Abstract
Background
The surveillance and effector functions of the immune system are critically dependent on the appropriate distribution of immune cells in the body. An acute or short-term stress response induces a rapid and significant redistribution of immune cells among different body compartments. Stress-induced leukocyte redistribution may be a fundamental survival response that directs leukocyte subpopulations to specific target organs during stress, and significantly enhances the speed, efficacy and regulation of an immune response. Immune responses are generally enhanced in compartments (e.g., skin) that are enriched with leukocytes, and suppressed in compartments that are depleted of leukocytes during/following stress. The experiments described here were designed to elucidate the: 1) Time-course, trajectory, and subpopulation-specificity of stress-induced mobilization and trafficking of blood leukocytes. 2) Individual and combined actions of the principal stress hormones, norepinephrine (NE), epinephrine (EPI), and corticosterone (CORT), in mediating mobilization or trafficking of specific leukocyte subpopulations. 3) Effects of stress/stress hormones on adhesion molecule, L-selectin (CD62L), expression by each subpopulation to assess its adhesion / functional / maturation status.
Methods
Male Sprague Dawley rats were stressed (short-term restraint, 2–120 min), or adrenalectomized and injected with vehicle (VEH), NE, EPI, CORT, or their combinations, and blood was collected for measurement of hormones and flow cytometric quantification of leukocyte subpopulations.
Results
Acute stress induced an early increase/mobilization of neutrophils, lymphocytes, helper T cells (Th), cytolytic T cells (CTL), and B cells into the blood, followed by a decrease/trafficking of all cell types out of the blood, except neutrophil numbers that continued to increase. CD62L expression was increased on neutrophils, decreased on Th, CTL, and natural killer (NK) cells, and showed a biphasic decrease on monocytes & B cells, suggesting that CD62L is involved in mediating the redistribution effects of stress. Additionally, we observed significant differences in the direction, magnitude, and subpopulation specificity of the effects of each hormone: NE increased leukocyte numbers, most notably CD62L−/+ neutrophils and CD62L− B cells. EPI increased monocyte and neutrophil numbers, most notably CD62L−/+ neutrophils and CD62L− monocytes, but decreased lymphocyte numbers with CD62L−/+ CTL and CD62L+ B cells being especially sensitive. CORT decreased monocyte, lymphocyte, Th, CTL, and B cell numbers with CD62L− and CD62L+ cells being equally affected. Thus, naïve (CD62L+) vs. memory (CD62L−) T cells, classical (CD62L+) vs. non-classical (CD62L−) monocytes, and similarly distinct functional subsets of other leukocyte populations are differentially mobilized into the blood and trafficked to tissues by stress hormones.
Conclusion
Stress hormones orchestrate a large-scale redistribution of immune cells in the body. NE and EPI mobilize immune cells into the bloodstream, and EPI and CORT induce traffic out of the blood possibly to tissue surveillance pathways, lymphoid tissues, and sites of ongoing or de novo immune activation. Immune cell subpopulations appear to show differential sensitivities and redistribution responses to each hormone depending on the type of leukocyte (neutrophil, monocyte or lymphocyte) and its maturation/functional characteristics (e.g., resident or inflammatory monocyte, naïve or central/effector memory T cell). Thus, stress hormones could be administered simultaneously or sequentially to induce specific leukocyte subpopulations to be mobilized into the blood, or to traffic from blood to tissues. Stress hormone-mediated changes in immune cell distribution could be clinically harnessed to: 1) Direct leukocytes to sites of vaccination, wound healing, infection, or cancer and thereby enhance protective immunity. 2) Reduce leukocyte traffic to sites of inflammatory/autoimmune reactions. 3) Sequester immune cells in relatively protected compartments to minimize exposure to cytotoxic treatments like radiation or localized chemotherapy. 4) Measure biological resistance/sensitivity to stress hormones in vivo. In keeping with the guidelines for Richter Award manuscripts, in addition to original data we also present a model and synthesis of findings in the context of the literature on the effects of short-term stress on immune cell distribution and function.
Keywords: psychoneuroimmunology, stress, immune cell trafficking, catecholamine and glucocorticoid stress hormones, surgical recovery, vaccine or cancer adjuvant, integrative immunology
INTRODUCTION
Regulated redistribution of immune cells among different body compartments is essential for effective immune surveillance and salubrious immune function (Butcher, 1990; Sprent and Tough, 1994). The blood is a critical compartment through which immune cells must pass in order to maintain their normal surveillance pathways and to rapidly reach sites of de novo immune activation (wounding, antigen or pathogen entry) (Springer, 1994). Therefore, the numbers and proportions of leukocytes in the blood provide an important representation of the state of distribution of leukocytes in the body and of the state of activation of the immune system. Numerous studies have shown that stress can significantly affect immune cell distribution and function (for review see: (Butts and Sternberg, 2008; Chrousos, 2010; Dhabhar, 2009b; Dhabhar and McEwen, 1997; Glaser and Kiecolt-Glaser, 2005). Stress has been defined as a constellation of events, consisting of a stimulus (stressor), that precipitates a reaction in the brain (stress perception), that in turn activates physiological fight-or-flight systems in the body (physiological stress response) (Dhabhar and McEwen, 1997). It is important to recognize that the only mechanism through which a stressor can affect the brain or body is by inducing biological changes in the organism, which highlights the critical importance of stress hormones and physiological stress response. While stress can be harmful when it is chronic or long lasting (Chrousos and Kino, 2007; Glaser and Kiecolt-Glaser, 2005; Irwin et al., 1990; McEwen, 1998), it is often overlooked that a stress response has salubrious adaptive effects in the short run (Dhabhar and McEwen, 2007; Dhabhar and Viswanathan, 2005; Rosenberger et al., 2009). Therefore, a major distinguishing characteristic of stress is duration: Acute or short-term stress has been defined as stress that lasts for a period of minutes to hours, and chronic stress as stress that persists for several hours per day for weeks or months (Dhabhar and McEwen, 1997).
It is well known that acute or short-term stress induces a large-scale redistribution of immune cells in the body (for review see: (Dhabhar, 1998, 2009a; Dhabhar, 2009b)). This redistribution is similar across many species, including humans, which suggests that it is evolutionarily significant and likely to confer an adaptive advantage (Dhabhar, 1998, 2009a; Dhabhar, 2009b). Given the rapid time course and large magnitude of stress-induced immune cell redistribution, these effects of stress are important to take into account while measuring immune function, therapeutically administering stress hormones, and collecting, analyzing, and interpreting experimental and clinical data. Surprisingly, in spite of its importance, immune cell redistribution has been a relatively underappreciated, and clinically untapped, effect of stress.
Although the functional and clinical ramifications of stress-induced leukocyte redistribution have yet to be fully appreciated and harnessed, numerous studies have elegantly examined the effects of stress (Bosch et al., 2003; Brosschot et al., 1994; Dhabhar et al., 1995b; Mills et al., 1995; Schedlowski et al., 1993b; Stefanski and Gr,ner, 2006), and exercise (Goebel and Mills, 2000; Hong et al., 2004; Nagatomi et al., 2000; Okutsu et al., 2008; Pedersen and Hoffman-Goetz, 2000) on selected leukocyte subpopulations. In a seeming contradiction, human studies have generally shown that acute stress increases blood immune cell numbers relative to resting state, while mouse and rat studies have shown that acute stress decreases blood immune cell numbers. However, these apparent contradictions may mainly be a matter of kinetics and arise when different studies examine different phases of the effects of acute stress on blood immune cell numbers. Therefore, the first series of results presented here comprehensively quantifies the effects of the early and late phases of stress on all major immune cell subpopulations. We test the hypothesis that the increase in blood immune cell numbers (which represents leukocyte mobilization into the blood) occurs early during short-term stress, while the decrease in blood immune cell numbers (which represents leukocyte traffic out of the blood and into tissues) occurs late during acute stress.
Numerous studies have also examined the effects of specific stress hormones on blood immune cell numbers (Benschop et al., 1996; Dale et al., 1974; Dhabhar et al., 1996; Fauci and Dale, 1974; Schedlowski et al., 1993a). Furthermore, it has been shown that adrenalectomy significantly reduces the magnitude of stress-induced decreases in blood leukocytes (Dhabhar et al., 1995b). Taken together, these studies suggest that the principal stress hormones, norepinephrine (NE), epinephrine (EPI) and corticosterone (CORT), that exert the widespread physiological effects of an acute stress response (Dhabhar and McEwen, 2001; Sapolsky et al., 2000), are also the major endocrine mediators of specific phases and redistribution profiles of psychological and physical (exercise) stressors on specific leukocyte subpopulations (Dhabhar and McEwen, 2001). EPI and NE have also been extensively studied as mediators of exercise-induced changes in immune cell distribution (Pedersen and Hoffman-Goetz, 2000). To our knowledge, however, no study has systematically and comprehensively elucidated the effects of stress hormones administered singly and in combination. Such elucidation of the combinatorial effects of stress hormones is important for understanding the differential contributions of NE, EPI, and CORT, that may come into effect as a result of different concentrations and combinations of these hormones being stimulated under different stress conditions (Kvetnansky et al., 1998; Pacak et al., 1998). Therefore, in the second series of results presented here we characterize and quantify individual and combined actions of NE, EPI and CORT, in mediating changes in all major leukocyte subpopulations. We hypothesized that specific stress hormones and their combinations would mediate distinct aspects of stress-induced leukocyte redistribution.
In both series of experiments we quantify changes in leukocyte expression of the adhesion molecule, L-selectin (CD62L) that mediates leukocyte rolling, the first step in the cascade of reactions that leads to leukocyte adhesion and transmigration, critical steps for leukocyte surveillance pathways and leukocyte response to immune activation / inflammation (Butcher and Picker, 1996; Khan et al., 2003; Wedepohl et al., 2011). Given its critical role in the adhesion cascade, we hypothesized that stress and stress hormones would change leukocyte CD62L expression. Additionally, the presence or absence of CD62L on an immune cell can be used to approximate its functional or maturation status. For example, CD62L+ Th and CTLs cells are thought to be either naïve or central memory T cells while CD62L- Th and CTLs are thought to be effector T cells (Seder and Ahmed, 2003). Similarly, CD62L+ monocytes are thought to be classical / inflammatory monocytes while CD62L- monocytes are thought to be non-classical monocytes (Gordon and Taylor, 2005; Tacke and Randolph, 2006). Therefore, we used the presence or absence of CD62L to characterize and quantify the differential effects of stress and stress hormones on specific functional/maturation phenotypes within each leukocyte subpopulation.
Studies such as these are important because they could conceivably lead to clinical applications that harness stress physiology to direct/enhance protective immune responses during vaccination, wound healing, infection, or cancer, to reduce leukocyte traffic to sites of inflammatory/autoimmune reactions, to sequester immune cells in certain compartments to minimize exposure to cytotoxic treatments like radiation or localized chemotherapy, and to monitor as a measure of stress hormone resistance/sensitivity. These studies also have important implications for experimental design and for the interpretation of experimental and clinical/diagnostic data. To our knowledge, these studies are the first to simultaneously quantify stress-induced changes in all major leukocyte subpopulations and their distinct functional subtypes, during the early (mobilization) as well as late (trafficking) phases of the leukocyte redistribution stress response. To our knowledge, these studies are also the first to quantify the combinatorial effects of simultaneously administering stress hormone combinations that mimic and elucidate the combined effects of NE, EPI, and CORT, which are the major mediators of the stress-induced changes in immune cell distribution. Based on data presented here as well as what is known in the literature, we propose a model explaining how stress hormones represent a “call to arms” and induce the body's “soldiers” (immune cells) to leave their “barracks” (marginated pool, spleen, bone marrow), travel through the “boulevards” (blood) and take up positions at ongoing or potential “battlefields” (e.g., skin) during or following stress.
MATERIALS & METHODS
Animals
Male Sprague Dawley rats (200–300 g) (Harlan Sprague Dawley, Indianapolis, IN) were used in all experiments. Animals were housed (3 per cage) in the accredited (American Association of Accreditation of Laboratory Animal Care) animal facilities of The Rockefeller University (New York, NY). Experiments were conducted according to protocols approved by The Rockefeller University Laboratory Animal Care and Use Committee. Animal rooms were maintained on a 12-h light-dark cycle (lights on at 7 am and off at 7 pm). All animals were given rat chow and tap water ad libitum.
Adrenalectomy
Bilateral adrenalectomy (ADX) was performed using standard aseptic surgical techniques on rats fully anesthetized with the inhalant, methoxyflurane (Metofane; Pitman-Moore, Washington Crossing, NJ). ADX animals were maintained on a low “normalizing” dose of corticosterone (20 μg/ml, Steraloids, Wilton, NH) administered through drinking fluid (animals were given two bottles, one with water and one with 3 % saline, described in: (Dhabhar and McEwen, 1999)). This was necessary for restoring permissive functions of corticosterone that are lost following ADX (Ambrose, 1964; De Kloet, 1995; Ingle, 1936, 1954). Corticosterone replacement normalizes basal levels of adrenocorticotropic hormone (ACTH), blood leukocyte numbers, and catecholamine hormones (Kvetnansky et al., 1993), all of which are abnormally high in ADX animals. Unlike constant replacement (via pellets or osmotic pumps), drinking water corticosterone also facilitates the normal termination of a stress-induced ACTH response (Akana et al., 1988; Jacobson et al., 1988), and simulates the circadian corticosterone rhythm as animals drink at the beginning of the active period.
