Abstract
Renal injury induced by brain death is characterized by ischemia and inflammation and limiting it is a therapeutic goal that could improve outcomes in kidney transplantation. Brain death resulted in decreased circulating nitrite levels and increased infiltrating inflammatory cell infiltration into the kidney. Since nitrite stimulates nitric oxide signaling in ischemic tissues, we tested whether nitrite therapy was beneficial in a rat model of brain death followed by kidney transplantation. Nitrite, administered over 2 hours of brain death, blunted the increased inflammation without affecting brain death-induced alterations in hemodynamics. Kidneys were transplanted after 2 hours of brain death and renal function followed over 7 days. Allografts collected from nitrite-treated brain dead rats showed significant improvement in function over the first 2 to 4 days post transplantation compared to untreated brain dead animals. Gene microarray analysis after 2 hours of brain death without or with nitrite therapy showed the latter significantly altered the expression of about 400 genes. Ingenuity Pathway analysis indicated multiple signaling pathways were affected by nitrite, including those related to hypoxia, transcription and genes related to humoral immune responses. Thus, nitrite-therapy attenuates brain death-induced renal injury by regulating responses to ischemia and inflammation, ultimately leading to better post-transplant kidney function.
Keywords: inflammation, nitric oxide, kidney, transplantation
Introduction
Brain dead (BD) donors account for a significant source (~60%) for all renal allografts transplanted in the US. Kidneys acquired from BD donors have inferior survival rates compared to living donors due to BD induced organ injury that comprises initially of massive acute cerebral injury, neurogenic shock, systemic vasoconstriction and hypoperfusion of multiple organs including the kidney 1–4. In addition to the tissue ischemia that ensues, electrolyte abnormalities and increased levels of pro-inflammatory cytokines post BD combine to result in inflammatory injury to the kidney that primes this organ for an exacerbation of ischemia-reperfusion (I/R) injury response post transplantation 5,6. This ‘two-hit’ process is a major contributor to poor kidney function and allograft survival post transplantation using organs from BD donors and has been demonstrated with other organ transplants. Limiting ischemic and inflammatory injury by treating the BD donor with corticosteroids, recombinant soluble P-selectin glycoprotein ligand, or erythropoietin have been shown to improve post transplantation renal function and survival in experimental models supporting the idea that therapeutics targeting the BD phase can be beneficial 7–9.
Nitric oxide (NO) is an important endogenous modulator of inflammation and at lower concentrations protects against inflammatory stress by multiple mechanisms including limiting leukocyte-adhesion, antioxidant activity, improving tissue oxygenation, and inhibiting cell death. This view is supported by studies showing that therapies that increase NO bioavailability confer protection against I/R injury in both experimental and clinical studies 10–17. Major limitations with NO-therapy are unwanted systemic vasodilator effects highlighting the lack of therapies that target NO-formation to specific tissues. Inorganic nitrite (NO2−) has long been considered an inert end product of aerobic NO metabolism. However, recent insights reveal the presence of biological mechanisms that result in the 1-electron reduction of nitrite back to NO and stimulation of NO-dependent signaling. Importantly, this reduction is focused in hypoxic (or ischemic)/acidic tissues resulting in targeted NO-formation. Proposed biochemical mechanisms include nitrite reduction by heme/ molybdenum containing metalloproteins (including xanthine oxidoreductase (XOR), hemoglobin and myoglobin) whose ability to reduce nitrite is dependent on lower oxygen tensions 18–22. Consistent with this concept several studies have shown the potential for nitrite therapy to stimulate NO-signaling only in ischemic tissues and mitigate I/R injury 11,13,23–28. Moreover, there is precedent for NO-based therapy to counter detrimental effects of BD. L-arginine therapy repletes NO-signaling and protected against BD dependent endothelial dysfunction and myocardial blood flow 29.
In this study, we evaluated the potential of nitrite to protect against I/R damage that occur to the kidney during BD and improve function post transplantation. Presented data show that nitrite therapy to the BD donor can be administered safely and improve function post transplantation via anti-inflammatory mechanisms.
Results
Brain death (2h) decreases circulating, but not renal nitrite levels
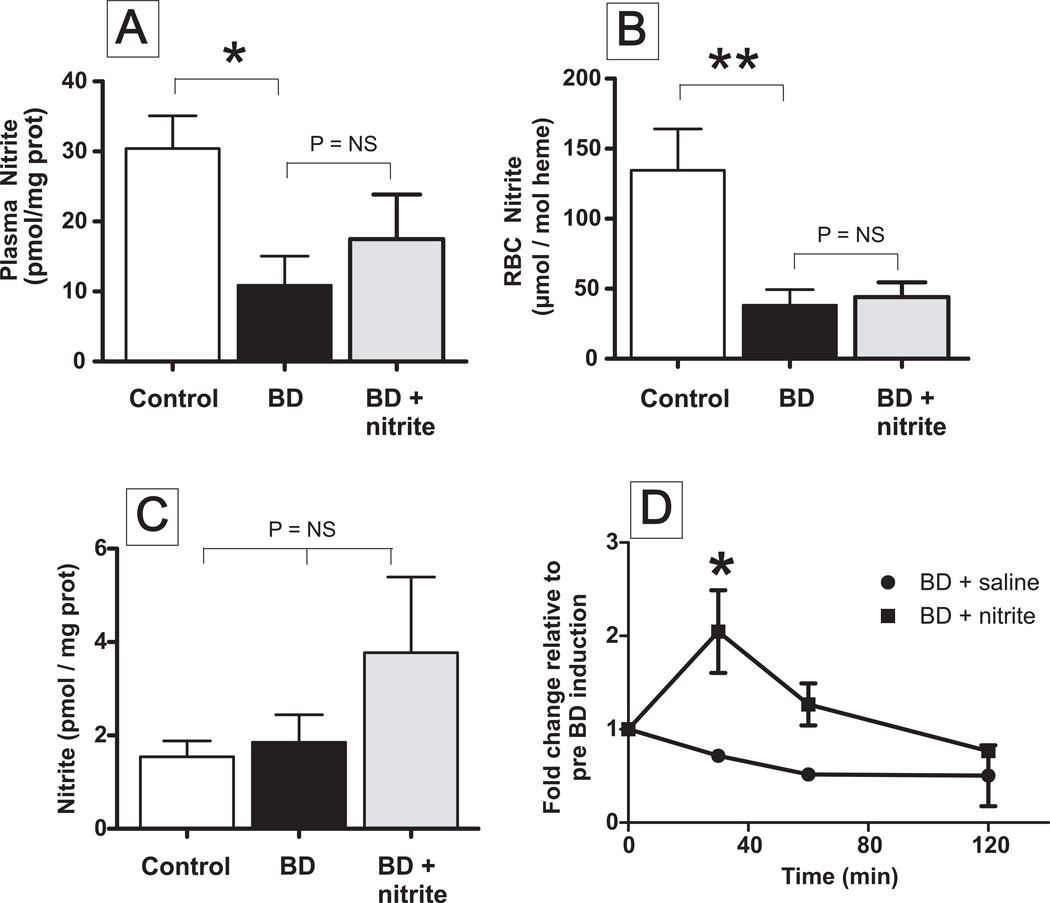
Nitrite levels decrease during ischemic stress and nitrite therapy has been shown to improve renal function after ischemia-reperfusion 13,25,28,30. Using nitrite + nitrate as an index for NO-production, previous studies have concluded that excessive NO formation does not occur during 2h BD in rats 31. Recent studies however, have suggested that nitrite is a more selective index of NO-formation in mammals 32. Figure 1 shows that circulating nitrite levels are decreased ~60–70% after 2h of BD. However, renal nitrite levels did not change after BD.
