Abstract
In order to obtain a global picture of how alveolar macrophages respond to influenza A virus (IAV) infection, we used a quantitative proteomics method to systematically examine protein expression in the IAV-infected primary human alveolar macrophages. Of the 1214 proteins identified, 43 were significantly up-regulated and 63 significantly down-regulated at > 95% confidence. The expression of an array of interferon (IFN)-induced proteins was significantly increased in the IAV-infected macrophages. The protein with the greatest expression increase was ISG15, an IFN-induced protein that has been shown to play an important role in antiviral defense. Concomitantly, quantitative real time PCR analysis revealed that the gene expression of type I IFNs increased substantially following virus infection. Our results are consistent with the notion that type I IFNs play a vital role in the response of human alveolar macrophages to IAV infection. In addition to the IFN-mediated responses, inflammatory response, apoptosis and redox state rebalancing appeared also to be major pathways that were affected by IAV infection. Furthermore, our data suggest that alveolar macrophages may play a crucial role in regenerating alveolar epithelium during IAV infection.
Keywords: Influenza A virus, IFN-α/β, quantitative proteomics, primary alveolar macrophage, protein expression, LC-MS/MS, SILAC
INTRODUCTION
Influenza A virus (IAV) infections represent a significant public health threat. Season outbreaks alone cause more than 200,000 hospitalizations and over 36,000 deaths annually in the United States1. In addition to typical seasonal infections, reassortant viruses have led to global pandemics, such as 1918 “Spanish flu”, 1957 “Asian flu” and 1968 “Hong Kong flu”. In 2009, a new type of influenza A (H1N1) viruses that emerged in Mexico quickly spread worldwide. These facts suggest that IAV is still a world-wide health threat and underline the urgency to understand the molecular mechanisms underlying virus-host interactions.
The innate immune response is the first line of defense of the host against a pathogen. Interactions between the viruses and host cells are complex and can be driven by both the pathogen and the host. While viruses usurp cellular processes for their own benefit, host cells mount a variety of defenses against the viral infection. The respiratory tract is the most common infective access path. Lungs are the main battlefield where the innate immune system fights back against IAV infection. Alveolar epithelial cells and resident macrophages located in lungs are candidate cells suspected to be mainly responsible for initiating the innate immune responses2.
Most experimental systems that study macrophage responses to virus infections use monocyte-derived macrophages (e.g., 3, 4). The monocyte-derived macrophages produced in vitro may respond differently to viral infections than the natural macrophages that have differentiated in vivo. In addition, several previous studies on determining the global responses of hosts to influenza infections mainly focused on using DNA microarrays to analyze gene expression changes at the mRNA level3, 5, 6. The actual effector molecules in cells are proteins, and the abundances of mRNAs usually do not reflect the levels of corresponding proteins7–9. Thus, to understand host-virus interactions it is important to examine the changes at the protein level. Mass spectrometry (MS) based quantitative proteomics has emerged as a powerful tool to study the mechanisms of viral infections10–13. In the present study, a quantitative proteomic method using stable isotope labeling with amino acids in cell culture (SILAC; also named AACT: amino acid-coded tagging)14, 15 was employed to systemically explore the responses of primary human alveolar macrophages to human influenza A/PR/8/34 (H1N1) virus infection.
EXPERIMENTAL PROCEDURES
Virus Growth
Human influenza A/PR/8/34 (H1N1) viruses and MDCK cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). MDCK cells were grown in Dulbecoo's modified Eagle medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 °C and 5% CO2. The A/PR/8/34 viruses were amplified in MDCK cells at 35 °C and 5% CO2 for 72 h in the virus growth medium (DMEM plus 10 mM HEPES and 0.125% BSA) supplemented with 2 mM L-glutamine, 1% penicillin-streptomycin, 1 mM sodium pyruvate, and 2 μg/ml TPCK-treated trypsin. Virus titers were determined by plaque assay on MDCK cells as described previously16.
Macrophage Culture, Proteome Labeling and Virus Infection
Alveolar macrophages were isolated from human lungs obtained through the National Disease Research Interchange (Philadelphia, PA) and the International Institute for the Advancement of Medicine (Edison, NJ) as described previously2. The donated lungs were not suitable for transplantation and thus were donated for medical research. The Committee for the Protection of Human Subjects at National Jewish Health has approved this research. Briefly, the lung was lavaged with HEPES-buffered saline and 2 mM EDTA, and the lavage fluid was centrifuged at 4 °C for 10 min. Red blood cells were lysed with Pharm Lyse (BD Biosciences, Franklin Lakes, NJ) and the macrophages were resuspended in DMEM. Cells were resuspended in 90% fetal bovine serum and frozen in liquid nitrogen until needed. Whereas one population of primary macrophages (20 million) was cultured in a 100 mm plate in the regular, unlabeled DMEM (light medium), a second population of macrophages (20 million) was grown in a separate plate in the labeled DMEM containing Arginine-13C6 and Lysine-13C615N2 (heavy medium) to isotopically label the proteome. After 12 days of culture, the stable isotope labeled macrophages were first washed twice with warm phosphate-buffered saline (PBS) and then infected with A/PR/8/34 viruses at a multiplicity of infection (MOI) of 0.5 for 1 h at 37 °C and 5% CO2. After removal of virus inoculums and washing the cells once with warm PBS, the macrophages were maintained in a virus growth medium at 37 °C and 5% CO2for 24 h2. The unlabeled macrophages were mock-treated with virus growth medium in place of viruses, and the other procedures were the same as described above. After 24 h, the two populations of macrophages were harvested, washed twice with cold PBS, and lysed for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Protein Fractionation, In-Gel Digestion and LC-MS/MS
The cell pellet from each cell population was resuspended in 100 μl modified RIPA buffer [50 mM HEPES, pH 7.5,150 mM NaCl,1.5 mM MgCl2,1 mM EDTA,10% glycerol, 1% Triton X-100,1% SDS and protease inhibitor cocktail (Roche, Indianapolis, IN)] and sonicated (Branson Digital Sonifer, Danbury, CT). The lysate was centrifuged at 16,000 g for 15 min at 4 °C. The concentration of the resulting total cellular protein was determined by an RC DC Kit (Bio-Rad, Hercules, CA). Equal amounts of protein from the two cell populations (labeled and unlabeled) (90 μg/each) were mixed and then fractionated by a 12% SDS-PAGE gel. After staining with Coomassie brilliant blue, the entire lane of the gel was cut into 15 slices. Gel slices were subjected to in-gel digestion with trypsin (Promega, Madison, WI)17, 18. The resulting peptides were analyzed by LC-MS/MS using a LTQ-Orbitrap XL mass spectrometer (ThermoFisher, San Jose, CA) operated in a data-dependent mode for tandem MS in the Proteomics Core Facility at the University of Arkansas for Medical School (Little Rock, AR) as described16, 19. Briefly, the peptides from in-gel digestion were dissolved in 20 μl 0.1% formic acid. In the LC-MS/MS analysis, peptides were separated by a Picofrit column (10 cm × 75 μm ID; New Objective, Woburn, MA) packed with Jupiter Proteo resin (Phenomenex, Torrance, CA), and the column was connected to a nanoLC-2D HPLC system (Eksigent, Dublin, CA). Solvent A was 0.5% acetonitrile and 0.1% formic acid, and solvent B was 75% acetonitrile and 0.1% formic acid. A nonlinear gradient started with a mixing of A:B = 98:2 and increased to A:B = 2:98 over 34 min. The flow rate was 250 nl/min. Full MS spectra were acquired in profile mode with a mass range of 375 to 1500 and resolution of 60000 in the Orbitrap analyzer. The 6 most abundant precursors from each survey scan were selected for subsequent fragmentation in collision-induced dissociation (CID), and MS/MS scan in the linear ion trap. Dynamic exclusion was enabled with a repeat count of 2, a repeat duration of 50 s, an exclusion list size of 500, and an exclusion duration of 65 s. MS/MS spectra were acquired in centroid mode using a normalized collision energy of 35% and a mass isolation window of 2 m/z. Singly charged ions were discarded.
