Abstract
Congenital muscular dystrophies (CMDs) with associated brain abnormalities are a group of disorders characterized by muscular dystrophy and brain and eye abnormalities that are frequently caused by mutations in known or putative glycotransferases involved in protein O-mannosyl glycosylation. Previous work identified α-dystroglycan as the major substrate for O-mannosylation and its altered glycosylation the major cause of these disorders. However, work from several labs indicated that other proteins in the brain are also O-mannosylated and therefore could contribute to CMD pathology in patients with mutations in the protein O-mannosylation pathway, however few of these proteins have been identified and fully characterized in CMDs. In this study we identify receptor protein tyrosine phosphatase ζ (RPTPζ) and its secreted variant, phosphacan, as another potentially important substrate for protein O-mannosylation in the brain. Using a mouse model of muscle-eye-brain disease lacking functional protein O-mannose β-1,2-N-acetylglucosaminyltransferase (POMGnT1), we show that RPTPζ/phosphacan is shifted to a lower molecular weight and distinct carbohydrate epitopes normally detected on the protein are either absent or substantially reduced, including HNK-1 reactivity. The spatial and temporal expression pattern of these O-mannosylated forms of RPTPζ/phosphacan and its hypoglycosylation and loss of HNK-1 glycan epitopes in POMGnT1 knockouts are suggestive of a role in the neural phenotypes observed in patients and animal models of CMDs.
Keywords: Receptor Protein Tyrosine Phosphatase β/ζ (RPTP β/ζ); Phosphacan; Protein O-mannose β-1,2-N-acetylglucosaminyltransferase 1 (POMGnT1); muscle-eye-brain disease (MEB); protein glycosylation; Human Natural Killer 1 (HNK-1)
1.1
Congenital muscular dystrophies (CMDs) with associated brain abnormalities are a group of devastating autosomal recessive disorders characterized by congenital muscular dystrophy, type II lissencephaly and eye anomalies (Dobyns et al., 1985, Haltia et al., 1997, Jimenez-Mallebrera et al., 2005, Reed, 2009). Abnormalities in protein O-mannosyl glycosylation, often arising from mutations in genes encoding functional or putative glycotransferase involved in O-mannosyl glycosylation, are causal to this group of disorders (Brockington et al., 2001a, Brockington et al., 2001b, Beltran-Valero de Bernabe et al., 2002, Schessl et al., 2006). Exemplary to this is muscle-eye-brain disease (MEB) which arises from mutations in the gene encoding the known glycotransferase protein O-mannose β-1,2-N-acetylglucosaminyltransferase 1 (POMGnT1) (Yoshida et al., 2001, Liu et al., 2006). POMGnT1 catalyzes the transfer of an N-acetylglucosamine saccharide on to O-mannose present on serine and threonine residues of glycoproteins (Yoshida et al., 2001).
The most well characterized protein substrate of O-mannosyl glycosylation is α-dystroglycan (α-DG), which forms a heterodimer with β-dystroglycan to function as a transmembrane cell-surface receptor for constituents of the extracellular matrix (ECM) present in the basal lamina of the developing and mature brain (Ervasti and Campbell, 1991, Ibraghimov-Beskrovnaya et al., 1992, Ervasti and Campbell, 1993, Montanaro et al., 1999). Proper protein O-mannosyl glycosylation of α-DG is essential to maintain its ligand binding properties and the subsequent integrity of the basement membrane (Kano et al., 2002, Michele et al., 2002, Kim et al., 2004). Disruption of pial basement membrane integrity consequently leads to overmigration of cortical neurons and type II lissencephaly along with other CNS pathologies (Michele et al., 2002). A number of studies have demonstrated that altered glycosylation of α-DG accounts for most of the obvious neural abnormalities (Moore et al., 2002, Satz et al., 2010), however a number of pieces of evidence indicate that additional protein substrates for protein O-mannosyl glycosylation exist and may also contribute to the phenotypes observed in these disorders. Glycomic analysis revealed brains of animals lacking α-DG have similar amounts of proteins with O-mannose initiated structures as wild-type animals (Stalnaker et al., 2011). Work also showed that up to 30% of all O-linked sugars in the brain are O-linked via mannose (Finne et al., 1979, Krusius et al., 1986, Chai et al., 1999, Kogelberg et al., 2001), a number that could not be accounted for by α-DG alone. Furthermore, O-mannosyl linked carbohydrates have also been described on particular cell-adhesion molecules (Bleckmann et al., 2009). Therefore identifying and characterizing novel protein substrates with altered glycosylation in these CMDs may provide important insights into the molecular basis of their complex phenotypes. Perhaps most importantly, future avenues of therapeutic intervention may be revealed in the yet-unidentified molecular underpinnings of these disorders.
Our previous work demonstrated that the monoclonal antibody, Cat-315, likely detects an HNK-1 epitope present on receptor protein tyrosine phosphatase ζ (RPTPζ), also referred to as RPTPβ, in the developing brain with biochemical properties that correlated with O-linked mannose structures (Matthews et al., 2002, Dino et al., 2006) and in a neuroblastoma cell line (Abbott et al., 2008). RPTPζ along with its secreted variant, phosphacan (Maurel et al., 1994), are primarily expressed in the CNS (Shitara et al., 1994, Maeda et al., 1995) and have been implicated in several key developmental neural processes including proliferation (Ida et al., 2006, Soh et al., 2007), differentiation (Canoll et al., 1996, Ranjan and Hudson, 1996, Meng et al., 2000, Soh et al., 2007) cell-adhesion and migration (Abbott et al., 2008), axonal guidance and neurite outgrowth (Grumet et al., 1996, Sakurai et al., 1997, Hayashi et al., 2005), myelination (Harroch et al., 2000, Harroch et al., 2002) and higher order cognitive function (Niisato et al., 2005). The extracellular domains of RPTPζ/phosphacan bind a wide array of ligands important for normal CNS development (for review see (Peles et al., 1998)) including pleiotrophin (Maeda et al., 1996, Meng et al., 2000), midkine (Maeda et al., 1999), tenascin (Grumet et al., 1994, Milev et al., 1995), NCAM, Ng-CAM (Maurel et al., 1994, Milev et al., 1994) and contactin (Sakurai et al., 1997). Previous work has demonstrated that carbohydrate modifications on RPTPζ modulate receptor-ligand binding which can in-turn influence downstream signaling and cellular behaviors (Milev et al., 1995, Peles et al., 1998, Maeda et al., 2003, Abbott et al., 2008). Therefore, altered O-mannosylation of RPTPζ/phosphacan could potentially have deleterious effects on neural development.
The work presented herein demonstrates that RPTPζ/phosphacan is hypoglycosylated in POMGnT1 knockout mice, an animal model of MEB. These data suggest that RPTPζ/phosphacan is a novel and major protein substrate for protein O-mannosyl glycosylation by POMGnT1 in vivo. Furthermore we demonstrate that in the developing brain RPTPζ/phosphacan bears the majority of HNK-1 reactivity in the soluble fraction, and most of these glycans on RPTPζ/phosphacan are O-linked via mannose. Given the CNS-enriched expression and interactions of RPTPζ/phosphacan and the known importance of the HNK-1 carbohydrate in development, our data argue for an important role of altered O-mannosyl glycosylation on RPTPζ/phosphacan and a potential contribution to the complex neurological phenotypes associated with CMDs.
1.2 EXPERIMENTAL PROCEDURES
1.2.1, Animals
Protocols for animal usage were approved by the Institutional Animal Care and Use Committee of Upstate Medical University. POMGnT1 knockout mice were generated by Lexicon Genetics Incorporated (The Woodlands, TX) (Liu et al., 2006). Large myodystrophy (Largemyd) mutant mice were acquired from Jackson Laboratories (Bar Harbor, ME). For timed pregnant breeding, the morning of plug discovery was designated E0. POMGnT1 and Largemyd genotyping was carried out as previously published (Browning et al., 2005, Liu et al., 2006). No differences were observed between wildtype and heterozygous animas, therefore both were used as littermate controls for knockout animals.
1.2.2, Antibodies
Cat-315 has been previously characterized (Lander et al., 1998, Dino et al., 2006). Mouse monoclonal anti-rat phosphacan (clone: 6B4) was purchased from Associates of Cape Cod, Inc. (East Falmouth, MA, USA). Rat monoclonal anti-DSD-1 (clone: 473HD) was purchased from both Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Chemicon (Billerica, MA, USA). Mouse monoclonal anti-phosphacan 5210 (Clone #122.2) and rabbit polyclonal anti-aggrecan were purchased from Chemicon. Rabbit polyclonal anti-PTPζ (H300) was purchased from Santa Cruz Biotechnology. Anti-brevican and anti-HNK-1 were purchased from BD-Biosciences, Pharmingen (San Diego, CA, USA). The 3F8 antibody developed by Dr. Margolis was obtained from the Developmental Studies Hybridoma Bank (developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA, USA). Table 1 summarizes the antibodies used in this study, their previously predicted epitopes and revisions to these epitopes identified through the experiments presented in this study.
Table 1. Description of antibodies.
Previously known antibody epitope specificity and modifications to these epitope specificities as suggested by the results presented herein.
| Antibody | Type | Previously known epitope specificity | Reference(s) | Epitope specificity based on results presented herein. |
|---|---|---|---|---|
| Cat-315 | Mouse Monoclonal IgM | Detects epitopes dependent on O-linked glycans attached to RPTPζ/phosphacan early in development and aggrecan in the adult brain, predicted to be O-mannosyl linked and HNK-1 reactive. | Matthews et al., 2002; Dino et al., 2006; Abbott et al., 2008 | Epitopes detected on RPTPζ/phosphacan require glycans that are likely O-mannosyl- linked. Epitopes detected on aggrecan are in PNs and are dependent on glycans likely not O-mannosyl linked. Likely HNK-1 reactive. |
| 6B4 | Mouse Monoclonal IgM | Detects carbohydrate-dependent epitopes on RPTPζ/phosphacan early in development and on aggrecan in the adult brain. | Maeda et al., 1994, 1995, Saitoh et al., 2008 | Epitopes detected on RPTPζ/phosphacan are likely dependent on O-mannosyl linked carbohydrates. While those detected on aggrecan are likely not O-mannosyl linked. |
| 3F8 | Mouse Monoclonal IgG | Detects carbohydrate epitopes on RPTPζ/phosphacan predicted to be on N-linked glycans. | Maurel et al., 1994; Garwood et al., 2003 | Epitopes detected on RPTPζ/phosphacan are likely dependent on O-mannosyl linked carbohydrates. |
| 473HD | Rat Monoclonal IgM | Sulfated CS/DS GAG motif on the long form of RPTPζ and phosphacan | Faissner et al., 1994; Ito et al., 2005 | As described. |
| 5210 (Clone #122.2) | Mouse Monoclonal IgM | Predicted to detect the protein core of RPTPζ/phosphacan and detect all isoforms of the protein. | Personal communication, Dr. Joel Levine | Epitopes detected on RPTPζ/phosphacan are dependent on glycans likely O- mannosyl and/or N-linked. |
| H300 | Rabbit Polyclonal IgG | Peptide antibody that detects the protein core of RPTPζ/phosphacan. | Manufacture’s specifications | As described. |
1.2.3, Preparation of homogenates, soluble fraction
Tissue homogenates with respective soluble and particulate fractions were prepared as previously described by Viapiano and colleagues (Viapiano et al., 2003). Briefly, the corticies from postnatal day (P) 4 and whole brains from adult POMGnT1 knockout and wildtype mice and whole brains from P1 Largemyd were homogenized in 10 volumes of 25mM Tris-HCl (pH=7.4) containing 0.32M sucrose and a protease inhibitor cocktail (EDTA-free Complete, Roche, Indianapolis, IN, USA). The homogenates were centrifuged at 950 × g for 10 min at 4°C, the resulting postnuclear supernatant was centrifuged again at either 20,000 or 100,000 × g for 60 min to obtain total soluble and particulate fractions.