Figure 1. Circulating nitrite levels are decreased during brain death.
Plasma (Panel A), Red blood cell (RBC) (Panel B) and kidney (Panel C) concentrations of nitrite were measured in rats after 2h of sham (control), brain death (BD) or BD + nitrite therapy. Data show means ± SEM, n= 3–5. *P < 0.05, **P<0.02 relative to control by t-test. Panel D shows time dependent changes in plasma nitrite levels in BD and BD ± nitrite therapy groups. Data show fold change relative to time 0 (pre BD induction) and are mean ± SEM (n=2–3). P < 0.01 by 2-way ANOVA and *P<0.01 by Bonferroni post test. (Plasma nitrite levels without normalization to protein ranged between 44 – 1140nM).
Hemodynamic effects of nitrite therapy during brain death
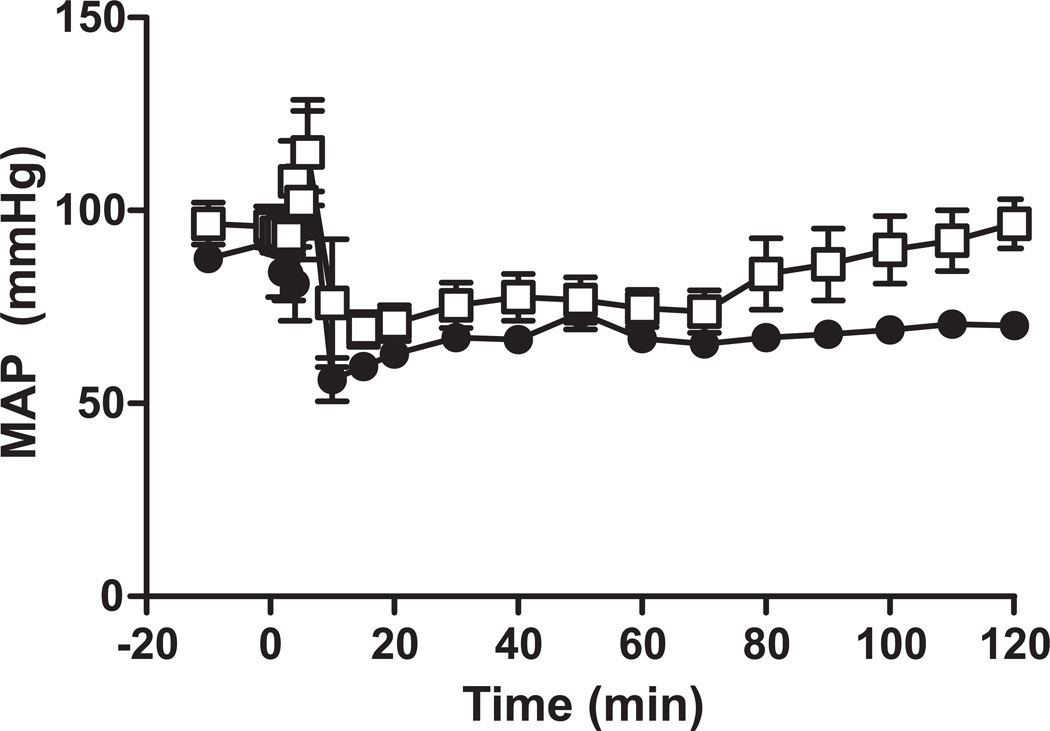
During induction of BD in this model, mean arterial pressure (MAP) increases and then rapidly (within 10mins) decreases and remains lower than before BD (Figure 2). Nitrite is a vasodilator and can promote hypotension. Initial studies therefore were designed to determine the optimal conditions for nitrite administration that would not exacerbate hypotension during brain death. Bolus administration of nitrite (0.1 mg/kg) led to a lower MAP compared to BD alone (not shown). We therefore tested an alternative nitrite administration protocol in which nitrite (0.1 mg) was administered over 2h of BD. Figure 2 shows that using this protocol, nitrite did not alter MAP compared to BD alone and was tested further for potential protective effects against BD induced kidney injury.
Figure 2. Effects of nitrite administration modality on mean arterial pressure (MAP) during brain death.
Rats were rendered brain dead (-●-) and then administered nitrite (0.1 mg/kg) as a bolus injection (-Δ-) or over 2h (-□-). No significant difference between BD alone or BD + nitrite administered over 2h by 2-way RM ANOVA was observed. Data show mean ± SEM (n=5) for BD alone or BD + continuous infusion of nitrite.
Nitrite therapy improves post-transplantation function of kidneys after brain death
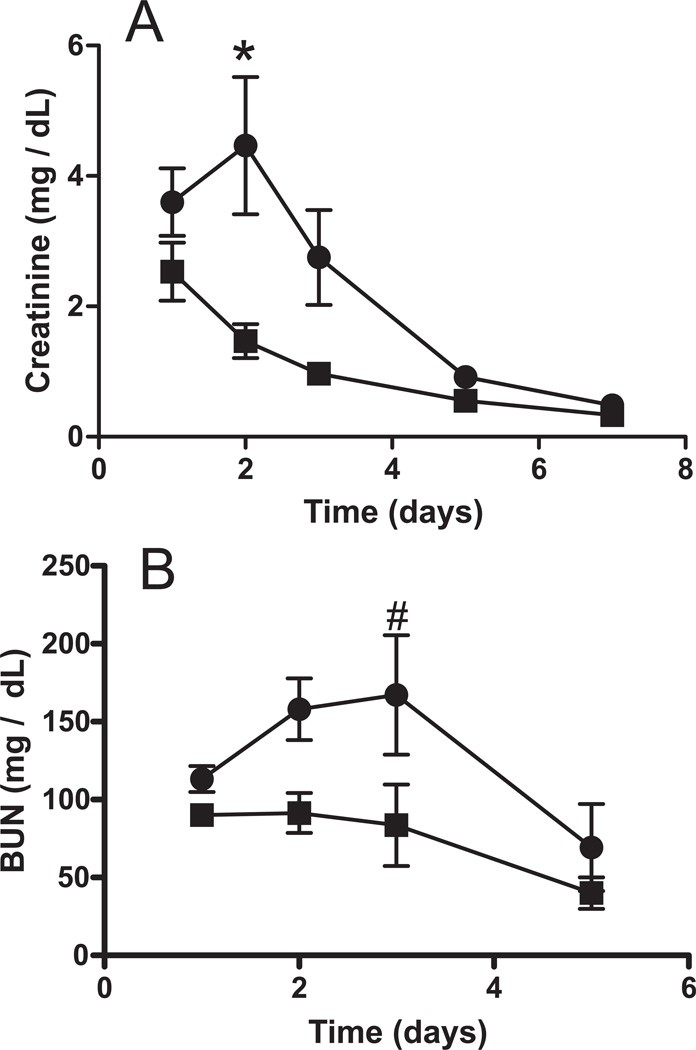
Kidneys from rats rendered BD for 2h which were administered either saline or nitrite were transplanted and renal function assessed by measuring plasma creatinine and BUN (blood urea nitrogen) as a function of time post transplantation. Figure 3 shows that after transplantation of a kidney after BD, plasma creatinine and BUN increased for 2–3 days and then started to decrease indicating an initial worsening of renal function followed by resolution of injury. After transplantation of kidneys isolated from BD rats treated with nitrite however, creatinine and BUN levels were lower and decreased at faster rate compared to the BD alone group. Figure 1A–C shows that protective effects of nitrite occurred without significant increased nitrite levels in the plasma, RBC or kidneys 2h after BD induction. However, Figure 1D shows that while circulating nitrite levels decrease from the onset of BD alone, with nitrite therapy, plasma nitrite levels are elevated for the initial 30–60min and then gradually decrease over the next 60 min.