Protein Identification, Quantification and Bioinformatics Analysis
Protein identification and quantification were performed with Maxquant (version 1.0.13.13)20, 21 and Mascot (version 2.2; Matrix Science, Boston, MA) by searching against a composite target-decoy International Protein Index (IPI) human protein database (version 3.52)22. Briefly, the MS error tolerance in the Mascot search was set to 15 ppm, and the MS/MS error tolerance in the Maxquant and Mascot searches was set to 0.65 Da. The minimum required peptide length was set to six amino acids, and a maximum of 2 missed cleavages was allowed. The variable modifications included acetylation at peptide N-terminus, phosphorylation on tyrosine/serine/threonine and oxidation on methionine. The false discovery rates (FDR) for peptide and protein identification were both set to 1%. The maximum posterior error probability (PEP) for peptides was set to 1 (meaning no additional filtering). Different from group FDR, which measures the error rate associated with a collection of peptide-spectrum matches, PEP is a local version of FDR and measures the probability of error for a single identification23. The protein abundance ratios (i.e., heavy/light ratios) were calculated using unique and razor peptides with at least three ratio counts. Razor peptides are non-unique peptides shared by different proteins within a group and are assigned to the protein group with most other peptides21, 24. The LC-MS/MS data were also used to search Swiss-Prot database (version 51.6) taxonomic field for virus using Mascot for identification of influenza viral proteins. Mass error tolerances for MS and MS/MS, and variable modifications used in the Mascot search for viral protein identifications were the same as described above. In viral protein identification, proteins with two or more peptides with an ion score above respective threshold score (p < 0.05) were considered as positive identifications. In case of singe peptide match at > 95% probability, the MS/MS spectra of the peptide were manually inspected.
The protein identification and quantification results from the Maxquant analysis were uploaded to an Excel file and sorted according to the values of “significance B”, a significance score for log protein ratios calculated on the protein subsets obtained by peptide intensity binning20. The proteins whose expression was significantly altered at > 95% confidence were considered to be IAV-regulated proteins. The IAV-regulated proteins were then imported in bioinformatics software IPA (Ingenuity Pathway Analysis; Ingenuity® Systems, Redwood City, CA), a bioinformatics tool based on information from published literature25, and functional groupings and network analyses were performed using the “Core analysis” module of the tool.
Western Blotting
Primary macrophages were cultured and infected with IAVs as described above. Twenty four hours postinfection, cells were harvested and lysed for Western blot analysis as described26. Anti-NS1 antibody was a gift from Dr. Stephen Ludwig, Münster University Hospital Medical School, Germany; anti-ISG15 and anti-CD44 antibodies were purchased from Cell Signaling Technology (Boston, MA); and anti-annexin I and anti-N-acylethanolamine-hydrolyzing acid amidase (NAAA) antibodies were from Santa Cruz Biotech (Santa Cruz, CA). In Western blot analysis, no repeat was performed for strong and clear results. However, if weak or ambiguous results occurred, at least three rounds of repeats with separate sample preparations were performed to confirm the consistency of the results.
Quantitative Real Time PCR (qRT-PCR)
Primary macrophages were cultured and infected with IAVs in the same way as for proteomics analysis. Twenty four hours postinfection, cells were harvested and total RNA was extracted for examination of type I interferon (IFN) gene expression by qRT-PCR as described16. Briefly, total RNA was isolated using RNeasy Mini Kit according to manufacturer's instructions (Qiagen, Valencia, CA). One μg of RNA was reverse transcribed using an iScript cDNA Synthesis Kit following the manufacturer's protocol (Bio-Rad, Hercules, CA), and the resulting cDNA was used for qRT-PCR analysis. The specific primers used in this study were: IFN-α forward: 5'-CTGAATGACTTGGAAGCCTG-3'; reverse: 5'-ATTTCTGCTCTGACAACCTC-3'; IFN-β forward: 5'-CGCCGCATTGACCATCTA-3'; reverse: 5'-GACATTAGCCAGGAGGTTCTCA-3'. Annexin I (internal control), forward: 5'-AAAGGTGGTCCCGGATCAG-3'; reverse: 5'-TTATGCAAGGCAGCGACATC-3'. mRNA abundance was measured using SYBR Green Supermix (Invitrogen, Carlsbad, CA) from three independent sample preparations. Relative gene expression of IFN-α/β was calculated according to the traditional 2−ΔΔCt method27.
RESULTS
Identification of the IAV-Regulated Cellular Proteins in the IAV-Infected Primary Alveolar Macrophages
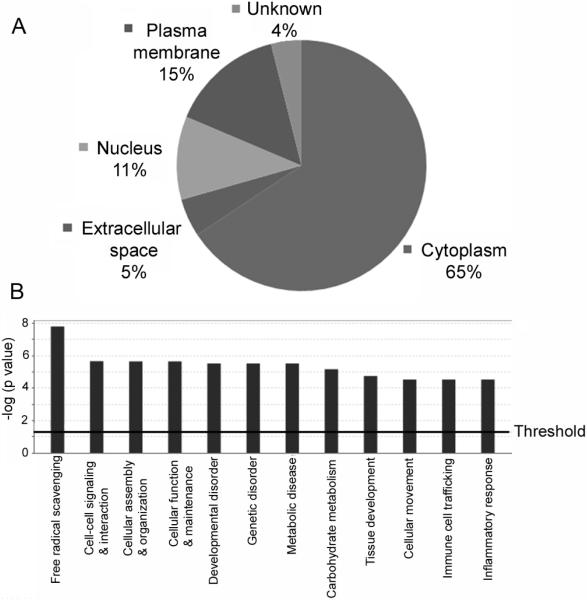
We used a SILAC-based quantitative proteomic approach to identify the cellular proteins that were regulated by IAV infection14, 15. During the period of in-vitro culture, the alveolar macrophages did not proliferate but stayed healthy as judged from cell shape and appearance. After 12 days of growing in the labeling medium, proteins in the macrophages reached more than 90% efficiency in isotope-coded amino acid incorporation (data not shown; also refer to Figs. 3A and 3B). For viral treatment, we infected the macrophages with influenza A/PR/8/34 viruses at an MOI of 0.5 and harvested the infected cells 24 h postinfection as described2 for proteomic analysis. At an MOI of 0.5, it was previously shown that more than 90% the alveolar macrophages were infected by virus, and there was no significant cytopathic effect at 24 h postinfection2. To make sure that the macrophages were infected by IAV, we first analyzed the mock- and virus-treated macrophages with Western blotting using an antibody against an IAV protein NS128. The results demonstrated that NS1 was expressed in the virus-infected cells but not in the mock-infected cells (Fig. 1), confirming IAV infection of the macrophages. After viral infection and proteomic analysis, a total of 1214 proteins were identified, and 875 of those proteins were quantified by Maxquant (version 1.0.13.13) and Mascot20, 21. Three hundred and thirty nine (339) proteins were identified but not quantified by the software because they did not meet the minimum requirements of at least three ratio counts for quantification. Among the quantified proteins, the expression of 106 proteins was statistically significantly altered (p < 0.05), 43 being up-regulated (Table 1) and 63 down-regulated (Table 2). Most of the IAV-regulated proteins were identified with multiple unique peptides and very low PEP (Tables 1 and 2), suggesting that those proteins were identified with very high confidence. Analysis of the 106 IAV-regulated proteins with the software IPA25 showed that 65% of the regulated proteins were soluble cytoplasmic proteins (Fig. 2A), whereas 5%, 11% and 15% of the regulated proteins were located in the extracellular space, nucleus and plasma membrane, respectively. Localization of the remaining 4% of regulated proteins could not be defined by the software (Fig. 2A). The most affected functional areas by IAVs were free radical scavenging, in which the expression of 15 proteins was significantly (p = 1.55E-08) affected by IAVs (Fig. 2B).
Fig. 3.
Representative peptide mass spectra from IAV-regulated proteins and Western blot validation of the expression of a subset of the IAV-regulated proteins that are functionally related to host cell immune responses. (A) A representative peptide mass spectrum from protein ISG15, which was up-regulated by IAVs. (B) A representative peptide mass spectrum from protein CD44, which was down-regulated by IAVs. The peptide mass spectra in A and B are displayed by software Xcalibur 2.0.7 SP1. (C) Western blot analysis of the expression of proteins ISG15, CD44, and NAAA (Tables 1 and 2; panels A and B). Primary alveolar macrophages were cultured and infected with IAVs as described in Fig. 1. Total protein from the mock- and IAV-infected cells was used for Western blot analysis with the indicated antibodies. Annexin I was used as the loading control. Equal amounts of total protein were loaded in each lane (40 μg/lane).