Stress
Acute stress was administered by placing animals (without squeezing or compression) in well-ventilated Plexiglas (Rohm and Haas, Philadelphia, PA) restrainers for 2 minutes to 2 hours as indicated in the figures. This procedure approximates stress that is largely psychological in nature due to the perception of confinement on part of the animal (for review see: (Berkenbosch et al., 1991; Glavin et al., 1994). Restraint activates the autonomic nervous system (Kvetnansky et al., 1993), and the hypothalamic-pituitary-adrenal axis (Dhabhar et al., 1993; Dhabhar et al., 1995a; Dhabhar et al., 1994; Plotsky and Meaney, 1993), and results in the activation of adrenal steroid receptors throughout the body (Dhabhar et al., 1995a; Plotsky and Meaney, 1993).
Experimental procedures
Effects of stress on catecholamine and glucocorticoid hormones (Figure 1): Rats (sample size, n = 5 or 6 per time point) were restrained for 0 (resting state baseline), 2, 4, 6, 30, and 120 minutes and euthanized at the end of each time period. Trunk blood was rapidly collected into EDTA tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) that were immediately placed on ice. Plasma was obtained via centrifugation and stored at - 70 °C until analysis. Based on previous studies that showed similar effects of stress on leukocyte redistribution during different phases of the circadian cycle (Dhabhar et al., 1995b), all experiments in the current study were started one hour after lights went on in the animal room. Effects of stress on blood immune cell numbers and CD62L expression (Figures 2–4): Rats (n = 3) were restrained in Plexiglas (Rohm and Haas, Philadelphia, PA) restrainers. Blood was rapidly collected into heparinized microfuge tubes from each rat by the tail clip method immediately after it was placed in the restrainer (0 minute, resting state baseline) and at 6, 15, 30, 60, amd 120 minutes. Tails were clipped only for collection of the first blood sample. No further clipping is necessary for subsequent sample collection. Animals were returned to their home cages for recovery after 2 h restraint stress. Blood was maintained at room temperature until total white blood cell counts were obtained and samples were stained for flow cytometric analysis. Effects of catecholamine and glucocorticoid hormones on blood immune cell numbers and CD62L expression (Figures 5–7): Different groups of adrenalectomized (ADX) rats were injected intraperitonealy with an aqueous solution of vehicle (VEH, 2-hydroxypropyl-β-cyclodextrin, Research Biochemicals International, Natick, MA, n = 3), norepinephrine (NE, 2 mg/kg, Research Biochemicals International, Natick, MA, n = 4), epinephrine (EPI, 0.3 mg/kg, Research Biochemicals International, Natick, MA, n = 3), corticosterone (CORT, 3 mg/kg, Steraloids, Wilton, NH, n=3), and the following combinations of the same concentrations of these hormones: NE +EPI (n = 4), NE + CORT (n = 4), EPI + CORT (n = 3), NE + EPI + CORT (n=4). The injected hormone concentrations were based on amounts used in other studies (Kradin et al., 2001; Meng et al., 1996; van Gool et al., 1984), and determined to induce circulating concentrations and effects that were within the range of a physiological stress response. Blood was rapidly collected by the tail clip method into heparinized microfuge tubes from each rat 2 hours after injection. Animals were returned to their home cages for recovery after blood collection. Blood was maintained at room temperature until total white blood cell counts were obtained and samples were stained for flow cytometric analysis.
Figure 1.
Acute stress induces rapid increases in circulating (plasma) norepinephrine, epinephrine, and corticosterone concentrations. Data are expressed as means ± SEM (n = 5 or 6 per time point). Statistically significant differences are indicated: * p < 0.05: relative to baseline (Fisher's LSD).
Figure 2.
Acute stress induces rapid and significant changes in WBC, monocyte, neutrophil, lymphocyte, and Th, CTL, B, and NK cell numbers in the blood. Leukocyte numbers (1000 /μl blood) were quantified at 0 (baseline) and 6, 15, 30, 60, and 120 minutes during restraint stress. An increase in immune cell numbers relative to baseline represents mobilization of leukocytes into the blood. A decrease from peak numbers or relative to baseline represents trafficking of cells out of the blood and into tissues. Percent change relative to baseline at peak and trough is indicated. Data are expressed as means ± SEM (n=3). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: relative to peak (Fisher's LSD).
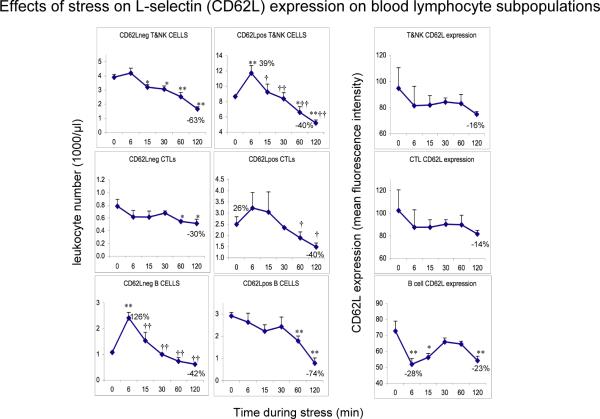
Figure 4.
Effects of acute stress on CD62L− and CD62L+ T&NK cell, CTL, and B cells numbers and CD62L expression on the same cell types. Leukocyte numbers (1000 /μl blood) were quantified at 0 (baseline) and 6, 15, 30, 60, and 120 minutes during restraint stress. CD62L surface expression is expressed as mean fluorescence intensity (MFI) as measured on the flow cytometer. An increase in immune cell numbers relative to baseline represents mobilization of leukocytes into the blood. A decrease from peak numbers or relative to baseline represents trafficking of cells out of the blood and into tissues. Percent change relative to baseline at peak and trough is indicated. Data are expressed as means ± SEM (n=3). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: relative to peak (Fisher's LSD).
Figure 5.
Effects of control (vehicle, VEH) or stress hormones, norepinephrine (NE), epinephrine (EPI), corticosterone (CORT), NE+EPI, NE+CORT, EPI+CORT, and NE+EPI+CORT (bars shown in this order) on WBC, monocyte, neutrophil, lymphocyte, and Th, CTL, B, and NK cell numbers in the blood. Leukocyte numbers (1000 /μl blood) were quantified 2 hours after vehicle or hormone injection in adrenalectomized animals. An increase in immune cell numbers relative to the VEH group represents mobilization of leukocytes into the blood. A decrease represents trafficking of cells out of the blood and into tissues. Data are expressed as means ± SEM (n=3 or 4 per treatment group). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: please see text for comparison groups (Fisher's LSD).
Figure 7.
Effects of control (vehicle, VEH) or stress hormones, norepinephrine (NE), epinephrine (EPI), corticosterone (CORT), NE+EPI, NE+CORT, EPI+CORT, and NE+EPI+CORT (bars shown in this order) on CD62L− and CD62L+ T&NK cell, CTL, and B cells numbers and CD62L expression on the same cell types. Leukocyte numbers (1000 /μl blood) were quantified 2 hours after vehicle or hormone injection in adrenalectomized animals. CD62L surface expression is expressed as mean fluorescence intensity (MFI) as measured on the flow cytometer. An increase in immune cell numbers relative to the VEH group represents mobilization of leukocytes into the blood. A decrease represents trafficking of cells out of the blood and into tissues. Data are expressed as means ± SEM (n=3 or 4 per treatment group). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: please see text for selected comparison groups (Fisher's LSD).
Quantification of plasma catecholamines and corticosterone
Norepinephrine (NE) and epinephrine (EPI) were quantified in EDTA plasma by HPLC with electrochemical detection in the laboratory of William Malarkey, at The Ohio State University. Standards and chemistry (Alumina extraction) were purchased from ChromSystems, Munich, Germany (U.S. affiliate Thermo-Alko, Beverly, MA). The HPLC Pump and detector were manufactured by ESA (ESA, Inc., Chelsford, MA). A Waters 717 plus autosampler was used to make injections (Waters Corporation, Milford, MA). C-18 Columns were purchased from the Waters Corporation. Intra-assay variation for norepinephrine and epinephrine was 3% and 6% respectively. Inter-assay variation for norepinephrine and epinephrine was 6% and 13% respectively. Sensitivity for norepinephrine was 15 pg/ml and for epinephrine was 6 pg/ml. Plasma catecholamine concentrations are expressed as pg/ml. Corticosterone was measured by radioimmunoassay using an RIA kit (Diagnostic Products Corporation, Los Angeles, CA). The antiserum is highly specific for rat corticosterone and has very low cross reactivity to other steroids. Intra-assay and inter-assay variation were 6.8% and 8.5% respectively. Assay sensitivity was 5.7 ng/ml of corticosterone. Plasma corticosterone concentrations are expressed as ng/ml.
Characterization and quantification of blood immune cell subpopulations
White blood cell (WBC) counts were obtained on a hematology analyzer (F800, Sysmex, McGraw Park, IL). Specific subtypes of immune cells were measured by immunofluorescent antibody staining of whole blood and subsequent analysis using three color flow cytometry (FACScan, Becton Dickinson, San Jose, CA) using a panel of leukocyte surface markers as previously described (Dhabhar et al., 1995b, 1996; Dhabhar et al., 1994). Neutrophil, monocyte, and lymphocyte differentials were obtained based on forward- versus side- scatter characteristics on the flow cytometer. Briefly, lymphocyte subsets were defined using 3-color panels consisting of the following monoclonal antibodies (Harlan, Indianapolis, IN): In the first panel we used FITC-conjugated OX-19 as a pan-T cell marker (Dallman et al., 1984), PE-conjugated W3/25, as a marker for helper T lymphocytes (Brideau et al., 1980), PE-Cy5-conjugated OX-8, as a marker for cytolytic T cells (CTL) (Barclay, 1981; Johnson et al., 1985). NK cells were identified as OX-19-OX-8+ cells. In a second panel, we used FITC-conjugated anti-CD62L antibody to identify L-selectin, PE-conjugated OX33, to identify B cells (Woollett et al., 1985), and PE-Cy5-conjugated OX-8 to identify CTLs. Antibody isotype controls were used to set the negative criterion analysis.
Statistical Analysis
Statistical analysis employed analysis of variance (ANOVA). Fixed block ANOVA was employed for repeated-measures analyses to adjust for effects of individual rats (Netter et al., 1985). Post hoc means comparisons employed Fisher's least significant difference (LSD) for assessment of statistical significance between key groups or time points (Milliken and Johnson, 1998). Where variances were unequal via a homogeneity test, Welch's correction was applied. Significant overall effects shown by ANOVAs are indicated together with post-hoc tests for significant differences for selected time point (e.g., baseline compared to stress, or peak compared to stress) comparisons or selected treatment group (VEH compared to hormone treatment, or highest hormone-induced change compared to other groups) comparisons. In some cases where there was no statistically significant overall effect, only the results of the post-hoc test are shown. Some observations that are discussed but were not statistically significant are indicated by the abbreviation “nss.” Actual p values are stated in the text for the overall ANOVAs, but rounded p values are given for all other comparisons in order to streamline presentation. Exact p values are also indicated in the few instances where trends (0.05 < p < 0.1) are mentioned so that the reader can gauge the strength of evidence for the trend. Means that differed significantly are indicated in the figures by symbols defined in the figure legends. Data are expressed as mean ± SEM in all figures. A computer statistics package was used for statistical analyses (SAS v9.2, SAS Institute, Cary, NC).
RESULTS
Acute stress-induced changes in norepinephrine (NE), epinephrine (EPI), and corticosterone (CORT)
Figure 1 shows stress-induced changes in NE, EPI, and CORT. Acute stress increased circulating concentrations of the three principal stress hormones, NE (resting = 3881 ± 550 pg/ml; stress = 5245 ± 659 pg/ml, nss), EPI (resting = 7537 ± 910 pg/ml; stress = 9846 ± 540 pg/ml, nss), and CORT (resting = 65 ± 31 ng/ml; stress = 791 ± 44 ng/ml, p < 0.05). Relative to resting state baseline levels, NE and EPI reached peak concentrations at 6 minutes after the beginning of stress (nss), while circulating CORT showed a significant increase starting at 2 minutes (p < 0.05), and remained significantly higher at all subsequent time points (p < 0.05), reaching peak concentrations at 30 minutes (p < 0.05).
Acute stress-induced changes in subtypes of blood monocytes, neutrophils, and lymphocytes
Figure 2 shows the time course of stress-induced changes in blood immune cell numbers at baseline (0 minutes) and 6, 15, 30, 60, and 120 minutes after the beginning of stress. Effects of stress were quantified as follows. 1) Total white blood cells (WBC): Fixed-block ANOVA showed that there was an overall effect of stress over time on blood WBC numbers (F = 8.03, p < 0.01). Compared to resting state baseline, WBC numbers increased at 6 minutes (p < 0.05), declined to become indistinguishable from baseline at 15 minutes (p = 0.86), and continued to decrease, reaching their lowest levels at 2 hours (p < 0.01). Compared to their peak levels at 6 minutes, WBC numbers were significantly lower at all other time points (p < 0.05). Since overall WBC numbers reflect the sum of the changes in leukocyte subpopulation numbers (that may change in different directions or to different extents in response to stress), we quantified stress-induced changes in all major immune cell subpopulations. 2) Monocytes: Monocyte numbers remained similar to baseline levels up to 30 minutes, and then began to decrease, reaching their lowest levels at 2 hours, however, these changes were not statistically significant. 3) Neutrophils: Compared to resting state baseline, neutrophil numbers increased at 6 minutes, remained at that level until 30 minutes, showed another increase at 60 minutes (trend, p = 0.07), and reached their highest levels at 2 hours (p < 0.05). 4) Lymphocytes: Fixed-block ANOVA showed that there was an overall effect of stress over time on blood lymphocyte numbers (F = 13.65, p < 0.001). Compared to resting state baseline, lymphocyte numbers increased at 6 minutes (p < 0.05), declined to become indistinguishable from baseline at 15 minutes (p = 0.89), and continued to decrease, reaching their lowest levels at 2 hours (p < 0.001). Compared to their peak levels at 6 minutes, lymphocyte numbers were significantly lower at all other time points (p < 0.05).