Figure 3. Nitrite treatment improves post-transplantation renal function after brain death.
Kidneys were isolated from BD rats treated with saline (-●-) or nitrite (-■-) and then transplanted. Data show serum creatinine (Panel A) and BUN (Panel B) levels in the recipient as a function of time post-transplantation. Data are means ± SEM (n=6) and significantly different (P < 0.0001 for creatinine and P< 0.005 for BUN) by 2-way ANOVA and *P<0.001 or #P<0.05 by Bonferroni post test relative to BD + nitrite.
Nitrite prevents brain death induced inflammation in the kidney but not lipid peroxidation
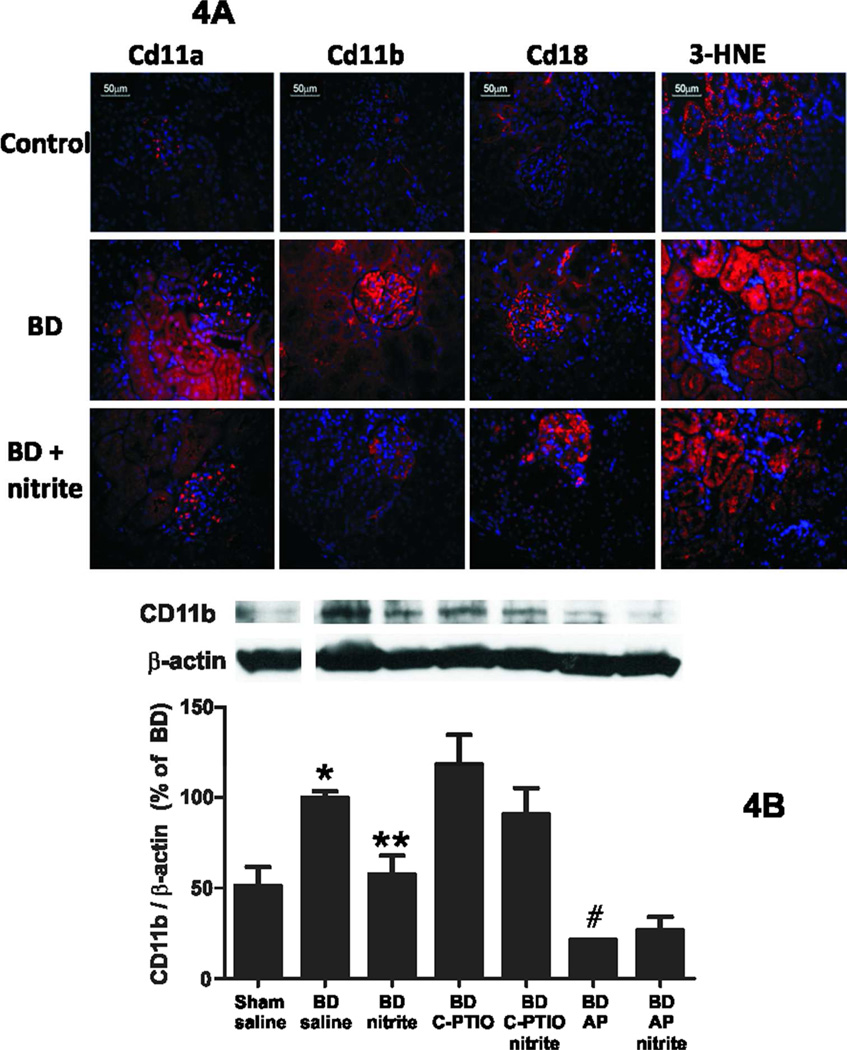
To evaluate the mechanisms underlying improved renal function post transplantation of kidneys collected from nitrite treated BD rats, expression of several markers / mediators of inflammation and oxidative stress were measured in kidneys collected after 2h of brain death. Figure 4 shows representative immunofluoresence staining for the adhesion molecules CD11a, CD11b, CD18 and the protein adduct of 3-HNE. All these markers increased after 2h BD and showed a tubular location consistent with increased inflammation and oxidative stress. Nitrite treatment decreased staining for CD11a and CD11b, further confirmed by significant decrease in CD11b measured by western blotting (Figure 4B). Interestingly, nitrite did not affect 3-HNE staining. No changes in expression of the pro-inflammatory peroxidase myeloperoxidase, the inflammatory marker 3-nitrotyrosine, or adhesion molecules ICAM-1, VCAM-1 were observed in BD exposed rats (not shown).
Figure 4. Nitrite treatment prevents BD induced increases in markers of leukocyte infiltration but not oxidative stress.
Panel A: Representative immunofluoresence images for Cd11a, Cd11b, Cd18 and 3-HNE (red staining) in control, BD (2h) or BD (2h) + nitrite. Blue staining represents nuclei stained with Hoechst 33342. Panel B: Western blot analysis for expression of CD11b / β-actin ratios. Data show mean ± SEM (n=2–6) and are normalized to BD alone group (set at 100%). P<0.001 by 1-way ANOVA with *P < 0.05 relative to sham, **P<0.05 relative to BD alone, #P<0.01 relative to BD alone by Tukey’s Multiple comparison post test.
Effect of C-PTIO and allopurinol on nitrite dependent protection against renal inflammation
Previous studies have shown that NO-scavenging (by C-PTIO) and/or inhibition of nitrite-reduction to NO by xanthine oxidoreductase (using allopurinol) prevents nitrite-dependent protection against ischemic tissue toxicity. Using CD11b expression as a marker, C-PTIO had no effect on BD induced renal injury and did not significantly reverse the protective effects of nitrite, although a trend towards a reversal is noted (Figure 4B). Allopurinol itself protected against BD induced renal inflammation. Nitrite had no additional effects and allopurinol did not reverse nitrite dependent inhibition of CD11b expression (Figure 4B).
