Abstract
Seasonal affective disorder (SAD), a major depressive disorder recurring in the fall and winter, is caused by the reduction of light in the environment, and its depressive symptoms can be alleviated by bright light therapy. Both circadian and monoaminergic systems have been implicated in the etiology of SAD. However, the underlying neural pathways through which light regulates mood are not well understood. The present study utilized a diurnal rodent model, Arvicanthis niloticus, to explore the neural pathways mediating the effects of light on brain regions involved in mood regulation. Animals kept in constant darkness received light exposure in early subjective day, the time when light therapy is usually applied. The time course of neural activity following light exposure was assessed using Fos as a marker in the following brain regions/cells: the suprachiasmatic nucleus (SCN), orexin neurons in the perifornical-lateral hypothalamic area (PF-LHA) and the dorsal raphe nucleus (DRN). A light-induced increase in Fos expression was observed in orexin neurons and the DRN, but not in the SCN. As the DRN is densely innervated by orexinergic inputs, the involvement of orexinergic signaling in mediating the effects of light on the DRN was tested in the second experiment. The animals were injected with the selective orexin receptor type 1 (OXR1) antagonist SB-334867 prior to the light exposure. The treatment of SB-334867 significantly inhibited the Fos induction in the DRN. The results collectively point to the role of orexin neurons in mediating the effects of light on the mood-regulating monoaminergic areas, suggesting an orexinergic pathway that underlies light-dependent mood fluctuation and the beneficial effects of light therapy.
Keywords: seasonal affective disorder, light therapy, diurnal grass rats, Fos, orexin neurons, dorsal raphe nucleus
Introduction
Seasonal affective disorder (SAD) is a major depressive disorder that recurs in the fall and winter due to the reduction of natural daylight in the environment (Rosenthal et al., 1984). To correct for the light deficiency, light therapy has been utilized and is known to be one of the most effective treatment methods for SAD (Rosenthal et al., 1984). Light is the most salient cue for entraining circadian rhythms (Pittendrigh, 1993). Therefore, it has been hypothesized that light therapy improves mood by resetting the circadian rhythms that become desynchronized with the environment due to light deficiency (Lewy, 2009). In addition to the circadian system, deficits in central monoaminergic systems, particularly serotoninergic transmission, have also been implicated in the pathogenesis of SAD and selective serotonin reuptake inhibitors (SSRIs) can be effective in treating SAD (Ruhrmann et al., 1998). However, the underlying neural pathways through which light regulates mood are still for the most part uncharted territory (Levitan, 2007).
A major barrier to progress for research in this area is the lack of adequate animal models (Cryan and Slattery, 2007, Pollak et al., 2010). Those readily available to researchers such as nocturnal mice and rats are not optimal for exploring the effects of light in regulating mood in diurnal humans due to the substantial differences between the diurnal and nocturnal animals in their circadian physiology and direct responses to light (Smale et al., 2003, Challet, 2007). For instance, the daily fluctuations in hypothalamic serotonin content are oppositely phased, with the peak level occurring during the day in diurnal species, and at night in nocturnal ones (Poncet et al., 1993, Cuesta et al., 2008). Moreover, light enhances activity and promotes wakefulness in diurnal species, but inhibits activity and induces sleep in nocturnal ones (Campbell and Dawson, 1990, Redlin, 2001). Therefore, a diurnal animal model is needed to elucidate the neural pathways mediating the effects of light on the circadian, arousal and monoaminergic systems, which could ultimately contribute to mood regulation.
In the present study, we utilized a diurnal rodent model, the Nile grass rats (Arvicanthis niloticus). These grass rats show diurnal patterns of general activity in the field and laboratory (McElhinny et al., 1997, Blanchong et al., 1999). When housed in winter-like lighting conditions, they show depression-like behaviors, supporting their potential for use as an animal model of SAD (Ashkenazy-Frolinger et al., 2010, Workman and Nelson, 2011. To explore the neural pathways mediating the effects of light on mood, an acute light pulse was administered in the early subjective day to grass rats housed in constant darkness. This light pulse was intended to mimic the acute effect of light therapy, which is generally carried out during early daytime (Terman and Terman, 2005, Terman, 2007). Using c-Fos as a marker, the brain responses were examined, in three interconnected brain regions/cell populations that could potentially be involved in mediating the effects of light on mood regulation: the principal circadian clock in the suprachiasmatic nucleus (SCN) (Stephan and Zucker, 1972), the wakefulness-promoting orexin neurons (Sakurai, 2007), and the largest serotonergic nucleus within the dorsal raphe (DRN) (Wiklund et al., 1981). The results provide insights about the neural pathways mediating the effects of light in brain regions that are involved in mood regulation.