In addition to the effects on the mixed lymphocyte population described above, the following effects of stress were observed for specific lymphocyte subpopulations. 1) Helper T (Th) cells: Fixed-block ANOVA showed that there was an overall effect of stress over time on blood Th numbers (F = 14.27, p < 0.001). Compared to resting state baseline, mean Th numbers increased at 6 minutes (p < 0.001), declined to become indistinguishable from baseline at 15 minutes (p = 0.15), and continued to decrease, reaching their lowest levels at 2 hours (nss). Compared to their peak levels at 6 minutes, Th numbers were significantly lower at all other time points (p < 0.01). Cytolytic T cells (CTL): Fixed-block ANOVA showed that there was an overall effect of stress over time on blood CTL numbers (F = 8.45, p < 0.01). Mean CTL numbers increased at 6 minutes (p < 0.01), declined to become indistinguishable from baseline at 15 minutes (p = 0.33), and continued to decrease, reaching their lowest levels at 2 hours (p = 0.06). Compared to their peak levels at 6 minutes, CTL numbers were significantly lower at all other time points (p < 0.05). 3) B cells: Fixed-block ANOVA showed that there was an overall effect of stress over time on blood B cell numbers (F = 22.31, p < 0.0001). Mean B cell numbers increased at 6 minutes (p < 0.05), declined to become indistinguishable from baseline at 15 minutes (p = 0.54), and continued to decrease, reaching their lowest levels at 2 hours (p < 0.0001). Compared to their peak levels at 6 minutes, B cell numbers were significantly lower at all other time points (p < 0.001). 4) NK cells: Fixed-block ANOVA showed that there was an overall effect of stress over time on blood NK cell numbers (F = 7.72, p < 0.01). Unlike T and B cells that increased at 6 minutes, mean NK cell numbers showed a rapid decrease at 6 minutes (p < 0.05), then showed a slight increase relative to the 6 minute time point at 15 and 30 minutes (nss), then decreased to reach their lowest levels at 2 hours (p < 0.001).
In summary, Figure 2 shows that stress induced an early (6 min) increase/mobilization of neutrophils, lymphocytes, helper T cells (Th), cytolytic T cells (CTL), and B cells into the blood, followed (at 60–120 min) by a decrease/trafficking of all cell types out of the blood, except neutrophil numbers that continued to increase while the stressor was present. The redistribution patterns shown by functional subsets (naïve, memory or effector lymphocytes, inflammatory or resident monocytes) within each subpopulation, and the hormonal mediators of mobilization and trafficking are elucidated below.
Acute stress-induced changes in blood CD62L expression on monocyte, neutrophil, and lymphocyte subpopulations
Given the critical role of CD62L in the adhesion cascade and as a maturational/functional status marker, we hypothesized that stress and stress hormones would increase or decrease leukocyte CD62L expression in relation to stress effects on immune cell distribution. Figure 3 shows stress-induced changes in numbers of CD62L− and CD62L+ cells, and the intensity of CD62L expression on monocytes and neutrophils. 1) Monocytes: Compared to resting state baseline, CD62L− monocytes, that are generally thought to be non-classical / resident cells (Gordon and Taylor, 2005; Tacke and Randolph, 2006) decreased steadily during stress, reaching their lowest levels at 2 hours although these differences were not statistically significant. In contrast, CD62L+ monocytes, which are thought to be inflammatory monocytes (Gordon and Taylor, 2005; Tacke and Randolph, 2006), increased slightly between 6 to 30 min, and then decreased to their lowest levels at 2 hours. These differences were also not statistically significant. With respect to CD62L expression on monocytes, fixed-block ANOVA showed that there was an overall effect of stress over time (F = 5.02, p < 0.05). Monocyte CD62L expression showed a biphasic decrease with troughs at 6 minutes (p < 0.01) and 2 hours (p < 0.01) and a peak in between, at 30 minutes (p < 0.05). 2) Neutrophils: Compared to resting state baseline, CD62L−neutrophil numbers showed a biphasic increase with peaks at 6 minutes (p < 0.05) and 2 hours (p = 0.06) and a trough in between at 30 minutes (p < 0.05). CD62L+ neutrophils showed a monophasic increase starting at 15 minutes and peaking at 1 hour, although these differences were not statistically significant. Neutrophil CD62L expression increased during stress, peaking at 2 hours (p < 0.05).
Figure 3.
Effects of acute stress on CD62L− and CD62L+ monocyte and neutrophil numbers and CD62L expression on monocytes and neutrophils. Leukocyte numbers (1000 /μl blood) were quantified at 0 (baseline) and 6, 15, 30, 60, and 120 minutes during restraint stress. CD62L surface expression is expressed as mean fluorescence intensity (MFI) as measured on the flow cytometer. An increase in immune cell numbers relative to baseline represents mobilization of leukocytes into the blood. A decrease from peak numbers or relative to baseline represents trafficking of cells out of the blood and into tissues. Percent change relative to baseline at peak and trough is indicated. Data are expressed as means ± SEM (n=3). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: relative to peak (Fisher's LSD).
Figure 4 shows stress-induced changes in numbers of CD62L− and CD62L+ cells, and the intensity of CD62L expression on T+NK, CTL, and B cells. Effects of stress were quantified as follows. 1) T and NK cells: Fixed-block ANOVA showed that there was an overall effect of stress over time on CD62L− cell numbers in the combined population of T cells plus NK cells (these cells could not be identified separately due to limitations of the flow cytometer, combinations of available antibodies, and blood volume) (F = 18.32, p < 0.0001). Compared to resting state baseline, CD62L− T+NK cells showed a significant decrease starting at 15 minutes (p < 0.05) and reaching their lowest levels at 2 hours (all p < 0.01). Fixed-block ANOVA also showed that there was an overall effect of stress over time on CD62L+ cell numbers of T+NK cells (F = 11.62, p < 0.001). Mean CD62L+ T plus NK cell numbers increased at 6 minutes (p < 0.01), declined to become indistinguishable from baseline at 15 minutes (p = 0.59), and continued to decrease, reaching their lowest levels at 2 hours (p < 0.01). Compared to their peak levels at 6 minutes, CD62L+ T plus NK cell numbers were significantly lower at all other time points (p < 0.05). CD62L expression on T+NK cells decreased during stress, reaching its lowest levels at 2 hours (p = 0.07). 2) CTLs: Compared to resting state baseline, CD62L− CTLs showed a decrease which reached statistical significance at 60 minutes and 2 hours (p < 0.05). Fixed-block ANOVA showed that there was an overall effect of stress over time on CD62L+ CTL numbers (F = 3.8, p < 0.05). Mean CD62L+ CTL numbers increased slightly at 6 and 15 minutes (nss), and then decreased, reaching their lowest levels at 2 hours (p = 0.06). Compared to their peak levels at 6 minutes, CD62L+ CTL numbers were significantly lower at 60 minutes and 2 hours (p < 0.05). CD62L expression on CTLs decreased during stress, reaching its lowest levels at 2 hours (p = 0.09). 3) B cells: Fixed-block ANOVA showed that there was an overall effect of stress over time on CD62L− B cell numbers (F = 15.95, p < 0.001). Compared to resting state baseline, mean CD62L− B cells reached peak levels at 6 minutes (p < 0.001) and then decreased reaching their lowest levels at 2 hours (p = 0.07). Compared to peak levels at 6 minutes, CD62L− B cell numbers decreased significantly at all other time points (all p < 0.01). Fixed-block ANOVA showed that there was an overall effect of stress over time on CD62L+ B cell numbers (F = 8.6, p < 0.01). CD62L+ B cell numbers decreased over time reaching their lowest levels at 60 minutes (p < 0.01) and 2 hours (p < 0.001). With respect to CD62L expression on B cells, fixed-block ANOVA showed that there was an overall effect of stress over time (F = 4.73, p < 0.05). Compared to resting state baseline, CD62L expression on B cells was lower at 6 minutes (p < 0.01) and 15 minutes (p < 0.05), indistinguishable from baseline at 30 and 60 minutes, and decreased again at 2 hours (p < 0.01).
In summary, cell surface expression of CD62L increased on neutrophils, decreased on Th, CTL, and natural killer (NK) cells, and showed a biphasic decrease on monocytes & B cells, suggesting that CD62L is involved in mediating the redistribution effects of stress. Moreover, significant differences were observed in the effects of stress on the magnitude and direction of changes in total, as well as CD62L− versus CD62L+ subtypes within each leukocyte subpopulation. This suggests, for example, that naïve (CD62L+) vs. memory (CD62L−) T cells, classical (CD62L+) vs. non-classical (CD62L−) monocytes, and similarly distinct functional subsets of other leukocyte populations are differentially affected by stress and stress hormones.
Stress hormone-induced changes in blood monocyte, neutrophil, and lymphocyte numbers
Numerous studies have examined the effects of specific stress hormones on blood immune cell numbers (Benschop et al., 1996; Dale et al., 1974; Dhabhar et al., 1995b, 1996; Fauci and Dale, 1974; Schedlowski et al., 1993a). Taken together, these studies suggest that the principal stress hormones, norepinephrine (NE), epinephrine (EPI) and corticosterone (CORT), are the major endocrine mediators of a stress-induced leukocyte redistribution (Dhabhar and McEwen, 2001). However, the combinatorial effects of simultaneously administering stress hormones remain to be investigated. We hypothesized that specific combinations of stress hormones would mediate distinct aspects of stress-induced leukocyte redistribution. To test this hypothesis, we characterized and quantified individual and combined actions of NE, EPI and CORT, in mediating changes in specific leukocyte subpopulations and their functional/maturation subsets as identified by the presence of absence of CD62L.
Figure 5 shows the effects of administering vehicle (VEH) control, NE, EPI, CORT, NE+EPI, NE+CORT, EPI+CORT and NE+EPI+CORT on leukocyte redistribution to adrenalectomized rats. Leukocyte subpopulations were quantified 2 hours after hormone administration. 1) WBC: One-way ANOVA showed that there was an overall effect of hormone treatment on blood WBC numbers (F = 19.71, p < 0.0001). Compared to the VEH group, mean WBC numbers were increased by NE (p < 0.05), and EPI (p < 0.05). NE-induced numbers were higher than those induced by EPI (p < 0.05). CORT administration significantly decreased WBC numbers (p < 0.05), and all catecholamine + CORT combinations resulted in WBC numbers between the high values induced by the catecholamines and the low values induced by CORT (all p < 0.05). 2) Monocytes: One-way ANOVA showed that there was an overall effect of hormone treatment on blood monocyte numbers (F = 2.85, p < 0.03). Mean monocyte numbers were increased by EPI (p < 0.05) and decreased by CORT (p < 0.05). Monocyte numbers were also decreased by all CORT containing combinations (nss). 3) Neutrophils: One-way ANOVA showed that there was an overall effect of hormone treatment on blood neutrophil numbers (F = 13.47, p < 0.0001). Compared to the VEH group, mean neutrophil numbers were significantly increased by NE (p < 0.05), EPI (p < 0.05) and all hormone combinations containing NE or EPI (p < 0.05). 4) Lymphocytes: One-way ANOVA showed that there was an overall effect of hormone treatment on blood lymphocyte numbers (F = 26.32, p < 0.0001). Compared to the VEH group, mean lymphocyte numbers were decreased by EPI (p < 0.05) and CORT (p < 0.05). CORT induced numbers were significantly lower than those induced by EPI (p < 0.05) indicating that CORT induced a larger decrease in blood lymphocyte numbers than EPI. Compared to the VEH and NE groups, all hormone combinations that contained EPI, CORT, or EPI+CORT, significantly decreased blood lymphocyte numbers (all p < 0.05).
The following effects of hormone treatment were observed for the specific lymphocyte subpopulations examined. 1) Helper T (Th) cells: One-way ANOVA showed that there was an overall effect of hormone treatment on blood Th cell numbers (F = 6.41, p < 0.001). Compared to the VEH group, mean Th cell numbers were unchanged by NE and EPI, decreased by CORT, and by all hormone combinations containing CORT (all p < 0.05). 2) Cytolytic T cells (CTL):One-way ANOVA showed that there was an overall effect of hormone treatment on blood CTL numbers (F = 16.2, p < 0.0001). Compared to the VEH group, mean CTL numbers were unchanged by NE, and decreased by EPI, CORT, and by all hormone combinations containing EPI or CORT (all p < 0.05). Interestingly, mean CTL numbers measured after administration of CORT or any combination containing CORT were significantly lower than those measured after administration of EPI or EPI+NE (p < 0.05). 3) B cells: One-way ANOVA showed that there was an overall effect of hormone treatment on blood B cell numbers (F = 110.32, p < 0.0001). Compared to the VEH group, mean B cell numbers were decreased slightly by NE (p < 0.05), and significantly by EPI, CORT, and by all hormone combinations containing EPI or CORT (all p < 0.05). Interestingly, mean B cell numbers measured after administration of CORT or any hormone combination containing CORT were significantly lower than those measured after administration of EPI or EPI+NE (p < 0.05). 4) NK cells: One-way ANOVA showed that there was an overall effect of hormone treatment on blood NK cell numbers (F = 3.43, p < 0.05). Compared to the VEH group, mean NK cell numbers were increased by CORT (p < 0.05), and decreased by NE+EPI, EPI+CORT, and NE+EPI+CORT although these changes were not statistically significant compared to the VEH group but were significantly lower than the CORT group (p < 0.05).