Nitrite-dependent changes in gene expression determined by microarray profiling
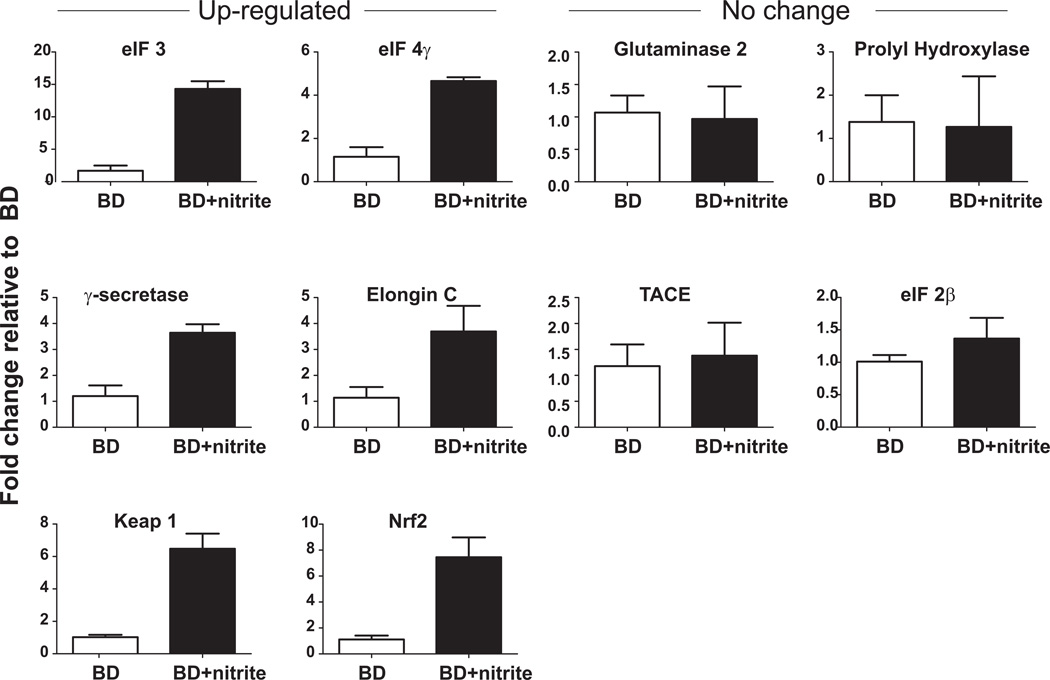
Supplementary Figure 1A shows a heat map showing that nitrite therapy altered the expression of 381 genes relative to BD alone, with 264 being down- and 117 up-regulated. Ingenuity Pathway Analysis (IPA) generated 19 networks (with >1 focus molecule identified) affected by nitrite therapy (Table 1) and consistent with recent reports showing nitrite therapy has diverse effects of gene expression profiles 33. Interestingly, the highest ranked network was related to the humoral immune response, an established key element of inflammatory tissue injury during BD in the kidney 34. Supplementary Figure 2 illustrates the top 5 canonical signaling pathways that are most significantly affected by nitrite therapy (and are elF2, Notch, HIF1α, Nrf2 / oxidative stress and elF4 / p70S6K respectively) and also indicate specific genes that maybe modulated by nitrite therapy. Supplementary Figure 1B lists the number of genes that are down- or up-regulated within these pathways. Figure 5 shows real time-PCR analyses of 9 selected genes encompassing the top 5 pathways and separates these into those that either did not change or were up-regulated by nitrite + BD relative to BD alone. Supplementary Table 1 compares mRNA array data with real time-PCR data and shows that nitrite dependent changes indicated by mRNA arrays were validated with 4 of the selected genes. Finally, since one of the genes validated to increase with nitrite therapy was Keap1, relative expression of Nrf2 was also determined. Figure 5 shows that this too was increased with nitrite therapy relative to BD alone.
Table 1.
| ID | Molecules in Network | Score | Focus Molecules |
Top Functions |
|---|---|---|---|---|
| 1 | ↓ADAM17, ↑ANXA13, Cbp, ↑COPS2, ↑COPS3, ↑DUSP5, ↑ECSIT, ↑HLA-C, ↑HLA-G, IFN alpha/beta, IFN Beta, ↑IRF3, ↑LITAF, ↑LRRFIP1, ↑LY6C1, MHC Class I (complex), MHC CLASS I (family), NFkB (complex), ↑NLRC4, Notch, ↑NUP98, ↓NUPL1, ↑PAK1IP1, ↓PDIA3, ↓PNPT1, ↑PTMA, ↑S100A6, ↑SEC13, ↑SLC2A5, sphingomyelinase, ↑SUGT1, Tap, ↓TFG, ↓TFPI, ↑ZMYND11 | 43 | 26 | Antigen Presentation, Antimicrobial Response, Humoral Immune Response |
| 2 | Akt, Ap1, ↑APEX1, ↑ASCC2, ↓ASS1, Ck2, ↑CPT1B, Cyclin A, ↓DARS, ↑DGCR6, ↑DHRSX, ↓DNMT1, ↓EED, ↑EEF1D, ↑EGLN2, Estrogen Receptor, ↑HBB (includes EG:3043), ↓JUN, ↑KLF7, ↓M6PR, ↓NFKBIB, oxidoreductase, ↑PDCD2, peptidase, ↑PI4KA, ↑PIP4K2A, ↓PSAP, Rar, ↓RORA, Rxr, ↑SLC19A1, ↓SNW1, ↑THRB, Thyroid hormone receptor, ↑VARS | 42 | 25 | Gene Expression, Tissue Morphology, Amino Acid Metabolism |
| 3 | Alpha tubulin, ↑BNIP3L, ↑CCT5, Creb, ↑DCN, ↑DUSP3, ↑EIF2B4, ERK1/2, FSH, ↓GEM, Gsk3, ↑GSK3B, ↑HSPA14, Insulin, ↑KLC1, Lh, ↑MAP4, p70 S6k, ↑PAF1, ↓PAH, PDGF BB, ↑PDPK1, Pi3-kinase, ↓POLR2B, PP1 protein complex group, PP2A, Ppp2c, ↑PPP2R2D, ↑RBBP5, ↑ROCK2, Rsk, ↑SLC6A6, ↑STIP1, ↓STK16, ↑TXK | 33 | 21 | Cell Morphology, Reproductive System Development and Function, Developmental Disorder |
| 4 | ↑C4ORF27, CDC5L, ↑COMMD9, ↑COQ6, ↑GLTSCR2, HNF1A, HNF4A, ITGB3BP, ↑KIAA1012, ↑MRPL15, ↑MRPL22, ↑MRPS11, ↓MRPS14, ↓MTHFS, POLRMT, ↑PRCC, SFRS2, ↓SLC35A3, ↑TFB2M, TGFB1, ↑TMEM140*, ↑TTC22, ↑ZNF410 | 27 | 16 | Gene Expression, Tissue Development, Endocrine System Development and Function |
| 5 | Alp, Alpha catenin, ↑ATF4, Caspase, ↑COL4A3, Collagen type I, Collagen type IV, ↑DDX31, ↑DDX52, ↑EDNRB, ↑HBXIP, ↑HES1, Holo RNA polymerase II, Hsp70, IL1, IL12 (complex), Immunoglobulin, Jnk, LDL, ↑MED29, ↑MED30, Mmp, ↓NCSTN, ↓NME3, ↑NOP58, PI3K, ↑PSEN1, ↑PTGES, RNA polymerase II, ↑TAF8, ↑TARDBP, ↑TCEB1, Tgf beta, ↓TMED2, Vegf | 26 | 18 | Nervous System Development and Function, Organ Development, Embryonic Development |
| 6 | ABCD3, ↑AP3B1, ↑BAT1, ↑BLOC1S1, BNIP1, ↑EMG1, ↑HDDC3, HNF4A, LSM3, ↑LSM4, LSM5, ↓LUC7L2, ↑MRPL43, NAPA, NMI, NSF, ↑PELO, ↑POLD4, POLE, POLL, ↑PPP6C, RFC3, SART3, SEC22B, ↑SFRS11, ↑SLC25A38, SOX10, ↑STK19, STX18, SUPT7L, TADA1, ↑TEAD3, ↑TMEM87B, ↑USE1, ZSCAN16 | 25 | 17 | Cellular Assembly and Organization, Molecular Transport, Protein Trafficking |
| 7 | ↑B3GNT1, ↑BANP, ↑BMPR2, Calcineurin protein(s), Cdc2, ↑EPS8, ↑EPS8L2, ERK, F Actin, ↑FLNB, Focal adhesion kinase, ↑HABP4, hCG, Ige, ↓IRX1, ↓JUNB, ↓LUC7L2, ↑MAP3K4, ↑MAPK14, ↑MAPKAPK2, Nfat (family), NGF, ↑NOP56, P38 MAPK, Pdgf, Pka, Pkc(s), ↑PLCGI, Rac, Ras, ↑RPA2, ↑RYR1, Sapk, TCR, VAV | 21 | 17 | Cell Morphology, Cellular Assembly and Organization, Genetic Disorder |