1.2.4, SDS-PAGE and Western blotting
Equal amounts of protein were electrophoresed on 5% or 7% SDS-polyacrylamide gels and transferred to nitrocellulose for blotting. Western blots were completed as described previously (Viapiano et al., 2003). In all cases brevican served as the loading control and HRP-conjugated secondary antibodies were employed and detected with supersignal west pico or femto chemilumensent substrate (Thermo Scientific, Rockford, IL, USA) and Kodak biomax MR film (Sigma-Aldrich, Saint Louis, MO, USA). Western blot quantification was carried out to determine relative intensity of resulting bands normalized to internal loading controls using ImageJ software.
1.2.5, Protein Deglycosylation
All samples analyzed by Western blotting were pretreated with chondroitinase ABC (chABC) to remove chondroitin sulfate glycosaminoglycan (CS-GAG). Protein samples were diluted to 1–2mg/mL in chABC buffer (40mM Tris-HCl, 40mM sodium acetate, protease inhibitor tablet, mini, EDTA free, pH=8.0) and treated with 0.2U/mL of chABC, protease free from Proteus vulgaris (Associates of Cape Cod, Inc.) and incubated for 8 hours at 37°C. For experiments in which CS-GAG, mucin-type O-linked and N-linked glycans were removed by enzymatic deglycosylation, 1.8mg/mL protein was incubated for 8 hours at 37°C in deglycosylation buffer (20mM Tris-HCl, 20mM sodium acetate, 25mM NaCl, pH= 7.0) with 0.2U/mL chABC, 0.2U/mL sialidase (Roche), 2,400,000U/mL O-Glycosidase (New England Biolabs, Ipswich, MA, USA), 20,000 U/mL PNGase F (New England Biolabs). For immunoprecipitation, samples were diluted to 1.5mg/mL in 40mM sodium phosphate buffer, pH 7.5, 25 mM NaCl, protease inhibitor tablet, mini, EDTA-free (Roche). Deglycosylation was performed by incubating samples for 2 hours at 37°C with 150U/mL bovine testicular hyaluronidase (which also removes CS-GAG chains) (Sigma Aldrich), 0.1U/mL sialidase (Roche), 2,400,000U/mL O-Glycosidase (New England Biolabs), 17,500 U/mL PNGase F (New England Biolabs) for 2 hours at 37°C.
1.2.6, Immunoprecipitation
Soluble samples from adult POMGnT1 wildtype, heterozygous and knockout animals were prepared as specified above. Ten volumes of Cat-315 hybridoma media was incubated with rat anti- mouse IgM sepharose beads (Invitrogen, Carlsbad, CA, USA) at 4°C overnight. For immunoprecipitation, samples were diluted to 1mg/mL in 25mM Tris, pH 8.0 with protease inhibitor tablet, mini, EDTA free (Roche). 500ug total protein was used for each IP reaction, protein samples and antibody/bead mixture were incubated for 5 hours, rotating at 4°C. Beads were washed several times in 25mM Tris, pH 8.0 and boiled under reducing conditions in 2X sample buffer. Starting material and immunoprecipitated material were electrophoresed on 5% SDS-polyacrylamide gels and processed for immunoblotting as described above.
1.2.7, Immunohistochemistry
Embryonic day (E) 15 and E18 embryonic brains were drop fixed in ice-cold 4% phosphate-buffered paraformaldehyde. P4 pups and adults were transcardially perfused with PBS prior to perfusion with 4% phosphate-buffered paraformaldehyde. In all cases, tissue was post-fixed overnight and cryoprotected by sinking in 30% phosphate-buffered sucrose with 0.2% sodium azide and cut on a cryostat. For E15, E18 and P4 brains, 14μM cryosections were mounted directly onto superfrost plus glass slides (Thermo Fisher Scientific, Waltham, MA). For adult animals, 40μM cryosections were collected as free floating sections in phosphate buffer with 0.2% sodium azide.
For immunostaining, sections were blocked with screening medium (DMEM, 5% FBS, 0.2% sodium azide with 1% Triton X-100) for 45 minutes at room temperature and then incubated in primary antibody overnight at 4°C. The following day, sections were washed and incubated with Alexa fluorescent-conjugated secondary antibodies (Molecular Probes, Invitrogen, Carlsbad, CA). In all cases, primary and secondary antibodies were diluted in screening media. Nuclei were stained with Hoechst 33342 (Sigma-Aldrich) diluted in 0.1M phosphate buffer with 1% Triton X-100.
1.2.8, Microscopy
Confocal images were collected on a Zeiss LSM 510 Laser Scanning Confocal Microscope (SUNY Upstate, Syracuse, NY 13210) and converted using Zeiss LSM 5 Image Browser Software. Final images were formatted and compiled into figures using Adobe Photoshop CS5.5.
1.3, RESULTS
1.3.1, The immunoreactivity of monoclonal antibodies Cat-315 and 6B4 are dramatically reduced in the cortex of embryonic and early postnatal POMGnT1 knockout animals
Previous work definitively demonstrated the monoclonal antibody Cat-315 prominently detects an epitope on aggrecan in the adult brain (Matthews et al., 2002), but an epitope on RPTPζ/phosphacan in the immature brain (Dino et al., 2006). Further characterization showed that the Cat-315 antibody detects an O-linked carbohydrate epitope (Matthews et al., 2002, Dino et al., 2006), which we hypothesized was likely O-linked via mannose. To test this hypothesis, Cat-315 reactivity was evaluated in POMGnT1 knockout mice.
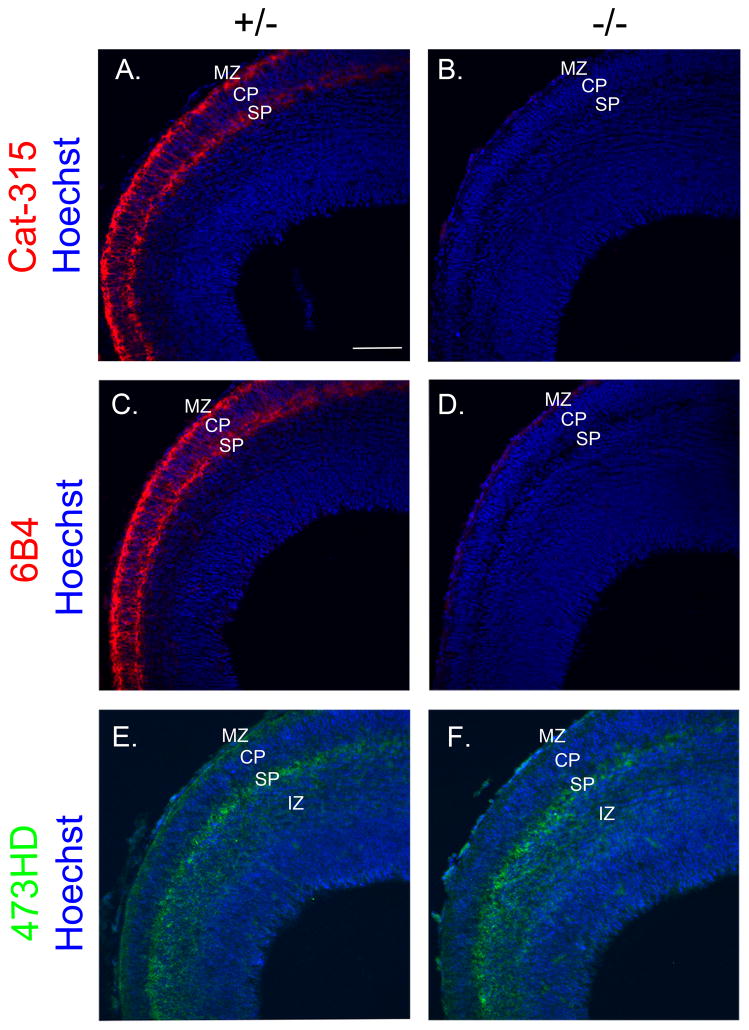
In the cortex of POMGnT1 heterozygous E15 mice, Cat-315 immunoreactivity was found in two intense bands in the marginal zone (MZ) and subplate (SP) but also diffusely in the cortical plate (CP) (Figure 1A), consistent with previous work on the expression of RPTPζ/phosphacan in the developing brain. Interestingly, Cat-315 reactivity was essentially eliminated in the cortex of POMGnT1 knockout mice (Figure 1B). These data support our hypothesis that the Cat-315 reactive epitope detects an O-linked glycan on RPTPζ/phosphacan that is likely an O-mannosyl-linked glycan.
FIGURE 1. Cat-315 and 6B4 staining are nearly eliminated in the cortex of E15 POMGnT1 knockout brains.
Coronal sections from E15 brains were immunostained, counter-stained with Hoechst nuclear stain (blue) and imaged by confocal microscopy. A, POMGnT1 heterozygous (+/−) control section stained for Cat-315 (red) showing prominent staining of the ECM between and surrounding cells in the MZ and SP and more diffuse radially oriented fibrillar staining in the CP. B, in a POMGnT1 knockout (−/−) section Cat-315 reactivity from the developing cortex of these animals is essentially eliminated. C, POMGnT1 +/− section showing 6B4 immunoreactivity throughout the MZ and SP. D, POMGnT1 −/− section showing the near complete elimination of 6B4 reactivity, with the exception of subtle residual staining which spans the medial to lateral aspect of the MZ. E, POMGnT1 +/− section stained for 473HD (green) showing antibody reactivity in the MZ, SP, and IZ of the E15 cortex. F, POMGnT1 −/− section stained for 473HD showing relatively similar spatial localization and intensity of antibody reactivity as its +/− counterpart. Bar, 100μM.
