Abstract
Background
Mild cognitive impairment is increasingly recognized as a construct in Parkinson’s disease (PD) and occurs in about 25% of non-demented PD patients. Although executive dysfunction is the most frequent type of cognitive deficit in PD, the cognitive phenotype of PD mild cognitive impairment (PD-MCI) is broad. PD-MCI subtypes are represented by amnestic and nonamnestic domain impairment as well as single- and multiple-domain impairment. However, it is unclear whether patients with different PD-MCI subtypes also differ in other clinical characteristics besides cognitive profile.
Methods
We studied 128 PD-MCI subjects at our Movement Disorders center, comparing clinical, motor, and behavioral characteristics across the PD-MCI subtypes.
Results
We found varying proportions of impairment subtypes: nonamnestic single-domain (47.7%), amnestic multiple-domain (24.2%), amnestic single-domain (18.8%), and nonamnestic multiple-domain (9.5%). Attentional/executive functioning and visuospatial abilities were the most frequently impaired domains. PD-MCI subtypes differed in their motor features with nonamnestic multiple-domain PD-MCI subjects showing particularly pronounced problems with postural instability and gait. Differences among PD-MCI subtypes in age, PD duration, medication use, mood or behavioral disturbances, or vascular disease were not significant.
Conclusions
In addition to differing cognitive profiles, PD-MCI subtypes differ in motor phenotype and severity but not in mood, behavioral, or vascular co-morbidities. Greater postural instability and gait disturbances in the nonamnestic multiple-domain subtype emphasize shared non-dopaminergic neural substrates of gait and cognition in PD. Furthermore, increased burden of cognitive dysfunction, rather than type of cognitive deficit, may be associated with greater motor impairment in PD-MCI.
Keywords: amnestic, dementia, gait, mild cognitive impairment, nonamnestic
Introduction
Mild cognitive impairment in PD (PD-MCI) has become increasingly recognized as a distinct entity that signifies a state of cognitive decline in clinically diagnosed PD patients that is not normal for age, but does not significantly impair functional activities, and does not meet criteria for PD dementia (PDD) 1–3. While rooted in studies of aging and Alzheimer’s Disease (AD), 4, 5 the construct of MCI recently has been applied to PD. In PD, MCI may represent the earliest stage of cognitive decline and a risk factor for PDD 6, 7, a frequent complication 8, 9 associated with poor outcomes 10, 11 and lacking effective treatments 12. Greater understanding of PD-MCI and its subtypes may lead to earlier detection of patients at risk of dementia and ultimately, therapies to halt or slow the progression of PD-MCI and PDD.
PD-MCI is frequent, occurring in about 25% of non-demented PD patients (range 19–55%) 1, 6, 13–21 and even in newly diagnosed, untreated PD patients 13, 16, 18. To date, many, but not all, PD-MCI studies have applied MCI criteria and subtyping proposed by Petersen et al 5 and Winblad et al 22. In the latter, MCI is further categorized into four subtypes depending on the presence of memory impairment and number of cognitive domains impaired: amnestic MCI single-domain, amnestic MCI multiple-domain, nonamnestic MCI single-domain, or nonamnestic MCI multiple-domain. Recently, PD-MCI diagnostic criteria have been developed by a Movement Disorder Society (MDS) Task Force 2. While nonamnestic single-domain impairment, particularly affecting executive function, predominates in PD-MCI, 6, 13, 15, 16, 18, 19 the PD-MCI cognitive phenotype is heterogeneous with some patients exhibiting posterior cortical-type profiles 7, and others, greater amnestic deficits 14, 23–25. This heterogeneity may reflect methodological differences between studies 1, 20, 21, but also differences in the neurobiological substrates of MCI subtypes.
Few studies, however, have examined whether PD-MCI subtypes differ in characteristics besides cognitive phenotype. Moreover, sample sizes of most PD-MCI cohorts have been relatively small (range 18–72), thereby precluding comparisons across subtypes, with the exception of one large multi-center study in which amnestic and nonamnestic multiple-domain PD-MCI had worse motor symptoms than those with single-domain PD-MCI 14. Differences in motor severity, mood or behavioral disorders, or other co-morbidities among PD-MCI subtypes would be important information to acquire because such differences may affect rates of progression and potentially influence treatment strategies.
Accordingly, the purpose of our study was to examine the clinical characteristics of PD-MCI subtypes (amnestic single-domain, amnestic multiple-domain, nonamnestic single-domain, nonamnestic multiple-domain) and determine whether PD-MCI subtypes, while distinct in their cognitive phenotype, differ regarding other clinical aspects and co-morbidities.
Methods
Subjects and evaluations
We studied 128 PD-MCI subjects drawn from a larger, prospective study involving a cross-sectional cohort of 350 consecutive PD patients evaluated at the Rush University Movement Disorders Center over a 2 ½-year period. All PD subjects met United Kingdom PD Society Brain Bank criteria 26 and were examined by movement disorders neurologists. We excluded those with atypical or secondary parkinsonism, known causes of dementia, and prior neurosurgery. The study was approved by the Rush University Institutional Review Board, Chicago, IL.