| 8 | ↓C10ORF2, DBN1, DDX56, ↑EG623169, EIF4A2, glutamyl-Se-methylselenocysteine, JRK, ↑LOC440733, LONP1, LYAR, MAGEB2, ↑MRPL23, MT-ND4, MT-ND5, MT-ND6, MT-ND4L, ↑MYO1C, ↑NDUFA10 (includes EG:678759), NDUFV2, PA2G4, ↓PGCP, phosphatidylinositol-3-phosphate, PIN4, PINX1, ↑RPL24, ↑RPL10A (includes EG:19896), ↑RPL13A, ↑RPL18A, ↑RPS11, selenomethylselenocysteine, SLC2A4, SNX1, ↑TXNDC12, ↑WDFY1*, WDR8 (includes EG:49856) | 19 | 14 | Cancer, Genetic Disorder, Neurological Disease |
| 9 | ANAPC13, ↑BAT1, ↓C16ORF80, ↑CDC16, CDC27, ↑DNAJC11, ↑DPEP1, DUSP15, EXTL3, ↑FAM110A, ↓FNBP4, FYN, GRB2, HTT, ISG20L2, ↓LUC7L2, MEPE, ↑OSBPL3, PAX3, ↑PDE10A, POP1, POP4, ↑RBM25 (includes EG:58517), RIN3, ↑RPP14, RPP21, RPP30, RPP38, RPP40, ↑RPS13, SAPS3, SUV39H2, SVIL, ↑TMEM102, YWHAG | 19 | 14 | Nervous System Development and Function, Organismal Development, Neurological Disease |
| 10 | AHSG, ANAPC2, ANAPC4, ↑ATOX1, BACE1, BMP1, ↑DAZAP1, ↑DCN, EIF4E, ↑ENTPD5, Eotaxin, Ferritin, FN1, ↑GNL1, ↑HCCS, Ifn, ITGB6, LGALS3BP, NAIP, ↑PAPPA, ↑PDRG1, PGF, PRG2, ↑PTMA, ↑SGCA, ↓SNRPA, SOCS, SOCS3, ↓ST6GAL1, STX6, SVIL, Talin, TNF, ↑VPS45, XIAP | 17 | 13 | Inflammatory Disease, Skeletal and Muscular Disorders, Cellular Response to Therapeutics |
| 11 | ↓AASS, ↑ACVR2B, AHNAK, ↑BANP, BUB1, CDH13, CDKN1A, ↑CNIH4, ↑CNKSR3, COL6A1, COL6A3, DHX36, ERBB2, ↑FAM110B, ↑GALNT3, ↓GOLGA5, IARS, ILK, LXN, ↑MAP4, MSTN, NET1, ↓PANK2, PARVA, RAB1A, Rb, ↓RFC4, RRAD, ↑S100A6, SPINT2, TGFB1, Type II Receptor, VPS39, YWHAZ, ↓ZNF593 | 17 | 13 | Genetic Disorder, Skeletal and Muscular Disorders, Skeletal and Muscular System Development and Function |
| 12 | ATXN1, beta-estradiol, ↑C13ORF33, ↑CDK11A, EDA, EIF3, EIF3B, EIF3CL, ↑EIF3D, EIF3G, ↑EIF3H, EIF3K, EIF3L, ↓EIF4G3, GAR1, GARS, GMFB, ↓KIAA2026, MBP, NARS, ↑NHP2, PNP, PTPRD, ↑PYGM, ↓R3HDM2, RCN2, RHPN2, ↑SAMD8, SAPS2, ↑SPOCK2, TGFBR1, TRAF6, VAMP1, ↓VAPA, ↑WFDC2 | 17 | 13 | Protein Synthesis, Digestive System Development and Function, Cell Death |
| 13 | ASCC3, ↓AUH, BATF, ↑C21ORF7, C3AR1, ↓C9ORF46, ↑CD248, CD3EAP, COL6A3, CYSLTR2, ↑DUSP11, GPX4, ↑HDLBP, IL4, IL13, IL15, IL19, ↑LAS1L, LGALS3BP, LSP1, MIR373, ↓MKRN1, ↓MRPS10, RELA, RETNLA, ↓RNF25, SFRS7, ↓SLC25A23, SPINT2, SPRR1A, ↓ST7, ↓SYAP1, TMEM9B, TNFSF4, XRCC6 | 17 | 13 | Cellular Growth and Proliferation, Tissue Development, Cell-To-Cell Signaling and Interaction |
| 14 | ↑DUSP5, FKBP1A, FKBP1B, GATA3, ↓GOLGA7, HRAS, IL5, ↑KCTD9, KLF3, MIR302A (includes EG:407028), ↓MYADM, peptidylprolyl isomerase, PIN4, PPIB, PPID, PPIF, ↑PPIL3, ↑RPS10, ↑SCYL1, SERPIND1, ↑SLU7, SRF, ↓ST7, TIMM10, TIMM17B, ↑TIMM8B, TOMM6, TOMM7, TOMM20L (includes EG:75266), TOMM40L, TOMM5 (includes EG:68512), ↓UPB1, ↓ZC3H8, ZDHHC9, ↑ZNF207 | 17 | 13 | Post-Translational Modification, Protein Folding, Cancer |
| 15 | ↑ACE2, AMPK, ↓ANGEL1, CHAF1A, CXCL1, ↑DCN, ↑DDX24, DPM2, DUSP1, ↑HDAC1L, HEXIM1, IL6, KIF11, MAP3K1, ↓MLF2, NOP2, phosphatidylinositol N-acetylglucosaminyltransferase, PIGA, PIGB, PIGC, ↓PIGH, PIGP, PIGQ, PIGY, RNase A, ↑RPL10A (includes EG:4736), ↓SALL1, ↑SP110, SPTAN1, TMEM9B, ↓TSSC4, VEGFA, ↓YIPF4, ZFP36, ↑ZNF692 | 17 | 13 | Tumor Morphology, Cellular Development, Hematological System Development and Function |
| 16 | APH1A (includes EG:51107), APH1B, ↓BCAS2, CAPN1, CCAR1, COMT, DDIT4, ↓ECHS1, ↓EI24, GLUL, ↓JMJD8, JMJD1C, LIG3, MIR34A (includes EG:407040), ↑NLRC4, NR3C1, PARP, ↓PARP2, ↓PARP4, ↑PHF17, ↑PLEKHFI, PSENEN, ↑RBBP6 (includes EG:5930), ↓SCAMP1, SNCAIP, SUPT16H, TEP1, TP53, TPT1 (includes EG:7178), Ube3, ↑UFC1, ↓UFSP2, VHL, XRCC1 | 16 | 13 | Post-Translational Modification, Cell Death, Small Molecule Biochemistry |
| 17 | ACTR6, AGT, AGTR1B, ARPC5, FOLR2, FOS, GNAO1, Immunoproteasome Pa28/20s, ↑L0C119358*, MOS, ↑MSLN, POMP, ↓PRELID1, Proteasome PA700/20s, PSMA, PSMA1, PSMA4, PSMA5, ↑PSMA7, PSMB2, PSMB3, PSMB4, ↑PSMB6, ↑PUS1, RARB, ↑RGS11, RPL7A (includes EG:6130), ↑SLC4A3, SNCG, STAT6, TOM1, ↑TWISTNB, ↑UBA52, ↑ZFAND2A | 13 | 11 | Drug Metabolism, Molecular Transport, Small Molecule Biochemistry |
| 18 | ABCD3, ACTC1, AFG3L1, ATP5B, ATP6V0B, ↑ATP6V1G2, ATPase, ↑BAT1, ↑C20ORF7, ↓CHMP7, ↑CHMP2A, CHMP4A, CHMP4B, CHMP4C, ↑CSAD, DDX1, DDX39, DNAJC14, ESCRT3, ESRRA, HLTF, MIRN324, MT-ATP8, MYH1, MYO9A, PPP2R4, RECQL5, RFC3, ↑SARNP, SKIV2L, ↓STAMBP, ↑THOC2, VPS24, VPS4B, ↓ZC3H4 | 12 | 10 | Molecular Transport, DNA Replication, Recombination, and Repair, Energy Production |
| 19 | 26s Proteasome, Actin, ATXN3, DNAJB2 (includes EG:3300), ↑GM10117, H2-LD, Histone h3, ↓HNRNPK, Hsp90, ID3, IKK (complex), Interferon alpha, ↓KEAP1, Mapk, MAPK6, MOS, ↑MYH14, NEB, PACRG, PLA2G6, ↑PPAN-P2RY11, RAB8B, ↑ROCK2, RPS3, SNCAIP, ↑TES, ↑TMCO6, Tropomyosin, TRPM7, Ubiquitin, UBQLN1, USP2, ↑USP3, USP18 | 10 | 9 | Cellular Assembly and Organization, Cell Cycle, Cellular Movement |
Figure 5. Effects of nitrite on BD (2h) induced changes in gene expression in the kidney.