Experimental Procedures
Animals and Experimental groups
The grass rats (Arvicanthis niloticus) were obtained from a laboratory colony established with animals from East Africa. Food (Prolab 2000 #5P06, PMI Nutrition LLC, MO) and water were provided ad libitum. Adult male and female grass rats (n=32) were kept in a 12:12h light/dark cycle (LD, 300lux/1lux). The time of lights-on was defined as Zeitgeber time (ZT) 0, and the time of lights-off was defined as ZT12. To explore the neural pathways mediating the effects of light, the animals were exposed to light during their subjective day. The animals were first housed in constant darkness (DD) for one day, and then received a light pulse (300 lux, 120min) on the second day of DD, starting at the projected ZT3. In experiment 1, to assess the time course of Fos induction, female grass rats (n=20) were sacrificed either right before (0min), or 30, 60 or 120 minutes after the beginning of the light pulse (LP, n=5/time point). In experiment 2, male grass rats (n=12) were treated the same way as the animals in experiment 1, but received an intraperitoneal injection of either a selective orexin receptor 1 (OXR1) antagonist SB-334867 (Tocris Bioscience, MN, USA) at 15 mg/kg dissolved in a 60:40 DMSO/saline solution, or vehicle only (n=6/treatment group) at projected ZT2 in the dark. The dose was determined on the basis of that used in studies of lab rats and mice (Ishii et al., 2004, Ito et al., 2009, Scott et al., 2011). The injections were given under dim red light. The animals received white light exposure (300 lux) starting at ZT3 and were then sacrificed 120min later. The sexes of the animals used for each experiment reflect the availability of animals of the appropriate age from our colony. We did not monitor reproductive conditions because female grass rats in our colony show no signs of estrous cycles in vaginal smears and no evidence of spontaneous estrous cycles in ovarian histology and mating behavior (T. L McElhinny and L. Smale unpublished observations). All experimental procedures were approved by the Michigan State University Animal Use and Care Committee.
Immunocytochemistry (ICC)
Animals were euthanized (pentobarbital, 200mg/kg, ip) and perfused transcardially using 50ml saline followed by 100ml 4% paraformaldehyde in 0.1 M phosphate buffer. After the perfusion, the brains were post-fixed, cryoprotected, and sectioned (40μm) using a cryostat (Leica, IL). Single or double label ICC was carried out as described in previous studies (Martinez et al., 2002, Yan and Silver, 2008, Castillo-Ruiz et al., 2010, Yan et al., 2010). Sections were first incubated with an antibody against c-Fos (1:10,000, sc-52, Santa Cruz Biotechnology, Inc, CA) for 48 hr at 4°C and processed with the avidin-biotin-immunoperoxidase technique using diaminobenzidine (DAB) enhanced with 4% Nickel Sulfate as the chromogen. The Fos-immunoreactive (ir) nuclei were stained into dark gray or black. For double-labeling with orexin or serotonin (5-HT), the sections were further incubated with the antibody against either orexin-A (1:20,000, s-19, Santa Cruz Biotechnology, Inc, CA) or 5-HT (1:10,000, Protos Biotech, NY) and processed with the avidin-biotin-immunoperoxidase technique using DAB as the chromogen. The orexin or 5-HT containing cell body and fibers were stained brown. Two alternate sets of the DRN containing sections were labeled with antibodies against orexin-A or 5-HT, respectively, to confirm the orexinergic innervation in the DRN. Following the ICC reaction, sections were mounted on slides, dehydrated with alcohol rinses, cleared with xylene, and coverslipped with Permount (Fisher Scientific, NJ, USA).