Fig. 1.

Western blot validation of viral infection of primary human alveolar macrophages. Two populations of primary alveolar macrophages were mocked-treated (control) or infected with IAV strain A/PR/8/34 at an MOI of 0.5. Twenty-four hours postinfection, the control and the infected cells were harvested, washed, and lysed for Western blot analysis using an antibody against viral protein NS1. The expression of NS1 in the infected cells but not in the mock-infected cell is shown. Annexin I was used as the loading control, because the expression of “classic” loading control protein in Western blotting – β-actin, was affected by IAV infection. Equal amounts of total protein were loaded in each lane (40 μg/lane).
Table 1.
Proteins that were significantly (p < 0.05) up-regulated in the IAV-infected macrophages
| Protein ID | UniProt ID | Name/Description | Gene symbol | H/L ratio* | Total peptide | Unique peptide | Sequence coverage (%)† | PEP‡ |
|---|---|---|---|---|---|---|---|---|
| IPI00375631 | P05161 | Interferon-induced 15 kDa protein | ISG15 | 26.70 | 3 | 3 | 17.6 | 1.30E-05 |
| IPI00643591 | B3KXW5 | AP-1 complex subunit gamma-1 | AP1G1 | 5.54 | 6 | 6 | 8.2 | 2.16E-20 |
| IPI00028564 | P32455 | Interferon-induced guanylate-binding protein 1 | GBP1 | 3.47 | 2 | 2 | 4.9 | 2.66E-12 |
| IPI00102864 | P52789 | Hexokinase-2 | HK2 | 2.88 | 6 | 1 | 6.9 | 3.15E-71 |
| IPI00847321 | F8W034 | Bax inhibitor 1 | TMBIM6 | 2.81 | 2 | 2 | 3.1 | 0.00064 |
| IPI00910047 | P08107 | Heat shock 70 kDa protein 1 | HSPA1A | 2.58 | 14 | 1 | 22.5 | 1.54E-203 |
| IPI00152505 | Q8TDB8 | Glucose transporter member 14 | GLUT14 | 2.49 | 2 | 2 | 2.9 | 0.02043 |
| IPI00796379 | F5H6I0 | Beta-2-microglobulin | B2M | 2.21 | 3 | 3 | 21.3 | 3.74E-12 |
| IPI00220007 | B4E1T5 | Apolipoprotein-L2 | APOL2 | 2.14 | 4 | 4 | 10.4 | 1.54E-07 |
| IPI00013455 | P30622 | CLIP1 protein | CLIP1 | 2.09 | 2 | 2 | 1.4 | 0.00539 |
| IPI00396286 | Q9NR20 | Dual specificity tyrosine-phosphorylation-regulated kinase 4 | DYRK4 | 2.03 | 1 | 1 | 1.3 | 0.03485 |
| IPI00554521 | P02794 | Ferritin heavy chain | FTH1 | 2.01 | 8 | 6 | 41.5 | 6.17E-59 |
| IPI00644748 | P30455 | MHC class I antigen | HLA-A | 2.01 | 6 | 1 | 22.9 | 1.75E-76 |
| IPI00007402 | O95373 | Importin-7 | IPO7 | 1.97 | 5 | 5 | 6 | 4.42E-47 |
| IPI00296190 | Q9BRX8 | Protein C10orf58 | C10orf58 | 1.97 | 4 | 4 | 21 | 6.52E-76 |
| IPI00747849 | P05026 | Sodium/potassium-transporting ATPase subunit beta-1 | ATP1B1 | 1.91 | 4 | 4 | 15.5 | 1.09E-28 |
| IPI00141318 | Q07065 | Cytoskeleton-associated protein 4 | CKAP4 | 1.85 | 7 | 7 | 15.4 | 7.15E-51 |
| IPI00033025 | E7EPK1 | Septin-7 | SEPT7 | 1.81 | 3 | 3 | 7.3 | 1.15E-14 |
| IPI00847322 | P04179 | Superoxide dismutase | SOD2 | 1.78 | 11 | 11 | 45.5 | 2.11E-142 |
| IPI00183666 | Q9Y5S1 | Transient receptor potential cation channel subfamily V member 2 | TRPV2 | 1.70 | 11 | 11 | 16.5 | 1.50E-71 |
| IPI00644037 | B3KSQ1 | Synaptic glycoprotein SC2 | GPSN2 | 1.70 | 3 | 3 | 8 | 3.36E-09 |
| IPI00216171 | P09104 | Gamma-enolase | ENO2 | 1.70 | 6 | 4 | 20 | 5.88E-98 |
| IPI00056357 | Q969H8 | UPF0556 protein C19orf10 | C19orf10 | 1.67 | 3 | 3 | 16.8 | 2.42E-20 |
| IPI00339384 | Q8TC12 | Retinol dehydrogenase 11 | RDH11 | 1.66 | 3 | 3 | 11.3 | 5.74E-23 |
| IPI00009253 | P54920 | Alpha-soluble NSF attachment protein | NAPA | 1.62 | 3 | 3 | 12.9 | 1.08E-27 |
| IPI00556385 | Q9BRR6 | ADP-dependent glucokinase | ADPGK | 1.62 | 3 | 3 | 7 | 1.45E-06 |
| IPI00018873 | P43490 | Nicotinamide phosphoribosyl transferase | NAMPT | 1.61 | 14 | 14 | 33.6 | 1.97E-172 |
| IPI00395887 | Q9H3N1 | Thioredoxin-related transmembrane protein 1 | TMX1 | 1.61 | 3 | 3 | 10.4 | 1.11E-18 |
| IPI00015476 | P43007 | Neutral amino acid transporter A | SLC1A4 | 1.61 | 2 | 2 | 3.9 | 2.28E-06 |
| IPI00306382 | O14828 | Secretory carrier-associated membrane protein 3 | SCAMP3 | 1.60 | 1 | 1 | 3.7 | 4.15E-17 |
| IPI00217563 | P05556 | Integrin beta-1 | ITGB1 | 1.59 | 10 | 10 | 12.5 | 1.58E-34 |
| IPI00604620 | P19338 | Nucleolin | NCL | 1.57 | 5 | 5 | 7.5 | 3.42E-32 |
| IPI00026530 | P49257 | Protein ERGIC-53 | ERGIC53 | 1.56 | 4 | 4 | 6.5 | 8.08E-14 |
| IPI00008485 | P21399 | Cytoplasmic aconitate hydratase | ACO1 | 1.55 | 17 | 17 | 19.7 | 2.87E-106 |
| IPI00303283 | P05106 | Integrin beta-3 | ITGB3 | 1.55 | 8 | 8 | 11.4 | 8.23E-66 |
| IPI00784119 | Q15904 | V-type proton ATPase subunit S1 | ATP6AP1 | 1.52 | 3 | 3 | 4.9 | 6.78E-05 |
| IPI00217766 | Q14108 | Lysosome membrane protein 2 | SCARB2 | 1.49 | 5 | 5 | 12.1 | 2.89E-33 |
| IPI00784090 | P50990 | T-complex protein 1 subunit theta | CCT8 | 1.48 | 13 | 13 | 28.8 | 1.25E-150 |
| IPI00216319 | Q04917 | 14-3-3 protein eta | YWHAH | 1.46 | 8 | 4 | 34.1 | 1.60E-75 |
| IPI00021766 | Q9NQC3 | Reticulon-4 | RTN4 | 1.46 | 5 | 5 | 6.5 | 6.52E-54 |
| IPI00006957 | Q9Y394 | Dehydrogenase/reductase SDR family member 7 | DHRS7 | 1.44 | 3 | 3 | 10 | 6.53E-14 |
| IPI00375676 | Q6S4P3 | Ferritin light chain | FTL | 1.44 | 9 | 9 | 35.6 | 5.80E-216 |
| IPI00012268 | Q13200 | 26S proteasome non-ATPase regulatory subunit 2 | PSMD2 | 1.43 | 6 | 6 | 7.8 | 1.07E-19 |
Ratio of IAV-infected cells versus control.
Coverage of all peptide sequences matched to the identified protein sequence (%).
PEP: posterior error probability.
Table 2.