In summary, NE increased neutrophil numbers, and EPI increased monocyte and neutrophil numbers. In contrast, EPI decreased lymphocyte numbers with CTL and B cells being particularly affected, and CORT decreased monocyte, lymphocyte, Th, CTL, and B cell numbers.
Stress hormone-induced changes in blood CD62L expression on monocyte, neutrophil, and lymphocyte subpopulations
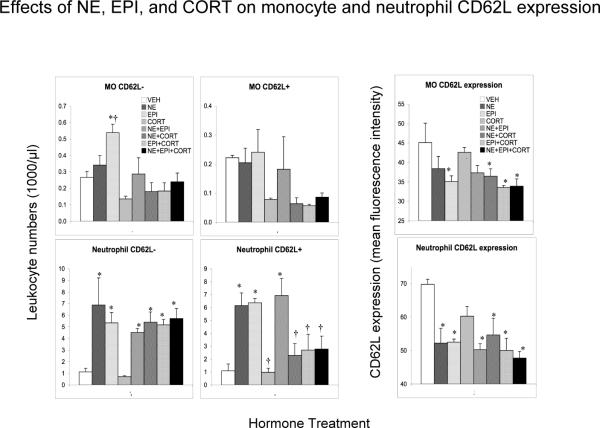
Figure 6 shows the effects of administration of vehicle (VEH) control, NE, EPI, CORT, NE+EPI, NE+CORT, EPI+CORT and NE+EPI+CORT on numbers of CD62L− and CD62L+ cells, and the intensity of CD62L expression on monocytes and neutrophils in ADX rats. 1) Monocytes: One-way ANOVA showed that there was an overall effect of hormone treatment on CD62L− monocyte numbers (F = 3.8, p < 0.01). Compared to the VEH group, CD62L− monocyte numbers were increased by EPI (p < 0.05) and decreased by CORT (nss). EPI induced numbers were higher than those of all other treatment groups (p < 0.05). There was no statistically significant overall effect of hormone treatment on CD62L+ monocytes although their cell numbers were lower than the VEH group in all CORT and CORT-combination groups (nss). With respect to CD62L expression on monocytes, one-way ANOVA showed that there was an overall effect of hormone treatment (F = 2.58, p < 0.05). Compared to the VEH group, monocyte CD62L expression was lower in the EPI group and in all catecholamine + CORT combination groups (p < 0.05). 2) Neutrophils: One-way ANOVA showed that there was an overall effect of hormone treatment on CD62L− neutrophil numbers (F = 3.54, p < 0.05). Compared to the VEH group, CD62L− neutrophils showed a massive mobilization (400–500% increase) in the NE, EPI groups, and all combinations containing NE or EPI (all p < 0.05), and were largely unaffected by CORT. One-way ANOVA also showed that there was an overall effect of hormone treatment on CD62L+ neutrophil numbers (F = 6.16, p < 0.001). Compared to the VEH group, CD62L+ neutrophils also showed massive mobilization (300–400%) in the NE, EPI and NE+EPI groups (all p < 0.05). However, all CORT-containing hormone combinations brought CD62L+ neutrophil numbers back to resting-state / VEH control levels, and CD62L+ neutrophil numbers were significantly lower in all CORT-containing groups relative to the NE, EPI or NE+EPI groups (all p < 0.05). These findings suggest that CD62L− neutrophils are more sensitive to catecholamine-induced mobilization into the bloodstream than to CORT-induced trafficking out oF = the blood, while CD62L+ neutrophils are sensitive to both, the mobilizing effects of catecholamines as well as the trafficking effects of CORT. With respect to CD62L expression on neutrophils, one-way ANOVA showed that there was an overall effect of hormone treatment (F = 4.21, p < 0.01). Compared to the VEH group neutrophil CD62L expression decreased in the NE and EPI groups, and all groups containing NE or EPI (all p < 0.05).
Figure 6.
Effects of control (vehicle, VEH) or stress hormones, norepinephrine (NE), epinephrine (EPI), corticosterone (CORT), NE+EPI, NE+CORT, EPI+CORT, and NE+EPI+CORT (bars shown in this order) on CD62L− and CD62L+ monocyte and neutrophil numbers and CD62L expression on monocytes and neutrophils. Leukocyte numbers (1000 /μl blood) were quantified 2 hours after vehicle or hormone injection in adrenalectomized animals. CD62L surface expression is expressed as mean fluorescence intensity (MFI) as measured on the flow cytometer. An increase in immune cell numbers relative to the VEH group represents mobilization of leukocytes into the blood. A decrease represents trafficking of cells out of the blood and into tissues. Data are expressed as means ± SEM (n=3 or 4 per treatment group). Statistically significant differences are indicated: * p < 0.05, and ** p < 0.01: relative to baseline (Fisher's LSD); † p < 0.05, and †† p < 0.01: please see text for selected comparison groups (Fisher's LSD).
Figure 7 shows hormone-induced changes in subpopulation-specific surface expression of CD62L and in numbers of CD62L− versus CD62L+ lymphocyte subpopulations. 1) T & NK cells: One-way ANOVA showed that there was an overall effect of hormone treatment on numbers of CD62L− T & NK cells (F = 11.69, p < 0.0001). Compared to the VEH group, CD62L− T and NK cell numbers were decreased by EPI (nss), CORT, and all CORT-containing combinations (p < 0.05). One-way ANOVA also showed that there was an overall effect of hormone treatment on numbers of CD62+ T & NK cells (F = 8.12, p < 0.01). Compared to the VEH group, CD62L+ T and NK cell numbers were decreased in the CORT and NE+EPI groups and all CORT-containing combinations (p < 0.05). With respect to CD62L expression on T & NK cells, one-way ANOVA showed that there was an overall effect of hormone treatment (F = 12.44, p < 0.0001). CD62L expression was decreased in the NE, EPI, and NE+EPI groups (p < 0.05). Although CD62L expression appeared to be unaffected by CORT, the NE+CORT and EPI+CORT groups showed CD62L expression at intermediate levels between those seen with NE or EPI alone and CORT alone, suggesting that CORT counteracted the NE or EPI induced reduction of CD62L on T and NK cells. 2) CTL: One-way ANOVA showed that there was an overall effect of hormone treatment on numbers of CD62L− CTLs (F = 4.69, p < 0.01). Compared to the VEH group, CD62L− CTLs cell numbers were decreased by EPI and all EPI-containing combinations (nss). One-way ANOVA showed that there was an overall effect of hormone treatment on numbers of CD62L+ CTLs (F = 20.28, p < 0.001). Compared to the VEH group, CD62L+ CTLs cell numbers were decreased by EPI, CORT, and all combinations containing EPI or CORT (p < 0.05). With respect to CD62L expression on CTLs, one-way ANOVA showed that there was an overall effect of hormone treatment (F = 2.53, p < 0.05). Compared to the NE group, CD62L expression on CTLs was lower in the CORT and all CORT-containing groups (p < 0.05). 3) B cells: One-way ANOVA showed that there was an overall effect of hormone treatment on numbers of CD62L−B cells (F = 25.45, p < 0.0001). Compared to the VEH group, CD62L− B cell numbers were increased by NE (p < 0.05), largely unaffected by EPI, or NE+EPI, and significantly decreased (60–80% decrease) by CORT and all CORT-containing combinations (p < 0.05). One-way ANOVA showed that there was an overall effect of hormone treatment on numbers of CD62L+B cells (F = 532.04, p < 0.0001). Compared to the VEH group, CD62L+ B cell numbers were decreased significantly by all hormone treatments (all p < 0.05), with the decrease being much more pronounced (60–95%) in the EPI or CORT-containing groups (all p < 0.05). With respect to CD62L expression on B cells, one-way ANOVA also showed that there was an overall effect of hormone treatment (F = 6.85, p < 0.01). Compared to the VEH group, CD62L expression on B cells was lower in the CORT (nss), NE+CORT (p < 0.05) and EPI+CORT (p < 0.05) groups, and unaffected by NE and EPI.
In summary, significant differences were observed in the effects of stress hormones on the magnitude and direction of changes in total, as well as CD62L− versus CD62L+ subtypes within each leukocyte subpopulation. This suggests, for example, that naïve (CD62L+) vs. memory (CD62L−) T cells, classical (CD62L+) vs. non-classical (CD62L−) monocytes, and similarly distinct functional subsets of other leukocyte populations are differentially affected by stress hormones.
DISCUSSION
Kinetics of stress-induced mobilization and trafficking of blood immune cells
It is important to recognize that a short-term increase in blood leukocytes (as seen during acute stress) reflects a mobilization of cells into the blood from certain compartments (e.g. marginated pool, spleen, bone marrow, lung, lymph nodes). In contrast, a short-term decrease in blood leukocyte numbers represents a trafficking of cells out of the blood to target organs such as the skin (Dhabhar and McEwen, 1996) and lung (Kradin et al., 2001), or sites of immune activation (Dhabhar and McEwen, 1997; Dhabhar and Viswanathan, 2005; Viswanathan and Dhabhar, 2005), or back to source compartments from which the cells were initially mobilized (Dhabhar, 1998; Stefanski, 2003). The experiments described here show that leukocytes are mobilized into the blood within minutes after the beginning of a stress response, following which, blood monocyte, lymphocyte, Th, CTL, B, and NK cell numbers decrease, while neutrophil numbers continue to increase.
It is important to appreciate the full kinetics of this response since an examination of the effects of stress on immune cell numbers at early time points would mainly reflect mobilization (increased numbers), while late time points would mainly reflect trafficking (decreased numbers). This is a likely explanation for the apparent contradiction between human studies that mainly show an increase in blood leukocyte numbers during/following acute stress, and rodent studies that show decreases. An elegant and interesting example comes from Schedlowski et al. who measured blood T cell and NK cell numbers as well as plasma catecholamine and cortisol levels in parachutists (Schedlowski et al., 1993b). Measurements were made 2 hours before, immediately after, and 1 hour after the jump. Results showed a significant increase in T cell and NK cell numbers immediately (minutes) after the jump that was followed by a significant decrease 1 hour after the jump. An early increase in plasma catecholamines preceded early increases in lymphocyte numbers whereas the more delayed rise in plasma cortisol preceded the late decrease in lymphocyte numbers. Importantly, changes in NK cell activity and antibody-dependent cell-mediated cytotoxicity closely paralleled changes in blood NK cell numbers, thus suggesting that changes in leukocyte numbers may be an important component of apparent changes in leukocyte “activity.”
It is also important to appreciate that the cessation of the stressor does not result in immediate cessation of the physiological stress response, so the immune cell redistribution cascade is likely to be proceed even after relatively brief stressors. The magnitude of the redistribution response is dependent on the duration and intensity/severity of stress (Dhabhar and McEwen, 1997). Interestingly, Rinner et al. have shown that a short stressor (1 min handling) induced an increase in mitogen-induced proliferation of T and B cells obtained from peripheral blood, while a longer stressor (2h immobilization) induced a decrease in the same proliferative responses (Rinner et al., 1992).
Stress-induced changes in leukocyte surface expression of CD62L suggest that this important adhesion molecule is at least partially involved in mediating the stress-induced redistribution of leukocytes in the body. Moreover, immune cell subpopulations appear to show differential sensitivities and redistribution responses depending on the type of leukocyte and its functional characteristics. For example, naïve (CD8+CD62L+) CTLs showed mobilization and trafficking during stress, while memory/effector (CD8+CD62L−) CTLs that are present in the blood under resting conditions traffic out to tissues. Similarly, inflammatory/classical (CD62L+) monocytes (Gordon and Taylor, 2005; Tacke and Randolph, 2006) show mobilization and trafficking, while resident/non-classical (CD62L−) monocytes (Gordon and Taylor, 2005; Tacke and Randolph, 2006) that are present in the blood traffic out to tissues during stress.
Furthermore, these findings show that the principal stress hormones act in concert to orchestrate leukocyte redistribution. While NE and EPI mobilize immune cells into the bloodstream, EPI and CORT enhance traffic of specific subpopulations out of the blood possibly to tissue surveillance pathways, secondary lymphoid tissues, and sites of ongoing inflammation, or de novo immune activation (if the stressor is accompanied by wounding or pathogen entry). Thus, different types of stressors (e.g., cold stress vs. immobilization vs. inflammatory pain vs. hypoglycemia), that are known to induce different relative increases in circulating concentrations of NE, EPI, and CORT (Kvetnansky et al., 1998; Pacak et al., 1998), are likely to have different effects on the kinetics and subpopulation specificity of changes in leukocyte distribution. Since exercise induces endocrine changes that are similar to stress (Pedersen and Hoffman-Goetz, 2000; Walsh et al., 2011), these findings are also likely to apply to the effects of exercise on immune cell distribution/function.
