Abstract
The cellular proto-oncogene c-Src is a nonreceptor tyrosine kinase involved in cell growth and cytoskeletal regulation. Despite being dysregulated in a variety of human cancers, its precise functions are not fully understood. Identification of the substrates of c-Src remains a major challenge, because there is no simple way to directly stimulate its activity. Here we combine the chemical rescue of mutant c-Src and global quantitative phosphoproteomics to obtain the first high resolution snapshot of the range of tyrosine phosphorylation events that occur in the cell immediately after specific c-Src stimulation. After enrichment by anti-phosphotyrosine antibodies, we identified 29 potential novel c-Src substrate proteins. Tyrosine phosphopeptide mapping allowed the identification of 382 nonredundant tyrosine phosphopeptides on 213 phosphoproteins. Stable isotope labeling of amino acids in cell culture-based quantitation allowed the detection of 97 nonredundant tyrosine phosphopeptides whose level of phosphorylation is increased by c-Src. A large number of previously uncharacterized c-Src putative protein targets and phosphorylation sites are presented here, a majority of which play key roles in signaling and cytoskeletal networks, particularly in cell adhesion. Integrin signaling and focal adhesion kinase signaling pathway are two of the most altered pathways upon c-Src activation through chemical rescue. In this context, our study revealed the temporal connection between c-Src activation and the GTPase Rap1, known to stimulate integrin-dependent adhesion. Chemical rescue of c-Src provided a tool to dissect the spatiotemporal mechanism of activation of the Rap1 guanine exchange factor, C3G, one of the identified potential c-Src substrates that plays a role in focal adhesion signaling. In addition to unveiling the role of c-Src in the cell and, specifically, in the Crk-C3G-Rap1 pathway, these results exemplify a strategy for obtaining a comprehensive understanding of the functions of nonreceptor tyrosine kinases with high specificity and kinetic resolution.
The discovery of c-Src (cellular, wild-type Src) as the proto-oncogene of v-Src (viral, mutant Src) has led to persistent interest in this nonreceptor protein-tyrosine kinase in studies of cell signaling. It is now known that c-Src is involved in regulating cellular growth, adhesion, motility, and invasion. c-Src is frequently overexpressed in human cancer, such as gastrointestinal, breast, ovarian, and other cancers (1), and it is considered a drug target. Despite its linkage to disease and breadth of functions, the specific roles of c-Src in signaling are still not fully understood.
A variety of biochemical and cellular approaches have been used to identify direct and indirect tyrosine-phosphorylated substrates of Src; many of these cellular substrate identification studies have used the hyperactive, dysregulated form of Src, v-Src (2, 3), which lacks normal down-regulation by C-terminal phosphorylation on Tyr-527, or constitutively active Src mutants (for example, Y527F) (4, 5). However, v-Src forms are rarely found in human cells, even in cancer (6). Instead, it would be informative to pursue these studies focusing on the cellular proto-oncogene c-Src.
Analyzing cellular protein-tyrosine phosphorylation targets of c-Src using a proteomics strategy would require an approach that can directly and specifically monitor c-Src kinase action rather than previously used indirect methods, such as growth factors activating growth factor receptor tyrosine kinases that indirectly stimulate c-Src (7). Related work has been done in this regard combining chemical genetics of kinases (8–10) and proteomics (11). For our goals, the challenge was to achieve specific and rapid activation of c-Src in living cells that will allow identification of substrates temporarily close to c-Src activation. An attractive strategy to pursue these objectives involves chemical rescue of mutant c-Src tyrosine kinase.
It has previously been shown that mutation of a highly conserved Arg (390 in c-Src) in protein-tyrosine kinases results in a dramatic reduction in catalytic activity (200–5000-fold), presumably because of the loss of a key hydrogen-bonding side chain responsible for orienting the substrate tyrosine phenol for phosphoryl transfer (12–14). A variety of di- and triamino compounds added to the enzyme reaction buffer have been shown to complement this defective kinase activity, the most efficient being imidazole (12–14). Structural and pH studies suggest that positively charged imidazolium occupies the unnatural cavity found in R/A mutant protein-tyrosine kinases and serves to rescue the catalytic function without significantly affecting c-Src substrate selectivity (14) (see Fig. 1A). Indeed, in the case of the Src kinase domain, treatment of R390A v-Src with imidazole stimulates catalytic activity by ∼100-fold to within 40% of the wild-type level and appears to have native substrate specificity and regulation in vitro (3). It was also shown that imidazole, a relatively nontoxic small molecule, could rescue R390A v-Src in cell culture (3).
Fig. 1.
c-Src chemical rescue by imidazole. A, proposed R390A c-Src chemical rescue mechanism by imidazole indicating interactions of imidazole with the catalytic base Asp-388 and Tyr from the substrate (based on Protein Data Bank entry 3GEQ). B, comparison of the phosphotyrosine profile of imidazole-treated cells compared with that of untreated cells. Tyrosine phosphorylation was induced by imidazole treatment (10 mm, 5 min) of SYF cells stably transfected with c-Src R390A and D388N SYF (negative control). The cells were subjected to lysis, and whole lysates were subjected to Western blotting with 4G10 anti-phosphotyrosine antibody.
By combining the specific and controlled activation of c-Src by chemical rescue with the ability of quantitative SILAC phosphoproteomics to provide the phosphorylation fingerprint of cells in different conditions, we have identified a number of potential novel c-Src targets and discuss the potential ramifications of these phosphorylation events in cell signaling. Chemical rescue has also served as a powerful tool to dissect the mechanism of activation of one of the identified targets (C3G) in a spatiotemporal manner.