The clinical evaluation assessed: demographics, medical co-morbidities, medications, and disease-related features including the Unified PD Rating Scale (UPDRS) Part III motor score, Hoehn and Yahr stage, and UPDRS Part I Thought Disorder score 27. PD medications were converted to levodopa equivalent daily doses (LEDD) 28. To identify associations of clinically distinct motor elements of the UPDRS Part III with PD-MCI subtypes, individual motor item scores were converted to six factors (i.e., axial functioning/gait, rest tremor, rigidity, left bradykinesia, right bradykinesia, and postural tremor), using previously published weighted factor loadings 29. Composite vascular risk factor scores (0–5) were calculated based on the dichotomized presence or absence of hypertension, hypercholesterolemia, diabetes mellitus, cardiovascular or cerebrovascular disease 30.
The neuropsychological evaluation included: 1) MiniMental State Examination (MMSE), 31 Hamilton Depression Rating Scale, 32 and individual cognitive tests grouped conceptually into following four cognitive domains – (a) attentional/executive function (Digit span forwards and backwards 33; oral version of the Symbol Digit Modalities Test 34, category fluency test of animal naming in 1 minute 35), (b) declarative memory (3 trials of word list learning and delayed recall from the Consortium to Establish a Registry for AD [CERAD] 35), (c) language (Boston Naming Test 36; Similarities 33), and (d) visuospatial function (Judgment of Line Orientation 37, intersecting pentagon drawing item from the MMSE using an ordinal 6 point scale 38, 39), 2) a semi-structured interview with the subject and/or informant, and 3) clinical impression of the subject’s general cognitive function.
Cognitive classification
Raw scores for cognitive tests were transformed to z-scores based upon normative data 40, 41. Cognitive domain scores were calculated by averaging z -scores for neuropsychological tests within specific domains, thereby accounting for any unequal distribution of tests per domain. Impairment was defined as a z-score of ≤ −1.5 for a given domain. The MMSE, except for intersecting pentagons, was used only for descriptive purposes.
PD subjects were classified as having PD-MCI if they had a decline in cognition as assessed during the neuropsychological evaluation, had a z-score of ≤ −1.5 on at least one of four cognitive domains, and did not meet MDS-PDD criteria 3. Subjective cognitive complaints were not required for the definition of PD-MCI but were endorsed by 83% of PD-MCI subjects. Of the 350 consecutive PD subjects, PD subjects with dementia (n=34) or normal cognition (n=188) were excluded.
Subtyping of PD-MCI subjects
Using these methods, PD-MCI subjects were categorized in one of the following four subtypes: amnestic single-domain (only memory domain impaired), amnestic multiple-domain (memory plus one or more other domains impaired), nonamnestic single-domain (one non-memory domain impaired), or nonamnestic multiple-domain (more than one non-memory domain impaired) 22.
Statistical analyses
Statistical analyses were performed using SPSS 18.0 (PASW 18, Chicago, IL).
Demographic- and disease-related variables were compared across PD-MCI subtypes using one-way analysis of variance (ANOVA) or for categorical variables, Kruskal-Wallis tests. Levene’s test was used to assess homogeneity of variance of the variables; continuous variables demonstrating unequal variances were analyzed using non-parametric tests. Analyses were adjusted for multiple comparisons using Bonferroni corrections, which are also valid for unequal samples. Single- and multiple-domain PD-MCI subtypes were compared using independent t-tests or Chi-square tests. Predictors of PD-MCI subtype were examined using multinominal logistic regressions with variables entered stepwise and nonamnestic single-domain subtype as the reference category. Statistical significance was set at p<0.05.