Stress-induced leukocyte redistribution – From Barracks, to Boulevards, to Battlefields
We present a model of stress-induced immune cell redistribution (Figure 8) that is based on the findings described here and those in the literature (Baum et al., 1996; Benschop et al., 1996; Dhabhar, 2009b; Dhabhar and McEwen, 1996, 1997; Engler et al., 2004). This model represents significant advances in our understanding of the hormone mediators, source organs, kinetics, direction, leukocyte subpopulation specificity, and target organs, of stress-induced changes in blood immune cell numbers (Dhabhar and McEwen, 1997). Furthermore, the model explains how stress-induced leukocyte redistribution may be one of nature's fundamental survival responses that directs specific leukocyte subpopulations to specific target organs during stress, and as result significantly enhances the speed, efficacy and regulation of an immune response. Since exercise induces endocrine changes that are similar to stress (Pedersen and Hoffman-Goetz, 2000; Walsh et al., 2011), this model is likely to explain the effects of exercise on immune cell distribution/function. Important aspects, components, and implications of this model are explained below:
Figure 8.
Model of stress-induced changes in leukocyte distribution. The model integrates the hormone mediators, source organs, kinetics, direction, leukocyte subpopulation specificity, and target organs, of stress-induced changes in blood immune cell numbers (Dhabhar and McEwen, 1997), and is based on data presented here as well as that published in the literature (for review see: (Dhabhar, 1998; Dhabhar, 2009b)). The stressor can be psychological (sensing a predator), physical (exercise / running from predator) or physiological (inflammation), and generally induces the release of norepinephrine (NE), epinephrine (EPI), and corticosterone (CORT). The adaptive/salubrious profile of blood lymphocyte and monocyte (MO) redistribution during stress involves a mobilization/increase (shaded dark upward diagonal) followed by a trafficking/decrease (shaded light downward diagonal) in cell numbers (Dhabhar, 2009b; Rosenberger et al., 2009), while neutrophils show only a biphasic mobilization. In general, early (2 to 30 minutes) during stress, NE and EPI mobilize monocytes, neutrophils, and lymphocytes into the blood from “barracks” like the spleen, marginated leukocyte pool, lung, bone marrow and lymph nodes. However, some leukocyte subpopulations (e.g., CD62L− CTLs or CD62L+ B cells) that are circulating in the blood under resting conditions, may traffic to tissues soon after the beginning of stress. Later (30 to > 120 minutes), overall lymphocyte and monocyte numbers decrease as cells traffic out of the blood and into target organs that include: 1) Active “battlefields” which are sites of wounding, antigen/pathogen entry, de novo immune activation, or ongoing inflammation. 2) Homeostatic surveillance and homing pathways within organs that form the main interfaces between the internal and external environments, i.e., the skin and the mucosal-epithelial linings of the oro-digestive and urogenital tracts. 3) Back to the “barracks” which are sites such as the spleen, lung, bone marrow, lymph nodes to which many immune cells may return during stress. Decreases in blood lymphocytes and monocytes, are largely driven by CORT, and for some subpopulations (CTL & B cells) also by EPI. from the bone marrow and may be driven by NE and EPI, and CORT. It is highly likely that leukocytes with specific functional and/or maturational characteristics (e.g., monocyte vs. neutrophil vs. lymphocyte, inflammatory vs. resident monocyte, naïve vs. memory lymphocyte, mucosa vs. bone marrow directed B cell) traffic to specific targets during stress. If the stress response is accompanied by immune activation then the stress-induced leukocyte redistribution ensures that more leukocytes are present to respond to challenge at sites of ongoing or potential inflammation either by already being present at the site of attack (e.g., inflammatory monocytes, effector T cells, and antibody secreting B cells) or by being available in the blood (e.g., mobilized neutrophils). Using “real world” metaphors, we have suggested that a short-term / fight-or-flight stress response prepares the body's “army” for battle, by inducing a redistribution of the body's “soldiers” from “barracks” to “boulevards” to actual or potential “battlefields” (Dhabhar, 1998; Dhabhar, 2009b). As a result, leukocyte redistribution response is one mechanism through which a short-term stress response facilitates immuno-enhancement in compartments that are enriched with immune cells during/following stress (Dhabhar, 2009b; Dhabhar and McEwen, 1996; Viswanathan et al., 2005; Viswanathan and Dhabhar, 2005).
General profile of stress-induced mobilization and trafficking of leukocytes
Early (2 to 30 minutes) during stress, blood monocyte, neutrophil, and lymphocyte numbers increase (shaded area with dark upward diagonal) as these cells are mobilized into the blood from sources like the spleen, marginated pool, lung bone marrow and lymph nodes. It is important to note that specific leukocyte subpopulations (e.g., CD62L− CTLs or CD62L+ B cells) that are circulating in the blood under resting conditions may traffic to tissues (and their circulating numbers decrease) immediately after the beginning of stress, but the overall increase in blood leukocyte numbers is due to the fact that a majority of cells are being mobilized into the blood. Later during stress (15 minutes to a few hours) monocyte and lymphocyte numbers decrease (shaded area with light downward diagonal) as cells traffic out of the blood and into target organs, while neutrophil numbers show a second wave of mobilization.
Source organs from which leukocytes are mobilized
The marginated leukocyte pool, spleen, lung, bone marrow, and perhaps certain lymph nodes are thought to be the source organs from which immune cells are mobilized into the blood early (2 – 30 minutes) during stress. These organs may serve as “barracks” where it makes physiological sense for a major proportion of immune cells (the body's “soldiers”) reside unless they are patrolling their normal surveillance pathways, or are called into action at sites of immune activation. Part of this mobilization may occur through catecholamine-driven splenic capsule contraction and subsequent extrusion of erythrocytes and leukocytes into the blood (Stewart and McKenzie, 2002). However, studies have shown that stress-induced leukocyte mobilization is also observed in splenectomized subjects (Baum et al., 1996; Schedlowski et al., 1996). This suggests that there are other sources such as the marginated leukocyte pool (which is likely to be a major source), lung, bone marrow and lymph nodes (Dhabhar and McEwen, 1997).
Target sites/organs to which leukocytes traffic during/following stress
Stress enhances leukocyte traffic to three major types of sites/organs: 1) Active “battlefields” which are sites of wounding, antigen/pathogen entry, de novo immune activation, or ongoing inflammation (Dhabhar and McEwen, 1996; Dhabhar et al., 2010; Viswanathan et al., 2005; Viswanathan and Dhabhar, 2005). Such sites may occur anywhere in the body including “immune privileged” organs like the brain if an inflammatory response has already begun in such organs. 2) Homeostatic surveillance and homing pathways within organs that form the main interfaces between the internal and external environments, i.e., the skin and the mucosal-epithelial linings of the oro-digestive and uro-genital tracts (Dhabhar and McEwen, 1996). It is likely, that short-term stress enhances leukocyte traffic through existing homeostatic surveillance pathways and mechanisms through which immune cells normally “guard” these tissues. For example, skin or gut homing T cells may home in larger numbers to skin or gut respectively during stress. In an elegant study, Kradin et al. (2001) have shown that EPI induces the trafficking of Th and NK cells from the spleen to blood and lung (Kradin et al., 2001). We suggest that the brain, which is also a site for immune cell traffic (Gottfried-Blackmore et al., 2009; Miller, 2010; Silver et al., 1996; Ziv et al., 2006), and where immune cells are now thought to play a role in facilitating homeostasis, neurogenesis, and function (Ziv et al., 2006), may also be a target of stress-induced leukocyte redistribution. 3) Back to the “barracks” which are sites such as the spleen, lung, bone marrow, and lymph nodes to which many immune cells may return during stress (Dhabhar, 1998). Leukocytes are likely to either traffic back to the “barracks” that they were mobilized from during stress, or to different “barracks.” For example, leukocytes mobilized from the spleen or lymph nodes during stress, may then traffic to bone marrow, or marginate in lung vasculature. Thus, a stress-induced redistribution of leukocytes (especially antigen-experienced lymphocytes, regulatory cells, or antigen presenting cells like macrophages and dendritic cells) among primary, secondary, or tertiary lymphoid organs may be an important component of efficient and effective immune activation, and a critical aspect of ensuring salubrious maintenance and regulation of an immune response.
If the stress response is brief (2–30 minutes), mobilized lymphocytes and monocytes return to resting state baseline numbers. If the stress response persists (30 minutes – few hours) blood lymphocyte and monocyte numbers decrease to below-baseline levels. Importantly, in either case, a decrease (relative to pre-stress baseline numbers or stress-mobilization peak numbers) in leukocytes indicates that immune cells have trafficked out of the blood as they remarginate within the vasculature of (or migrate through) target organs described above. Importantly, it is possible that target organs are different for immune cells (e.g., CD62L− CTLs and CD62L+ B cells) that were already in the blood at baseline and immediately traffic out of the blood in response to stress, from target organs of immune cells that were first mobilized and then trafficked by stress. Further research is needed to characterize and quantify the different dimensions discussed above of target organs to which leukocyte subtypes traffic during stress.
Hormone specificity of mobilization versus trafficking
Norepinephrine (NE) and epinephrine (EPI) released early during a stress response mobilize lymphocytes, monocytes, and neutrophils into the blood from “barracks” like the marginated leukocyte pool, spleen, lung, bone marrow, and lymph nodes. In contrast, decreases in blood lymphocytes and monocytes, which represent cell trafficking out of the blood and into tissues, are largely driven by corticosterone (CORT), and for some subpopulations (CTL & B cells) also by EPI. Blood neutrophil numbers generally do not decrease during stress, but show a biphasic increase with a second mobilization seen at about 30 – 60 minutes after the beginning of stress. This second mobilization likely comes from the bone marrow, may involve immature neutrophils, and may be driven by NE and EPI, and CORT. Thus, the principal stress hormones exert specific effects on different leukocyte subtypes and different phases of stress-induced redistribution. This suggests that different types of stressors (e.g., cold stress vs. immobilization vs. inflammatory pain vs. hypoglycemia), that are known to induce different relative increases in circulating concentrations of NE, EPI, and CORT (Kvetnansky et al., 1998; Pacak et al., 1998), are likely to have different effects on the kinetics and subpopulation specificity of changes in leukocyte distribution.
Functional and maturation characteristics of leukocytes determine where they go during stress
It is highly likely that leukocytes with specific functional and/or maturational characteristics (e.g., naïve vs. memory lymphocyte, inflammatory vs. resident monocyte, neutrophil vs. lymphocyte or monocyte) traffic to specific targets during stress. We hypothesize that skin homing T cells traffic to skin in larger numbers during stress, gut homing T cells traffic to gut, etc. Moreover, with respect to B cells, it has been suggested that IgA secreting plasma cells traffic preferentially to mucosal surfaces, IgG secreting plasma cells traffic preferentially to sites of inflammation, and both types traffic to bone marrow (Kunkel and Butcher, 2003). We hypothesize that stress enhances the delivery of specific populations of B cells to these sites. The stress-induced increase (and absence of significant decrease) in blood neutrophil numbers is consistent with their functional properties because neutrophils generally enter tissues only if they sense active inflammation, and once this happens neutrophils launch inflammatory cascades that ultimately end in their death (generally through apoptosis) at the site of inflammation. Thus, unlike monocyte and lymphocyte subsets that traffic to tissues during stress, neutrophil numbers only increase in the blood. This increase can still contribute to immuno-enhancement by making more neutrophils available to respond at sites of immune activation if the stressor is accompanied by wounding or antigen/pathogen entry. If the stress response is not accompanied by immune activation, blood leukocyte numbers return to resting state levels within about 3 hours after cessation of stress (Dhabhar et al., 1995b). However, if the stress response is accompanied by immune activation then the stress-induced leukocyte redistribution ensures that more leukocytes are present to respond to challenge at sites of ongoing or potential inflammation either by being available in the blood (e.g., mobilized neutrophils) or already infiltrating the site of attack (e.g., inflammatory monocytes, effector T cells, and antibody secreting B cells). As a result, this redistribution response enhances immune function in compartments that are enriched with immune cells during/following stress (Dhabhar, 2009b; Dhabhar and McEwen, 1996; Dhabhar et al., 2010; Viswanathan et al., 2005; Viswanathan and Dhabhar, 2005).
Adhesion molecules and chemokines
It is highly likely that stress-induced changes in immune cell distribution are mediated by stress hormone-induced induce changes in conformation and/or expression of adhesion molecules, including but not limited to CD62L, on leukocytes and/or endothelial cells together with changes in endothelial cell/tissue chemokine expression (please see below for further discussion).
Effects of chronic stress
It has been shown that chronic stress induces a significant reduction in resting state numbers of circulating lymphocytes and induces a loss of immune cell responsivity to acute stress-induced redistribution (Dhabhar and McEwen, 1997). Thus, chronic stress decreases the number of “troops” that are available and also reduces their ability to traffic to “battlefields” during stress. Moreover, the functional consequences of these effects of chronic stress are indicated by a significant suppression of skin immunity (Dhabhar and McEwen, 1997; Dhabhar et al., 2012; Saul et al., 2005). It is likely that several elements of the proposed model are adversely affected during chronic stress, and may include a loss of sensitivity of the system to stress hormones. Interestingly, a dysregulation of the circadian corticosterone rhythm is observed around the time that deleterious effects of chronic stress begin to manifest themselves (Dhabhar and McEwen, 1997). The mechanisms by which chronic stress adversely affects acute stress-induced leukocyte redistribution need to be investigated further.