Proteins that were significantly (p < 0.05) down-regulated in the IAV-infected macrophages
| Protein ID | UniProt ID | Name/description | Gene symbol | H/L ratio* | Total peptide | Unique peptide | Sequence coverage (%)† | PEP‡ |
|---|---|---|---|---|---|---|---|---|
| IPI00024083 | Q02083 | N-acylethanolamine-hydrolyzing acid amidase | NAAA | 0.06 | 3 | 3 | 8.4 | 2.33E-06 |
| IPI00216402 | Q16695 | Histone H3.3 | H3F3A | 0.15 | 4 | 4 | 21.3 | 1.44E-05 |
| IPI00027745 | P08236 | Beta-glucuronidase | GUSB | 0.16 | 9 | 9 | 16.4 | 3.68E-37 |
| IPI00453473 | P62805 | Histone H4 | HIST1H4A | 0.17 | 7 | 7 | 51.5 | 1.84E-45 |
| IPI00554538 | O14773 | Tripeptidyl-peptidase 1 | TPP1 | 0.17 | 6 | 6 | 14.8 | 1.29E-84 |
| IPI00021785 | P10606 | Cytochrome c oxidase subunit 5B, mitochondrial | COX5B | 0.19 | 2 | 2 | 17.8 | 0.00739 |
| IPI00217465 | P16403 | Histone H1.2 | HIST1H1C | 0.21 | 6 | 6 | 23.5 | 7.08E-35 |
| IPI00305064 | P16070 | CD44 antigen | CD44 | 0.22 | 3 | 3 | 5.1 | 3.73E-39 |
| IPI00299024 | P80723 | Brain acid soluble protein 1 | BASP1 | 0.22 | 2 | 2 | 18.5 | 1.93E-15 |
| IPI00022975 | P20292 | Arachidonate 5-lipoxygenase-activating protein | ALOX5AP | 0.23 | 3 | 3 | 17.4 | 5.31E-13 |
| IPI00843910 | P04066 | Tissue alpha-L-fucosidase | FUCA1 | 0.23 | 3 | 3 | 9 | 1.50E-69 |
| IPI00008223 | P54727 | UV excision repair protein RAD23 homolog B | RAD23B | 0.24 | 4 | 4 | 16.4 | 4.04E-36 |
| IPI00296141 | Q9UHL4 | Dipeptidyl-peptidase 2 | DPP2 | 0.24 | 3 | 3 | 6.9 | 8.80E-09 |
| IPI00006579 | P13073 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | COX4I1 | 0.25 | 5 | 5 | 33.7 | 6.02E-20 |
| IPI00023407 | P55160 | Nck-associated protein 1-like | NCKAP1L | 0.25 | 2 | 2 | 2.6 | 8.49E-28 |
| IPI00016255 | Q6P4A8 | Phospholipase B-like 1 | PLBD1 | 0.25 | 7 | 7 | 16.6 | 1.59E-75 |
| IPI00029997 | O95336 | 6-phosphogluconolactonase | PGLS | 0.27 | 2 | 2 | 16.3 | 1.03E-10 |
| IPI00383581 | Q14697 | Neutral alpha-glucosidase AB | GANAB | 0.28 | 25 | 1 | 28 | 1.61E-300 |
| IPI00418446 | Q13510 | N-acylsphingosine amidohydrolase 1 | ASAH1 | 0.29 | 22 | 22 | 44.5 | 8.78E-157 |
| IPI00893918 | B0V043 | Valyl-tRNA synthetase | VARS | 0.29 | 3 | 3 | 3.6 | 2.64E-09 |
| IPI00008787 | P54802 | Alpha-N-acetylglucosaminidase | NAGLU | 0.29 | 14 | 14 | 24.1 | 3.85E-172 |
| IPI00019988 | P51688 | N-sulphoglucosamine sulphohydrolase | SGSH | 0.29 | 4 | 4 | 8.8 | 1.85E-19 |
| IPI00794461 | Q99877 | Histone H2B type 1-N | HIST1H2BN | 0.29 | 8 | 2 | 38 | 1.78E-38 |
| IPI00216456 | Q93077 | Histone H2A | HIST1H2AC | 0.29 | 3 | 1 | 20.7 | 7.93E-33 |
| IPI00007047 | P05109 | S100-A8 | S100A8 | 0.29 | 3 | 3 | 26.9 | 5.43E-05 |
| IPI00156689 | Q99536 | Synaptic vesicle membrane protein VAT-1 homolog | VAT1 | 0.31 | 11 | 11 | 34.9 | 4.39E-223 |
| IPI00298406 | Q16836 | Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial | HADH | 0.31 | 2 | 2 | 6.4 | 0.00011 |
| IPI00021840 | P62753 | 40S ribosomal protein S6 | RPS6 | 0.32 | 4 | 4 | 18.1 | 1.26E-41 |
| IPI00031708 | P16930 | Fumarylacetoacetase | FAH | 0.32 | 3 | 3 | 7.9 | 3.71E-13 |
| IPI00027462 | P06702 | S100-A9 | S100A9 | 0.32 | 3 | 3 | 30.7 | 3.54E-11 |
| IPI00479186 | P14618 | Pyruvate kinase isozymes M1/M2 | PKM2 | 0.33 | 26 | 3 | 43.5 | 0 |
| IPI00183695 | P60903 | S100-A10 | S100A10 | 0.33 | 4 | 4 | 35.1 | 2.29E-07 |
| IPI00888051 | P47914 | Ribosomal protein L29 (RPL29) pseudogene | RPL29 | 0.34 | 1 | 1 | 6.1 | 2.11E-10 |
| IPI00793375 | Q9NQW7 | Xaa-Pro aminopeptidase 1 | XPNPEP1 | 0.34 | 5 | 5 | 10.2 | 3.47E-35 |
| IPI00550364 | Q96G03 | Phosphoglucomutase-2 | PGM2 | 0.35 | 8 | 8 | 14.2 | 1.75E-25 |
| IPI00019971 | E7EQD5 | Syntaxin-binding protein 2 | STXBP2 | 0.36 | 5 | 5 | 10.4 | 1.07E-23 |
| IPI00293088 | P10253 | Lysosomal alpha-glucosidase | GAA | 0.37 | 16 | 16 | 18.3 | 2.70E-97 |
| IPI00001466 | Q9HC35 | Echinoderm microtubule-associated protein-like 4 | EML4 | 0.37 | 3 | 3 | 3.2 | 7.73E-09 |
| IPI00004845 | Q9UFN0 | Protein NipSnap homolog 3A | NIPSNAP3A | 0.37 | 4 | 4 | 14.5 | 7.43E-13 |
| IPI00003817 | P52566 | Rho GDP-dissociation inhibitor 2 | ARHGDIB | 0.39 | 6 | 6 | 42.3 | 8.84E-63 |
| IPI00017726 | Q99714 | 3-hydroxyacyl-CoA dehydrogenase type-2 | HADH2 | 0.39 | 5 | 5 | 22.2 | 7.07E-22 |
| IPI00027851 | P06865 | Beta-hexosaminidase subunit alpha | HEXA | 0.39 | 10 | 10 | 21.7 | 2.85E-145 |
| IPI00017342 | P84095 | Rho-related GTP-binding protein RhoG | RHOG | 0.39 | 3 | 3 | 18.8 | 0.00176 |
| IPI00398780 | P07203 | Glutathione peroxidase 1 | GPX1 | 0.40 | 7 | 7 | 35.5 | 3.12E-47 |
| IPI00060200 | Q96C23 | Aldose 1-epimerase | GALM | 0.40 | 2 | 2 | 6.7 | 0.01461 |
| IPI00900293 | B2ZZ83 | Filamin B | FLNB | 0.40 | 15 | 8 | 5.5 | 6.80E-70 |
| IPI00219301 | P29966 | Myristoylated alanine-rich C-kinase substrate | MARCKS | 0.40 | 2 | 2 | 9.6 | 1.77E-13 |
| IPI00376119 | P22694 | cAMP-dependent protein kinase catalytic subunit beta | PRKACB | 0.41 | 2 | 1 | 5.3 | 0.00016 |
| IPI00026156 | P14317 | Hematopoietic lineage cell-specific protein | HCLS1 | 0.41 | 1 | 1 | 2.1 | 0.01522 |
| IPI00005969 | P52907 | F-actin-capping protein subunit alpha-1 | CAPZA1 | 0.42 | 3 | 2 | 14 | 1.70E-33 |
| IPI00470529 | B4E3D4 | Transmembrane glycoprotein NMB | GPNMB | 0.42 | 4 | 4 | 8.1 | 4.42E-72 |
| IPI00021812 | Q09666 | Neuroblast differentiation-associated protein AHNAK | AHNAK | 0.42 | 94 | 94 | 35.9 | 0 |
| IPI00003482 | Q16698 | 2,4-dienoyl-CoA reductase, mitochondrial | DECR1 | 0.45 | 5 | 5 | 14.9 | 2.71E-20 |
| IPI00019376 | Q9NVA2 | Septin-11 | SEPT11 | 0.46 | 5 | 5 | 14.9 | 1.20E-82 |
| IPI00003918 | P36578 | 60S ribosomal protein L4 | RPL4 | 0.46 | 5 | 5 | 11.9 | 2.93E-23 |
| IPI00011107 | P48735 | Isocitrate dehydrogenase [NADP], mitochondrial | IDH2 | 0.47 | 11 | 9 | 23.5 | 2.81E-108 |
| IPI00017704 | Q14019 | Coactosin-like protein | COTL1 | 0.49 | 6 | 6 | 26.1 | 1.45E-09 |
| IPI00299150 | P25774 | Cathepsin S | CTSS | 0.49 | 5 | 5 | 15.4 | 2.89E-48 |
| IPI00514587 | Q5T5C7 | Seryl-tRNA synthetase, cytoplasmic | SARS | 0.49 | 5 | 5 | 11.6 | 3.68E-23 |
| IPI00219446 | P30086 | Phosphatidylethanolamine-binding protein 1 | PEBP1 | 0.49 | 7 | 7 | 43.9 | 3.24E-165 |
| IPI00219077 | P09960 | Leukotriene A-4 hydrolase | LTA4H | 0.50 | 23 | 23 | 46.8 | 0 |
| IPI00847766 | P08865 | 40S ribosomal protein SA | RPSA | 0.52 | 8 | 8 | 34 | 1.95E-160 |
| IPI00025307 | Q02318 | Sterol 26-hydroxylase, mitochondrial | CYP27A1 | 0.53 | 18 | 18 | 39 | 2.20E-52 |
Ratio of IAV-infected cells versus control.