Results
Cognitive profile of PD-MCI subjects
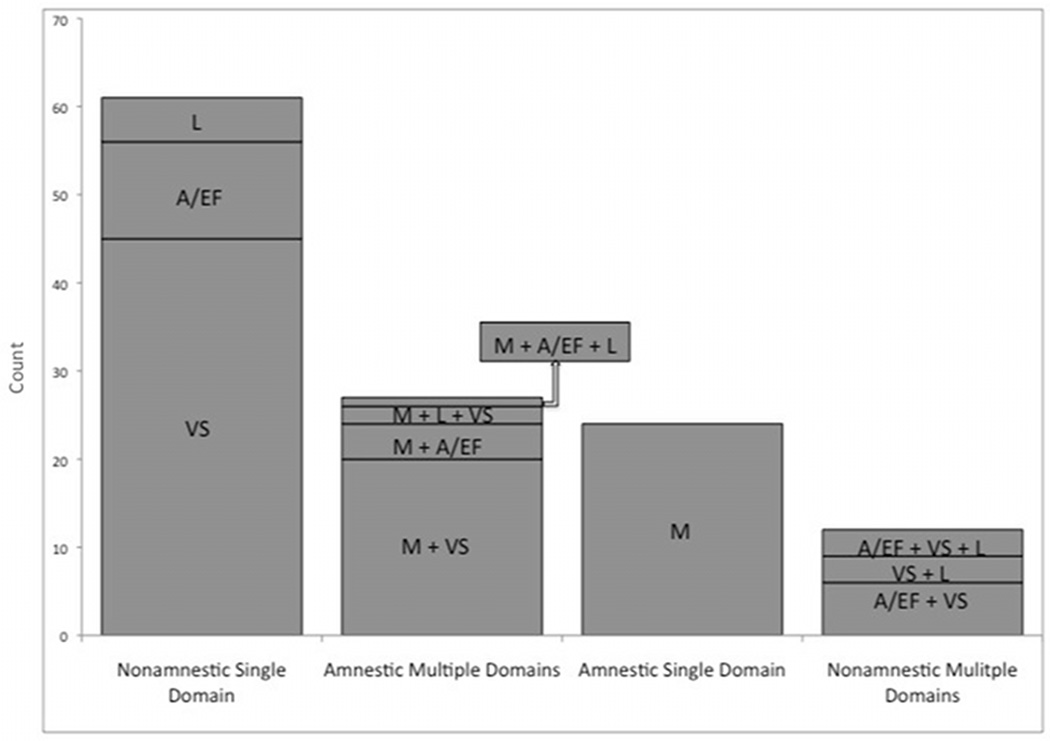
All four MCI subtypes were represented: 47.7% had nonamnestic single-domain impairment, 24.2% amnestic multiple-domain impairment, 18.8% amnestic single-domain impairment, and 9.5% nonamnestic multiple-domain impairment. Overall, nonamnestic deficits predominated, occurring in 57%. Two-thirds of the PD-MCI group had single-domain impairment, either amnestic or nonamnestic subtypes. Within nonamnestic single-domain impairment, visuospatial deficits were frequent (73.8%), followed by attentional/executive dysfunction (18%), and language deficits (8.2%). When multiple domains were impaired, 76.7% had two domains involved, 23.3% had three domains affected, but none had all four domains impaired (Figure 1).
Figure 1.
Comparison of PD-MCI subtypes
There were no significant differences across PD-MCI subtypes regarding age, gender, or education (Table 1). PD duration, LEDD, vascular risk factors, and UPDRS Part III motor scores also did not differ significantly among PD-MCI subtypes, though mean UPDRS motor scores were higher in both multiple-domain groups compared to single-domain subtypes. Motor function as measured by Hoehn and Yahr stage, however, differed significantly among PD-MCI subtypes (χ2 [3, N=128]=8.15, p=0.04). More advanced Hoehn and Yahr stages occurred in nonamnestic multiple-domain PD-MCI subjects, compared to amnestic single-domain PD-MCI (p=0.036, corrected for multiple comparisons). Moreover, axial functioning/gait, one of the six clinically distinct UPDRS factors, differed significantly among PD-MCI subtypes (F [3, 117] 2.73, p=0.047), with worse axial function in nonamnestic multiple-domain subjects compared to nonamnestic single-domain subjects (p=0.05) (Table 2).
Table 1.
Clinical characteristics of PD-MCI subtypes
| Amnestic Single- domain N=24 |
Amnestic Multiple- domain N=31 |
Nonamnestic Single- domain N=61 |
Nonamnestic Multiple- domain N=12 |
p value |
|
|---|---|---|---|---|---|
| Age, years | 72.79 (10.57) | 71.81 (6.83) | 70.51 (10.88) | 76.50 (6.01) | 0.24 |
| Male, n (%) | 16 (66.7) | 20 (64.5) | 32 (52.5) | 8 (66.7) | 0.51 |
| Education, years | 15.50 (3.67) | 14.39 (3.01) | 14.26 (2.48) | 15.25 (3.02) | 0.37 |
| PD duration, years | 5.54 (4.13) | 7.06 (4.04) | 7.64 (5.16) | 9.04 (7.18) | 0.19 |
| UPDRS total motor score | 28.43 (11.46) | 31.83 (11.07) | 29.97 (12.08) | 36.42 (11.07) | 0.24 |
| Hoehn & Yahr stage (median, range) | 2.0, 2–4 | 2.0, 2–5 | 3, 1–4 | 3, 2–5 * | 0.04 |
| Vascular risk factor score (median, range) | 1.0, 0–5 | 1.0, 0–4 | 1.0, 0–4 | 1.5, 0–3 | 0.57 |
| LEDD, mg/d | 375.63 (308.42) | 524.84 (368.89) | 550.33 (552.65) | 753.96 (438.20) | 0.14 |
| Agonist, n (%) | 6 (25.0) | 10 (32.3) | 24 (39.3) | 4 (33.3) | 0.65 |
| Mood medication (antidepressant/anxiolytic), n (%) | 8 (33.3) | 14 (45.2) | 21 (34.4) | 0 (0) + | 0.05 |
| Sleep medication, n (%) | 4 (16.7) | 5 (16.1) | 9 (14.8) | 3 (25.0) | 0.86 |
| Antipsychotic, n (%) | 3 (12.5) | 3 (9.7) | 1 (1.6) | 1 (8.3) | 0.21 |
| Cognitive medication, n (%) | 2 (8.3) | 7 (22.6) | 4 (6.6) | 0 (0) | 0.06 |
Data presented as mean (SD), unless otherwise noted.