Quantitative analysis of ICC results
For quantification, images of sections through the SCN, the perifornical-lateral hypothalamic area (PF-LHA) in the tuberal hypothalamus and the dorsal raphe nucleus (DRN) were captured using a CCD video camera (CX9000, MBF bioscience, Williston, Vermont, USA) attached to a light microscope (Zeiss, Gottingen, Germany). In the SCN, the number of Fos-ir nuclei was counted bilaterally in 3 mid-SCN sections using the NIH Image J program. The counting regions for the SCN were delineated as in previous studies (Ramanathan et al., 2006). The average of the counts from the 3 bilateral regions was used to represent the value for each animal. Images captured from PF-LHA or DRN were manually counted. The number of orexin neurons and the orexin/Fos double-labeled neurons were counted on 4 images per animal at the PF-LHA region as described in a previous study (Martinez et al., 2002). The average number of Fos positive cells and the percentage of double-labeled cells were used to represent the value for each animal. In the DRN, the 5-HT staining was used as regional markers and the images were captured around the clusters of 5-HT neurons across the rostrocaudal extent from level 1 to level 4 (Janusonis and Fite, 2001). Fos-ir nuclei were counted on 6 to 8 images per animal and the average number was used to represent the value of each animal. The effect of time following the LP (experiment 1) was assessed using one-way ANOVA followed by Tukey post-hoc comparison. The effect of drug treatment (experiment 2) was assessed using t-test. In all cases, differences were considered significant when p< 0.05.
Results
Time course of Fos-ir following light exposure during subjective day
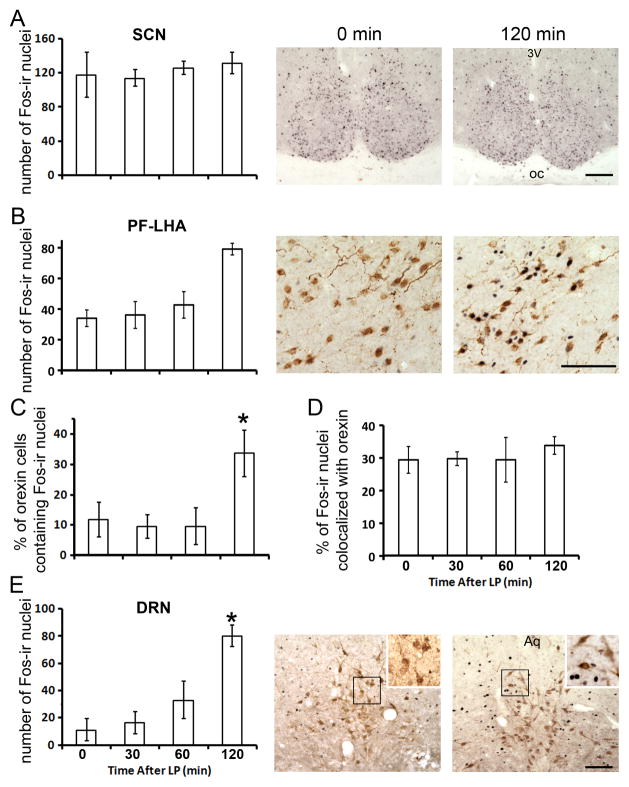
Following the light exposure, the time course of neural activity was assessed in the SCN, PF-LHA and DRN (Fig. 1). In the SCN (Fig. 1A), no apparent changes in the number of Fos-ir nuclei was observed from the beginning through the end of the 120 min light exposure (one-way ANOVA, F3,19=0.26, p>0.05). In the PF-LHA of the tuberal hypothalamus, the number of Fos-ir nuclei increased over time following the light exposure (Fig. 1B, one-way ANOVA, F3,19=7.03, p<0.05). The significant increase was found at 120min (post-hoc Tukey test, p<0.05). There were no changes in the number of orexin-ir cells (one-way ANOVA, F3,19=2.06, p>0.05, data not shown). Analysis of Fos and orexin double-labeled cells revealed a significant increase (from 10% to 30%) in the proportion of orexin neurons containing Fos (Fig. 1C, one-way ANOVA, F3,19=6.79, p<0.05). The proportion of Fos-ir nuclei colocalized with orexin remained unchanged over the time course (Fig. 1D, one-way ANOVA, F3,19=0.25, p>0.05), suggesting that the increase in Fos-ir was not restricted to orexin-ir neurons. In the DRN (Fig. 1E), the number of Fos-ir also increased over time (one-way ANOVA, F3,19=15.38, p<0.05), and a significant increase was observed at 120 min (post-hoc Tukey test, p<0.05). However, almost no Fos-ir nuclei were found co-localized with 5-HT cells in the DRN (Fig. 1E).