Coverage of all peptide sequences matched to the identified protein sequence (%).
PEP: posterior error probability.
Fig. 2.
The cellular distribution and functional classification of the IAV-regulated proteins. (A) The cellular distribution of the IAV-regulated proteins. (B) The top 12 functional categories that were significantly (p < 0.05) affected by IAV infection. The cellular distribution and classification analyses were performed using bioinformatics software IPA. The top 12 functional areas were ranked by p-value, which is a measure of significance of the changes in a specific category induced by IAV infection. The threshold value shown in panel B corresponds to the significance at 95% confidence.
The expression of multiple proteins involved in the responses to inflammation or infection in macrophages was altered by the IAV infection. For example, CAP-Gly domain-containing linker protein 1 (CLIP1) is a microtubule-binding protein that plays an important role in efficient phagocytosis in activated macrophages29, 30, and its expression in the IAV-infected macrophages was found to be up-regulated in the present study (Table 1). S100A8 and S100A9 belong to family of S100 calcium-binding proteins and are predominantly expressed in neutrophils, monocytes and activated macrophages31. The two proteins form heterodimer and possess proinflammatory and anti-oxidant activities31. In the present study, the expression of S100A8 and S100A9 as well as a third S100 family member S100A10 was all significantly (p < 0.05) suppressed by IAV infection (Table 2). The expression of all identified histones was suppressed by IAV infection (Table 2). Suppression of histones is possibly a strategy IAV uses to shut down the normal physiological activities of host cells, thus facilitating virus survival, a mechanism that has been observed in Herpes simplex virus infection32.
Identification of Influenza Viral Proteins in the IAV-Infected Primary Alveolar Macrophages
The eight RNA segments of the IAV viral genome code for 11 viral proteins, and nine of them are packed in the influenza virion33. A subset of the viral proteins has recently been detected by LC-MS/MS in the nucleolar fractions prepared from IAV-infected human embryonic kidney 293T cells11. In order to understand how the 11 viral proteins were expressed in the IAV-infected primary human alveolar macrophages, we searched the Swiss-Prot database (version 51.6) taxonomic field for virus using the LC-MS/MS data to identify influenza viral proteins. Five viral proteins (NS1, M1, HA, NP and PA) were identified (Table 3). As expected, all peptides for the five viral proteins were identified as Arg-13C6 or Lys-13C615N2 labeled peptides, and no unlabeled counterparts were identified in the Mascot search, which corresponds to the presence of label in virus-infected cells, but not in uninfected cells. NS1, HA and NP proteins were detected in multiple gel slices (Table 3). NS1 is the most abundant viral protein in the IAV-infected cells34, 35, and glycoprotein HA and the nuclear protein NP were predicted to be highly abundant in the influenza virion36. The wide distributions of HA and NP proteins in SDS-PAGE gel slices have also been observed in other studies and a potential cause for this was smearing of those abundant proteins in the gel33. We were not able to identify viral proteins PB1, PB2, NA, M2, NS2 and PB1-F2 from the infected macrophages. Viral proteins M2 and NS2 are synthesized from the mRNAs spliced from those that code for M1 and NS1, respectively, and the steady-state amounts of the spliced mRNAs are only a fraction of their counterparts in the infected cells37, 38. It is reasonable to speculate that the six viral proteins that could not be detected by LC-MS/MS in the present study might be expressed at substantially lower levels in the infected macrophages than the five detected viral proteins (Table 3).
Table 3.
Influenza viral proteins identified in the IAV-infected primary alveolar macrophages
| Protein name | Protein description | Swiss-Prot ace. no. | Mass (Da) | Gel slice no* | No. peptide† | Sequence coverage (%) | Mascot protein score | Mascot identity score |
|---|---|---|---|---|---|---|---|---|
| NS1 | Non-structural protein 1 | P03496 | 25851 | 1–5, 7, 8 | 5–11 | 37–56 | 91–493 | 20–34 |
| M1 | Matrix protein 1 | P03485 | 27875 | 5 | 2‡ | 16 | 75 | 20–32 |
| HA | Hemagglutinin precursor | P03452 | 63341 | 6–8, 11–13, 15 | 4–9 | 7–23 | 33–156 | 23–33 |
| NP | Nucleoprotein | P03466 | 56111 | 11, 14, 15 | 3–19 | 13–35 | 33–511 | 28–35 |
| PA | RNA-directed RNA polymerase subunit P2 | P03433 | 82535 | 12 | 2§ | 12 | 34 | 30–33 |
Numbered consecutively from the bottom to the top of a 12% SDS-PAGE gel.
The range of peptide number if a protein appeared in multiple gel slices; same for sequence coverage, Mascot protein score and Mascot identity score.
One peptide had a Mascot ion score above the identity score, and a low-ion-score peptide was assigned to the M1 protein with that peptide; the two peptides were also matched to M1 protein sequences in IAV stains A/Fowl plague virus/Rostock/1934 H7N1 and A/Fowl plague virus/Weybridge H7N7.
One peptide had a Mascot ion score above the identity score, and a low-ion-score peptide was assigned to the PA protein with that peptide; the two peptides were also matched to PA protein sequence in IAV stain A/WS/1933 H1N1.
Validation of the Expression of the IAV-Regulated Proteins
In order to make sure that our proteomic identifications and quantifications were genuine, we used Western blotting to validate a subset of proteins that are closely related to IAV infection. Consistent with our proteomic data (Figs. 3A and 3B; Tables 1 and 2), Western blot results demonstrated that the expression of ISG15 and CD44 was substantially increased and decreased respectively by IAV infection (Fig. 3C, two top rows). Similarly, Western blot results also confirmed the altered expression of NAAA (compare the indicated row in Fig. 3C with the H/L ratio in Table 2). ISG15 is an IFN-induced protein and plays crucial roles in host antiviral defense39. CD44 and NAAA proteins are known to be important in mediating inflammatory responses40–42. In the Western blot analysis, we found that the expression of “classic” loading control protein in Western blotting - β-actin, was moderately decreased by IAVs in the macrophages (Supplementary Fig. S1, Supporting Information). Thus it could not serve as the loading controls in this study. However, our proteomic data demonstrated that the expression of annexin I was not significantly affected by IAV infection (with an infected/uninfected H/L ratio of 1.18; p = 0.49). Consistent with this, the Western blot analysis showed that the expression of annexin I was not affected by IAV infection (Fig. 3C, row at the bottom; Supplementary Fig. S1, Supporting Information). In the present study, we used annexin I as the loading control in Western blot and qRT-PCR analyses.