Abbreviations: LEDD, levodopa equivalent daily doses; UPDRS, Unified Parkinson’s Disease Rating Scale.
Nonamnestic multiple-domain vs. Amnestic single-domain PD-MCI, p=0.04;
Nonamnestic multiple-domain vs. Amnestic multiple-domain PD-MCI, p=0.03
Table 2.
UPDRS factor scores for PD-MCI subtypes
| Amnestic Single-domain N=24 |
Amnestic Multiple- domain N=31 |
Nonamnestic Single-domain N=61 |
Nonamnestic Multiple- domain N=12 |
p value | |
|---|---|---|---|---|---|
| Axial functioning/gait | 5.90 (2.87) | 6.89 (3.54) | 5.97 (3.32) | 8.84 (4.12) * | 0.05 |
| Rest tremor | 1.48 (1.82) | 1.78 (2.22) | 1.43 (2.22) | 1.17 (2.05) | 0.83 |
| Rigidity | 5.17 (2.41) | 4.96 (2.41) | 5.35 (2.57) | 6.08 (1.72) | 0.59 |
| Left side bradykinesia | 3.96 (2.41) | 4.79 (2.02) | 4.29 (1.90) | 5.53 (1.83) | 0.12 |
| Right side bradykinesia | 3.54 (1.86) | 4.27 (2.02) | 4.01 (2.14) | 4.84 (1.49) | 0.30 |
| Postural tremor | 1.13 (1.20) | 1.14 (1.16) | 1.27 (1.18) | 0.74 (1.13) | 0.56 |
Data presented as mean (SD), unless otherwise noted.
Nonamnestic multiple-domain vs. nonamnestic single-domain, p=0.05
Regarding non-motor features, PD-MCI subtypes did not differ in depression or psychosis. Amnestic multiple-domain PD-MCI subjects were treated more frequently with antidepressants or anxiolytics, compared to nonamnestic multiple-domain PD-MCI subjects. As defined, PD-MCI subtypes differed significantly in cognitive and MMSE scores (F [3, 124] 18.86, p<0.0001), with the lowest MMSE scores in nonamnestic multiple-domain PD-MCI subjects (Table 3, Supplemental table). There was a trend for greater use of cognitive medications (i.e., cholinesterase inhibitors or memantine) in amnestic multiple-domain PD-MCI subjects (χ2 [3, N=128]=7.49, p=0.06).
Table 3.
Neuropsychological features and cognitive domain scores for PD-MCI subtypes
| Amnestic Single- domain N=24 |
Amnestic Multiple- domain N=31 |
Nonamnestic Single- domain N=61 |
Nonamnestic Multiple- domain N=12 |
p value | |
|---|---|---|---|---|---|
| MMSE | 27.88 (1.92) * | 26.39 (1.91) | 27.87 (1.48) ^ | 25.08 (3.45) | <0.0001 |
| Cognitive complaints, n (%) | 18 (75) | 31 (100)† | 48 (78.7) | 9 (75) | 0.04 |
| Hamilton Depression rating scale | 5.61 (3.24) | 7.61 (4.36) | 7.39 (4.16) | 7.33 (3.75) | 0.26 |
| UPDRS Thought disorder score (median, range) | 0, 0–3 | 0.5, 0–4 | 0.5, 0–3 | 0.5, 0–1 | 0.84 |
| Cognitive domain z-scores | |||||
| Attentional/Executive Function | −0.70 (0.58) | −1.17 (0.57) | −0.82 (0.70) | −1.58 (0.50) | <0.0001 |
| Memory | −1.95 (0.31) | −1.97 (0.40) | −0.60 (0.59) | −0.82 (0.54) | <0.0001 |
| Language | 0.89 (1.23) | 0.25 (1.48) | 0.55 (1.33) | −1.47 (2.44) | 0.02 |
| Visuospatial | −0.78 (0.71) | −2.95 (3.13) | −1.91 (1.66) | −2.77 (0.95) | <0.0001 |
Data presented as mean (SD), unless otherwise noted.
Abbreviations: MMSE, MiniMental State Examination; UPDRS, Unified Parkinson’s Disease Rating Scale.
Amnestic single-domain vs. Amnestic multiple-domain, p=0.03; vs. Nonamnestic multiple-domain, p<0.0001;
Nonamnestic single-domain vs. Amnestic multiple-domain, p=0.004; vs. Nonamnestic multiple-domain, p<0.0001;
Amnestic multiple-domain vs. Amnestic single-domain, p=0.02; vs. Nonamnestic single-domain, p=0.04; vs. Nonamnestic multiple-domain, p=0.02
We also compared single- and multiple-domain PD-MCI subtypes to examine differences related to degree of cognitive dysfunction rather than qualitative categorization as amnestic or nonamnestic. Compared to single-domain PD-MCI subjects, multiple-domain PD-MCI subjects had significantly worse MMSE scores (t [126] = 5.11, p<0.001) and more frequent cognitive complaints (t [126] = 2.17, p=0.03). Multiple-domain PD-MCI subjects had worse motor function as measured by Hoehn and Yahr stage (t [126] = 1.96, p=0.05) and UPDRS factors reflecting axial/gait function (t [119] = 2.31, p=0.02) and left-sided bradykinesia (t [119] = 2.09, p=0.04).