We next asked whether the loss of O-mannosyl glycans on RPTPζ/phosphacan altered its distribution in the brain. To investigate this, tissue was stained with the 6B4 antibody, which specifically detects RPTPζ/phosphacan in the developing brain (Maeda et al., 1994). Similar to Cat-315, 6B4 has also been suggested to detect a carbohydrate epitope (Maeda et al., 1995). Additionally, we stained with the rat monoclonal antibody 473HD, which detects CS-GAG epitopes specific to RPTPζ/phosphacan in the embryonic brain (Faissner et al., 1994, Ito et al., 2005). We predicted that neither of these epitopes would be altered in the POMGnT1 knockout animals. Similar to Cat-315, staining with 6B4 in heterozygous POMGnT1 animals revealed two prominent bands of immunoreactivity in the MZ and SP (Figure 1C). Surprisingly, however, 6B4 reactivity was essentially eliminated in POMGnT1 knockout brains, suggesting it too detects an O-mannosyl glycan on RPTPζ/phosphacan (Figure 1D). 473HD staining in the heterozygous animals intensely stained the MZ and SP and diffusely stained the intermediate zone (IZ) (Figure 1E). Importantly, unlike Cat-315 and 6B4 staining, staining with 473HD appeared essentially unaltered in the cortex of knockout animals, indicating that the distribution of RPTPζ/phosphacan is not dramatically changed despite its altered glycosylation (Figure 1F).
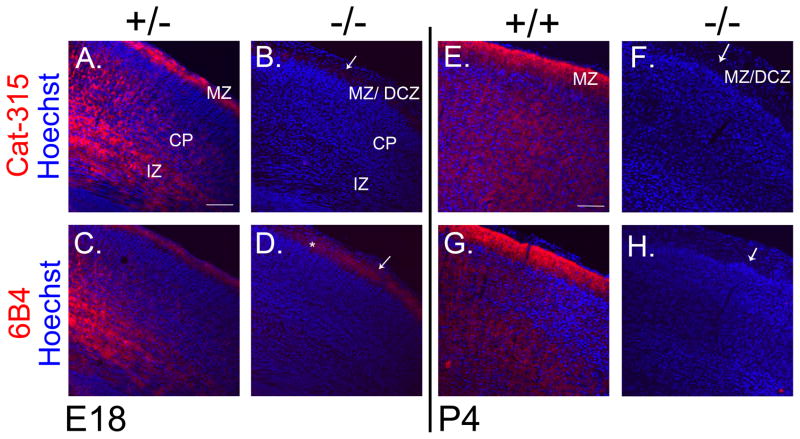
We next determined whether the expression and glycosylation of RPTPζ/phosphacan was affected in late embryonic (E18) and early postnatal (P4) development, which is roughly coincident with the peak of RPTPζ/phosphacan expression (Maeda et al., 1995). At E18, Cat-315 and 6B4 reactivity in the cortex of heterozygous POMGnT1 mice was distributed throughout the MZ, CP and IZ (Figure 2A, C). However, their reactivities were dramatically reduced in the cortex of knockout animals, with the exception of subtle residual staining which remained in the MZ/diffuse cell zone (DCZ) and was most prominent for 6B4 (Figure 2B, D). At P4, the immunoreactivity of Cat-315 and 6B4 were found throughout cortical layers and most highly in the MZ but were essentially absent in the knockout cortex (Figure 2E, F, G, H).
FIGURE 2. Cat-315 and 6B4 staining are dramatically reduced in the cortex of E18 and P4 POMGnT1 knockout brains.
Coronal sections from E18 and P4 brains were immunostained and counter-stained with Hoechst nuclear stain (blue) and imaged with confocal microscopy. A, POMGnT1 +/− section at E18 stained for Cat-315 (red) showing prominent ECM staining in the MZ, CP and IZ. B, POMGnT1 −/− sections shows the near complete elimination of Cat-315 reactivity from the cortex of these animals. C, POMGnT1 +/− at E18 section stained for 6B4 (red) shows prominent staining in the MZ, CP and IZ, which is very similar to the localization of Cat-315 reactivity at this age. D, POMGnT1 −/− sections stained for 6B4 shows the near complete elimination of reactivity from the cortex with the exception of subtle residual staining which remains in the MZ/DCZ (denoted by *). E, wildtype (+/+) section at P4 stained for Cat-315 (red) shows prominent ECM staining most intensely in the MZ and throughout cortical layers. F, POMGnT1 −/− section shows the absence of Cat-315 reactivity from the cortex at P4. G, +/+ section stained for 6B4 (red) shows high levels of reactivity in the MZ and throughout the cortical layers. H, POMGnT1 −/− sections shows the essential elimination of 6B4 reactivity form the cortex of these animals. Arrows in POMGnT1 −/− images point to characteristic abnormalities in cortical lamination formed at the MZ, which results from neuronal overmigration leading to the formation of the DCZ. Bar, 100μM.
1.3.2, RPTPζ/phosphacan is hypoglycosylated in the cortex of POMGnT1 knockout mice
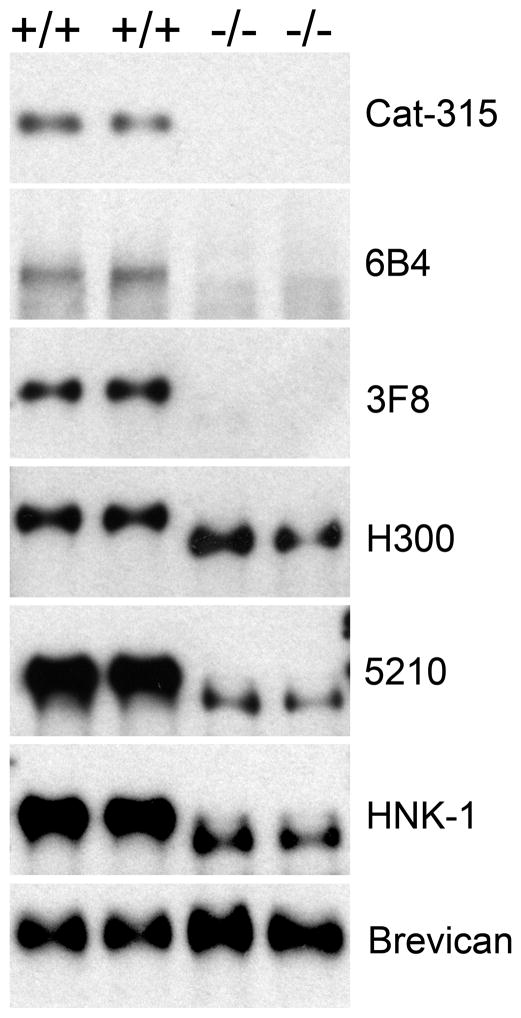
Our immunohistochemical analysis clearly demonstrated the loss of carbohydrate epitopes on RPTPζ/phosphacan. We hypothesized this represented the direct loss of O-mannosyl glycans that were reactive for Cat-315 and 6B4 antibodies. To investigate this possibility in more detail, we turned to Western blot analyses. Consistent with published data, immunoblot analysis of samples from the soluble fraction of cortical homogenate from wildtype P4 brains revealed high molecular weight bands (greater than 250 kD) that were reactive for Cat-315 and 6B4. These bands were absent in POMGnT1 knockout samples (Figure 3), providing further evidence that the carbohydrate epitopes recognized by Cat-315 and 6B4 are likely O-mannose linked.
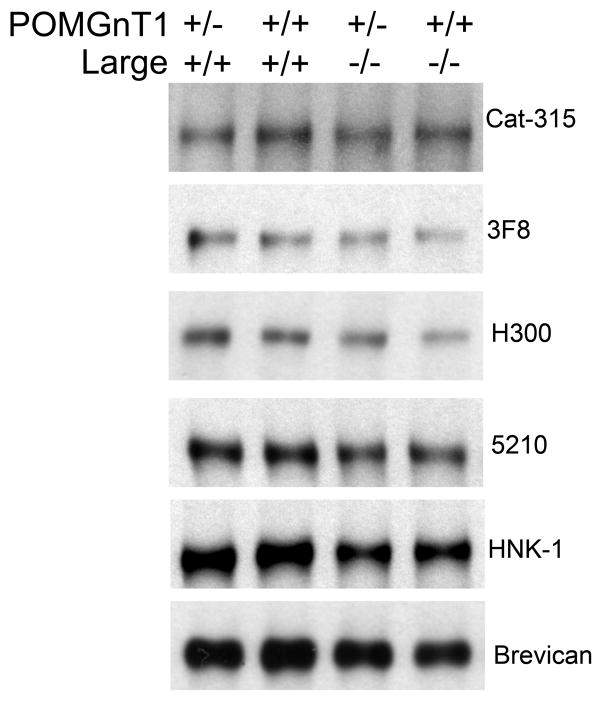
FIGURE 3. RPTPζ/phosphacan is abnormally glycosylated and has reduced reactivity for HNK-1 glycans in the cortex of P4 POMGnT1 knockout animals.
The corticies of P4 POMGnT1 +/+ or −/− animals were homogenized and processed for subcellular fraction, the resulting soluble fraction was subject to chABC treatment and prepared for Western blot analysis. Immunoblot analysis with 5% SDS- polyacrylamide gels showed bands reactive for antibodies Cat-315, 6B4, 3F8, H300, 5210 and HNK-1 in +/+ samples that were greater than 250kD. In POMGnT1 −/− samples, reactivity for Cat-315, 6B4, and 3F8 were essentially eliminated. Additionally, H300 reactive bands were shifted to a lower molecular weight but maintained consistent levels of reactivity relative to +/+ controls. The reactivity of 5210 and HNK-1 antibodies were shifted to a lower molecular weight and were dramatically decreased in intensity in −/− brains relative to +/+ controls. An antibody against the full-length form of brevican was used as the loading control, which ran around 150kD and showed consistent levels of reactivity across +/+ and −/− samples.
While there are a limited number of antibodies directed against RPTPζ/phosphacan that work well for immunohistochemical analysis, there is a greatly expanded array of antibodies that detect RPTPζ/phosphacan on immunoblots. Therefore to further explore the altered glycosylation of RPTPζ/phosphacan, we employed three additional well-established antibodies, 3F8, H300 and 5210. 3F8 is a monoclonal antibody, which has been previously shown to recognize a carbohydrate epitope present on RPTPζ/phosphacan (Maurel et al., 1994). The specificity of the 3F8 antibody for RPTPζ/phosphacan has been clearly demonstrated, as it was utilized to purify phosphacan to homogeneity which led to its original identification and cloning of the gene (Maurel et al., 1994). 3F8 detected a high molecular weight band in wildtype samples. Surprisingly, 3F8 reactivity was also absent from knockout samples (Figure 3). Our interpretation of these data is that 3F8 also detects an O-mannosyl glycan on RPTPζ/phosphacan, however these findings raised the question whether RPTPζ/phosphacan was absent in POMGnT1 knockout mice.