Fig. 1.
Light-induced Fos expression in the SCN, PF-LHA and DRN. (A), number of Fos-ir nuclei and representative images in the SCN. (B), number of Fos-ir and representative images in the PF-LHA. (C), the percent of orexin cells double-labeled with Fos and (D), Fos double-labeled with orexin in the PF-LHA. (E), number of Fos-ir nuclei in the DRN and representative images at the rostral-caudal level 1–2 of the DRN. Higher magnification images are shown in the insets. Animals were treated with a light pulse (LP, 300lux), and analyzed prior to the LP (0 min) and 30, 60 and 120 min after. Results are displayed as mean ± SEM. * indicated p<0.05. 3v, 3rd ventricle; Aq, aqueduct; oc, optic chiasm. Scale bar, 100μm.
Orexinergic signaling mediates light-induced Fos-ir in the DRN
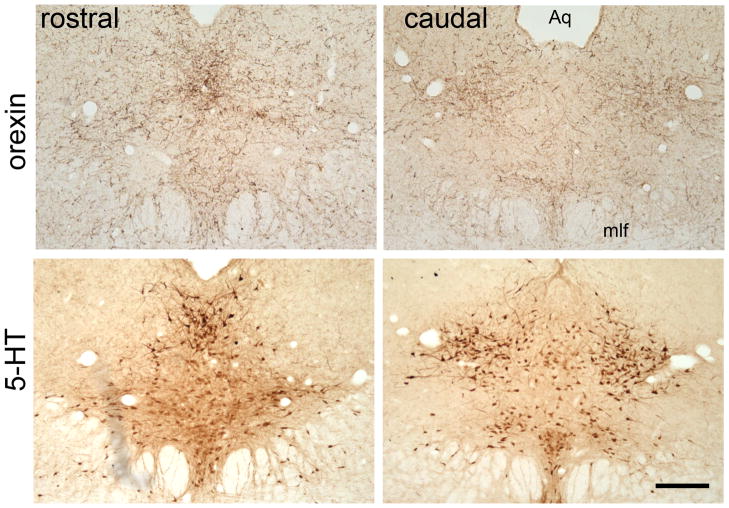
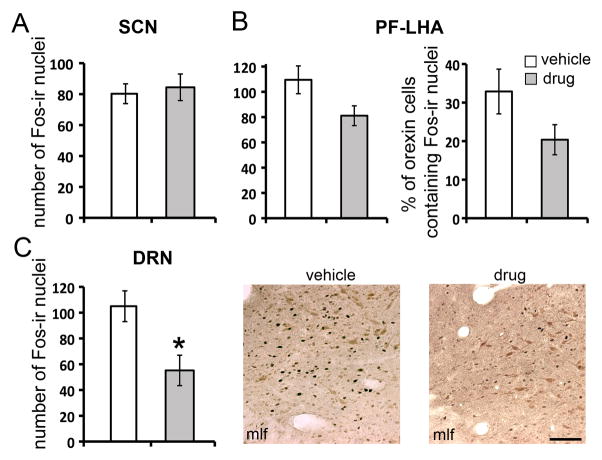
Consistent with the previous findings (Nixon and Smale, 2007), the DRN of our grass rats was densely innervated by orexinergic fibers (Fig. 2). To assess the role of orexinergic signaling in mediating the effects of light, light-induced Fos-ir was analyzed following the treatment of SB-334867, an orexin receptor 1 (OXR1) antagonist (Fig. 3). In the SCN (Fig. 3A), there was no significant difference in the number of Fos-ir nuclei between the animals treated with the drug or vehicle prior to the LP (t-test, t10=0.3, p>0.05). In the PF-LHA (Fig. 3B), although not statistically significant, there was a trend of a reduction in the number of Fos-ir cells (t-test, t10=2.35, p=0.069) and in the percentage of Fos-positive orexin cells (t-test, t10=2.06, p=0.11) following the treatment of OXR1 antagonist. No changes were found in the number of orexin-ir cells or the proportion of Fos-ir nuclei colocalized with orexin (data not shown). In the DRN (Fig. 3C), treatment with OXR1 antagonist significantly inhibited the Fos-ir resulting a near 50% reduction in the number of Fos-ir nuclei (t-test, t10=−2.9, p<0.05).