Predictors of PD-MCI subtype
We assessed predictors of PD-MCI subtypes using multinominal logistic regression models with age, PD duration, LEDD, Hamilton Depression Rating score, vascular risk factor score, and motor severity (Hoehn and Yahr staging, UPDRS motor factors, or UPDRS total motor score) as predictors and PD-MCI subtype as the dependent variable. Hoehn and Yahr stage contributed significantly to the model (χ2 = 10.58, df 3, p = 0.01), with significant differences in nonamnestic multiple-domain PD-MCI compared to amnestic single-domain PD-MCI (Wald 8.89, p=0.003). Of the UPDRS motor factors, only axial functioning/gait contributed significantly (χ2 = 8.33, df 3, p = 0.04), with significant differences between nonamnestic multiple-domain and amnestic single-domain PD-MCI (Wald 5.92, p=0.02). Age, PD duration, LEDD, depression, vascular factors, or UPDRS total motor scores did not predict PD-MCI subtype.
Discussion
The main finding of our study was that PD-MCI subtypes differed in motor stage and axial functioning/gait, in addition to cognitive phenotype. Our findings suggest a link between nonamnestic multiple-domain PD-MCI and motor impairment, particularly axial functioning/gait disturbances. Few studies have examined PD-MCI subtypes and their motor features, and to our knowledge, none has described discrete UPDRS motor factors. In the largest reported PD-MCI cohort (n=1346) drawn from eight centers, amnestic and nonamnestic multiple-domain PD-MCI subtypes had worse motor symptoms than single-domain PD-MCI subjects, but additional details regarding motor phenotype were not presented 14. Other studies included small PD-MCI cohorts. In a longitudinal study of 38 PD-MCI subjects, at baseline examination, those with multiple nonamnestic domains “slightly impaired” (n=15) had higher mean Hoehn and Yahr stages than those with amnestic impairment (n=6) 6. In a retrospective chart review of 38 PD-MCI subjects, comparison of single-domain vs. multiple-domain, or amnestic vs. nonamnestic subtypes did not reveal statistically significant differences in motor characteristics, though details were not given and subject numbers, small 19.
Greater motor severity and worse cognitive function have been associated with shared neural substrates, increased dementia risk, and earlier dementia onset 6–8, 42, 43. Specifically, the axial motor phenotype, characterized by postural instability and gait disturbance, may be linked to cognitive impairment in PD 44, 45. Cholinergic deficits in the pedunculopontine nucleus and neocortex may underlie PD-related postural instability and gait disturbances 46, 47 and in basal forebrain nuclei, prefrontal and temporal regions, may impair attentional/executive function and memory in PD 46, 48. Increased fall risk in PD may be associated with worse performance on frontal lobe tasks 49, 50; PD patients with attentional/executive dysfunction demonstrate variable gait and slower speeds when simultaneously walking and performing cognitive tasks 51, 52.
Vascular risk factors, which have been associated with cognitive and gait impairment, were low in our PD-MCI cohort overall. Nonamnestic multiple-domain PD-MCI had higher median scores, though not statistically significant. White matter lesions on brain magnetic resonance imaging (MRI) 53–55 have been associated with increased gait variability 56 and falls 57 in older adults and in one PD study, the postural instability/gait difficulty-dominant phenotype 54. Neuroimaging may help elucidate the relationships between PD-MCI subtypes, gait impairment, and vascular burden.
The cognitive profile of our PD-MCI cohort featured predominantly nonamnestic deficits and single-domain phenotype, similar to other studies 1, 13, 15, 16, 18. Of the nonamnestic domains impaired, attentional/executive function and visuospatial abilities were primarily affected. The high rate of visuospatial impairment may signify greater posterior-cortical impairment, which in some studies confers an increased dementia risk 7, 58, 59. In our cohort, both multiple-domain PD-MCI subtypes had prominent visuospatial deficits. Multiple-domain PD-MCI subtypes demonstrated a greater burden of cognitive dysfunction (i.e., lower MMSE and other cognitive scores) compared to single-domain PD-MCI, and thereby reflect a gradient of cognitive severity and a progressive distribution of neuroanatomical regions involved in cognitive deficits. Similarly, greater postural instability may signal advancing motor severity and non-dopaminergic involvement. Of particular interest in our cohort, however, was that the two multiple-domain PD-MCI subtypes differed in motor profile, with only nonamnestic multiple-domain PD-MCI exhibiting significantly worse axial functioning/gait. Distinct cognitive and motor phenotypes within multiple-domain subtypes may indicate different contributions from PD, coexistent AD, vascular disease, or other neuropathologies. Longitudinal follow-up studies of our PD-MCI cohort will permit investigations of cognitive and motor progression.