Therefore we next evaluated H300 antibody reactivity, a peptide antibody that detects the protein core of RPTPζ/phosphacan. Again we detected a large molecular weight band in wildtype samples, but unlike other antibodies we also detected a band in samples from knockouts of similar intensity that was shifted downward in molecular weight (Figure 3). These data suggest that RPTPζ/phosphacan in POMGnT1 knockouts is expressed at normal levels but is shifted in molecular weight due to hypoglycosylation. To further confirm these findings we investigated the antibody reactivity of 5210, which is believed to detect all forms of RPTPζ/phosphacan (Dr. Joel Levine, personal communication). 5210 detected bands in both control and knockout samples, and similar to H300, the bands were shifted downward in knockout samples. However, surprisingly there was also a dramatic decrease in the intensity of 5210 reactive bands (Figure 3), suggesting that the epitope detected by 5210 is at least partially glycosylation dependent. Overall we identified 4 antibodies against carbohydrate epitopes on RPTPζ/phosphacan that are absent or dramatically reduced in POMGnT1 knockout mice, likely indicating that they are detecting O-mannosyl glycans.
As noted above, from our previous work we hypothesized that the Cat-315 epitope belongs within the family of HNK-1 (Human Natural Killer-1) glycans, also known as CD34. Therefore we next investigated if HNK-1 reactivity was altered in the POMGnT1 knockouts. As expected, we detected an HNK-1-reactive band at the same molecular weight as RPTPζ/phosphacan in wildtype samples, which was shifted downward and less intense in the POMGnT1 knockout samples (Figure 3), similar to the results with the 5210 antibody. Our findings with both 5210 and HNK-1 immunoblotting led us to the hypothesis that 5210 and HNK-1 terminal carbohydrates on RPTPζ/phosphacan are present on O-mannosyl linked glycans.
1.3.3, HNK-1 carbohydrates on RPTPζ/phosphacan are O-mannose and N-linked in the early postnatal cortex
Previous studies have described HNK-1 terminal carbohydrate structures on both N-linked and O-linked glycans, of which most O-linked HNK-1 bound glycans were found to be O-mannose linked (Yuen et al., 1997). Since residual HNK-1 reactivity on RPTPζ/phosphacan was observed in POMGnT1 knockout samples lacking O-mannosyl linked glycans, we sought to determine how these residual HNK-1 glycans were linked by utilizing enzymatic deglycosylation. Similarly we reasoned that if 5210 was detecting a carbohydrate epitope we should be able to eliminate the residual reactivity through enzymatic deglycosylation.
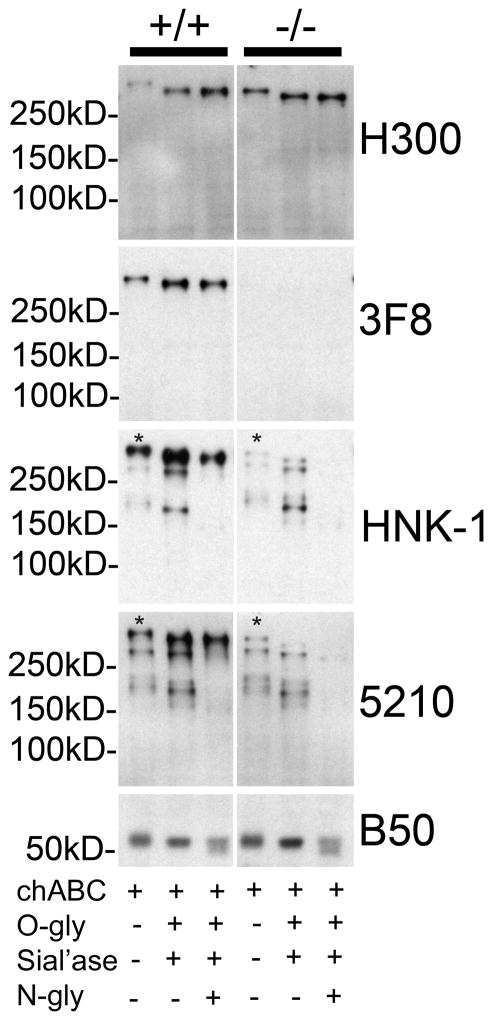
Samples were deglycosylated with chABC alone or in combination with O-glycanse and sialidase (to remove CS-GAG and mucin-type O-linked glycans) or with O-glycanse and sialidase and N-glycanse (to remove CS-GAG, mucin-type O-linked and N-linked sugars). All samples were then subsequently analyzed by Western blot analysis. Immunoblot analysis with H300 showed expected results, wherein enzymatic deglycosylation shifted the apparent molecular weight of RPTPζ/phosphacan downward in both wildtype and knockout samples (Figure 4). Similarly, 3F8 reactivity showed a comparable shift in apparent molecular weight with deglycosylation in wildtype samples, but was absent in knockout samples as expected. Therefore these results show that RPTPζ/phosphacan was effectively deglycosylated by our treatments.
FIGURE 4. Residual HNK-1 glycan reactivity on RPTPζ/phosphacan in the cortex of P4 POMGnT1 knockout animals is N-linked.
Soluble fractions from P4 POMGnT1 +/+ and −/− cortical homogenates were subject to enzymatic protein deglycosylation with chABC and combinations of O-glycosidase (O-gly), sialidase (Sial’ase), and N-glycanase (N-gly) and then subject to Western blot analysis. Electrophoresis on 7% SDS- polyacrylamide gels and immunoblotting of +/+ samples with H300 revealed a reactive band that when treated with chABC alone ran well above 250kD and was the same molecular weight as the 3F8 reactive band in the corresponding sample. Enzymatic deglycosylation with chABC, O-gly, sial’ase resulted in a shift toward a lower molecular weight and an increase in the intensity of H300 reactivity in both +/+ and −/− samples. The same was also observed for 3F8 in +/+ samples, while no 3F8 reactivity was detected in POMGnT1 −/− samples. High molecular weight bands reactive for HNK-1 and 5210 which resolved at the same molecular weight as H300 and 3F8 reactive bands in +/+ samples treated with chABC (denoted with an *), corresponding to phosphacan/RPTPζ, were decreased in intensity in POMGnT1 −/− samples. Enzymatic deglycosylation with a combination of chABC, O-gly, sial’ase, and N-gly resulted in a near complete elimination of these bands (*) in POMGnT1 −/− samples in comparison to chABC, O-gly, sial’ase alone. Additionally, numerous bands of lower molecular weight also reactive for HNK-1 and 5210 were detected in chABC treated wildtype samples and found to be unaltered in POMGnT1 −/− samples. The reactivity of these lower molecular weight bands, which likely detect proteins other than RPTPζ/phosphacan, were essentially eliminated with the treatment of chABC, O-gly, sial’ase, and N-gly relative to chABC, O-gly, sial’ase alone. Subsequently, a single band corresponding to RPTPζ/phosphacan in the +/+ samples remained and essentially all reactive bands in POMGnT1 −/− samples were nearly eliminated, as faint residual bands were detected at longer exposures in these samples (not shown). The B50 antibody against the cleavage fragment of brevican served as the internal control for both total protein concentration and enzymatic deglycosyaltion.
Next we analyzed how deglycosylation altered the reactivity of HNK-1 and 5210 antibodies. In wildtype samples both HNK-1 and 5210 detected a similar pattern of bands perhaps suggesting they detect a similar epitope. Both detected the high molecular weight band representing RPTPζ/phosphacan and two other bands, one just above 250 kD and another between 250 and 150 kD that likely represent reactivity with other proteins. Reactivity for these lower molecular weight bands seemed to be consistent in wildtype and knockout samples and increased in intensity with both antibodies when deglycosylated with O-glycanase and sialidase. However, their reactivities were dramatically reduced with N-glycanase essentially leaving RPTPζ/phosphacan reactive bands. Similar results were noted in knockout samples where N-glycanase nearly eliminated all remaining reactivity (Figure 4), however at significantly longer exposures subtle bands were still detected (data not shown), which could be attributed to our experimental methodology and the absence of detergent from enzymatic reactions. Consistent with previous work (Maeda et al., 1994), our data show that RPTPζ/phosphacan is a major soluble HNK-1 reactive protein in the P4 brain and the majority of HNK-1 reactive glycans that decorate it are seemingly O-linked via mannose. Additionally, the majority of residual reactivity on RPTPζ/phosphacan in knockout samples for both HNK-1 and 5210 represent N-linked glycans. Finally our data suggest that 5210 does not detect RPTPζ/phosphacan core protein but a carbohydrate epitope on RPTPζ/phosphacan that bears notable resemblance to the HNK-1 epitope.
1.3.4, Glycosylation of RPTPζ/phosphacan is not significantly altered in Largemyd mutant mice
In order to further characterize the nature of the glycan modifications on RPTPζ/phosphacan, we turned to Largemyd mutant mice. Large is a putative glycotransferase involved in the extension of O-mannosyl linked glycans, which has also been implicated in CMDs with associated brain abnormalities (Longman et al., 2003). In the brains of Largemyd mutant mice, which lack Large function, α-DG is hypoglycosylated and has substantially reduced immunoreactivity for IIH6C4 and VIA4, antibodies that are believed to recognize undefined O-mannosidically linked carbohydrate epitopes, and laminin binding capabilities (Grewal et al., 2001, Michele et al., 2002). Recent work suggested that Large is involved in catalyzing a phosphoryl glycan branch of alternating xylose and glucuronoic acid residues on O-mannosyl linked glycans (Yoshida-Moriguchi et al., 2010, Inamori, 2012). Therefore we sought to determine if glycans on RPTPζ/phosphacan are modified by Large and if the absence of Large function has similar effects on the reactivity for our array of carbohydrate antibodies against RPTPζ/phosphacan.
Immunoblot analysis on the soluble fraction from P1 Largemyd mutant and wildtype whole brain homogenate was performed. The immunoreactivity of antibodies against carbohydrate epitopes on RPTPζ/phosphacan, that were essentially eliminated in early postnantal POMGnT1 brains, were not detectably altered in Largemyd mutant samples. Specifically, Cat-315, 3F8, 5210, and HNK-1 reactivities and molecular weights were similar in Largemyd mutant samples and respective controls (Figure 5). Moreover, the molecular weight of RPTPζ/phosphacan core protein as detected by H300 was seemingly unchanged (Figure 5). Taken together these findings suggest that Cat-315, 3F8, 5210, and HNK-1 epitopes on RPTPζ/phosphacan are unaffected in the brains of Largemyd mutant mice. The absence of a detectable shift in the molecular weight of RPTPζ/phosphacan core protein as detected by H300 suggests that RPTPζ/phosphacan is not substantially glycosylated by the enzymatic activity of Large.
FIGURE 5. The glycosylation of RPTPζ/phosphacan is seemingly normal in Large myodysotrophy mutant mice.