Fig. 2.
Orexinergic innervations in the DRN. Orexin and 5-HT staining in the rostral and caudal portion of the DRN in adjacent sections. Aq, aqueduct; mlf: medial longitudinal fasciculus. Scale bar, 250μm.
Fig. 3.
Effects of orexinergic signaling on Fos expression in the SCN (A), PF-LHA (B) and DRN (C). (A), number of Fos-ir nuclei in the SCN. (B), number of Fos-ir nuclei and the percent of orexin cells double-labeled with Fos in the PF-LHA. (C), number of Fos-ir nuclei in the DRN and representative images showing Fos/5-HT double-label in the lateral DRN at rostral-caudal level 3. Animals were treated with vehicle or OXR1 antagonist SB-334867 before exposed to the LP (120min, 300lux). Results are displayed as mean ± SEM. * indicated p<0.05. mlf: medial longitudinal fasciculus. Scale bar, 100μm.
Discussion
The results from the present study show that in diurnal grass rats, light exposure during subjective daytime increases neural activity in the PF-LHA and in the DRN as measured by Fos-ir. In the PF-LHA, increased Fos-ir was co-localized with orexinergic cells. Furthermore, blocking the orexinergic signaling using an OXR1 antagonist inhibits the light-induced neural activity in the DRN. The results suggest that in a diurnal brain, light induces excitatory responses in the 5-HTergic DRN through activating orexinergic pathways.
Light information is received by the retina and conveyed to hypothalamic, thalamic, and brain stem nuclei through non-image forming pathways (Youngstrom et al., 1991, Hattar et al., 2006). Direct and indirect retinal inputs to the SCN have been well established in all rodent species studied including the grass rats (Smale and Boverhof, 1999). In contrast to the profound Fos induction at night seen in both nocturnal and diurnal rodents (Nelson and Takahashi, 1991, Mahoney et al., 2001), no significant changes in Fos expression was observed in the SCN following light exposure at ZT3 in grass rats (Fig. 1A). However, a lack of acute Fos induction in the SCN during the subjective day does not mean that the SCN function is not influenced by the ambient lighting conditions. For example, in lab rats, two days of constant darkness significantly reduces the amplitude of the clock gene Per2 rhythm in the SCN, indicating an effect of daytime light exposure on the clock functioning within the SCN (Yan et al., 1999). Also, as the responsiveness of the SCN to light is phase dependent, light exposures at times during the subjective day other than ZT3 might be more effective in activating the SCN neurons (Mahoney et al., 2001). Thus, the lack of an acute response to light in the SCN should be interpreted cautiously, without dismissing the influence of the circadian clock on arousal and monoaminergic systems.
A significant increase of Fos expression was observed in the PF-LHA and within orexin neurons after the LP at ZT3 (Fig. 1B, C). Orexin is a neuropeptide that has been implicated in the regulation of the sleep/wake cycle, as revealed by the animals with deficiencies in genes encoding orexin or its receptors (reviewed in Sakurai, 2007). Retinal inputs can be seen in the lateral hypothalamus where orexin neurons are located (Hattar et al., 2006). Furthermore, the orexin neurons receive indirect retinal inputs from other hypothalamic nuclei including the SCN, which can potentially mediate the effects of light on orexin neurons (Abrahamson et al., 2001, Deurveilher and Semba, 2005, Sakurai et al., 2005). In mice, dark pulses (arousal cues for nocturnal species), activate orexin neurons (Marston et al., 2008). In contrast, the orexin neurons of diurnal grass rats appear to be activated by light (present study). The present study found that 10–15% of the orexin cells were Fos-positive prior to (0min) and at 30 or 60 min post LP (Fig. 1B). However, at 120 min following the LP (ZT5), about 30% of the orexin neurons were found Fos-positive. This value is much higher than the baseline level in the present study as well as the daytime levels reported in a previous study on the neural activity of orexin neurons in grass rats (Martinez et al., 2002). It has been shown that the Fos-ir is high throughout the day, with 10–15% Fos-positive orexin neurons from ZT23 to 11, and low at night with less than 5% Fos-positive orexin cells at ZT17 and 20 (Martinez et al., 2002). Therefore, the elevated Fos-ir in orexin cells at 120 min (30%) is unlikely due to an endogenous rise at this time, but is rather caused by the LP.