With growing recognition of PD-MCI as a construct and pre-dementia state, a greater understanding of PD-MCI subtypes, their neurobiology, and progression is paramount to advancing our treatments for cognitive decline. In non-PD populations, MCI subtypes may differ in etiologies and conversion rates to dementia. Amnestic subtypes (in non-PD) are more likely to convert to AD; nonamnestic subtypes typically develop non-AD dementias or depression 4, 5, 60, 61. MCI subtypes vary in their progression; some MCI patients even improve to normal cognition at follow-up, while others remain stable or decline. Higher rates of improvement in nonamnestic single-domain impairment MCI and higher rates of decline in multiple-domain impairment or amnestic subtypes have been reported at follow-up 60, 62–64. Additionally, MCI subtypes may have different functional consequences 65, 66, with worse financial management abilities in amnestic MCI and worse performance on health and safety measures in nonamnestic MCI.
In PD, there is increasing evidence from neuroimaging, genetics, and neuropathology that different cognitive phenotypes reflect different neurobiological substrates. Compared to amnestic MCI patients without PD, amnestic PD-MCI (single- and multiple-domain impairment) patients exhibited prefrontal and temporal lobe atrophy 67 and hypoperfusion in parieto-occipital regions on neuroimaging studies 68. Single-domain PD-MCI patients also demonstrated prefrontal and parietal lobe atrophy, regions similarly affected in multiple-domain PD-MCI but to a greater extent 67, 69. Genetic studies suggest dissociations between fronto-striatal and posterior-cortical dysfunction in PD. Catechol-O-methyl transferase Val158Met gene polymorphisms, linked to executive functions, were not associated with dementia over 5-year follow-up of an incident PD cohort 58, whereas microtubule-associated protein tau H1/H1 genotypes, associated with posterior-cortical functions, were 58 70. The neurochemistry of cognitive processes, however, is complex, involving dopaminergic and non-dopaminergic neurotransmitters with direct, indirect, and modulating effects. Classic dopaminergic deficits associated with PD executive function may depend on disease duration and stage; 71 executive functions also have monoaminergic and cholinergic influences 72, 73, and dopamine plays a role in learning and memory 74. With neuropathological examination of eight PD-MCI cases (mixed subtypes) revealing mixed Lewy bodies, AD, and cerebrovascular pathologies 75, future biomarker and post-mortem studies of nonamnestic and amnestic PD-MCI subtypes are needed to delineate the neural substrates of these heterogeneous clinical phenotypes and identify cases with co-existent PD and AD pathology.
Our study’s strengths include a large, well-defined PD cohort, diagnosis by Movement Disorder specialists, and detailed motor and cognitive characterizations including factors (e.g., medications, mood, disorders, and vascular disease), which can affect cognitive function. As diagnostic criteria specifically for MCI in PD have only been recently developed 2, we utilized well-recognized classification schema for MCI and its subtyping 5, 22 and excluded PDD using currently recommended MDS-PDD criteria 3. Our rationale for amnestic/nonamnestic MCI subtyping was rooted not only in historical AD and MCI literature and PD-MCI studies to date, but also in our investigation of distinct clinical and neurobiological substrates in amnestic and nonamnestic phenotypes. With large PD-MCI cohorts and collaborative efforts, future studies can compare PD-MCI subtypes across individual cognitive domains and using recently proposed MDS PD-MCI criteria.
Limitations include our university setting and relatively highly educated subjects, which may diminish the direct extrapolation of our findings to a general population. A number of neuropsychological decisions (e.g., selection and number of tests administered, classification of tests in cognitive domains, and cut-off scores for defining MCI) were anchored in frequently used neuropsychological schemas and our own experience, 1, 20, 21 and thus, could lead to over- or under-estimations of PD-MCI and its subtypes. In the evolving area of PD-MCI, definitive guidelines for these issues have not yet been established, and recently published MDS PD-MCI criteria await validation. In the absence of large samples suitable for factor analyses of cognitive domains 76, we followed general neuropsychological principles and previous literature in classifying neuropsychological tests into cognitive domains. With this methodology, our concept of an executive system encompassed executive functions and attentional roles 77–79. Neuropsychological tests can be grouped into cognitive domains differently; some tests have overlapping features (e.g., executive components of visuospatial tests), and others may be sensitive to deficits in more than one area (e.g., category fluency and temporal and frontal dysfunction). Also, although our PD-MCI cohort was relatively large (n=128), the nonamnestic multiple-domain subtype was the smallest. Although we assessed and controlled for this statistically, non-balanced groups may affect results, and findings related to the smaller group will require replication in larger samples. Lastly, our motor evaluation focused specifically on the UPDRS Part III (total score and component factor analyses) and Hoehn and Yahr staging, but future studies incorporating dual-tasking paradigms or other motor analyses may clarify the motor associations of attentional/executive dysfunction.