Real time-PCR analyses of indicated genes was performed. Data show fold change relative to BD alone and are mean ± SEM, n=3. For all up-regulated genes, P <0.05 by t-test.
Discussion
Brain dead donors comprise a significant resource of kidney allografts for transplantation. A major limitation of organs collected from BD donors is the enhanced sensitivity of the organ to injury post-transplantation when compared to organs collected from living donors. This is important since the extent of this injury is inversely related to organ function and graft viability and provides a target for therapeutic intervention to both limit BD induced injury and prevent secondary transplantation induced renal dysfunction. Underlying BD induced injury is increased ischemia and inflammation. Recent studies have shown that rather than an inert metabolite of aerobic NO metabolism, at low concentrations (nM-µM), nitrite can serve as a source for NO specifically under hypoxic and/or acidic conditions. This concept has been demonstrated by i) decreased nitrite levels during ischemic stress with the concomitant increase in NO and NO-protein adducts 13,35–38, ii) stimulation of NO-signaling in hypoxic, but not normoxic tissues 24,39and iii) nitrite therapy protecting against I/R injury in diverse organs systems by NO-formation 11–13,21,40,41. This together with decreased circulating nitrite levels during BD (Figure 1), consistent with ischemic stress, provided the rationale for testing nitrite therapy to attenuate BD induced renal injury and improve function post-transplantation.
Decreased circulating nitrite levels could be due to endothelial dysfunction as previously reported with BD 29, and/or increased consumption which has also been reported in the setting of ischemic tissue injury 13. In fact data showing that nitrite levels in the circulation rise and then fall despite continual administration over 2h of BD (Figure 1D) suggest that increased nitrite consumption is occurring. Renal nitrite levels did not change with BD suggesting that with the relatively mild (only 2h BD) model used herein, ischemic stress in the circulation is more prevalent. Further studies evaluating nitrite therapy using longer BD times that also more severely compromise organ function are clearly required.
Most previous studies have shown nitrite-protection against IR injury to be mediated by NO-formation. In this model, nitrite targets NO formation only in the ischemic tissue where it can stimulate multiple signaling responses that result in inhibition of IR injury including attenuating inflammation and oxidative stress, inhibiting cell death, promoting blood flow and regulating mitochondrial respiration 20,40. Importantly, all of these potential effects could play a role in the observed protective effects of nitrite during BD. Based on BD causing decreased nitrite only in the intravascular compartment, we posit that the protective effects of nitrite are mediated primarily in the circulation and perhaps by improving renal blood flow or attenuating leukocyte activation, which could explain decreased CD11a and CD11b levels in the kidney. Co-administration of C-PTIO did not reverse nitrite dependent protection suggesting NO-independent mechanisms are operating to limit kidney injury. Also, a role for XOR was difficult to assess since allopurinol itself prevented BD induced injury more so than nitrite suggesting increased reactive oxygen species production plays a role in BD induced organ dysfunction, a conclusion supported by increased lipid peroxidation in the kidney (Figure 4A).
Despite the lack of understanding of where nitrite is exerting protective effects, it is clear that nitrite therapy affects gene expression profile in the kidney after BD. Previous studies have shown changes in the expression of 60–90 genes using microarray analysis in kidneys 6h after BD 34. Interestingly, differential profiles of genes being up-or down- regulated are observed in the kidney post BD depending on whether hemodynamics are controlled or not, and encompassed genes that affected metabolism/transport, immune/inflammatory cell activation, growth/fibrosis and defense/repair processes. We utilized gene arrays to gain insights into potential mechanisms by which nitrite protected against BD-dependent renal injury and specifically those that are not revealed by analysis of candidate markers of inflammation and oxidative stress. IPA of networks affected by nitrite compared to BD alone, showed that many processes were potentially affected (Table 1) including gene expression and modulation of humoral immune response, the latter being consistent with nitrite inhibiting inflammatory stress.
Within the top 5 canonical pathways that were most significantly affected by nitrite therapy, only a small percentage of total genes were in fact modulated by nitrite (4–10%) suggesting a specificity of responses. Real time-PCR analysis was performed on selected genes encompassing these pathways. Supplementary Table 1 shows ~50% concordance between changes in gene expression mediated by nitrite determined by array and real time-PCR analysis. Importantly however, at least one gene from each of the top 5 pathways identified as changing in response to nitrite therapy was validated by real time-PCR (Figure 5). Two of the top 5 pathways relate to expression of eukaryotic initiation factors with nitrite therapy up-regulating eIF4γ. The exact consequences of such changes are difficult to predict, but are consistent with high diversity of genes affected by nitrite 33 as indicated in Table 1. The second pathway was related to Notch signaling an increase of which has recently been shown to facilitate tubular repair after acute renal injury 42. Nitrite therapy increased expression of γ-secretase suggesting increased Notch cleavage and subsequent stimulation gene transcription will occur. Potential effects of nitrite on HIF1α and Nrf2 (pathways 3 and 4 respectively) are clearly of interest with respect to known mechanisms of BD induced renal injury. Interestingly, nitrite therapy up-regulated elongin-C, the predicted consequence is limited activation of HIF1α and may represent an affect of nitrite in countering BD induced ischemic stress. One potential mechanism is possible improvement in renal blood flow via reactions with hemoglobin 43,44. Moreover, nitrite therapy up-regulated the expression of Keap-1 and Nrf2. Keap 1 binds Nrf2 in the cytosol, preventing the latter from inducing a variety of stress response genes that protect cells from oxidative and inflammatory stimuli. While relatively little is known on how expression levels of Keap1 and Nrf2 mRNA affects responses to oxidative / inflammatory stimuli, this data suggests that nitrite may affect oxidative and nitrosative signaling via these key transcription factors. Further studies evaluating how protein levels and transcriptional activity of the aforementioned mediators (identified from array and RT-PCR studies), change during BD injury in the presence or absence of nitrite therapy, are a necessary next step to further define mechanisms of nitrite protection.