Whole brains from P1 +/+ and Largemyd mutant mice (−/−) that were either +/+ or +/− for POMGnT1 were homogenized and processed for subcellular fractionation. The resulting soluble fraction was analyzed by Western blot analysis for Cat-315, 3F8, H300, 5210, HNK-1, and brevican. In all cases, large molecular weight bands positive for Cat-315, 3F8, H300, 5210 and HNK-1 resolved well above 250kD. An antibody detecting the full-length form of brevican was used as a loading control and reactive bands were detected around 150kD. Quantification of reactive bands revealed no alteration in their intensities when normalized to brevican for all antibodies with respect to genotype. Additionally, the molecular weight of H300 in Large myodystrophy mutant mice was not unaltered from controls samples.
1.3.5, Cat-315 and 6B4 reactive perineuronal nets are present in the barrel cortex of adult POMGnT1 knockout animals
The Cat-315 antibody was originally discovered due to its ability to specifically detect a substructure of the neural matrix in the adult brain called the perineuronal net (PN), which envelop a subset of neuronal cells and exhibit a characteristic reticular structure with holes at points of synaptic contact (for review see (Celio et al., 1998)). While both Cat-315 and 6B4 have been shown to primarily detect carbohydrate epitopes on RPTPζ/phosphacan in the early developing brain these antibodies largely detect carbohydrate epitopes on aggrecan and PNs in the adult brain (Matthews et al., 2002; McRae et al., 2007; Saitoh et al., 2008). Given that the immunoreactivity of both Cat-315 and 6B4 on RPTPζ/phosphacan were dramatically reduced in the cortex of embryonic and early postnatal POMGnT1 knockout animals, the reactivity of these antibodies were evaluated in the cortex of adult POMGnT1 knockout animals to determine if their carbohydrate epitopes present on aggrecan were also lost in the absence of O-mannosyl linked glycans.
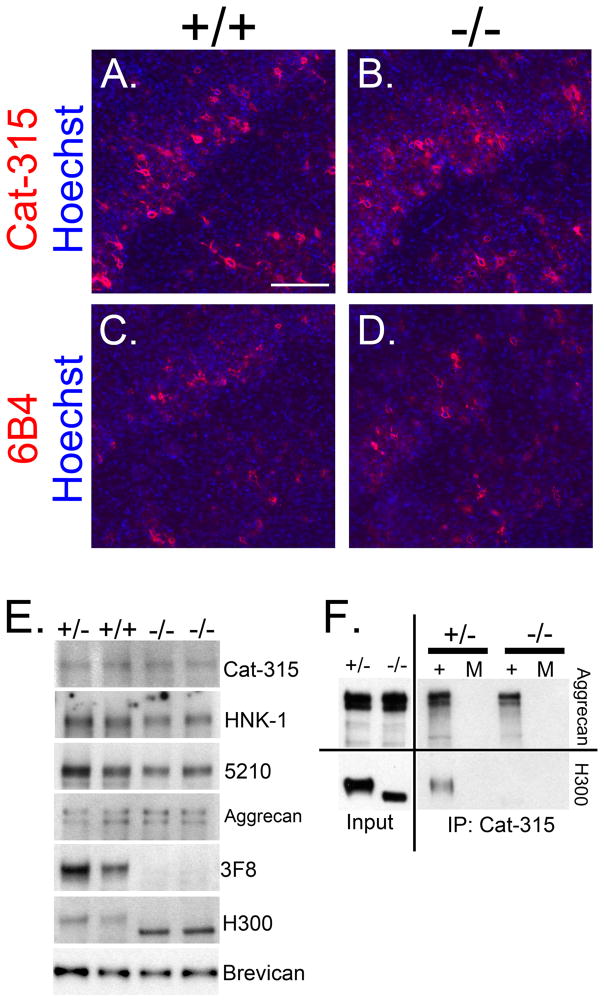
Immunohistochemical analysis with Cat-315 and 6B4 in adult wildtype brains showed the typical reticular PN staining in the cortex surrounding a subset of interneurons (Figure 6A, C). However, surprisingly, reactivity in the POMGnT1 knockouts appeared unaltered (Figure 6B, D). Western blot analysis on the soluble fraction isolated from adult wildtype and POMGnT1 heterozygous and knockout animals, confirmed that Cat-315, HNK-1 and 5210 immunoreactivities in adult POMGnT1 knockout animals were indeed unaltered, as was the molecular weight of aggrecan (Figure 6E). Immuno-reactive bands for all antibodies were present at molecular weights well above 250kD. Importantly, carbohydrate and protein core antibodies against RPTPζ/phosphacan showed similar trends as the studies in younger brains. In adult POMGnT1 knockout mice, 3F8 immunoreactivity was essentially absent, which was also accompanied by a molecular weight shift in H300 reactive bands relative to wildtype and heterozygous samples (Figure 6E). These results suggest that the molecular weight of aggrecan along with the Cat-315, HNK-1 and 5210 epitopes are largely unaffected, while the hypoglycosylation of RPTPζ/phosphacan is maintained in the brains of adult POMGnT1 knockout mice.
FIGURE 6. Cat-315 and 6B4 reactive perineuronal nets are present in the cortex of adult POMGnT1 knockout animals.
Coronal sections from the barrel cortex of adult POMGnT1 +/+ and −/− samples were immunostained and counter-stained for Hoechst nuclear stain (blue). A, POMGnT1 +/+ section stained for Cat-315 (red) shows reactivity that is localized to PNs in layer IV and VI. B, POMGnT1 −/− sections stained for Cat-315 shows abnormal cortical lamination but the presence of Cat-315 reactive PNs. C, POMGnT1 +/+ section stained for 6B4 (red) shows reactivity that is localized to PNs. D, POMGnT1 −/− section shows abnormal cortical lamination but the presence of 6B4 (red) reactive PNs. Bar, 100μM. E, chABC treated soluble adult brain homogenates from POMGnT1 +/+, +/− and −/− mice were analyzed by Western blot analysis for Cat-315, HNK-1, 5210, aggrecan, 3F8, H300 and brevican reactivity. Reactivity and molecular weight for Cat-315, HNK-1, 5210 and aggrecan reactive bands appeared unaffected in the POMGnT1 −/− tissue relative to controls (+/+ and +/−). In contrast, 3F8 reactivity was absent in POMGnT1 −/− samples and the apparent molecular weight of the H300 bands were shifted downwards. Reactive bands are isolated from the same region of the gel, as reactivity for Cat-315, HNK-1, 5210, aggrecan, 3F8 and H300 were all present above 250kD. As in previous figures, brevican reactive bands were located at 150kD. F, adult brain soluble fractions from +/− and −/− animals were subject to immunoprecipitation (IP) with the Cat-315 antibody. Immunoprecipitated material was then detected with antibodies against either aggrecan or H300. Aggrecan reactive bands were pulled down with the Cat-315 antibody and this reactivity was essentially unaltered in −/− samples compared to +/− controls. In contrast, a band reactive for H300 was only detected in +/− samples and absent from −/− samples. (+) denotes the inclusion of the Cat-315 antibody in IP reaction, (M) indicates mock control IP reactions lacking Cat-315 antibody.
Both phosphacan and aggrecan have been detected in PN structures (Haunso et al., 1999, Deepa et al., 2006), however previous work has suggested that the Cat-315 antibody primarily detects aggrecan in PNs (Matthews et al., 2002). Therefore, to show that maintained Cat-315 reactivity in the adult POMGnT1 knockout cortex was primarily from the presence of reactive epitopes on aggrecan and not RPTPζ/phosphacan, we performed immunoprecipitation with the Cat-315 antibody and immunoblotted with antibodies against either aggrecan or RPTPζ/phosphacan (H300). Reactive bands with similar intensities were observed with the anti-aggrecan antibody at high molecular weights (above 250 kDa) in both control and POMGnT1 knockout samples. In contrast, a high molecular weight band reactive for H300 was detected in control samples but absent from knockout samples (Figure 6F). These results show that the Cat-315 epitope on aggrecan is unaltered in POMGnT1 knockout brains, while the same epitope on RPTPζ/phosphacan is absent in animals lacking O-mannosyl glycans. Our interpretation of these findings is that Cat-315 and likely 6B4 detect O-mannosyl glycans on RPTPζ/phosphacan in the developing brain and to a lesser extent in the adult brain, but detect carbohydrate epitopes that are not O-mannosyl linked on aggrecan in the adult brain. These findings exemplify the highly heterogeneous and complex nature of protein glycosylation in the CNS.
1.4, DISCUSSION
1.4.1, Abnormal glycosylation of RPTPζ/phosphacan in models of CMDs with associated brain abnormalities
The congenital nature of CMD disorders, which stem from abnormalities in prenatal development provide a significant challenge to both diagnosing and treating affected individuals at the point when defects occur (Brasseur-Daudruy, 2011). Therefore, fully understanding the molecular pathomechanisms leading to the complex disease phenotypes of these disorders is imperative to future efforts in diagnostic testing and therapeutic intervention.
While this study primarily focused on a model system of MEB caused by mutations in POMGnT1, mutations in related known or putative glycostransferases also involved in O-mannosyl glycosylation give rise to associated disorders with highly overlapping phenotypes. While the abnormal glycosylation of α-DG is a commonality to all of these disorders and clearly a primary cause of most of the identified phenotypes (Grewal et al., 2001, Michele et al., 2002), a complete knowledge of affected glycoproteins and their functional contribution to the pathomechanisms underlying these disorders are crucial for the full identification of potential avenues of therapeutic intervention.
In accordance with this, the findings presented herein represent the first in vivo evidence that the glycosylation of RPTPζ/phosphacan is altered in the brains of animal models of MEB lacking functional POMGnT1 activity. Together with previous findings, these data suggest RPTPζ/phosphacan is a substrate for protein O-mannosyl glycosylation by POMGnT1 in vivo and raise the possibility that RPTPζ/phosphacan contributes to the pathogenesis of these CMDs.
1.4.2, The abnormal glycosylation of RPTPζ/phosphacan in the developing brain may contribute to cortical malformations
Type II lissencephaly resulting from breaches in the pial basement membrane and subsequent neuronal overmigration is a common cortical malformation that has been observed in CMD patients and animal models (Haltia et al., 1997, Michele et al., 2002, Liu et al., 2006, Hu et al., 2007); for review see (Reed, 2009). While previous work has shown that animal models lacking α-DG recapitulate this phenotype (Moore et al., 2002, Satz et al., 2010), the expression pattern of O-mannosylated forms of RPTPζ/phosphacan suggests that its abnormal glycosylation may also contribute to these cortical malformations.