Psychological, physical, and inflammatory stressors
It is important to note that the major endocrine mediators of a stress response, EPI, NE, CORT, can be induced by psychological (Dhabhar et al., 1993), physical (e.g exercise (Pedersen and Hoffman-Goetz, 2000)), and physiological (e.g., immune activation) (Dunn, 1993; Elenkov et al., 2005; Kusnecov and Goldfarb, 2005; Silverman et al., 2005; Sternberg et al., 1989) stressors (Kvetnansky et al., 1998; Pacak et al., 1998). Our findings suggest that different types of stressors (e.g., cold stress, immobilization, inflammatory pain, hypoglycemia), that are known to induce different relative increases in circulating concentrations of NE, EPI, and CORT (Kvetnansky et al., 1998; Pacak et al., 1998), may have different effects on the kinetics and subpopulation specificity of changes in leukocyte distribution.
The fact that inflammation can activate the stress axis (Dunn, 1993; Elenkov et al., 2005; Kusnecov and Goldfarb, 2005; Silverman et al., 2005; Sternberg et al., 1989) implies that under certain conditions, an immune response may also tap into the stress leukocyte redistribution cascade to deliver more leukocytes to the site of immune activation, or to regulate/resolve inflammation. It must be noted that immune system induced activation of the stress axes is generally thought to induce a regulatory loop by which stress hormones prevent immune responses from becoming chronically activated or autoimmune (Butts and Sternberg, 2004; Sternberg et al., 1989; Tonelli et al., 2001). However, it is known that under certain conditions, physiological levels of endogenous glucocorticoids have immunoenhancing effects (Dhabhar and McEwen, 1999) while under other conditions similar hormone levels are immuno-suppressive. To reconcile these differences it has been hypothesized that these differential effects are achieved by differences in overall stress hormone sensitivity or receptivity of the immune response being affected, or by differences in the duration of hormone exposure (Dhabhar, 2009b; Dhabhar and McEwen, 2001). At the very beginning of an immune response, certain components such as leukocyte trafficking, antigen presentation, helper T cell function, leukocyte proliferation, cytokine and chemokine function, and effector cell function may be receptive to stress and stress hormone-mediated immunoenhancement. In contrast, at a later, more advanced stage of an immune response these components may be more receptive to immunosuppression. While this hypothesis needs to be tested through further experiments, studies have shown important temporal differences in the sensitivity of immune reactions to the enhancing or suppressive effects of physiologic concentrations of stress hormones (Wiegers et al., 1995; Wiegers and Reul, 1998). Studies have also shown that while acute administration of stress concentrations of endogenous glucocorticoids is immuno-enhancing, chronic administration of the same concentrations is immuno-suppressive (Dhabhar and McEwen, 1999).
Effects on integrated immune function
Stress-induced changes in immune cell distribution are accompanied by enhancement of immune function in organs to which immune cells traffic during stress. Such enhancement has been observed in mice (Dhabhar et al., 2000; Dhabhar et al., 2010; Dhabhar and Viswanathan, 2005; Saint-Mezard et al., 2003; Viswanathan et al., 2005), rats (Dhabhar and McEwen, 1996, 1999), hamsters (Bilbo et al., 2002), and human subjects (Edwards et al., 2007; Rosenberger et al., 2009) (for review see: (Dhabhar, 2009b; Dhabhar and McEwen, 2007; Edwards et al., 2007)). In addition to enhancing immunity by increasing immune cell numbers in specific compartments, acute stress also enhances leukocyte function (Viswanathan et al., 2005).
Adaptive significance of stress-induced immune cell redistribution
Using “real world” metaphors, we have suggested that a short-term / fight-or-flight stress response prepares the body's “army” for battle, by inducing a redistribution of the body's “soldiers” from “barracks” to “boulevards” to actual or potential “battlefields” (Figure 8). The net result of these effects of stress is a significant enhancement of in vivo immune function in compartments like the skin and skin draining lymph nodes. Aspects of stress-induced immune cell redistribution are similar across many species, including humans, which suggests that this response is evolutionarily significant and likely to confer an adaptive advantage (Dhabhar, 1998, 2009a; Dhabhar, 2009b). In nature, the stress-response and immune activation are intimately linked as one often follows the other (e.g., predator chases prey, fight-or-flight response is mounted, prey is wounded; or animal falls and is wounded, pain pathways and inflammatory pathways are activated, and the stress response is initiated). Moreover, in many cases the brain can perceive and predict a stressor-induced immune challenge, while the immune system cannot (e.g., an impala's immune system has no way of knowing that a lion is lurking in the grass and is about to pounce, but it's brain does). Therefore, it was suggested that just as the stress response activates the cardiovascular and musculoskeletal systems for fight-or-flight, it may also prepare the immune system for impending challenges (Dhabhar, 2009b; Dhabhar and McEwen, 1997; Dhabhar et al., 1995b; Dhabhar et al., 1994). Stress-induced mobilization of leukocytes into the blood may contribute to immuno-enhancement by making more leukocytes available to rapidly respond to any site of immune activation in the body. In addition, stress-induced leukocyte trafficking to target organs ensures that leukocytes are already present and ready at potential battlefields should the body's defenses be breached. In addition to these changes in leukocyte distribution, acute stress and stress hormones have also been shown to enhance immune cell function (Dhabhar and McEwen, 1996, 1999; Dhabhar et al., 2010; Dhabhar and Viswanathan, 2005; Viswanathan et al., 2005; Wiegers et al., 1993; Wiegers et al., 1994). Immune function is generally enhanced in organs that are enriched with leukocytes during stress.
A maximally protective immune response is likely to occur when the traffic and function of the most relevant immune cells work in synchrony to enhance immunity in the appropriate compartment(s) where the immune battle needs to be fought. It is important to recognize that the acute stress response is a critical physiological mechanism that could act like a multi-dimensional endogenous adjuvant to ensure that leukocytes are present in the right place, at the right time, and in the right state of activation for immune challenges that are likely to be coupled with stress under natural conditions (e.g, skin, oro-digestive tract, and urogenital tract damage that may be incurred during predation, feeding, and intercourse respectively). Examples of target organs for such stress-induced leukocyte trafficking and immuno-enhancement include: skin and other tissues that can be wounded by a predator or attacker (stressor), microbes or abrasions in the oro-digestive tract following feeding (which induces a postprandial physiological stress response), or genital tract pathogen entry and/or abrasions during sexual intercourse (which also induces a physiological stress response).
Hormonal mechanisms
Immune cells are known to express receptors for catecholamine and glucocorticoid hormones (Ader, 2007) which means that the biological machinery is in place for these hormones to exert effects on immune cell distribution and function. The effects of catecholamine hormones on immune cell distribution and function through specific subtypes of alpha- and beta-adrenergic receptors have been discussed in excellent reviews (Benschop et al., 1996; Kavelaars, 2002; Kohm and Sanders, 2000; Sanders, 2006) and the catecholamine-related results presented here are in agreement with elegant studies by Engler et al. who used catecholamine antagonists to eliminate stress-induced changes in specific leukocyte subpopulations (Engler et al., 2004). Moreover, studies have shown that adrenalectomy reduces the magnitude of stress-induced decreases in blood leukocytes, suggesting that EPI and CORT are mediators of the effects of stress (Dhabhar et al., 1995b; Engler et al., 2004). Acute administration of corticosterone (which acts at Type I and Type II adrenal steroid receptors) or RU28362 (Type II adrenal steroid receptor agonist) to adrenalectomized animals reproduces the late effects of stress, while aldosterone (Type I adrenal steroid receptor agonist) has little effect suggesting that the late effects of stress are mediated through the Type II glucocorticoid receptor (Dhabhar et al., 1996). There is also the important possibility that some early and late effects of glucocorticoids on immune cells may be mediated through faster-acting non-classical / alternative signaling pathways (for review see: (Orchinik, 1998; Song and Buttgereit, 2006)). Such non-genomic pathways of glucocorticoid signaling have also been reported in immune cells (Boldizsar et al., 2010) and are likely to mediate some of the effects of stress and stress steroids on immune cell distribution and function. While the role of catecholamine and glucocorticoid hormones has been largely examined separately, the present study is an important step towards putting together the effects of combinations of these hormones as would occur during a physiological stress response. Further investigation is needed that utilizes more combinations, concentrations and durations of exposure, and receptor-specific agonists to elucidate the molecular mechanisms by which stress hormones act in concert to produce rapid and large-scale changes in immune cell distribution. The role of potential interactions among signaling pathways triggered by adrenergic receptors, and classical and non-classical glucocorticoid receptors also needs to be examined.
Adhesion molecules and chemokines
We suggest that stress hormones induce changes in leukocyte distribution by acting through many of the same mechanisms (chemokines and adhesion molecules) that are involved in mediating immune cell distribution and trafficking through normal surveillance pathways, or to sites of immune activation. However, it is also possible that some adhesion molecules or chemokines may be particularly/uniquely responsive to stress hormones, and it would be important and useful to identify these. Numerous studies have examined the effects of stress on leukocyte-endothelial cell adhesion molecule expression on circulating immune cells (Bauer et al., 2001; Goebel and Mills, 2000; Hong et al., 2004; Mills et al., 2003; Okutsu et al., 2008; Rainer et al., 1999; Redwine et al., 2003; Shephard, 2003). While a review of these studies is beyond the scope of this manuscript, taken together, they suggest that changes in leukocyte adhesion molecule conformation/expression may mediate aspects of both the mobilization and trafficking effects of stress and stress hormones. In support of a role for CD62L in mediating the immuno-enhancing effects of short-term stress, a recent study has shown that the acute stress-induced enhancement of skin cell-mediated immunity is significantly decreased in CD62L deficient mice (Bae et al., 2011). Other studies have suggested that glucocorticoid hormones suppress neutrophil CD62L mRNA expression and that this induces a decrease in cell-surface protein expression of CD62L that is correlated with a glucocorticoid-induced neutrophilia (Weber et al., 2001). Additionally, Redwine et al. (2003) have shown that acute stress increases PBMC chemotaxis and adhesion molecule (Mac-1) expression in human subjects (Redwine et al., 2003). It is important to note that in addition to changes on leukocytes, changes in endothelial cell adhesion molecule conformation/expression and/or endothelial expression of chemokines and/or leukocyte expression of chemokine receptors, are likely to be important mediators of the effects of stress- or stress hormone-induced leukocyte redistribution. The role of chemokines and adhesion molecules in mediating stress-induced changes in immune cell distribution needs to be further investigated.
Functional/maturation specificity of stress-induced changes in leukocyte distribution
We hypothesized that acute stress-induced leukocyte redistribution may be a fundamental physiological mechanism for enabling specific leukocyte subtypes to traffic to tissues where they may be most effective and/or required during stress (e.g., wounding by a predator). For example, naïve T cells may traffic in larger numbers to lymph nodes where they are likely to be activated by antigen presenting cells carrying primary antigen. In contrast, memory cells may traffic to tissues such as the skin or the mucosal/epithelial lining of the gut where they are likely to encounter antigens to which they have been previously exposed. There are many markers/indices through which the functional characteristics and states of activation or maturation of leukocytes can be assessed. While these need to be further investigated in the context of stress, here we used CD62L expression as a first step towards elucidating the characteristics of immune cell subpopulations that redistribute during stress. Among the monocyte population, CD62L+ monocytes are thought to be classical / inflammatory monocytes while CD62L− monocytes are non-classical/resident monocytes (Gordon and Taylor, 2005; Tacke and Randolph, 2006). Here we report that inflammatory monocytes showed mobilization and trafficking, while resident/non-classical monocytes appeared mainly to traffic out of the blood during stress. Similarly, naïve (CD8+CD62L+) CTLs show mobilization and trafficking, while memory/effector (CD8+CD62L−) CTLs only traffic out of the blood. With respect to B cells, we observed that CD62L− B cells show mobilization and trafficking, while CD62L+ B cells only traffic out of the blood during stress. Drawing from the discussion of Kunkel & Butcher (2003), we suggest that these changes perhaps reflect differences in B cell populations that may be targeted to sites of ongoing or potential inflammation (mucosal surfaces), versus sites of maintenance (e.g., bone marrow, lymph nodes, or spleen). Other studies have shown that T cell subtypes that are more likely to traffic to sites of inflammation (Bosch et al., 2003), effector-memory CTLs (Campbell et al., 2009), and tissue-directed memory-type gamma-delta T cells (Anane et al., 2010), are selectively mobilized during stress in human subjects. Taken together, these studies support the idea that the mobilization and trafficking patterns of functionally distinct leukocyte subsets within each leukocyte subpopulation are differentially affected by stress.
