Tuttle BS, Jacobson JL. The influence of maternal drinking and drug use on the quality of the home environment of school-aged children. Alcoholism: Clinical and Experimental Research. 2000;24:1187–1197. [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, Del Campo M, Manning MA, Prewitt LM, Chambers CD. Fetal alcohol spectrum disorders: Extending the range of structural defects. American Journal of Medical Genetics. Part A. 2010;152A:2731–2735. doi: 10.1002/ajmg.a.33675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Benirschke K. Evaluation of the cranial base in amnion rupture sequence involving the anterior neural tube: Implications regarding recurrence risk. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76:688–691. doi: 10.1002/bdra.20299. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Halverson PT. Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychology. 2000;6:115–128. doi: 10.1076/chin.6.2.115.7058. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Mulvaney S, Tanner Halverson P. Learning following prenatal alcohol exposure: Performance on verbal and visual multitrial tasks. Archives of Clinical Neuropsychology. 2003;18:33–47. [PubMed] [Google Scholar]
- Lambek R, Tannock R, Dalsgaard S, Trillingsgaard A, Damm D, Thomsen PH. Validating neuropsychological subtypes of ADHD: how do children with and without an executive function deficit differ? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51:895–904. doi: 10.1111/j.1469-7610.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. Oxford University Press; New York: 2004. [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å , Autti-Rämö I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP, the CIFASD Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology. 2009;31:71–75. doi: 10.1016/j.ntt.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. The American Journal of Drug and Alcohol Abuse. 2008a;34:423–431. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN. Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcoholism: Clinical and Experimental Research. 2008b;32:1388–1397. doi: 10.1111/j.1530-0277.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125:75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami AY, Huang-Pollock CL, Pfiffner LJ, McBurnett K, Hangai D. Social skills differences among attention-deficit/hyperactivity disorder types in a chat room assessment task. Journal of Abnormal Child Psychology. 2007;35:509–521. doi: 10.1007/s10802-007-9108-5. [DOI] [PubMed] [Google Scholar]
- Miller M, Hinshaw SP. Does childhood executive function predict adolescent functional outcomes in girls with ADHD? Journal of Abnormal Child Psychology. 2010;38:315–326. doi: 10.1007/s10802-009-9369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Broaddus JE, Rosenstein LD, Medina DE, Soderberg C. Neuropsychological test performance of children with ADHD relative to test norms and parent behavioral ratings. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2002;17:671–689. [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pressman LJ, Loo SK, Carpenter EM, Asarnow JR, Lynn D, McCracken JT, McGough JJ, Lubke GH, Yang MH, Smalley SL. Relationship of family environment and parental psychiatric diagnosis to impairment in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:346–354. doi: 10.1097/01.chi.0000192248.61271.c8. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends in Cognitive Sciences. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Blondis TA, Irwin M, Stein M. Adaptive functioning in children with attention-deficit hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine. 1994;148:1137–1142. doi: 10.1001/archpedi.1994.02170110023004. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. Journal of Studies on Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Pliszka S, Liotti M. Executive functioning in children with attention-deficit/hyperactivity disorder: Combined type with and without a stimulant medication history. Neuropsychology. 2008;22:329–340. doi: 10.1037/0894-4105.22.3.329. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Butcher B. Executive functioning in children with Asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of Autism and Developmental Disorders. 2010;40:1017–1027. doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Survey Form Manual. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales, 2nd edition: Survey forms manual. AGS Publishing; Circle Pines, MN: 2005. [Google Scholar]
- SPSS . SPSS 19.0 for Mac OS X. Chicago: 2010. [Google Scholar]
- Stavro GM, Ettenhofer ML, Nigg JT. Executive functions and adaptive functioning in young adult attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2007;13:324–334. doi: 10.1017/S1355617707070348. [DOI] [PubMed] [Google Scholar]
- Stein MA, Szumowski E, Blondis TA, Roizen NJ. Adaptive skills dysfunction in ADD and ADHD children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1995;36:663–70. doi: 10.1111/j.1469-7610.1995.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Willms J, Spohr H-L. Long-term psychopathological and cognitive outcome of children with fetal alcohol syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:990–994. doi: 10.1097/00004583-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome. Diagnosis, epidemiology, prevention, and treatment. 1996:213. [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Final report: Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) University of Washington Publication Services; Seattle, WA: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: Adolescent data from a population-based prospective study. Alcoholism: Clinical and Experimental Research. 1994;18:248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: Effects on child IQ and learning problems at age 7 1/2 years. Alcoholism: Clinical and Experimental Research. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, do Rosario-Campos MC, Scahill L, Katsovich L, Pauls DL, Peterson BS, King RA, Lombroso PJ, Findley DB, Leckman JF. Adaptive, emotional, and family functioning of children with obsessive-compulsive disorder and comorbid attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2005;162:1125–1132. doi: 10.1176/appi.ajp.162.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research. 1998;22:528–533. [PubMed] [Google Scholar]
- Thorell LB, Wahlstedt C. Executive functioning deficits in relation to symptoms of ADHD and/or ODD in preschool children. Infant and Child Development. 2006;15:503–518. [Google Scholar]
- Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: Performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2009;15:53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. American Journal on Mental Retardation. 1998;103:12–18. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstedt C, Thorell LB, Bohlin G. ADHD symptoms and executive function impairment. Early predictors of later behavioral problems Developmental Neuropsychology. 2008;33:160–178. doi: 10.1080/87565640701884253. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: Views from developmental psychology. Developmental Neuropsychology. 1988;4:199–230. [Google Scholar]
- Whaley SE, O’Connor MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcoholism: Clinical and Experimental Research. 2001;25:1018–1024. [PubMed] [Google Scholar]