RPTPζ knockout mice display abnormalities in myelination (Harroch et al., 2000, Harroch et al., 2002) and impaired learning and memory (Niisato et al., 2005), raising the possibility that abnormal glycosylation of RPTPζ/phosphacan may contribute in-part to these phenotypes which have also been described in O-mannosyl CMD patients (for review see (Muntoni and Voit, 2004)). The relatively mild phenotypes of RPTPζ knockout mice are in stark contrast to the wide array of interactions RPTPζ/phosphacan has with key extracellular and intracellular components of nervous system development. However, the possibility that abnormal glycosylation of RPTPζ/phosphacan could lead to a gain of function and exhibit far more deleterious effects than eliminating the protein alone cannot be discounted.
In support of this, Abbott and colleagues demonstrated highly Cat-315-reactive RPTPζ led to receptor dimerization and subsequent inactivation of intracellular phosphatase domains, a mechanism that was mediated by binding of glycosylated RPTPζ to galectin-1 (Abbott et al., 2008). Consequently the authors observed increased tyrosine phosphorylation of β-catenin resulting in its destabilization along with increased cell migration and decreased cell-cell adhesion (Abbott et al., 2008). Therefore, the absence of key glycan epitopes on RPTPζ/phosphacan may impinge on β-catenin signaling and influence key cellular events during neural development including cell migration and adhesion. The potential implications of findings by Abbott and colleagues in relation to those presented here are unclear in so much that O-mannosylated forms of RPTPζ/phosphacan were present in large quantities in the soluble fraction from subcellular fractionation, suggesting that we were detecting primarily phosphacan in our analyses. Future work to assess isoform-specific glycosylation will reveal if the glycosylation of RPTPζ is altered by the absence of O-mannosyl glycosylation and begin to unravel the individual function of abnormally glycosylated isoforms of RPTPζ/phosphacan.
1.4.3, Multiple antibodies against RPTPζ/phosphacan detect likely O-mannosyl glycan carbohydrate epitopes
In our previous studies we hypothesized that the Cat-315 epitope was an O-linked, terminal HNK-1 saccharide that correlated with biochemical characteristics consistent with O-mannose linked epitopes (Matthews et al., 2002, Dino et al., 2006, Abbott et al., 2008). Our findings presented herein strongly support that Cat-315 likely detects an O-mannose linked epitope on RPTPζ/phosphacan early in development by demonstrating the essential elimination of Cat-315 reactive RPTPζ/phosphacan in vivo from the cortex of embryonic and early postnantal POMGnT1 knockout animals relative to controls. Taken together, our data supports the hypothesis that the Cat-315 epitope on RPTPζ/phosphacan is the direct result of deficient O-mannosyl glycosylation.
Surprisingly, we also observed the essential elimination of reactivity of two other known anti- RPTPζ/phosphacan antibodies, 6B4 and 3F8, and a substantial reduction in the reactivity of 5210 in the cortex of POMGnT1 knockout animals. While 6B4 and 3F8 were known carbohydrate antibodies, the reactivity of 5210 with carbohydrate epitopes had not been previously described. However, our studies clearly demonstrated using the peptide antibody, H300, that in POMGnT1 knockouts RPTPζ/phosphacan was present at normal levels but shifted in molecular weight, leading us to the conclusion that these antibodies detect a carbohydrate-dependent epitope.
Previous work has suggested that the 6B4 epitope is similar biochemically to Cat-315 (Maeda et al., 1995). The 3F8 antibody was used in the initial isolation and identification of RPTPζ/phosphacan, it has been reported by other groups that 3F8 detects an N-linked epitope on RPTPζ/phosphacan (Garwood et al., 1999, Garwood et al., 2003). In contrast in our work, the absence of 3F8 reactivity from POMGnT1 knockouts and our inability to alter its reactivity with N-glycanase suggests that 3F8 detects a carbohydrate epitope on RPTPζ/phosphacan that is O-mannose linked. We cannot, however rule out that reaction conditions used for our deglycosylation experiments, which were non-denaturing, could also result in the incomplete digestion of buried N-linked glycans. Understanding this discrepancy will be a focus of future work. The antibody 5210 is predicted to detect the protein core and therefore its reduction in POMGnT1 knockouts was a surprise. Further characterization of the 5210 epitope through enzymatic protein deglycosylation revealed that this epitope is indeed carbohydrate dependent and can likely be found on O- and N-linked structures. While there are alternative explanations for the absence of 6B4, 3F8 and altered 5210 reactivity in POMGnT1 knockouts including the indirect disruption of carbohydrate epitopes present on other carbohydrate linkages in the absence of O-mannosyl linked glycans, the most straight-forward explanation for these finding are simply that these antibodies detect epitopes present on RPTPζ/phosphacan that are O-mannose linked.
While Cat-315 and 6B4 reactivity were essentially eliminated in the cortex of embryonic and early postnatal POMGnT1 knockouts, the reactivity of these antibodies for PNs in the adult brain was unaffected. Previous work has shown that both Cat-315 and 6B4 primarily detect carbohydrates on RPTPζ/phosphacan early in development (Dino et al., 2006; Maeda et al., 1994) and on aggrecan in the adult brain (Matthews et al., 2002, McRae et al., 2007, Saitoh et al., 2008). Therefore, while the reactivity of these antibodies for RPTPζ/phosphacan was eliminated, surprisingly it was essentially unaffected on aggrecan localized to PN structures. Further work will be required to determine the exact epitope these antibodies are detecting and how these carbohydrates are linked to aggrecan. Overall this work highlights the micro-heterogeneity of glycosylation in the nervous system in both the complex elaboration of O-glycans on RPTPζ/phosphacan and the conservation of specific terminal epitopes on different glycans.
1.4.4, Decreased HNK-1 immunoreactive RPTPζ/phosphacan in the early postnatal cortex of POMGnT1 knockout mice
Our findings demonstrate that HNK-1 reactivity on RPTPζ/phosphacan was significantly diminished in the cortex of early postnatal POMGnT1 knockout brains relative to wildtype controls, and suggest that RPTPζ/phosphacan is a highly abundant soluble substrate for HNK-1 modifications in the P4 brain. We hypothesize that these modifications are primarily O-mannose linked, which is in agreement with our previously published findings (Matthews et al., 2002; Dino et al., 2006).
HNK-1 carbohydrate structures are predominately expressed in the brain and have previously been described on RPTPζ/phosphacan (Maeda et al., 1994) along with a wide array of other cell adhesion and ECM molecules (McGarry et al., 1983, Morita et al., 2009a); for review see (Morita et al., 2008). The HNK-1 carbohydrate epitope is comprised of a 3-sulfated terminal glucuronic acid residue linked to the β-1,3 position of a galactose (Bakker et al., 1997, Terayama et al., 1997, Ong et al., 1998). HNK-1 structures have been shown to influence cell adhesion (Schmidt and Schachner, 1998), proliferation (Yagi et al., 2010), motility (Abbott et al., 2008), synapse formation (Morita et al., 2009b) and higher order cognitive functioning (Yamamoto et al., 2002a, Yoshihara et al., 2009). Previous work has demonstrated that animals with reduced HNK-1 reactivity have impaired synaptic plasticity and spatial learning (Yamamoto et al., 2002b, Yoshihara et al., 2009). Thus, suggesting that the terminal HNK-1 glycan structure is involved in higher order nervous system function in vivo.
Previous work has also shown that protein substrates in addition to RPTPζ/phosphacan are also likely decorated with O-mannosyl linked HNK-1 saccharides, such as CD-24 (Bleckmann et al., 2009). While our analyses did not identify abnormalities in HNK-1 reactive bands corresponding to proteins other than RPTPζ/phosphacan in POMGnT1 knockout brains, it is likely that additional abnormally glycosylated protein substrates are also present in the brains of these animals. Future work to specifically evaluate the glycosylation of other known protein substrates of O-mannosyl glycosylation, like CD-24, will likely yield important information regarding the molecular underpinnings of O-mannosyl CMDs
1.4.5, The absence of O-mannosyl glycans on RPTPζ/phosphacan does not affect its expression
Despite the clear loss of carbohydrate epitopes on RPTPζ/phosphacan in POMGnT1 knockout animals relative to controls, our preliminary studies suggest that its expression was not substantially altered. Both by immunohistochemistry with the 473HD antibody at E15 and by Western blot analysis with the core peptide antibody, H300, we show that both the spatial localization and overall expression levels of RPTPζ/phosphacan in POMGnT1 knockouts are comparable to that in controls. The absence of a core peptide antibody against RPTPζ/phosphacan that can be utilized for immunohistochemistry studies limit our abilities to evaluate both the spatial and cellular localizations of the hypoglycosylated protein in POMGnT1 knockout animals in detail and this will be a focus of future work.
1.4.6, The glycosylation of RPTPζ/phosphacan is unaffected in Largemyd mutant brains
Despite the profound effect the absence of POMGnT1 had on the glycosylation of RPTPζ/phosphacan, we found no effect in Largemyd mutant mice. These findings indicate that RPTPζ/phosphacan likely does not exhibit abnormal glycosylation in all models of CMDs with associated brain abnormalities. Subtractive analysis of phenotypes from Largemyd mutants and POMGnT1 knockout mice may provide useful in determining the function of hypoglycosylated RPTPζ/phosphacan. Furthermore, differential glycosylation of α-dystroglycan and RPTPζ/phosphacan by Large suggests a level of exclusivity in the enzymatic activity of Large for α-dystroglycan and emphasizes structural differences in O-mannosyl linked glycans on α-dystroglycan and RPTPζ/phosphacan. Large, fukutin and fukutin related protein (FKRP) have been suggested to participate in the assembly of a common carbohydrate moiety on α-dystroglycan, which is required for proper laminin binding (Yoshida-Moriguchi et al., 2010, Kuga et al., 2012). In combination with our data that suggests RPTPζ/phosphacan is not modified by Large, the circumstantial evidence supports the hypothesis that RPTPζ/phosphacan also would not be a likely substrate for fukutin or FKRP. However, it is highly likely that RPTPζ/phosphacan is also abnormally glycosylated in protein O-mannosyltransferase 1 and 2 (POMT1/POMT2) knockout animals, in so much that these enzymes catalyze the attachment of the initial O-mannose saccharide which is prerequisite for further O-mannosyl glycan elaboration by downstream enzymes like POMGnT1 (Manya et al., 2004).