The current studies evaluated nitrite therapy during BD itself and not post-transplantation dependent inflammatory injury. However, numerous studies have shown protective effects of nitrite during organ transplantation and/or IR injury and in most of these instances nitrite is effective independent of how it is administered (dietary, intraperitoneal, intra-organ injection or intravenous) 11,13,14,28,45. With respect to the kidney however, the route of administration of nitrite appears to play an important role. Intraperitoneal or intravenous administration did not protect against renal I/R injury 46, whereas topical administration does 25,30. The mechanistic basis for this difference remains unclear. Irrespective, intravenous nitrite administration does protect the kidney from BD induced injury. How nitrite is administered during BD is also important when assessing safety. A key element in BD induced kidney injury is hypotension as demonstrated by experimental studies showing decreased injury when hypotension is avoided 4. Indeed, clinically the management of hypotension is by administration of fluid and/or vasoconstrictors. Nitrite is a vasodilator which raises concerns about using this anion as a therapeutic. Initial studies testing bolus nitrite administration decreased MAP compared to BD alone resulting in mortality. However, administration of nitrite as for the duration of BD did not result in additional hypotension and did not promote mortality. These data show that in the setting of BD, nitrite can be administered safely and protects against BD induced renal injury, however the route of administration is critical.
Limitations of the current study include the lack of precise mechanistic insights into how nitrite prevents BD induced renal injury. Moreover, we note that a relatively mild (2h) BD injury model was employed (the severity of BD injury increases with increased time of BD 4). Despite these limitations, the data presented herein support the feasibility of NO based and specifically nitrite based therapy for limiting BD induced renal injury.
In summary, we have shown that nitrite can be administered safely in a rat brain death model and was able to mitigate the brain death induced ischemia reperfusion injury to renal grafts and improve post transplant renal function. Nitrite administration during 2h BD results in decreased markers of infiltrating inflammatory cells in the kidney and more importantly, resulted in kidneys whose function was significantly improved after transplantation compared to an allograft obtained from a BD alone rat. These data suggest that nitrite prevents ischemic / inflammatory tissue injury during the BD phase which results in a kidney that is more resistant to subsequent IR injury post transplantation. Whether nitrite dependent protection occurs by affecting inflammation, tissue perfusion or the kidney responses to ischemic and inflammatory stress is not clear. Gene array studies clearly suggest however, that modulation of renal responses to stress are likely to play a role.
Methods
Animals
Male Lewis rats, aged 8–12 weeks and weighing 250-300 g, were used. Animals were fed a normal NaCl diet (AIN-76A) diet (Dyets, Inc., Bethlehem, PA) as previously described47 and allowed free access to water. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Brain Dead Model
Brain death was produced by balloon inflation of a Fogarty catheter introduced into the subdural space through an occipital burr hole as described in detail in Supplementary material. Briefly, after anesthesia rats were intubated and mechanically ventilated with intermittent positive pressure for 2h. Arterial blood pressure was monitored continuously. All animals received 0.9 ml of lactated Ringer solution intravenously for 30 min during the 2-h period. A 1-mm hole was drilled through the skull 0.3 cm lateral to the sagittal suture and then a No. 3 Fogarty catheter inserted through the burr hole. For raising the intracranial pressure gradually, the balloon was inflated with 40 µl/min saline until respiration ceased. The absence of reflexes, apnea, and maximally dilated and fixed pupils confirmed the condition. The balloon was kept inflated during the entire 2hr follow-up after which left and right kidneys were harvested. Left kidney was used for transplantation and right kidney for mRNA or tissue collection for subsequent analyses. MAP was controlled and maintained between 60–80mmHg by addition of lactated ringers solution and Hespan as necessary. We note that with bolus administration of nitrite, fluid administration was not able to increase MAP (see Figure 2).
Nitrite therapy
Sodium nitrite (Sigma, St. Louis, MO) was dissolved in sterile PBS and administered intravenously either as a bolus injection (0.1 mg/kg, in a 100 µl volume) or by injecting 133 µl every 10min (over 2hr, first dose starting 10min after induction of BD) resulting in 0.0083 mg/kg/injection or 0.1 mg/kg over 2h. Saline was used as a control for nitrite. Nitrite administration was started 10min after BD induction. C-PTIO and allopurinol were administered at 1 mg/Kg after induction of BD.
Kidney Transplant
Kidney transplantation surgery (Lewis to Lewis syngeneic transplantation) was performed according to the method previously described 48 and described in detail in supplementary material.
Creatinine and BUN Assay
Serum creatinine and BUN levels were assayed using a VetACE biochemistry machine (Alfa Wasserman, West Caldwell, NJ).
Histology and Immunofluoresence
Paraffin embedded kidney sections were deparaffinized and stained with indicated antibodies as described in supplementary material.
Western Blotting
Western blotting was performed as described in supplementary material.
Nitrite measurements
Nitrite was measured in the blood and kidneys as previously described and in supplementary material.16
RNA Isolation for Gene Analysis
Detailed methods are provided in supplementary information. Briefly kidneys were cut into 3cm × 3cm segments and flash frozen in liquid N2. Total RNA was then isolated using a protocol based on the interaction of phenol and guanidine with cellular components. Gene expression analysis was performed using the Rat Ref-12 BeadChip and iScan system from Illumina, Inc. (San Diego, CA). The Rat Ref-12 BeadChips contain sequences representing approximately 22,000 curated and putative genes and ESTs. Quality standards for hybridization, labeling, staining, background signal, and basal level of housekeeping gene expression for each chip were verified. After scanning the probe array, the resulting image was analyzed using the GenomeStudio software (Illumina, Inc., San Diego, CA). Samples were normalized using a quantile procedure, and differential gene expression control and treatment groups was performed via a custom Illumina analysis designed to eliminate background noise from the analysis by subtracting out the signal from negative beads (p<0.05). Gene lists were not adjusted for multiple testing.
Ingenuity Pathway Analysis
The data set was analyzed by using Ingenuity Pathways Analysis (IPA) software (ver 8.7: Ingenuity® Systems, www.ingenuity.com). The data set contained gene identifiers and corresponding expression values and was uploaded into the application. Each identifier was mapped to its corresponding object in Ingenuity’s Knowledge Base. Fold change for the target molecules was used to display the genes whose expression was up- or down-regulated. These Network Eligible molecules were overlaid onto a global molecular network developed from information contained in Ingenuity’s Knowledge Base. Networks of Network Eligible Molecules were then algorithmically generated based on their connectivity. The Functional Analysis identified the biological functions that were most significant to the data set. Right-tailed Fisher’s exact test was used to calculate a p-value determining the probability that each biological function assigned to that data set is due to chance alone. Canonical pathways analysis identified the pathways from the Ingenuity Pathways Analysis library of canonical pathways that were most significant to the data set. The significance of the association between the data set and the canonical pathway was measured in 2 ways: 1) A ratio of the number of molecules from the data set that map to the pathway divided by the total number of molecules that map to the canonical pathway is displayed. 2) Fisher’s exact test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone. Finally, we note that gene array analysis of control vs. BD alone, or control vs. nitrite alone groups revealed significant and distinct gene expression profiles changes compared to BD vs. BD + nitrite (not shown). Our focus here was on understanding the therapeutic potential of nitrite and hence we limit discussion to gene expression changes in BD + nitrite vs. BD alone groups only.
Real time-PCR
primer sequences and real time-PCR analyses were performed as described in supplementary data.