Limitations and future directions
We have presented a series of findings and proposed a model for integrating the mechanisms and functional consequences of stress-induced changes in leukocyte distribution. However, further research is needed to more specifically identify molecular mechanisms, and to quantify the maturation/functional characteristics of individual leukocyte subpopulations in terms of the kinetics and magnitude of their redistribution in response to stress and stress hormones. Our studies would also have benefited from being able to examine CD62L expression separately on Th and NK cells the way it was done on CTL and B cells. We also recognize the limitation of using only one marker (CD62L) to elucidate leukocyte adhesion/maturation/functional characteristics, and a more complete investigation of functional/maturation markers is warranted. Another important limitation arises from the small sample sizes used in these experiments. We recognize that small sample sizes can increase the risk of false positive findings, however, it must be noted that the patterns of changes in immune cell numbers described here are in agreement with those from preclinical (Dhabhar and McEwen, 1997; Dhabhar et al., 1995b, 1996; Fauci, 1975; Kradin et al., 2001) and human subjects studies (Altemus et al., 2001; Atanackovic et al., 2004; Fauci and Dale, 1974; Rosenberger et al., 2009; Schedlowski et al., 1993b), suggesting that the effects described here are representative and robust. A fourth limitation is the fact that for the hormone administration study only one time point (2 hours) was examined. This time point was chosen keeping in mind that for this series of studies we chose to examine the net effects of NE, EPI, and CORT as they were likely to occur during the full time course of acute stress effects including the late time period when leukocytes are known to traffic out of the blood. Based on pilot experiments (data not shown) the 2-hour time point was determined to be optimal. Future studies should be designed to quantify hormonal effects at earlier time points and to examine the effects of different doses, and sequential hormone administration to more closely mimic hormonal changes observed during stress. The effects of stress and stress hormones on the direction and magnitude of distribution of dendritic cells (Rossi and Young, 2005), and gamma-delta T cells (Scotet et al., 2008), which are also critical for effective immune function, need to be carefully examined. However, it is of interest to note that an acute stressor experienced during immunization enhances dendritic cell maturation and trafficking to lymph nodes (Viswanathan et al., 2005). Clearly, while these and other studies have provided important information, further research is needed to explore the potential of harnessing the physiological stress response in a clinically beneficial manner.
Implications for experimental and clinical measurements
Since stress is a ubiquitous aspect of life for most organisms, and can be induced even by seemingly innocuous events, the significant effects of stress on immune cell distribution are important to take into account while conducting preclinical or clinical studies, and while interpreting experimental or clinical/diagnostic data. For example, given the known responsivity of immune cells to stress and stress hormones, leukocyte distribution and function are likely to be significantly changed (from resting state conditions) by a study participant or patient anxiously rushing in for a blood draw, or by transporting mice from the vivarium to the lab bench before measurements are made. The effects of such “hidden” or “unappreciated” stressors on immune cell distribution and function, measured in blood or elsewhere in the body, are likely to be significant and to confound the interpretation of experimental or clinical/diagnostic data. For example, drawing blood from a subject at the peak of leukocyte mobilization (or trough induced by trafficking) can lead to significantly higher (or lower) immune cell numbers and function being measured in the blood. In either case, the measurements would not be representative of the resting state that is critical to accurately quantify regardless of whether or not stress is the focus of the study. Moreover, there are significant inter-individual differences in the effects of stress (Hellhammer and Wade, 1993; Marsland et al., 2002), especially in case of unrecognized and unintended stressors that are also uncontrolled. Thus, stress-induced changes are likely to introduce data variability and “noise” that could make data analysis and interpretation difficult and in some cases may completely mask important effects. Therefore, it is highly advisable to minimize (if not eliminate) unintended stressors and to try and record/quantify them as far as possible while making physiological/immune measurements.
Even for experiments studying controlled stressors, it may be important to bear in mind the effects of stress on the leukocyte composition of the compartment in which an immune parameter is being measured. This could also help with interpreting data showing stress-induced changes in functional assays such as lymphocyte proliferation or NK activity. For example, it has been shown that acute stress induces a redistribution of leukocytes from the blood to the skin and that this redistribution is accompanied by a significant enhancement of skin cell mediated immunity (Dhabhar and McEwen, 1996; Dhabhar et al., 2010; Dhabhar and Viswanathan, 2005). In seemingly contradicting results, acute stress has been shown to suppress splenic IgM production (Zalcman and Anisman, 1993), and splenic and peripheral blood responses to T cell mitogens (Cunnick et al., 1990). However, it is important to note that in contrast to the skin and skin draining lymph nodes that are enriched with immune cells following acute stress, peripheral blood and spleen, are relatively depleted of leukocytes (Dhabhar, 1998). Thus, it is likely that the stress-induced decrease in blood and spleen leukocyte numbers contributes to the acute stress-induced suppression of immune function in these compartments. The immune cell subpopulation heterogeneity of the effects of stress means that using equal numbers of cells in functional assays may not sufficiently control for stress-induced changes in cell numbers in different compartments.
Potential clinical applications
It is important to note that if the pattern of change in blood immune cell numbers shown in Figure 8 is observed during the stress of surgery, it predicts (and perhaps partially mediates) enhanced post-surgical recovery. Interestingly, patients who do not show this pattern of immune cell redistribution during the stress of surgery, do not recover as well as patients who do. This is one illustration of the functional and clinical importance of stress-induced changes in immune cell distribution. The overall findings described here are important because they could lead to useful clinical applications: First, behavioral (e.g., acute psychosocial or virtual reality-induced stressors) or pharmacological (e.g., stress hormones injected in sequence or in combination) interventions could be designed to harness stress physiology to induce changes in leukocyte distribution to facilitate the enhancement (during vaccination, surgery/wound healing, or cancer) or suppression (during pro-inflammatory and autoimmune reactions) of immune function in specific compartments (e.g., skin, oro-digestive, and urinary-genital tracts). Second, changes in stress hormones and leukocyte distribution during naturally stressful medical procedures (e.g., surgery or vaccination) could be quantified and used as prognostic indicators or predictors of the effectiveness of the ensuing immune response (e.g., post-surgical healing and recovery, or vaccine efficacy). In fact, a recent prospective study demonstrated that a priori defined adaptive changes in blood leukocyte distribution during the stress of undergoing surgery, predicted enhanced post-surgical recovery, from 1-week to 1-year following surgery (Rosenberger et al., 2009). Third, an increase or decrease in leukocyte responsiveness to stress and stress hormone induced changes in distribution could be used as a rapidly and conveniently quantifiable, in vivo clinical measure of enhancement or reduction of physiological sensitivity to specific stress hormones. For example, if administration of physiological concentrations of cortisol induced a smaller than normal decrease in blood lymphocyte numbers, it might indicate increased glucocorticoid resistance. In fact, studies have shown that chronic stress induces a significant loss of immune cell responsivity to acute stress-induced redistribution (Dhabhar and McEwen, 1997) thus indicating a potential increase in glucocorticoid resistance with chronic stress. Fourth, stress- or hormone-mediated interventions could conceivably be used to sequester immune cells within specific compartments where they more likely to be shielded from cytotoxic treatments like radiation or localized chemotherapy. We suggest that the potential clinical applications of the findings described here would benefit from a systematic identification of the mechanisms mediating stress-induced changes in immune cell distribution, the target organs to which cells traffic during stress, the hormone receptor subtypes involved, the adhesion molecules and chemokines involved, and the functional/maturation/activation characteristics of the affected leukocyte subpopulations.
Conclusion
These studies take an important step towards comprehensively elucidating the kinetics, subpopulation specificity, and hormonal mechanisms mediating stress-induced changes in blood leukocyte distribution. To our knowledge these studies are the first to characterize and quantify numbers of specific immune cell populations and functional/maturation subtypes within the major leukocyte populations, during a complete time course of early and late changes induced by an acute or short-term stress response, and to examine the combinatorial effects of the three principal stress hormones in mediating the blood leukocyte mobilization and trafficking seen during acute stress. These studies are important in light of the significant effects of stress and stress hormones on immune cell distribution and the consequent effects of leukocyte distribution on immune function, the importance of the blood as a readily accessible compartment for diagnostic measurements as well as therapeutic manipulations, and the considerable potential of harnessing these stress-immune effects for clinical application. The relevance of these findings is further highlighted by the fact that stress is a ubiquitous aspect of life for most if not all organisms, the fact that a stress response is likely to be evoked by most experimental/medical manipulations, and the fact that stress hormones and their agonists and antagonists are widely used to treat a large number of clinical conditions.
ACKNOWLEDGEMENT
We thank Maryse Aubourg for help with some of the experiments, and Sue Moseley for conducting the catecholamine assays. These studies and the writing of this manuscript would not have been possible without support from The John D. & Catherine T. MacArthur Foundation (BSM, FSD), The DeWitt Wallace Foundation Fellowship (FSD), The Dana Foundation (FSD), NIH grants AI48995, AR46299, CA107498, and startup support from the Carl & Elizabeth Naumann Fund (FSD).
ROLE OF FUNDING SOURCE Funding sources played no role in study design or any other process related to this manuscript except for providing the greatly-appreciated financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST The authors declare no conflicts of interest with regards to this manuscript.
CONTRIBUTORS FSD designed the study, conducted the experiments, collected data, worked with statistical analyst, interpreted results, and wrote the manuscript. WBM conducted catecholamine analyses and reviewed the manuscript. EN conducted statistical analyses and reviewed the manuscript. BSM worked with FSD to interpret results, and reviewed and edited numerous drafts of the manuscript.
REFERENCES
- Ader R. Psychoneuroimmunology IV. Academic Press; San Diego: 2007. [Google Scholar]
- Akana SF, Jacobson L, Cascio CS, Shinsako J, Dallman MF. Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology. 1988;122:1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein R. Stress-induced changes in skin barrier function in healthy women. J Investigative Dermatology. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- Ambrose CT. The requirement for hydrocortisone in antibody-forming tissue cultivated in serum-free medium. J. Exp. Med. 1964;119:1027–1049. doi: 10.1084/jem.119.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anane LH, Edwards KM, Burns VE, Zanten J.J.C.S.V.v., Drayson MT, Bosch JA. Phenotypic characterization of [gamma][delta] T cells mobilized in response to acute psychological stress. Brain, behavior, and immunity. 2010;24:608–614. doi: 10.1016/j.bbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Atanackovic D, Kroger H, Serke S, Deter HC. Immune parameters in patients with anxiety or depression during psychotherapy. Journal of affective disorders. 2004;81:201–209. doi: 10.1016/S0165-0327(03)00165-4. [DOI] [PubMed] [Google Scholar]
- Bae SJ, Shimizu K, Yozaki M, Yamaoka T, Akiyama Y, Yoshizaki A, Muroi E, Hara T, Ogawa F, Sato S. Involvement of L-selectin in contact hypersensitivity responses augmented by auditory stress. Am J Pathol. 2011;176:187–197. doi: 10.2353/ajpath.2010.090322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunol. 1981;42:593–600. [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Perks P, Lightman SL, Shanks N. Are adhesion molecules involved in stress-induced changes in lymphocyte distribution? Life Sci. 2001;69:1167–1179. doi: 10.1016/s0024-3205(01)01200-0. [DOI] [PubMed] [Google Scholar]
- Baum M, Geitner T, Liesen H. The role of the spleen in the leucocytosis of exercise: consequences for physiology and pathophysiology. International journal of sports medicine. 1996;17:604–607. doi: 10.1055/s-2007-972902. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain, Behavior, & Immunity. 1996;10:77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, Wolvers DA, Derijk R. Neuroendocrine and immunological mechanisms in stress-induced immunomodulation. J. Steroid Biochem. & Mol. Biol. 1991;40:639–647. doi: 10.1016/0960-0760(91)90286-e. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldizsar F, Talaber G, Szabo M, Bartis D, Palinkas L, Nemeth P, Berki T. Emerging pathways of non-genomic glucocorticoid (GC) signalling in T cells. Immunobiology. 2010;215:521–526. doi: 10.1016/j.imbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Berntson GG, Cacioppo JT, Dhabhar FS, Marucha PT. Acute stress evokes selective mobilization of T cells that differ in chemokine receptor expression: A potential pathway linking immunologic reactivity to cardiovascular disease. Brain, Behavior and Immunity. 2003;17:251–259. doi: 10.1016/s0889-1591(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Brideau RJ, Carter PB, McMaster WB, Mason DW, Williams AF. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur. J. Immunol. 1980;10:609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Benschop RJ, Godaert GL, Olff M, De Smet M, Heijnen CJ, Ballieux RE. Influence of life stress on immunological reactivity to mild psychological stress. Psychosom. Medicine. 1994;56:216–224. doi: 10.1097/00006842-199405000-00007. [DOI] [PubMed] [Google Scholar]
- Butcher EC. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990;136:3–11. [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Butts C, Sternberg E. Different approaches to understanding autoimmune rheumatic diseases: the neuroimmunoendocrine system. Best practice & research. 2004;18:125–139. doi: 10.1016/j.berh.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cellular immunology. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJCSV, Drayson MT, Bosch JA. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain, behavior, and immunity. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and Sex Versus Immunity and Inflammation. Sci. Signal. 2010;3:pe36. doi: 10.1126/scisignal.3143pe36. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress (Amsterdam, Netherlands) 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- Cunnick JE, Lysle DT, Kucinski BJ, Rabin BS. Evidence that shock-induced immune suppression is mediated by adrenal hormones and peripheral beta-adrenergic receptors. Pharmacol., Biochem., & Behav. 1990;36:645–651. doi: 10.1016/0091-3057(90)90270-r. [DOI] [PubMed] [Google Scholar]