1.4.7, Theoretical Model of HNK-1 linked glycan structure on RPTPζ/phosphacan
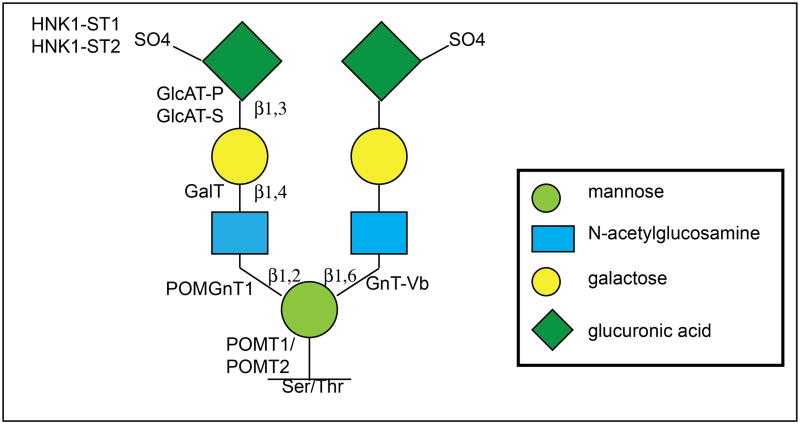
Taken together, previous published findings and those presented here provide the bases for a theoretical model of O-mannosyl linked HNK-1 glycan structures on RPTPζ/phosphacan (Figure 7). Wherein, O-mannosyl glycosylation by POMGnT1 at the β-1,2 position of the primary mannose and subsequent glycosylation by HNK-1 synthesizing glycotransferases results in the elaboration of a terminal HNK-1 structure. Our previous work suggested that additional elaboration of the mannose at the β-1,6 position by GnT-Vb could result in the creation of a bi-antennary HNK-1 branched glycan structure which is dependent on preceding POMGnT1 modifications (Abbott et al., 2008, Stalnaker et al., 2011), however glycan analysis is necessary to definitively conclude this. Our findings which suggest RPTPζ/phosphacan glycosylation is seemingly normal in the brains of Largemyd mutant mice suggest that RPTPζ/phosphacan is not endogenously modified by Large (Figure 7). Therefore in POMGnT1 knockout mice, both β-1,2 and presumptive β-1,6- mannose modifications would be absent thus eliminating HNK-1 reactivity from O-mannosyl glycans on RPTPζ/phosphacan.
FIGURE 7. Theoretical schematic diagram of O-mannosyl linked HNK-1 glycan structures on RPTPζ/phosphacan in the early postnatal cortex.
O-mannosyl glycosylation by POMGnT1 at the β1,2 position of the primary mannose and further modifications by HNK-1 synthesizing enzymes results in the elaboration of a terminally sulfated HNK-1 structure. POMGnT1 activity, which is prerequisite for GnT-Vb modifications at the β1,6 position of the primary mannose, could then facilitate GnT-Vb mediated glycan modifications of terminal HNK-1 structures at the β1,6 position resulting in a bi-antennary HNK-1 glycan structure. Additionally, RPTPζ/phosphacan is likely not a primary substrate of glycosylation by Large. Consequential to an absence of POMGnT1 activity, terminal HNK-1 structures are not elaborated on O-mannosyl linked glycans present on RPTPζ/phosphacan in the cortex of POMGnT1 knockout mice.
1.4.8, CONCLUSIONS
In summary, herein we reported that RPTPζ/phosphacan is hypoglycosylated in animal models of MEB lacking functional POMGnT1. Theses results suggest that RPTPζ/phosphacan is a significant substrate for protein O-mannosyl glycosylation in vivo, displaying the loss of several carbohydrate epitopes including HNK-1. Additionally, this work identifies the epitopes of several previously uncharacterized carbohydrate antibodies against RPTPζ/phosphacan as likely detecting O-mannosyl-linked glycans. While our results clearly demonstrate RPTPζ/phosphacan is hypoglycosylated in POMGnT1 knockout mice, additional evidences also suggest that abnormal glycosylation of the protein does not affect its overall expression or spatial localization. We also evaluated the glycosylation of RPTPζ/phosphacan is Largemyd mutant mice and found that carbohydrate epitopes that were absent or altered in the brains of POMGnT1 mice were seemingly unaffected in these animals. These results suggest that abnormal glycosylation of RPTPζ/phosphacan is not present in all CMD models, which provide insight into the O-mannosyl glycan structures on RPTPζ/phosphacan and highlights the specificity of glycotransferase activity by Large. Finally, we show that select carbohydrate epitopes absent from the cortex of embryonic and early postnatal POMGnT1 animals are unchanged in the adult cortex localized to aggrecan in PN structures, while the hypoglycosylation of RPTPζ/phosphacan is maintained. These results represent the first in vivo data showing the hypoglycosylation of RPTPζ/phosphacan in the absence of POMGnT1 and the possibility that abnormally glycosylated RPTPζ/phosphacan may contribute to the neural pathology of O-mannosyl CMDs.
Highlights.
A novel hypoglycosylated protein in POMGnT1 knockout brains, a model of CMDs.
RPTPζ/phosphacan is a likely substrate for O-mannosylation by POMGnT1 in vivo.
HNK-1 glycans on RPTPζ/phosphacan are diminished in POMGnT1 knockouts.
Glycosylation of RPTPζ/phosphacan is unaffected in LARGE knockouts.
Acknowledgments
The authors would like to thank Peng Zhang for animal genotyping and Wendi Burnette for technical assistance. This work was funded by NIH/NINDS grant # NS069660 to R.T.M and grant # HD060458 and NS066582 to H.H.
Glossary
- Receptor protein tyrosine phosphatase ζ (RPTPζ)/phosphacan
A chondroitin sulfate proteoglycan with CNS enriched expression that is encoded by the PTPRZ1 gene. The full-length form of the protein functions as a receptor protein tyrosine phosphatase, while alternative splicing gives rise to a secreted form called phosphacan. While the full length-form of the receptor (RPTPζ) and secreted variant (phosphacan) are considered to be long-isoforms, short-isoforms of each variant have also been described. The extracellular portion of the protein is highly decorated with chondroitin sulfate glycosaminoglycan chains and is a major constituent of the neural extracellular matrix
- O-mannosyl glycosylation
The process wherein serine or threonine residues of glycoproteins are elaborated with O-linked mannose saccharides that receive additional carbohydrate modifications
- Protein O-mannose β-1,2-N-acetylglucosaminyltransferase 1 (POMGnT1)
A glycotransferase that catalyzes the transfer of an N-acetylglucosamine saccharide on to O-mannose present on serine and threonine residues of glycoproteins
Abbreviations
- RPTPζ
receptor protein tyrosine phosphatase ζ
- POMGnT1
protein O-mannose β-1,2-N-acetylglucosaminyltransferase 1
- CMDs
congenital muscular dystrophies
- MEB
muscle-eye-brain disease
- α-DG
α-dystroglycan
- ECM
extracellular matrix
- chABC
chondroitinase ABC
- CS-GAG
chondroitin sulfate glycosaminoglycan
- HNK-1
human natural killer-1
- PNs
perineuronal nets
- POMT1/POMT2
protein O-mannosyltransferase 1 and 2
- +/+
wildtype
- +/−
heterozygous
- −/−
knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chrissa A. Dwyer, Email: wrighchr@upstate.edu.
Eric Baker, Email: embake@gmail.com.
Huaiyu Hu, Email: huh@upstate.edu.
Russell T. Matthews, Email: matthewr@upstate.edu.
References
- Abbott KL, Matthews RT, Pierce M. Receptor tyrosine phosphatase beta (RPTPbeta) activity and signaling are attenuated by glycosylation and subsequent cell surface galectin-1 binding. J Biol Chem. 2008;283:33026–33035. doi: 10.1074/jbc.M803646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker H, Friedmann I, Oka S, Kawasaki T, Nifant’ev N, Schachner M, Mantei N. Expression cloning of a cDNA encoding a sulfotransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. J Biol Chem. 1997;272:29942–29946. doi: 10.1074/jbc.272.47.29942. [DOI] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann C, Geyer H, Lieberoth A, Splittstoesser F, Liu Y, Feizi T, Schachner M, Kleene R, Reinhold V, Geyer R. O-glycosylation pattern of CD24 from mouse brain. Biol Chem. 2009;390:627–645. doi: 10.1515/BC.2009.044. [DOI] [PubMed] [Google Scholar]
- Brasseur-Daudruy MVPH, Ickowicz V, Eurin D, Verspyck E. Walker-Warburg syndrome diagnosed by findings of typical ocular abnormalities on prenatal ultrasound. Pediatr Radiol. 2011 doi: 10.1007/s00247-011-2242-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001a;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry CA, Bushby K, Voit T, Blake DJ, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001b;10:2851–2859. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- Browning CA, Grewal PK, Moore CJ, Hewitt JE. A rapid PCR method for genotyping the Large(myd) mouse, a model of glycosylation-deficient congenital muscular dystrophy. Neuromuscul Disord. 2005;15:331–335. doi: 10.1016/j.nmd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTP-beta are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996;44:199–215. doi: 10.1002/(SICI)1097-4547(19960501)44:3<199::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 1999;263:879–888. doi: 10.1046/j.1432-1327.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- Dino MR, Harroch S, Hockfield S, Matthews RT. Monoclonal antibody Cat-315 detects a glycoform of receptor protein tyrosine phosphatase beta/phosphacan early in CNS development that localizes to extrasynaptic sites prior to synapse formation. Neuroscience. 2006;142:1055–1069. doi: 10.1016/j.neuroscience.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Kirkpatrick JB, Hittner HM, Roberts RM, Kretzer FL. Syndromes with lissencephaly. II: Walker-Warburg and cerebro-oculo-muscular syndromes and a new syndrome with type II lissencephaly. Am J Med Genet. 1985;22:157–195. doi: 10.1002/ajmg.1320220118. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Clement A, Lochter A, Streit A, Mandl C, Schachner M. Isolation of a neural chondroitin sulfate proteoglycan with neurite outgrowth promoting properties. J Cell Biol. 1994;126:783–799. doi: 10.1083/jcb.126.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J, Krusius T, Margolis RK, Margolis RU. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 1979;254:10295–10300. [PubMed] [Google Scholar]
- Garwood J, Heck N, Reichardt F, Faissner A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 2003;278:24164–24173. doi: 10.1074/jbc.M211721200. [DOI] [PubMed] [Google Scholar]
- Garwood J, Schnadelbach O, Clement A, Schutte K, Bach A, Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J Neurosci. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during developments: interactions with adhesion molecules. Perspect Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- Haltia M, Leivo I, Somer H, Pihko H, Paetau A, Kivela T, Tarkkanen A, Tome F, Engvall E, Santavuori P. Muscle-eye-brain disease: a neuropathological study. Ann Neurol. 1997;41:173–180. doi: 10.1002/ana.410410208. [DOI] [PubMed] [Google Scholar]
- Harroch S, Furtado GC, Brueck W, Rosenbluth J, Lafaille J, Chao M, Buxbaum JD, Schlessinger J. A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nat Genet. 2002;32:411–414. doi: 10.1038/ng1004. [DOI] [PubMed] [Google Scholar]