Determination of Type I IFNs in the IAV-Infected Macrophages
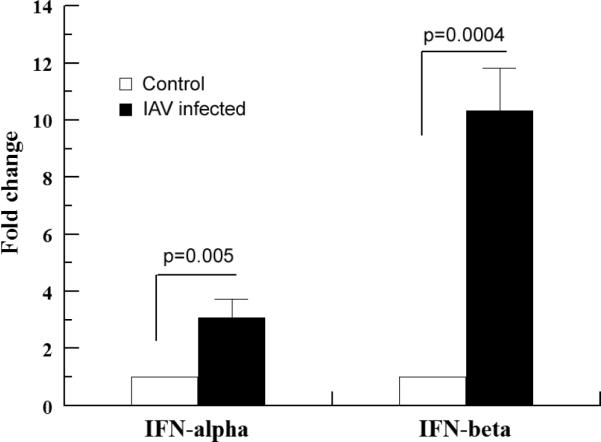
Although we have detected the changes in the expression of an array of IFN-inducible proteins after IAV infection (Tables 1 and 2; also see below), we were not able to detect IFNs by LC-MS/MS. In order to make sure the expression of IFNs was indeed altered by IAV infection in the macrophages, we examined the expression of IFN-α by Western blotting using an antibody against IFN-α (Santa Cruz Biotech; sc-80996). No IFN-α protein bands were detected in both the untreated and the A/PR/8/34-infected (with an MOI of 0.5 and 24 h postinfection culture) macrophages. However, when human lung epithelial A549 cells were infected with influenza A/PR/8/34 viruses using the same conditions as for macrophages, an IFN-α protein band became visible 12 h postinfection in the Western blotting (data not shown). These results suggest that the most likely explanation for the failure of LC-MS/MS detection of IFNs in macrophages is that the levels of these proteins in macrophages were too low to detect. We then used qRT-PCR to detect the IFN-α and IFN-β mRNAs, a method that is commonly used in examining type I IFN expression in virus infected macrophages43. As shown in Fig. 4, the abundances of IFN-α and IFN-β mRNA increased by approximately 3- and 10-fold respectively by IAV infection. IFN-α and IFN-β belong to type I IFNs and bind to the IFN-α receptor, so they share the same downstream signaling pathways. A bigger change in the expression of IFN-β than IFN-α (Fig. 4) suggests that the former may play a more important role in inducing immune responses in the alveoli during IAV infections. This result is in contrast with the results obtained from mice infected with Newcastle disease viruses or Vesicular stomatitis viruses43. In that study, with a knock-in mouse model, it appeared that IFN-α was the major type I IFN induced after the pulmonary virus infection. The contradiction suggests that human alveolar macrophages may respond differently to viral infection from mouse alveolar macrophages. Alternatively, it may also imply that different RNA viruses induce different type I IFNs. In short, the results from the qRT-PCR analysis revealed that IAV infection induced robust type I IFN transcription and that IFN-β was the major IFN induced in human alveolar macrophages (at least at the time of 24 h postinfection). Together with the proteomic data, which showed that the expression of multiple IFN-inducible proteins was affected by IAV infection (Tables 1 and 2; also see below), the transcription data on IFN expression obtained in this section suggest that it is highly likely IAV infection induced type I IFN protein expression in the human alveolar macrophages despite the fact that we were not able to detect the changes with LC-MS/MS and Western blotting.
Fig. 4.
IAV infection induces IFN gene expression in primary alveolar macrophages. Macrophages were cultured and infected with IAVs as described in Fig. 1. Total RNA extracted from the mock- and IAV-infected cells was used for IFN gene expression determination by qRTPCR. The values were means ± SE of three independent experiments. Annexin I was used as the internal loading control.
Multiple Protein Networks Are Affected by IAV Infection and IAV-Induced IFNs Trigger the Expression of An Array of Antiviral Proteins
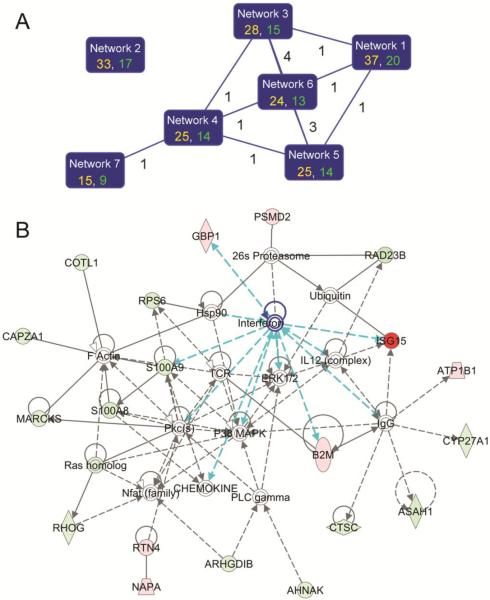
In order to get a global picture of protein expression in response to IAV infection, we analyzed the 106 IAV-regulated proteins with bioinformatics software IPA25. A total of 10 protein networks were identified by IPA to be significantly (p < 0.05) affected by IAV infection. Six of the top 7 networks shared at least one common “focus molecules” (i.e., significantly regulated molecules) between 2 networks, suggesting they were highly interconnected (Fig. 5A; Supplementary Table S1, Supporting Information). The remaining networks were identified with the minimum score, contained only one focus molecule in each network, and were not connected to each other. The cutoff score, which corresponded to identification at 95% confidence, was 2. The network that received the highest score (a score of 37) was a network with IFNs at the center of the network (Fig. 5B), again suggesting IFNs play a critical role in the responses of primary human alveolar macrophages to IAV infection. In this network, IFNs were found to influence the expression of multiple proteins, including ISG15, GBP1, B2M and S100A9 (potentially also S100A831) in primary alveolar macrophages (Fig 5B; Tables 1 and 2).
Fig. 5.
Protein networks that are significantly (p < 0.05) regulated by IAV infection. The IAV-regulated proteins were imported in the bioinformatics software IPA and analyzed by the tool. (A) The top 7 protein networks that were identified at the greater than 95% confidence. In each network, the identification score (in yellow) and the number of “focus molecules” (significantly regulated molecules) in the network (in green) are shown. The cutoff score for the 95% confidence level identification was 2. A line between the two networks indicates that they are interconnected by sharing one or more focus molecules, and the number of the shared molecules is shown by the line. (B) A functional antiviral network with type I IFNs at its center. The functional network with the highest score was an antiviral network with type I IFNs at its center. The proteins in red and green were those proteins whose expression was significantly up- or down-regulated (p < 0.05) by IAV infection, respectively. The color intensities correspond to the degree of expression alterations. Proteins in white are those proteins that are available in the IPA database but were not detected in the present study. The shape of symbols denotes the molecular class of the proteins. A solid line indicates a direct molecular interaction, whereas a dashed line indicates an indirect molecular interaction. The lines in blue highlight the interactions between IFNs and their related molecules in this network.
DISCUSSION
IFNs are known to trigger the innate immune response, resulting in the induction of more than 300 IFN-stimulated genes (ISGs)44. However, relatively few of these ISGs have been directly implicated in the antiviral state. In the present study, ISG15, a 15 kDa ubiquitin-like protein, was induced markedly with the highest fold-change in primary human alveolar macrophages by IAV infection (Table 1). We have confirmed the induction of ISG15 expression with Western blotting (Fig. 3). The ISGylation is a three-step enzymatic cascade, and all enzymes identified in the ISGylation pathways are coordinately induced by IFNs39. Over 150 proteins have been identified as putative ISGylation targets. Some of them are involved in the IFN pathway, including retinoic acid inducible gene 1(RIG-1), Mx1, protein kinase R (PKR), STAT1, and JAK145. Antiviral activities associated with ISGylation in vitro or in vivo have been reported for both DNA and RNA viruses, including IAVs46, 47. However, certain viral proteins, such as NS1 protein from influenza B viruses, can deconjugate ISG15 from its target proteins or bind to ISG15 directly to prevent the generation of ISGylation, thus impair its antiviral activity48. The dramatic induction of ISG15 by IAV infection suggests that this protein may play a vital role in defending the host from IAV infection. Several recent studies have demonstrated that ISG15 is a key player in host antiviral defense in IAV infections39.