- Dale DC, Fauci AS, Wolff SM. Alternate day prednisone leukocyte kinetics and susceptibility to infections. N. Engl. J. Med. 1974;291:1154–1158. doi: 10.1056/NEJM197411282912203. [DOI] [PubMed] [Google Scholar]
- Dallman MJ, Thomas ML, Green JR. MRC OX-19: A monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur. J. Immunol. 1984;14:260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Steroids, stability and stress. Frontiers in neuroendocrinology. 1995;16:416–425. doi: 10.1006/frne.1995.1015. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Annals of the New York Academy of Sciences. 1998;840:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. A Hassle a Day May Keep the Pathogens Away: The Fight-Or-Flight Stress Response and the Augmentation of Immune Function. Integrative and comparative biology. 2009a;49:215–236. doi: 10.1093/icb/icp045. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009b;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J. Immunology. 1996;156:2608–2615. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: A potential role for leukocyte trafficking. Brain Behavior & Immunity. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. PNAS, USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional Effects Of Stress & Glucocorticoid Hormones On Immune Function: Possible Explanations For Paradoxical Observations. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Third Edition Academic Press; San Diego: 2001. pp. 301–338. [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional effects of stress on immune function: Possible explanations for salubrious as well as harmful effects. In: Ader R, editor. Psychoneuroimmunology IV. Elsevier Academic Press; San Diego: 2007. pp. 723–760. [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels, and corticosteroid-binding globulin levels -- a comparison between Sprague Dawley, Fischer 344, and Lewis rats. Brain research. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J. Neuroimmunol. 1995a;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution -- dynamics and hormonal mechanisms. J. Immunology. 1995b;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution -- role of adrenal steroid hormones. J. Immunology. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav. Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-Induced Enhancement of Skin Immune Function: A Role For IFNg. PNAS, USA. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Saul AN, Daugherty C, Holmes TH, Bouley DM, Oberyszyn TM. Short-Term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain, behavior, and immunity. 2010;24:127–137. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt DF, Oberyszyn TM. High anxiety is associated with higher chronic stress burden, lower protective immunity, and increased cancer progression. PLoS ONE. 2012 doi: 10.1371/journal.pone.0033069. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Viswanathan K. Short-Term Stress Experienced At The Time Of Immunization Induces A Long-lasting Increase In Immunological Memory. American journal of physiology. 2005;289:R738–744. doi: 10.1152/ajpregu.00145.2005. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Infection as a stressor: a cytokine-mediated activation of the hypothalamo-pituitary-adrenal axis? Ciba Foundation symposium. 1993;172:226–239. doi: 10.1002/9780470514368.ch11. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Carroll D, Drayson M, Ring C. The acute stress-induced immunoenhancement hypothesis. Exercise and sport sciences reviews. 2007;35:150–155. doi: 10.1097/JES.0b013e3180a031bd. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, Stefanski V. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. Journal of neuroimmunology. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and B lymphocytes to the bone marrow. Immunol. 1975;28:669–680. [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Dale DC. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J. Clin. Invest. 1974;53:240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Paré WP, Sandbak T, Bakke H-K, Murison R. Restraint stress in biomedical research: an update. Neurosci. & Biobehav. Rev. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ. Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and density. Psychosom Med. 2000;62:664–670. doi: 10.1097/00006842-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20918–20923. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer DH, Wade S. Endocrine correlates of stress vulnerability. Psychotherapy and psychosomatics. 1993;60:8–17. doi: 10.1159/000288675. [DOI] [PubMed] [Google Scholar]
- Hong S, Farag NH, Nelesen RA, Ziegler MG, Mills PJ. Effects of regular exercise on lymphocyte subsets and CD62L after psychological vs. physical stress. J Psychosom Res. 2004;56:363–370. doi: 10.1016/S0022-3999(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Ingle DJ. Resistance of the rat to histamine shock after destruction of the adrenal medulla. Merck, General Physiology. 1936;11:57–61. [Google Scholar]
- Ingle DJ. Permissibility of hormone action. A review. Acta Endocrinologica. 1954;17:172–186. [PubMed] [Google Scholar]
- Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin CJ, Grant I. Reduction of immune function in life stress and depression. Biol. Psychiatry. 1990;27:22–30. doi: 10.1016/0006-3223(90)90016-u. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of the adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- Johnson P, Gagnon J, Barclay AN, Williams AF. Purification, chain separation, and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985;4:2359–2545. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of [alpha]-1 adrenergic receptors in the immune system. Brain, behavior, and immunity. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Khan AI, Landis RC, Malhotra R. L-Selectin Ligands in Lymphoid Tissues and Models of Inflammation. Inflammation. 2003;27:265–280. doi: 10.1023/a:1026056525755. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunology today. 2000;21:539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- Kradin R, Rodberg G, Zhao LH, Leary C. Epinephrine yields translocation of lymphocytes to the lung. Exp Mol Pathol. 2001;70:1–6. doi: 10.1006/exmp.2000.2342. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Goldfarb Y. Neural and Behavioral Responses to Systemic Immunologic Stimuli: A Consideration of Bacterial T Cell Superantigens. Current Pharmaceutical Design. 2005;11:1039–1046. doi: 10.2174/1381612053381602. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein DS, Kopin IJ. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993;133:1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS. Stressor specificity of peripheral catecholaminergic activation. Advances in pharmacology (San Diego, Calif. 1998;42:556–560. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity, and susceptibility to infectious disease. Phsyiology & Behavior. 2002;77:711–716. doi: 10.1016/s0031-9384(02)00923-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meng X, Brown JM, Ao L, Banerjee A, Harken AH. Norepinephrine induces cardiac heat shock protein 70 and delayed cardioprotection in the rat through alpha 1 adrenoceptors. Cardiovascular research. 1996;32:374–383. doi: 10.1016/0008-6363(96)00078-8. [DOI] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain, behavior, and immunity. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken GA, Johnson DE. Analysis of messy data, volume I: Designed experiments. Chapman & Hall CRC; Boca Raton, FL: 1998. [Google Scholar]
- Mills PJ, Berry CC, Dimsdale JE, Ziegler MG, Nelesen RA, Kennedy BP. Lymphocyte subset redistribution in response to acute experimental stress: effects of gender, ethnicity, hypertension, and the sympathetic nervous system. Brain, Behav., Immun. 1995;9:61–69. doi: 10.1006/brbi.1995.1006. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Farag NH, Hong S, Kennedy BP, Berry CC, Ziegler MG. Immune cell CD62L and CD11a expression in response to a psychological stressor in human hypertension. Brain, behavior, and immunity. 2003;17:260–267. doi: 10.1016/s0889-1591(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Nagatomi R, Kaifu T, Okutsu M, Zhang X, Kanemi O, Ohmori H. Modulation of the immune system by the autonomic nervous system and its implication in immunological changes after training. Exercise immunology review. 2000;6:54–74. [PubMed] [Google Scholar]
- Netter J, Wasserman W, Kutner MH. Applied linear statistical models. Irwin; Homewood, IL: 1985. [Google Scholar]
- Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain, behavior, and immunity. 2008;22:1066–1071. doi: 10.1016/j.bbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Orchinik M. Glucocorticoids, stress, and behavior: shifting the timeframe. Hormones and behavior. 1998;34:320–327. doi: 10.1006/hbeh.1998.1488. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye's doctrine of nonspecificity. The American journal of physiology. 1998;275:R1247–1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiological reviews. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content, and stress-induced release in adult rats. Brain Res. Molec. Brain. Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rainer TH, Lam N, Cocks RA. Adrenaline upregulates monocyte L-selectin in vitro. Resuscitation. 1999;43:47–55. doi: 10.1016/s0300-9572(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Redwine L, Snow S, Mills P, Irwin M. Acute psychological stress: effects on chemotaxis and cellular adhesion molecule expression. Psychosom Med. 2003;65:598–603. doi: 10.1097/01.psy.0000079377.86193.a8. [DOI] [PubMed] [Google Scholar]
- Rinner I, Schauenstein K, Mangge H, Porta S, Kvetnansky R. Opposite effects of mild and severe stress on in vitro activation of rat peripheral blood lymphocytes. Brain, Behav., Immun. 1992;6:130–140. doi: 10.1016/0889-1591(92)90013-e. [DOI] [PubMed] [Google Scholar]
- Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, Dhabhar FS. Surgery stress induced immune cell redistribution profiles predict short- and long-term postsurgical recovery: A prospective study. Journal of Bone and Joint Surgery. 2009;91:2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Young JW. Human Dendritic Cells: Potent Antigen-Presenting Cells at the Crossroads of Innate and Adaptive Immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- Saint-Mezard P, Chavagnac C, Bosset S, Ionescu M, Peyron E, Kaiserlian D, Nicolas JF, Berard F. Psychological stress exerts an adjuvant effect on skin dendritic cell functions in vivo. J Immunol. 2003;171(8):4073–4080. doi: 10.4049/jimmunol.171.8.4073. [DOI] [PubMed] [Google Scholar]
- Sanders VM. Interdisciplinary research: noradrenergic regulation of adaptive immunity. Brain, behavior, and immunity. 2006;20:1–8. doi: 10.1016/j.bbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Nat Cancer Institute. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M, Falk A, Rohne A, Wagner TOF, Jacobs R, Tewes U, Schmidt RE. Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. J. Clin. Immunol. 1993a;13:344–351. doi: 10.1007/BF00920243. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Hosch W, Oberbeck R, Benschop RJ, Jacobs R, Raab HR, Schmidt RE. Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol. 1996;156:93–99. [PubMed] [Google Scholar]
- Schedlowski M, Jacobs R, Stratman G, Richter S, Hädike A, Tewes U, Wagner TOF, Schmidt RE. Changes of natural killer cells during acute psychological stress. J. Clin. Immunol. 1993b;13:119–126. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- Scotet E, Nedellec S, Devilder MC, Allain S, Bonneville M. Bridging innate and adaptive immunity through gammadelta T-dendritic cell crosstalk. Front Biosci. 2008;13:6872–6885. doi: 10.2741/3195. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature immunology. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003;33:261–284. doi: 10.2165/00007256-200333040-00002. [DOI] [PubMed] [Google Scholar]
- Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends in neurosciences. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, Biron CA, Miller AH. Immune Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Viral Infection. Viral Immunology. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Molecular and cellular endocrinology. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress affects migration of blood T cells into lymphoid organs. J Neuroimmunology. 2003;138:17–24. doi: 10.1016/s0165-5728(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Gr,ner S. Gender difference in basal and stress levels of peripheral blood leukocytes in laboratory rats. Brain, behavior, and immunity. 2006;20:369–377. doi: 10.1016/j.bbi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc. Natl. Acad. Sci. USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart IB, McKenzie DC. The human spleen during physiological stress. Sports medicine (Auckland, N.Z. 2002;32:361–369. doi: 10.2165/00007256-200232060-00002. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Tonelli L, Webster JI, Rapp KL, Sternberg E. Neuroendocrine responses regulating susceptibility and resistance to autoimmune/inflammatory disease in inbred rat strains. Immunological reviews. 2001;184:203–211. doi: 10.1034/j.1600-065x.2001.1840118.x. [DOI] [PubMed] [Google Scholar]
- van Gool J, Boers W, Sala M, Ladiges NCJJ. Glucocorticoids and catecholamines as mediators of acute-phase proteins, especially rat a-macrofoetoprotein. The Biochemical journal. 1984;220:125–132. doi: 10.1042/bj2200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. International immunology. 2005;17:1059–1069. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. PNAS, USA. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Pyne DB, Nieman DC, Dhabhar FS, Shephard RJ, Oliver SJ, Bermon S, Kajeniene A. Position statement. Part two: Maintaining immune health. Exercise immunology review. 2011;17:64–103. [PubMed] [Google Scholar]
- Weber PS, Madsen SA, Smith GW, Ireland JJ, Burton JL. Pre-translational regulation of neutrophil L-selectin in glucocorticoid-challenged cattle. Veterinary immunology and immunopathology. 2001;83:213–240. doi: 10.1016/s0165-2427(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Wedepohl S, Beceren-Braun F, Riese S, Buscher K, Enders S, Bernhard G, Kilian K, Blanchard V, Dernedde J, Tauber R. l-Selectin - A dynamic regulator of leukocyte migration. European Journal of Cell Biology. 2011 doi: 10.1016/j.ejcb.2011.02.007. doi: DOI: 10.1016/j.ejcb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Croiset G, Reul JMHM, Holsboer F, DeKloet ER. Differential effects of corticosteroids on rat peripheral blood T lymphocyte mitogenesis in vivo and in vitro. Am. J. Physiol. 1993;265:E825–E830. doi: 10.1152/ajpendo.1993.265.6.E825. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Labeur MS, Stec IE, Klinkert WE, Holsboe r.F., Reul JM. Glucocorticoids accelerate anti-T cell receptor-induced T cell growth. J. Immunol. 1995;155:1893–1902. [PubMed] [Google Scholar]
- Wiegers GJ, Reul JMHM. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. TiPS. 1998;19:317–321. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Reul JMHM, Holsboer F, De Kloet ER. Enhancement of rat splenic lymphocyte mitogenesis after short term exposure to corticosteroids in vitro. Endocrinology. 1994;135:2351–2357. doi: 10.1210/endo.135.6.7988417. [DOI] [PubMed] [Google Scholar]
- Woollett GR, Barclay AN, Puklavec M, Williams AF. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur. J. Immunol. 1985;15:168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Anisman H. Acute and chronic stressor effects on the antibody response to sheep red blood cells. Pharmacol., Biochem., Behav. 1993;46:445–452. doi: 10.1016/0091-3057(93)90377-6. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]