- Harroch S, Palmeri M, Rosenbluth J, Custer A, Okigaki M, Shrager P, Blum M, Buxbaum JD, Schlessinger J. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunso A, Celio MR, Margolis RK, Menoud PA. Phosphacan immunoreactivity is associated with perineuronal nets around parvalbumin-expressing neurones. Brain Res. 1999;834:219–222. doi: 10.1016/s0006-8993(99)01596-6. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Mizusaki MJ, Kamei K, Harada S, Miyata S. Chondroitin sulfate proteoglycan phosphacan associates with parallel fibers and modulates axonal extension and fasciculation of cerebellar granule cells. Mol Cell Neurosci. 2005;30:364–377. doi: 10.1016/j.mcn.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hu H, Yang Y, Eade A, Xiong Y, Qi Y. Breaches of the pial basement membrane and disappearance of the glia limitans during development underlie the cortical lamination defect in the mouse model of muscle-eye-brain disease. J Comp Neurol. 2007;502:168–183. [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, Oohira A. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J Biol Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- Inamori K-i. Dystroglycan Function Requires Xylosyl- and Glucuronyltransferase Activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology. 2005;15:593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62:809–823. doi: 10.1007/s00018-004-4510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, Topaloglu H, Endo T, Yoshikawa H, Toda T. Deficiency of alpha-dystroglycan in muscle-eye-brain disease. Biochem Biophys Res Commun. 2002;291:1283–1286. doi: 10.1006/bbrc.2002.6608. [DOI] [PubMed] [Google Scholar]
- Kim DS, Hayashi YK, Matsumoto H, Ogawa M, Noguchi S, Murakami N, Sakuta R, Mochizuki M, Michele DE, Campbell KP, Nonaka I, Nishino I. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in alpha-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- Kogelberg H, Chai W, Feizi T, Lawson AM. NMR studies of mannitol-terminating oligosaccharides derived by reductive alkaline hydrolysis from brain glycoproteins. Carbohydr Res. 2001;331:393–401. doi: 10.1016/s0008-6215(01)00051-9. [DOI] [PubMed] [Google Scholar]
- Krusius T, Finne J, Margolis RK, Margolis RU. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 1986;261:8237–8242. [PubMed] [Google Scholar]
- Kuga A, Kanagawa M, Sudo A, Chan YM, Tajiri M, Manya H, Kikkawa Y, Nomizu M, Kobayashi K, Endo T, Lu QL, Wada Y, Toda T. Absence of post-phosphoryl modification in dystroglycanopathy mouse models and wild-type tissues expressing non-laminin binding form of alpha-dystroglycan. J Biol Chem. 2012;287:9560–9567. doi: 10.1074/jbc.M111.271767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C, Zhang H, Hockfield S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J Neurosci. 1998;18:174–183. doi: 10.1523/JNEUROSCI.18-01-00174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1) Mech Dev. 2006;123:228–240. doi: 10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, Sewry CA, Brown SC, Muntoni F. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- Maeda N, Hamanaka H, Oohira A, Noda M. Purification, characterization and developmental expression of a brain-specific chondroitin sulfate proteoglycan, 6B4 proteoglycan/phosphacan. Neuroscience. 1995;67:23–35. doi: 10.1016/0306-4522(94)00069-h. [DOI] [PubMed] [Google Scholar]
- Maeda N, Hamanaka H, Shintani T, Nishiwaki T, Noda M. Multiple receptor-like protein tyrosine phosphatases in the form of chondroitin sulfate proteoglycan. FEBS Lett. 1994;354:67–70. doi: 10.1016/0014-5793(94)01093-5. [DOI] [PubMed] [Google Scholar]
- Maeda N, He J, Yajima Y, Mikami T, Sugahara K, Yabe T. Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule. J Biol Chem. 2003;278:35805–35811. doi: 10.1074/jbc.M305530200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci U S A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry RC, Helfand SL, Quarles RH, Roder JC. Recognition of myelin-associated glycoprotein by the monoclonal antibody HNK-1. Nature. 1983;306:376–378. doi: 10.1038/306376a0. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Meyer-Puttlitz B, Margolis RK, Margolis RU. Complex-type asparagine-linked oligosaccharides on phosphacan and protein-tyrosine phosphatase-zeta/beta mediate their binding to neural cell adhesion molecules and tenascin. J Biol Chem. 1995;270:24650–24653. doi: 10.1074/jbc.270.42.24650. [DOI] [PubMed] [Google Scholar]
- Montanaro F, Lindenbaum M, Carbonetto S. alpha-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol. 1999;145:1325–1340. doi: 10.1083/jcb.145.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Morita I, Kakuda S, Takeuchi Y, Itoh S, Kawasaki N, Kizuka Y, Kawasaki T, Oka S. HNK-1 glyco-epitope regulates the stability of the glutamate receptor subunit GluR2 on the neuronal cell surface. J Biol Chem. 2009a;284:30209–30217. doi: 10.1074/jbc.M109.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita I, Kakuda S, Takeuchi Y, Kawasaki T, Oka S. HNK-1 (human natural killer-1) glyco-epitope is essential for normal spine morphogenesis in developing hippocampal neurons. Neuroscience. 2009b;164:1685–1694. doi: 10.1016/j.neuroscience.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Morita I, Kizuka Y, Kakuda S, Oka S. Expression and function of the HNK-1 carbohydrate. J Biochem. 2008;143:719–724. doi: 10.1093/jb/mvm221. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul Disord. 2004;14:635–649. doi: 10.1016/j.nmd.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Niisato K, Fujikawa A, Komai S, Shintani T, Watanabe E, Sakaguchi G, Katsuura G, Manabe T, Noda M. Age-dependent enhancement of hippocampal long-term potentiation and impairment of spatial learning through the Rho-associated kinase pathway in protein tyrosine phosphatase receptor type Z-deficient mice. J Neurosci. 2005;25:1081–1088. doi: 10.1523/JNEUROSCI.2565.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong E, Yeh JC, Ding Y, Hindsgaul O, Fukuda M. Expression cloning of a human sulfotransferase that directs the synthesis of the HNK-1 glycan on the neural cell adhesion molecule and glycolipids. J Biol Chem. 1998;273:5190–5195. doi: 10.1074/jbc.273.9.5190. [DOI] [PubMed] [Google Scholar]
- Peles E, Schlessinger J, Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase beta: implications for intercellular signaling. Trends Biochem Sci. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Hudson LD. Regulation of tyrosine phosphorylation and protein tyrosine phosphatases during oligodendrocyte differentiation. Mol Cell Neurosci. 1996;7:404–418. doi: 10.1006/mcne.1996.0029. [DOI] [PubMed] [Google Scholar]
- Reed UC. Congenital muscular dystrophy. Part II: a review of pathogenesis and therapeutic perspectives. Arq Neuropsiquiatr. 2009;67:343–362. doi: 10.1590/s0004-282x2009000200035. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Matsui F, Chiba Y, Kawamura N, Keino H, Satoh M, Kumagai N, Ishii S, Yoshikawa K, Shimada A, Maeda N, Oohira A, Hosokawa M. Reduced expression of MAb6B4 epitopes on chondroitin sulfate proteoglycan aggrecan in perineuronal nets from cerebral cortices of SAMP10 mice: a model for age-dependent neurodegeneration. J Neurosci Res. 2008;86:1316–1323. doi: 10.1002/jnr.21582. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Lustig M, Nativ M, Hemperly JJ, Schlessinger J, Peles E, Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta. J Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, Hoshi T, Moore SA, Campbell KP. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schessl J, Zou Y, Bonnemann CG. Congenital muscular dystrophies and the extracellular matrix. Semin Pediatr Neurol. 2006;13:80–89. doi: 10.1016/j.spen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Schmidt JT, Schachner M. Role for cell adhesion and glycosyl (HNK-1 and oligomannoside) recognition in the sharpening of the regenerating retinotectal projection in goldfish. J Neurobiol. 1998;37:659–671. doi: 10.1002/(sici)1097-4695(199812)37:4<659::aid-neu13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Shitara K, Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Brain-specific receptor-type protein-tyrosine phosphatase RPTP beta is a chondroitin sulfate proteoglycan in vivo. J Biol Chem. 1994;269:20189–20193. [PubMed] [Google Scholar]
- Soh BS, Song CM, Vallier L, Li P, Choong C, Yeo BH, Lim EH, Pedersen RA, Yang HH, Rao M, Lim B. Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells. Stem Cells. 2007;25:3029–3037. doi: 10.1634/stemcells.2007-0372. [DOI] [PubMed] [Google Scholar]
- Stalnaker SH, Aoki K, Lim JM, Porterfield M, Liu M, Satz JS, Buskirk S, Xiong Y, Zhang P, Campbell KP, Hu H, Live D, Tiemeyer M, Wells L. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 2011;286:21180–21190. doi: 10.1074/jbc.M110.203281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama K, Oka S, Seiki T, Miki Y, Nakamura A, Kozutsumi Y, Takio K, Kawasaki T. Cloning and functional expression of a novel glucuronyltransferase involved in the biosynthesis of the carbohydrate epitope HNK-1. Proc Natl Acad Sci U S A. 1997;94:6093–6098. doi: 10.1073/pnas.94.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viapiano MS, Matthews RT, Hockfield S. A novel membrane-associated glycovariant of BEHAB/brevican is up-regulated during rat brain development and in a rat model of invasive glioma. J Biol Chem. 2003;278:33239–33247. doi: 10.1074/jbc.M303480200. [DOI] [PubMed] [Google Scholar]
- Yagi H, Yanagisawa M, Suzuki Y, Nakatani Y, Ariga T, Kato K, Yu RK. HNK-1 epitope-carrying tenascin-C spliced variant regulates the proliferation of mouse embryonic neural stem cells. J Biol Chem. 2010;285:37293–37301. doi: 10.1074/jbc.M110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Oka S, Inoue M, Shimuta M, Manabe T, Takahashi H, Miyamoto M, Asano M, Sakagami J, Sudo K, Iwakura Y, Ono K, Kawasaki T. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J Biol Chem. 2002a;277:27227–27231. doi: 10.1074/jbc.C200296200. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Oka S, Saito-Ohara F, Inazawa J, Kawasaki T. Molecular cloning and genomic analysis of mouse glucuronyltransferase involved in biosynthesis of the HNK-1 epitope. J Biochem. 2002b;131:337–347. doi: 10.1093/oxfordjournals.jbchem.a003108. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Sugihara K, Kizuka Y, Oka S, Asano M. Learning/memory impairment and reduced expression of the HNK-1 carbohydrate in beta4-galactosyltransferase-II-deficient mice. J Biol Chem. 2009;284:12550–12561. doi: 10.1074/jbc.M809188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen CT, Chai W, Loveless RW, Lawson AM, Margolis RU, Feizi T. Brain contains HNK-1 immunoreactive O-glycans of the sulfoglucuronyl lactosamine series that terminate in 2-linked or 2,6-linked hexose (mannose) J Biol Chem. 1997;272:8924–8931. doi: 10.1074/jbc.272.14.8924. [DOI] [PubMed] [Google Scholar]