We conclude that besides the heterogeneous cognitive phenotype of PD-MCI, PD-MCI subtypes may be distinguished by their motor profile. These findings suggest the association of nonamnestic multiple-domain PD-MCI and greater axial/gait dysfunction. Longitudinal studies of large, well-defined PD-MCI cohorts will be needed to determine the progression and prognoses of different PD-MCI subtypes and to compare effects of increased burden of cognitive dysfunction (i.e., multiple-domains affected) and type of cognitive deficit (i.e., amnestic or nonamnestic deficits) on the risk of dementia.
Supplementary Material
Acknowledgements
This study was supported by grants K23NS060949 (JGG) from the National Institutes of Health and from the Parkinson’s Disease Foundation.
Appendix
Full Financial Disclosures of all Authors for the Past Year: Information concerning all sources of financial support and funding for the preceding twelve months, regardless of relationship to current manuscript. List sources or “none”:
Jennifer G. Goldman, MD, MS:
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: Movement Disorders Society | Royalties: none |
| Grants: NIH K23NS060949 | Other: Parkinson’s Disease Foundation |
Holly Weis, MD:
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Lutheran General Hospital |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Glenn Stebbins, PhD:
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: IMPAX Laboratories, Inc.; Ceregene, Inc.; Biovail Technologies, LTD; Santhera Pharmaceuticals; i3 | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH, Michael J. Fox Foundation for Parkinson’s Research, American Cancer Society, Fragile X Foundation | Other: Editorial Board, Journal of Clinical and Experimental Neuropsychology |
Bryan Bernard, PhD:
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Christopher G. Goetz, MD:
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: Addex Pharma SA, Asubio, Biovail Technologies, Cleveland Medical Devices, CNS Therapeutics, Curry Rockerfeller Group, Decision Resources, Dixon Group, ICON Clinical Research, Impax Pharmaceuticals, Ingenix (i3 Research), Intec Pharmaceuticals, Kenes International, Medical Education Global Solutions, Ono Pharmaceuticals, Oxford Biomedica, Santhera, United Bioscience Corporation, UCB. |
Expert Testimony: none |
| Advisory Boards: Addex Pharma SA, Asubio, Biovail Technologies, Cleveland Medical Devices, CNS Therapeutics, Curry Rockerfeller Group, Decision Resources, Dixon Group, ICON Clinical Research, Impax Pharmaceuticals, Ingenix (i3 Research), Intec Pharmaceuticals, Kenes International, Medical Education Global Solutions, Ono Pharmaceuticals, Oxford Biomedica, Santhera, United Bioscience Corporation, UCB. |
Employment: Rush University Medical Center |
| Partnerships | Contracts: None |
| Honoraria: Movement Disorder Society, American Academy of Neurology, University of Miami, University of Pennsylvania, University of Montreal. Neurological Society. |
Royalties: Royalties: Oxford University Press, Elsevier Publishers, Wolters Kluwer Health, Lippincott, Wilkins and Williams. |
| Grants: Funding from NIH, Michael J. Fox Foundation, NIH. Dr. Goetz directs the Rush Parkinson’s Disease Research Center that receives support from the Parkinson’s Disease Foundation. He directs the translation program for the MDSUPDRS and UDysRS and receives funds from the MDS for this effort. |
Other: none |
Footnotes
Financial Disclosure/Conflict of Interest: Dr. Goldman has received grant/research support from NIH K23NS060949 and the Parkinson’s Disease Foundation.
Jennifer G. Goldman, MD, MS: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique
Holly Weis, MD: 1) Research project: C. Execution; 3) Manuscript: B. Review and Critique
Glenn Stebbins, PhD: 1) Research project: A. Conception, B. Organization; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
Bryan Bernard, PhD: 1) Research project: C. Execution; 3) Manuscript: B. Review and Critique
Christopher G. Goetz, MD: 1) Research project: A. Conception, B. Organization; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
References
- 1.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD-MCI. Mov Disord. 2011 doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012 doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 7.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 9.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 10.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 11.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson's disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118–123. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 12.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 13.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 14.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 16.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 17.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson's disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 19.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson's disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16(3):177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson's disease. Mov Disord. 2011;26(4):629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- 21.Troster AI. A Precis of Recent Advances in the Neuropsychology of Mild Cognitive Impairment(s) in Parkinson's Disease and a Proposal of Preliminary Research Criteria. J Int Neuropsychol Soc. 2011:1–14. doi: 10.1017/S1355617711000257. [DOI] [PubMed] [Google Scholar]
- 22.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.Appollonio I, Grafman J, Clark K, Nichelli P, Zeffiro T, Hallett M. Implicit and explicit memory in patients with Parkinson's disease with and without dementia. Arch Neurol. 1994;51(4):359–367. doi: 10.1001/archneur.1994.00540160053008. [DOI] [PubMed] [Google Scholar]
- 24.Higginson CI, Wheelock VL, Carroll KE, Sigvardt KA. Recognition memory in Parkinson's disease with and without dementia: evidence inconsistent with the retrieval deficit hypothesis. J Clin Exp Neuropsychol. 2005;27(4):516–528. doi: 10.1080/13803390490515469. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17(4):195–200. [PubMed] [Google Scholar]
- 26.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 28.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 29.Stebbins GT, Goetz CG. Factor structure of the Unified Parkinson's Disease Rating Scale: Motor Examination section. Mov Disord. 1998;13(4):633–636. doi: 10.1002/mds.870130404. [DOI] [PubMed] [Google Scholar]