Statistical Analysis
Data are presented as mean±SEM. The t test was used for comparisons between two groups. For the comparisons that involved more than two groups, ANOVA and the Tukey/Bonferroni post test were used for analysis, with statistical significance considered at p<0.05
Supplementary Material
Acknowledgements
Sources of support: This work was supported by grants from the JDRF (5-2008-187), ADA (1-08-IN-27) to SK, 5R1 DK071300 to JAT, HL092624 to RPP and the NIDDK funded UAB-UCSD O’Brien Core Resource for Acute Kidney Injury Research (1P30DK079337) to AA
Footnotes
Disclosure
RPP is listed as a coinventor on a patent for the use of sodium nitrite for the treatment of cardiovascular diseases.
References
- 1.Pratschke J, Neuhaus P, Tullius SG. What can be learned from brain-death models? Transpl Int. 2005 Jan;18(1):15–21. doi: 10.1111/j.1432-2277.2004.00018.x. [DOI] [PubMed] [Google Scholar]
- 2.Pratschke J, Wilhelm MJ, Kusaka M, Laskowski I, Tilney NL. A model of gradual onset brain death for transplant-associated studies in rats. Transplantation. 2000 Feb 15;69(3):427–430. doi: 10.1097/00007890-200002150-00020. [DOI] [PubMed] [Google Scholar]
- 3.van Der Hoeven JA, Ter Horst GJ, Molema G, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000 Dec;232(6):804–813. doi: 10.1097/00000658-200012000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Hoeven JA, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003 Nov;64(5):1874–1882. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 5.Schuurs TA, Morariu AM, Ottens PJ, et al. Time-dependent changes in donor brain death related processes. Am J Transplant. 2006 Dec;6(12):2903–2911. doi: 10.1111/j.1600-6143.2006.01547.x. [DOI] [PubMed] [Google Scholar]
- 6.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009 Apr;53(4):425–435. doi: 10.1111/j.1399-6576.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 7.Gasser M, Waaga AM, Kist-Van Holthe JE, et al. Normalization of brain death-induced injury to rat renal allografts by recombinant soluble P-selectin glycoprotein ligand. J Am Soc Nephrol. 2002 Jul;13(7):1937–1945. doi: 10.1097/01.asn.0000019401.12257.c4. [DOI] [PubMed] [Google Scholar]
- 8.Pratschke J, Kofla G, Wilhelm MJ, et al. Improvements in early behavior of rat kidney allografts after treatment of the brain-dead donor. Ann Surg. 2001 Dec;234(6):732–740. doi: 10.1097/00000658-200112000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijboer WN, Ottens PJ, van Dijk A, van Goor H, Ploeg RJ, Leuvenink HG. Donor pretreatment with carbamylated erythropoietin in a brain death model reduces inflammation more effectively than erythropoietin while preserving renal function. Crit Care Med. 2010 Apr;38(4):1155–1161. doi: 10.1097/CCM.0b013e3181cf6e78. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Hines I, Zibari G, Grisham MB. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch Biochem Biophys. 2009 Apr 15;484(2):232–237. doi: 10.1016/j.abb.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemiareperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007 Jul 15;75(2):327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005 May;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006 Apr;290(4):F777–F778. doi: 10.1152/ajprenal.00470.2005. [DOI] [PubMed] [Google Scholar]
- 16.Lang JD, Jr, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007 Sep;117(9):2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox-Robichaud A, Payne D, Hasan SU, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998 Jun 1;101(11):2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladwin MT, Raat NJ, Shiva S, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006 Nov;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 19.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009 Jan 20;42(1):157–167. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 20.van Faassen EE, Bahrami S, Feelisch M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009 Sep;29(5):683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009 Dec;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008 Feb;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 23.Hendgen-Cotta UB, Merx MW, Shiva S, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar D, Branch BG, Pattillo CB, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripatara P, Patel NS, Webb A, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007 Feb;18(2):570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 26.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004 Sep 14;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007 Sep 3;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiva S, Wang X, Ringwood LA, et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006 Sep;2(9):486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 29.Szabo G, Soos P, Heger U, et al. L-arginine improves endothelial and myocardial function after brain death. Transplantation. 2006 Jul 15;82(1):108–112. doi: 10.1097/01.tp.0000225778.49388.f5. [DOI] [PubMed] [Google Scholar]
- 30.Milsom AB, Patel NS, Mazzon E, et al. Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric Oxide. 2010 Feb 15;22(2):141–148. doi: 10.1016/j.niox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Halejcio-Delophont P, Hoshiai K, Fukuyama N, Nakazawa H. No evidence of NO-induced damage in potential donor organs after brain death. J Heart Lung Transplant. 2001 Jan;20(1):71–79. doi: 10.1016/s1053-2498(00)00207-2. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006 Jan 15;40(2):295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Pattillo CB, Fang K, Pardue S, Kevil CG. Genome expression profiling and network analysis of nitrite therapy during chronic ischemia: possible mechanisms and interesting molecules. Nitric Oxide. 2010 Feb 15;22(2):168–179. doi: 10.1016/j.niox.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuurs TA, Gerbens F, van der Hoeven JA, et al. Distinct transcriptional changes in donor kidneys upon brain death induction in rats: insights in the processes of brain death. Am J Transplant. 2004 Dec;4(12):1972–1981. doi: 10.1111/j.1600-6143.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 35.Bryan NS, Fernandez BO, Bauer SM, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005 Oct;1(5):290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 36.Bryan NS, Rassaf T, Maloney RE, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feelisch M, Rassaf T, Mnaimneh S, et al. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002 Nov;16(13):1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 38.Heiss C, Lauer T, Dejam A, et al. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006 Feb 7;47(3):573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 39.Pattillo CB, Bir S, Rajaram V, Kevil CG. Inorganic nitrite and chronic tissue ischaemia: a novel therapeutic modality for peripheral vascular diseases. Cardiovasc Res. 2010 Oct 19; doi: 10.1093/cvr/cvq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvert JW, Lefer DJ. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric Oxide. 2010 Feb 15;22(2):91–97. doi: 10.1016/j.niox.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefer DJ. Emerging role of nitrite in myocardial protection. Arch Pharm Res. 2009 Aug;32(8):1127–1138. doi: 10.1007/s12272-009-1804-y. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Li S, Abedin MJ, et al. Effect of Notch activation on the regenerative response to acute renal failure. Am J Physiol Renal Physiol. 2010 Jan;298(1):F209–F215. doi: 10.1152/ajprenal.00451.2009. [DOI] [PubMed] [Google Scholar]
- 43.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003 Dec;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 44.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NOdependent hypoxic vasodilation. Blood. 2006 Jan 15;107(2):566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan J, Nakao A, Sugimoto R, et al. Orally administered nitrite attenuates cardiac allograft rejection in rats. Surgery. 2009 Aug;146(2):155–165. doi: 10.1016/j.surg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Basireddy M, Isbell TS, Teng X, Patel RP, Agarwal A. Effects of sodium nitrite on ischemia-reperfusion injury in the rat kidney. Am J Physiol Renal Physiol. 2006 Apr;290(4):F779–F786. doi: 10.1152/ajprenal.00334.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sanders PW, Gibbs CL, Akhi KM, et al. Increased dietary salt accelerates chronic allograft nephropathy in rats. Kidney Int. 2001 Mar;59(3):1149–1157. doi: 10.1046/j.1523-1755.2001.0590031149.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen B, Kapturczak MH, Joseph R, et al. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007 May;7(5):1112–1120. doi: 10.1111/j.1600-6143.2007.01772.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.