Light-induced Fos expression was also observed in the DRN (Fig. 1E). The DRN is heavily innervated by orexin neurons in nocturnal lab rats (Peyron et al., 1998) and in diurnal grass rats (Nixon and Smale, 2007; present study). Although direct retinal projections in DRN have been reported in a few rodent species including Mongolian gerbils, Chilean degus and lab rats (Shen and Semba, 1994, Kawano et al., 1996, Fite et al., 1999, Fite and Janusonis, 2001), we have not observed any retinal innervations in the DRN of the grass rats (Shuboni et al, unpublished results). This is consistent with the results showing no projection from melanopsin-containing retinal ganglion cells in DRN in mice (Hattar et al., 2006). These results collectively suggest that the SCN and orexin neurons could be part of the route through which light information is transmitted to the DRN (Fig. 2). The involvement of orexinergic signaling in light-induced neural activity in the DRN was further supported by our results from animals treated with the orexin receptor antagonist SB-334867 (Fig. 3). This compound is a selective OXR1 antagonist, which has been widely used in rodent species (Rodgers et al., 2001, Ishii et al., 2004, Scott et al., 2011). Injections of SB-334867 in mice have been shown to block the antidepressant effects of orexin (Ito et al., 2008, Ito et al., 2009). Applying SB-334867 to brain slices from lab rats inhibits the orexin-receptor mediated excitatory effects in the DRN (Soffin et al., 2004). Our results show that SB-334867 inhibits light-induced increases of neural activity in the DRN, thus supporting the involvement of orexinergic signaling in the mediation of this effect of light. SB-334867 treated animals showed a near 50% reduction of Fos in the DRN, which was still higher than the baseline level prior to light exposure. This partial reduction could be due to the relatively low dose of the antagonist used here or may reflect the involvement of other subtypes of orexin receptors (i.e. OXR2) or mechanisms independent from orexinergic signaling. It is noteworthy that although not statistically significant, a trend of reduction of Fos-ir in SB-334867 treated animals was also observed in the PF-LHA (Fig. 3B). OXR1 has been found in the LHA and co-localized with neurons expressing melanin-concentrating hormone or orexin (Hervieu et al., 2001, Backberg et al., 2002). Injecting orexin into the LHA results in a local induction of Fos-ir (Mullett et al., 2000). These results collectively suggest that the OXR1 antagoinst inhibited the Fos-ir in the PF-LHA through the local circuitry (Burt et al., 2011). Although the OXR1 is also expressed in the SCN (Hervieu et al., 2001, Backberg et al., 2002), no significant changes in Fos-ir were found in the SCN of SB-334867 treated animals (Fig. 3A), suggesting that the effect of the drug was rather selective.
The results from the present study revealed that in diurnal grass rats, daytime light exposure activates orexin neurons, supporting the hypothesis that orexinergic signaling mediates the light-induced neuronal activation in the DRN. Constrained by the availability of animals from the breeding colony, females were used to characterize the time course of Fos induction following the LP (Fig. 1), while males were used to determine the effects of the orexinergic pathway (Fig. 3). Sex differences could be a potential confounding factor for interpreting the results, since circadian and arousal systems are influenced by sex and gonadal hormones (Mong et al., 2011). However, the effects of light observed in the present study were very robust and unlikely to be affected by sex difference. It should also be acknowledged that the orexin neurons are widely distributed in the tuberal hypothalamus (Marchant et al., 2012). Functional distinction between orexin neurons in different sub regions has been reported in nocturnal rodents; e.g. cells in the lateral hypothalamus are related to reward, while those in the perifornical area and dorsomedial hypothalamus are related to stress and arousal (Harris and Aston-Jones, 2006). To get an overall picture on how the orexin neurons respond to LP in grass rats, the present study focused on the PF-LHA, which contains the highest proportion of orexin cells in this species (Martinez et al., 2002). Future work will aim to elucidate the region-specific role of orexin neurons in mediating the effects of light on mood regulating brain regions i.e. the DRN. In the DRN, few, if any, Fos-ir nuclei were found to be co-localized with 5-HT neurons. The lack of activation in this group of neurons appears to contradict the results that in humans, serotonin production directly correlates with prevailing bright sunlight exposure (Lambert et al., 2002). Sunlight is hundreds of fold brighter than the fluorescent light used in the present study. Therefore, the low intensity of light exposure used here may have limited the orexinergic responses necessary for activation of 5-HTergic neurons. It is also possible that the 5-HT neurons are activated independently from Fos expression, which would require other types of markers to detect.