The alveolar epithelium is composed of type I (covers 95% of the alveolar surface) and type II (covers the remaining 5% surface) alveolar epithelial cells. Type I alveolar epithelial cells are fully differentiated cells and do not proliferate, whereas type II epithelial cells can proliferate and differentiate into type I epithelial cells under certain circumstance such as lung injury49. In addition, resident alveolar macrophages are located in the alveoli and physically in close contact with alveolar epithelial cells50, 51. The resident alveolar macrophages are normally quiescent within the alveolus. When macrophages are infected or stimulated by foreign antigen, they respond by increased phagocytosis activity and secretion of cytokines and chemokines52. One important protein secreted by alveolar macrophages is transforming growth factor β (TGF-β), a potent inhibitor of epithelial cell proliferation. However, at the early stages of bleomycin-induced lung injury in rats, while alveolar macrophages produce and secret large quantities of biologically active TGF-β, the proliferation of type II alveolar epithelial cells is induced instead of suppressed53. One potential cause for this contradiction is the controlled expression of TGF-β receptors in the epithelial cells54. Results in the present study support an alternative and/or complementary mechanism underlying the interactions between alveolar macrophages and alveolar epithelial cells. Although we were not able to detect TGF-β proteins by LC-MS/MS in the present study, we found and validated that the expression of CD44 was markedly suppressed in the IAV-infected macrophages (Fig. 3 and Table 2). CD44 is a cell adhesion molecule that is expressed on the surface of many immune cells. CD44 has been shown to play a critical role in promoting the activation of TGF-β42, 55. Thus it is tempting to speculate that the decreased expression of CD44 during IAV infection could suppress the activation of TGF-β, which in turn would allow the robust proliferation of type II alveolar epithelial cells during IAV infection to repair the damaged type I epithelial cells. If this scenario is true, alveolar macrophages may play a crucial role in regenerating alveolar epithelium during IAV infection by allowing proliferation of type II alveolar epithelial cells, and the interaction between macrophages and type II alveolar epithelial cells in the alveoli during IAV infection may mimics the situation in bleomycin-induced lung injury in rats51, 54.
In the present study, we found that the expression of NAAA and CD44 was markedly suppressed by IAV infection (Fig. 3 and Table 2). Both of these proteins are involved in the regulation of inflammation. Palmitoylethanolamide (PEA) is a naturally occurring lipid amide and inhibits inflammatory responses by activating peroxisome proliferator-activated receptor-α (PPAR-α)56. PEA is preferentially hydrolyzed by NAAA, a member of the choloylglycine hydrolase family and highly expressed in alveolar macrophages57. Selective NAAA inhibitor increases PEA levels in activated inflammatory cells and impairs inflammatory reactions41. On the other hand, binding of CD44 to its ligand, hyaluronan, is known to suppress inflammatory responses40, 42. While reduced expression of NAAA could result in increased levels of cellular PEA and thus the suppression of the inflammatory response (a viral strategy for survival in host cells), decreased expression of CD44 would lead to enhanced inflammatory responses (an antiviral defense). These results suggest that the altered expression of NAAA and CD44 in the IAV-infected macrophages may reflect a battlefield in the area of inflammation between the macrophages and IAVs.
IAV infection is known to induce oxidative stress in host cells, and a more oxidized cellular environment is important for virus replication58–60. The significant changes in the expression of a large number of proteins in the functional area of free radical scavenging (Fig. 2B) imply that the virus-infected macrophages were under high level of oxidative stress and that the infected macrophages might counterbalance the altered redox state by adjusting the expression of the free radical scavenging proteins. Our results coincide with the results obtained using monocyte-derived macrophages, which revealed that IAV infection affected the expression of multiple proteins involved in the regulation of cellular reactive oxygen species in the IAV-infected macrophages4.
Induction of apoptosis of the infected cells is an important strategy host cells employ to limit virus replication and serves as an innate defense mechanism against viral infections4. However, viruses have evolved various strategies to modulate apoptosis in infected cells to delay the cell apoptosis61. Bax inhibitor-1 is a highly preserved transmembrane protein. It inhibits cell apoptosis through stimulating the anti-apoptotic function of Bcl-2 or suppressing the pro-apoptotic effect of Bax62. In the present study, we found that the expression of Bax inhibitor-1 was significantly up-regulated by the infection of IAVs (Table 1). The increased expression of Bax inhibitor-1 could suppress apoptosis of the infected host cells and hence favors viral replication.
In summary, through a SILAC based quantitative proteomics analysis, we profiled the global protein expression in primary human alveolar macrophages after they were infected with IAVs. The results demonstrated that the expression of an array of IFN-induced proteins was induced by IAV infection. Our results support the notion that induction of IFNs plays a central role in the host antiviral defense against IAV attack in the infected alveolar macrophages. In addition, several other battlefields between IAVs and the host were also revealed by our proteomic data. For example, alveolar macrophages mounted antiviral defenses through enhancing inflammatory responses via suppressing the expression of CD44. In the mean time, IAVs counteracted host antiviral defenses by modulating the expression of NAAA and Bax inhibitor 1 to inhibit the inflammatory response and apoptotic processes of the host cells to establish a cellular environment that favors virus replication and survival. Our results also suggest that alveolar macrophages may play a crucial role in regenerating alveolar epithelium during IAV infection.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Stephan Ludwig (University of Muenster, Muenster, Germany) for kindly providing the mouse monoclonal anti-NS1 antibody. This work was supported by an NIH grant 3P20RR015569-10S2 (Y. Du) and a Summer research grant from the Arkansas INBRE Program (C.J. Funk), supported by grants from the National Center for Research Resources (5P20RR016460-11) and the National Institute of General Medical Sciences (8 P20 GM103429-11) from the NIH.