- 30.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. Development of a rating scale for primary depressive illness. Brit Jrl Soc Clin Psych. 1967;6:278–297. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Weschler D. Weschler Adult Intelligence Scale-III. New York: The Psychological Corporation; 1991. [Google Scholar]
- 34.Smith A. Symbol Digits Modality Test. Los Angeles: Western Psychological Services; 1973. [Google Scholar]
- 35.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test Philadelphia Lea & Febiger. 1983. [Google Scholar]
- 37.Benton ALSA, Hamsher K DeS, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2nd ed. ed. New York: Oxford University Press; 1994. [Google Scholar]
- 38.Bourke JCC, Stephen B, Dennis M. A comparison of clock and pentagon drawing in Alzheimer's disease. Int J Geriatr Psychiatry. 1995;10:703–705. [Google Scholar]
- 39.Cormack FAD, Ballard C, Tovee M. Pentagon drawing and neuropsychological performance in Dementia with Lewy Bodies, Alzheimer's disease, Parkinson's disease and Parkinson's disease with dementia. Int J Geriatr Psychiatry. 2004;19:371–377. doi: 10.1002/gps.1094. [DOI] [PubMed] [Google Scholar]
- 40.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 41.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 42.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21(8):1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 43.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77(5):585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59(9):1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 45.Verbaan D, Marinus J, Visser M, et al. Cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78(11):1182–1187. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. 2011;26(14):2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 47.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 49.Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(2):110–115. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord. 2009;24(9):1280–1289. doi: 10.1002/mds.22561. [DOI] [PubMed] [Google Scholar]
- 51.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson's disease. J Geriatr Psychiatry Neurol. 2003;16(1):53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 52.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 53.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36(1):56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 54.Lee SJ, Kim JS, Lee KS, et al. The severity of leukoaraiosis correlates with the clinical phenotype of Parkinson's disease. Arch Gerontol Geriatr. 2009;49(2):255–259. doi: 10.1016/j.archger.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 56.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3–4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40(1):175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 58.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease; 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 59.Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Mov Disord. 2008;23(7):998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 60.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76(11):1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81(5):541–546. doi: 10.1136/jnnp.2008.171066. [DOI] [PubMed] [Google Scholar]
- 63.Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3- year follow-up period. Dement Geriatr Cogn Disord. 2009;27(5):418–423. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ritchie K, Ancelin ML, Beaino E, et al. Retrospective identification and characterization of mild cognitive impairment from a prospective population cohort. Am J Geriatr Psychiatry. 2010;18(8):692–700. doi: 10.1097/JGP.0b013e3181df4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bangen KJ, Jak AJ, Schiehser DM, et al. Complex activities of daily living vary by mild cognitive impairment subtype. J Int Neuropsychol Soc. 2010;16(4):630–639. doi: 10.1017/S1355617710000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim KR, Lee KS, Cheong HK, Eom JS, Oh BH, Hong CH. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(3):278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- 67.Lee JE, Park HJ, Song SK, Sohn YH, Lee JD, Lee PH. Neuroanatomic basis of amnestic MCI differs in patients with and without Parkinson disease. Neurology. 2010;75(22):2009–2016. doi: 10.1212/WNL.0b013e3181ff96bf. [DOI] [PubMed] [Google Scholar]
- 68.Nobili F, Abbruzzese G, Morbelli S, et al. Amnestic mild cognitive impairment in Parkinson's disease: a brain perfusion SPECT study. Mov Disord. 2009;24(3):414–421. doi: 10.1002/mds.22381. [DOI] [PubMed] [Google Scholar]
- 69.Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 Pt 2):1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seto-Salvia N, Clarimon J, Pagonabarraga J, et al. Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch Neurol. 2011;68(3):359–364. doi: 10.1001/archneurol.2011.17. [DOI] [PubMed] [Google Scholar]
- 71.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 72.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(Suppl 1):i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 73.Bohnen NI, Muller ML, Kotagal V. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bethus I, Tse D, Morris RG. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30(5):1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler CH, Caviness JN, Sabbagh MN, et al. Heterogeneous neuropathological findings in Parkinson's disease with mild cognitive impairment. Acta Neuropathol. 2010;120(6):827–828. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacCallum R, Widaman K, Preacher K, Hong S. Sample size in factor analysis: the role of model error. Multivariate Behavioral Research. 2001;36(4):611–637. doi: 10.1207/S15327906MBR3604_06. [DOI] [PubMed] [Google Scholar]
- 77.Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139(1):5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 78.Baddeley A, Della Sala S. Working memory and executive control. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1397–1403. doi: 10.1098/rstb.1996.0123. discussion 1403-1394. [DOI] [PubMed] [Google Scholar]
- 79.Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1405–1411. doi: 10.1098/rstb.1996.0124. discussion 1411-1402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.