Current theories and the clinical practice on SAD and light therapy focus on the effects of light on entraining circadian rhythms (Terman and Terman, 2005, Lewy et al., 2007, Lewy, 2009). In addition to entraining the circadian system, light can also influence behavior and physiology directly by acting on neural pathways regulating sleep/wakefulness (Dijk and Archer, 2009). In the present study, we found that light activates the orexinergic systems in the diurnal grass rats. The results support the idea that the activation of the wakefulness promoting orexinergic system also underlies the therapeutically effects of light on SAD. Although the present study focused on the DRN, orexin neurons project to and directly regulate all monoaminergic neurons that are involved in mood regulation (Tsujino and Sakurai, 2009). Therefore, the orexinergic pathway is well positioned to mediate the effects of light on monoaminergic system that ultimately contribute to light-dependent mood fluctuations seen in the grass rats and in SAD patients. A better understanding of how light affects brain function, particularly in diurnal species, will help promote awareness of “light hygiene” and lead to more effective treatment protocols for SAD.
Highlights.
Daytime light exposure activated orexin neurons in diurnal grass rats.
Orexingergic signaling mediates light-induced neural activity in the DRN.
The orexinergic pathway may be responsible for light-dependent mood changes.
Acknowledgments
WA performed the experiment, analyzed the data and wrote the manuscript. GL performed the experiment and analyzed the data. LY designed the experiment and wrote the manuscript. All authors have approved the final draft of the manuscript. We thank Drs Antonio A. Nunez and Laura Smale for helpful comments on the manuscript. We also thank Jennifer Kott for technical assistant. GL is supported by the Provost Undergraduate Research Initiative Awards from College of Social Science of MSU. This work is supported by NSF grant (IOS1051919) and NIH grant (R03MH093760).
A comprehensive list of abbreviations
- 5-HT
serotonin
- DAB
diaminobenzidine
- DD
constant darkness
- DRN
dorsal raphe nucleus
- ICC
immunocytochemistry
- LD
light/dark cycle
- LP
light pulse
- OXR
orexin receptor
- OXR1
orexin receptor type 1
- PF-LHA
perifornical-lateral hypothalamic area
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
- SSRI
selective serotonin reuptake inhibitors
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods. 2010;186:165–170. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- Burt J, Alberto CO, Parsons MP, Hirasawa M. Local network regulation of orexin neurons in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2011;301:R572–580. doi: 10.1152/ajpregu.00674.2010. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Dawson D. Enhancement of nighttime alertness and performance with bright ambient light. Physiol Behav. 1990;48:317–320. doi: 10.1016/0031-9384(90)90320-4. [DOI] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Nixon JP, Smale L, Nunez AA. Neural activation in arousal and reward areas of the brain in day-active and night-active grass rats. Neuroscience. 2010;165:337–349. doi: 10.1016/j.neuroscience.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cuesta M, Mendoza J, Clesse D, Pevet P, Challet E. Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Exp Neurol. 2008;210:501–513. doi: 10.1016/j.expneurol.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7:e1000145. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite KV, Janusonis S. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus) Brain Res. 2001;895:139–145. doi: 10.1016/s0006-8993(01)02061-3. [DOI] [PubMed] [Google Scholar]
- Fite KV, Janusonis S, Foote W, Bengston L. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J Comp Neurol. 1999;414:469–484. doi: 10.1002/(sici)1096-9861(19991129)414:4<469::aid-cne4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Rodgers RJ. Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, Hanawa T. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–732. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Nagai T, Oikawa T, Yamada H, Hanawa T. A possible mechanism underlying an antidepressive-like effect of Kososan, a Kampo medicine, via the hypothalamic orexinergic system in the stress-induced depression-like model mice. Biol Pharm Bull. 2009;32:1716–1722. doi: 10.1248/bpb.32.1716. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Fite KV. Diurnal variation of c-Fos expression in subdivisions of the dorsal raphe nucleus of the Mongolian gerbil (Meriones unguiculatus) J Comp Neurol. 2001;440:31–42. doi: 10.1002/cne.1368. [DOI] [PubMed] [Google Scholar]