Footnotes
Supporting Information Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- (2).Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J. Immunol. 2009;182(3):1296–304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lee SM, Gardy JL, Cheung CY, Cheung TK, Hui KP, Ip NY, Guan Y, Hancock RE, Peiris JS. Systems-level comparison of host-responses elicited by avian H5N1 and seasonal H1N1 influenza viruses in primary human macrophages. PLoS One. 2009;4(12):e8072. doi: 10.1371/journal.pone.0008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Lietzen N, Ohman T, Rintahaka J, Julkunen I, Aittokallio T, Matikainen S, Nyman TA. Quantitative subcellular proteome and secretome profiling of influenza A virus-infected human primary macrophages. PLoS Pathog. 2011;7(5):e1001340. doi: 10.1371/journal.ppat.1001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fornek JL, Korth MJ, Katze MG. Use of functional genomics to understand influenza-host interactions. Adv. Virus Res. 2007;70:81–100. doi: 10.1016/S0065-3527(07)70003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sakabe S, Iwatsuki-Horimoto K, Takano R, Nidom CA, Le MT, Nagamura-Inoue T, Horimoto T, Yamashita N, Kawaoka Y. Cytokine production by primary human macrophages infected with highly pathogenic H5N1 or pandemic H1N1 2009 influenza viruses. J. Gen. Virol. 2011;92:1428–1434. doi: 10.1099/vir.0.030346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18(3-4):533–7. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- (8).Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 1999;17(10):994–9. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- (9).Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev. Genomics Hum. Genet. 2001;2:343–72. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- (10).Coombs KM, Berard A, Xu W, Krokhin O, Meng X, Cortens JP, Kobasa D, Wilkins J, Brown EG. Quantitative proteomic analyses of influenza virus-infected cultured human lung cells. J. Virol. 2010;84(20):10888–906. doi: 10.1128/JVI.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Emmott E, Wise H, Loucaides EM, Matthews DA, Digard P, Hiscox JA. Quantitative proteomics using SILAC coupled to LC-MS/MS reveals changes in the nucleolar proteome in influenza A virus-infected cells. J. Proteome Res. 2010;9(10):5335–45. doi: 10.1021/pr100593g. [DOI] [PubMed] [Google Scholar]
- (12).Munday DC, Emmott E, Surtees R, Lardeau CH, Wu W, Duprex WP, Dove BK, Barr JN, Hiscox JA. Quantitative proteomic analysis of A549 cells infected with human respiratory syncytial virus. Mol. Cell. Proteomics. 2010;9(11):2438–59. doi: 10.1074/mcp.M110.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ringrose JH, Jeeninga RE, Berkhout B, Speijer D. Proteomic studies reveal coordinated changes in T-cell expression patterns upon infection with human immunodeficiency virus type 1. J. Virol. 2008;82(9):4320–30. doi: 10.1128/JVI.01819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics. 2002;1(5):376–86. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- (15).Zhu H, Pan S, Gu S, Bradbury EM, Chen X. Amino acid residue specific stable isotope labeling for quantitative proteomics. Rapid Commun. Mass Spectrom. 2002;16(22):2115–23. doi: 10.1002/rcm.831. [DOI] [PubMed] [Google Scholar]
- (16).Wang Y, Zhou J, Ruan C, Du Y. Inhibition of Type I Interferon Production via Suppressing IKK-Gamma Expression: A New Strategy for Counteracting Host Antiviral Defense by Influenza A Viruses? J. Proteome Res. 2012;11(1):217–23. doi: 10.1021/pr200894t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Du Y, Zhou J, Fan J, Shen Z, Chen X. Streamline proteomic approach for characterizing protein-protein interaction network in a RAD52 protein complex. J. Proteome Res. 2009;8(5):2211–7. doi: 10.1021/pr800662x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Du Y, Gu S, Zhou J, Wang T, Cai H, Macinnes MA, Bradbury EM, Chen X. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell. Proteomics. 2006;5(6):1033–44. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- (19).Byrum S, Mackintosh SG, Edmondson RD, Cheung WL, Taverna SD, Tackett AJ. Analysis of Histone Exchange during Chromatin Purification. J. Integr. OMICS. 2011;1(1):61–65. doi: 10.5584/jiomics.v1i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26(12):1367–72. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- (21).Cox J, Matic I, Hilger M, Nagaraj N, Selbach M, Olsen JV, Mann M. A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat. Protoc. 2009;4(5):698–705. doi: 10.1038/nprot.2009.36. [DOI] [PubMed] [Google Scholar]
- (22).Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat. Methods. 2005;2(9):667–75. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- (23).Kall L, Storey JD, MacCoss MJ, Noble WS. Posterior error probabilities and false discovery rates: two sides of the same coin. J. Proteome Res. 2008;7(1):40–4. doi: 10.1021/pr700739d. [DOI] [PubMed] [Google Scholar]
- (24).Nesvizhskii AI, Aebersold R. Interpretation of shotgun proteomic data: the protein inference problem. Mol. Cell. Proteomics. 2005;4(10):1419–40. doi: 10.1074/mcp.R500012-MCP200. [DOI] [PubMed] [Google Scholar]
- (25).Thomas S, Bonchev D. A survey of current software for network analysis in molecular biology. Hum. Genomics. 2010;4(5):353–60. doi: 10.1186/1479-7364-4-5-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, Chen X, Liu KJ, Hu CA. PUMA overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res. 2005;65(5):1647–54. doi: 10.1158/0008-5472.CAN-04-1754. [DOI] [PubMed] [Google Scholar]
- (27).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- (28).Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proc. Natl. Acad. Sci. U. S. A. 2008;105(11):4381–6. doi: 10.1073/pnas.0800482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Binker MG, Zhao DY, Pang SJ, Harrison RE. Cytoplasmic linker protein-170 enhances spreading and phagocytosis in activated macrophages by stabilizing microtubules. J. Immunol. 2007;179(6):3780–91. doi: 10.4049/jimmunol.179.6.3780. [DOI] [PubMed] [Google Scholar]
- (30).Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J. Cell Biol. 2008;183(7):1287–98. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol. Cell Biol. 2010;88(1):41–9. doi: 10.1038/icb.2009.88. [DOI] [PubMed] [Google Scholar]
- (32).Cliffe AR, Knipe DM. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 2008;82(24):12030–8. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Shaw ML, Stone KL, Colangelo CM, Gulcicek EE, Palese P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008;4(6):e1000085. doi: 10.1371/journal.ppat.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lazarowitz SG, Compans RW, Choppin PW. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971;46(3):830–43. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- (35).Skehel JJ. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- (36).Inglis SC, Carroll AR, Lamb RA, Mahy BW. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- (37).Lamb RA, Choppin PW, Chanock RM, Lai CJ. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc. Natl. Acad. Sci. U. S. A. 1980;77(4):1857–61. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lamb RA, Lai CJ, Choppin PW. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc. Natl. Acad. Sci. U. S. A. 1981;78(7):4170–4. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Skaug B, Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143(2):187–90. doi: 10.1016/j.cell.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm. Allergy Drug Targets. 2009;8(3):208–20. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- (41).Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(49):20966–71. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296(5565):155–8. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- (43).Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27(2):240–52. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- (44).Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 1998;95(26):15623–8. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. U. S. A. 2005;102(29):10200–5. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Hsiang TY, Zhao C, Krug RM. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J. Virol. 2009;83(12):5971–7. doi: 10.1128/JVI.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, Garcia-Sastre A, Leib DA, Pekosz A, Knobeloch KP, Horak I, Virgin H. W. t. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. U. S. A. 2007;104(4):1371–6. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Versteeg GA, Hale BG, van Boheemen S, Wolff T, Lenschow DJ, Garcia-Sastre A. Species-specific antagonism of host ISGylation by the influenza B virus NS1 protein. J. Virol. 2010;84(10):5423–30. doi: 10.1128/JVI.02395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Penney DP. The ultrastructure of epithelial cells of the distal lung. Int. Rev. Cytol. 1988;111:231–69. doi: 10.1016/s0074-7696(08)61736-2. [DOI] [PubMed] [Google Scholar]
- (50).Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J. Exp. Med. 1989;170(3):727–37. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Khalil N, O'Connor RN, Flanders KC, Shing W, Whitman CI. Regulation of type II alveolar epithelial cell proliferation by TGF-beta during bleomycin-induced lung injury in rats. Am. J. Physiol. 1994;267(5 Pt 1):L498–507. doi: 10.1152/ajplung.1994.267.5.L498. [DOI] [PubMed] [Google Scholar]
- (52).Lambrecht BN. Alveolar macrophage in the driver's seat. Immunity. 2006;24(4):366–8. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- (53).Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J. Clin. Invest. 1993;92(4):1812–8. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Khalil N, Parekh TV, O'Connor RN, Gold LI. Differential expression of transforming growth factor-beta type I and II receptors by pulmonary cells in bleomycin-induced lung injury: correlation with repair and fibrosis. Exp. Lung Res. 2002;28(3):233–50. doi: 10.1080/019021402753570527. [DOI] [PubMed] [Google Scholar]
- (55).Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J. Immunol. 2009;183(4):2232–41. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67(1):15–9. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- (57).Tsuboi K, Takezaki N, Ueda N. The N-acylethanolamine-hydrolyzing acid amidase (NAAA) Chem. Biodivers. 2007;4(8):1914–25. doi: 10.1002/cbdv.200790159. [DOI] [PubMed] [Google Scholar]
- (58).Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 2003;34(7):928–36. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- (59).Nencioni L, Sgarbanti R, De Chiara G, Garaci E, Palamara AT. Influenza virus and redox mediated cell signaling: a complex network of virus/host interaction. New Microbiol. 2007;30(4):367–75. [PubMed] [Google Scholar]
- (60).Beck MA. The influence of antioxidant nutrients on viral infection. Nutr. Rev. 1998;56(1 Pt 2):S140–6. doi: 10.1111/j.1753-4887.1998.tb01632.x. [DOI] [PubMed] [Google Scholar]
- (61).Koyama AH, Fukumori T, Fujita M, Irie H, Adachi A. Physiological significance of apoptosis in animal virus infection. Microbes Infect. 2000;2(9):1111–7. doi: 10.1016/s1286-4579(00)01265-x. [DOI] [PubMed] [Google Scholar]
- (62).Reimers K, Choi CY, Bucan V, Vogt PM. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr. Mol. Med. 2008;8(2):148–56. doi: 10.2174/156652408783769562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.