- Kawano H, Decker K, Reuss S. Is there a direct retina-raphe-suprachiasmatic nucleus pathway in the rat? Neurosci Lett. 1996;212:143–146. doi: 10.1016/0304-3940(96)12795-6. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci. 2007;9:315–324. doi: 10.31887/DCNS.2007.9.3/rlevitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep. 2009;11:459–465. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS. The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci. 2007;9:291–300. doi: 10.31887/DCNS.2007.9.3/alewy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M, Bult A, Smale L. Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. J Biol Rhythms. 2001;16:149–162. doi: 10.1177/074873001129001854. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, McNally GP. The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci. 2012;69:581–597. doi: 10.1007/s00018-011-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N, Piggins HD. Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol Brain. 2008;1:19. doi: 10.1186/1756-6606-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–96. doi: 10.1016/s0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett MA, Billington CJ, Levine AS, Kotz CM. Hypocretin I in the lateral hypothalamus activates key feeding-regulatory brain sites. Neuroreport. 2000;11:103–108. doi: 10.1097/00001756-200001170-00021. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster. J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: classical strategies and new directions. Ann Med. 2010;42:252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- Poncet L, Denoroy L, Jouvet M. Daily variations in in vivo tryptophan hydroxylation and in the contents of serotonin and 5-hydroxyindoleacetic acid in discrete brain areas of the rat. J Neural Transm Gen Sect. 1993;92:137–150. doi: 10.1007/BF01244873. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Martinez GS, Schwartz MD, Smale L. Temporal and spatial distribution of immunoreactive PER1 and PER2 proteins in the suprachiasmatic nucleus and peri-suprachiasmatic region of the diurnal grass rat (Arvicanthis niloticus) Brain Res. 2006;1073–1074:348–358. doi: 10.1016/j.brainres.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Redlin U. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int. 2001;18:737–758. doi: 10.1081/cbi-100107511. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Kasper S, Hawellek B, Martinez B, Hoflich G, Nickelsen T, Moller HJ. Effects of fluoxetine versus bright light in the treatment of seasonal affective disorder. Psychol Med. 1998;28:923–933. doi: 10.1017/s0033291798006813. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222:289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Semba K. A direct retinal projection to the dorsal raphe nucleus in the rat. Brain Res. 1994;635:159–168. doi: 10.1016/0006-8993(94)91435-4. [DOI] [PubMed] [Google Scholar]
- Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. J Comp Neurol. 1999;403:190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Soffin EM, Gill CH, Brough SJ, Jerman JC, Davies CH. Pharmacological characterisation of the orexin receptor subtype mediating postsynaptic excitation in the rat dorsal raphe nucleus. Neuropharmacology. 2004;46:1168–1176. doi: 10.1016/j.neuropharm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity are eliminated by suprachiasmatic lesions. Proc Natl Acad Sci USA. 1972;54:1521–1527. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11:497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–663. doi: 10.1017/s1092852900019611. quiz 672. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Wiklund L, Leger L, Persson M. Monoamine cell distribution in the cat brain stem. A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol. 1981;203:613–647. doi: 10.1002/cne.902030405. [DOI] [PubMed] [Google Scholar]
- Workman JL, Nelson RJ. Potential animal models of seasonal affective disorder. Neurosci Biobehav Rev. 2011;35:669–679. doi: 10.1016/j.neubiorev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Yan L. Structural and functional changes in the suprachiasmatic nucleus following chronic circadian rhythm perturbation. Neuroscience. 2011;183:99–107. doi: 10.1016/j.neuroscience.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Day-length encoding through tonic photic effects in the retinorecipient SCN region. Eur J Neurosci. 2008;28:2108–2115. doi: 10.1111/j.1460-9568.2008.06493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Youngstrom TG, Weiss ML, Nunez AA. Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain Res Bull. 1991;26:403–411. doi: 10.1016/0361-9230(91)90014-b. [DOI] [PubMed] [Google Scholar]