EXPERIMENTAL PROCEDURES
Cell Culture and SILAC
Src-, Yes-, and Fyn-triple knockout mouse embryonic fibroblast cells (hereafter referred to as SYF cells) (ATCC) stably transfected with either R390A c-Src or D388N c-Src constructs as previously described (3) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) qualified fetal bovine serum (Invitrogen), 1% (v/v) of penicillin-streptomycin-glutamine (Invitrogen) and 300 μg/ml of hygromycin B (Roche Applied Science) and incubated at 37 °C in the presence of 5% CO2. R390A c-Src SYF MEF cells (hereafter referred to as R390A/SYF MEF cells) were adapted to the SILAC1 medium. A detailed protocol for SILAC medium preparation can be obtained from http://www.silac.org. Arginine- and lysine-free Dulbecco's modified Eagle's media were bought from Invitrogen. c-Src R390A stably transfected SYF cells were cultured in Arg-/Lys-free Dulbecco's modified Eagle's medium supplemented with light (12C6 Arg/12C6 Lys) amino acids from Sigma or heavy (13C6 Arg/13C6 Lys) amino acids from Cambridge Isotope Laboratories, Inc. Both media contained 10% qualified fetal bovine serum (Invitrogen), 1% penicillin-streptomycin-glutamine (Invitrogen) and 300 μg/ml of hygromycin B (Roche Applied Science). The cells were adapted to the SILAC medium by growing them in these media for at least five replication cycles, as described earlier (15). When starved, the cells were cultured in the same medium lacking serum. The Tyr(P) proteomes were assessed from cells actively growing and motile at ∼75% confluency. Untransfected SYF cells and wild-type MEFs were grown as above without hygromycin treatment.
Cell Treatment and Peptide Preparation
Thirty 150-mm-diameter plates of R390A/SYF cells (per condition) were serum-starved for 16 h. Cells grown in 13C6 Arg/13C6 Lys-containing medium (heavy medium) were treated with 10 mm imidazole, pH 7.5, for 5 min, whereas cells grown in 12C6 Arg/12C6 Lys-containing medium (light medium) were left unstimulated and used as a control. Both cells sets were then washed once with ice-cold PBS (Invitrogen) and immediately lysed in urea lysis buffer (20 mm HEPES, pH 8.0, 9 m urea, 1 mm sodium orthovanadate, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate) by three bursts of ultrasonication (duty cycle 40%, output control at 4, on Sonifier 250; Branson) at 4 °C. After sonication, the lysate was cleared by centrifugation at 20,000 × g for 15 min, and the supernatant was collected. Three biological replicates were conducted. Protein concentrations were calculated by Bradford (supplemental Fig. 1A). As a further loading control, equal amounts of lysates (50 μg) were mixed with SDS loading buffer and boiled at 95 °C for 5 min, analyzed by SDS-PAGE, and stained with colloidal Coomassie (supplemental Fig. 1A).
Tyrosine Phosphopeptide Enrichment
Phosphotyrosine protein profile with and without imidazole treatment was analyzed by immunoaffinity purification of phosphoproteins with 4G10 anti-phosphotyrosine antibody followed by Western blot using 4G10 anti-phosphotyrosine antibody (supplemental Fig. 1B). “Heavy” and “light” lysates were mixed in protein concentration ratios 1:1. As a result, a total amount of 35 mg (first biological replicate), 50 mg (second biological replicate), and 52 mg (third biological replicate) were generated for phosphotyrosine peptide profiling. Two of the three biological replicates had two technical replicates with respect to LC-MS/MS. LC-MS analysis of sample aliquots were performed to observe the equal mixing of heavy and light cell lysate proteins using a Micromass quadrupole time of flight mass spectrometer Ultima API (Waters) (supplemental Fig. 1C).
Proteins were reduced with DTT at a final concentration of 4.5 mm and alkylated with 10 mm iodoacetamide. The lysates were diluted to a final concentration of 2 m urea, 20 mm HEPES, pH 8.0. 1/100 (v/v) of 1 mg/ml trypsin-tosylphenylalanyl chloromethyl ketone (Worthington Biochemical Corporation) was added to the diluted protein extracts, and digestion was performed overnight at 25 °C. The lysates were analyzed before and after digestion as a control (supplemental Fig. 1D) using SDS-PAGE and colloidal Coomassie staining.
The protein digests were acidified with TFA at a final concentration of 1%. The acidified peptide solutions were centrifuged at 1,800 × g for 5 min to remove the precipitate. The clear supernatants were loaded onto Sep-Pak C18 cartridges (Waters, catalogue number WAT051910) previously wetted with 100% acetonitrile and equilibrated with 0.1% TFA. Acidified peptide samples were passed through cartridge by gravity and the cartridge cartridges were then washed with 0.1% TFA. The peptides were eluted by 40% ACN, 0.1% TFA. The eluted peptide solutions were lyophilized to remove residual TFA completely.
Immunoaffinity Purification of Tyrosine Phosphopeptides
Anti-phosphotyrosine antibody-based immunoaffinity purification (IAP) of phosphopeptides was carried out according to manufacturer protocol (16). Lyophilized peptides from each of the fractions (three biological replicates and two technical replicates) were dissolved with 1.4 ml of IAP buffer (50 mm MOPS, pH 7.2, 10 mm sodium phosphate, 50 mm NaCl) aided by shaking and brief sonication. The solution was cleared by centrifugation for 5 min at 1,800 × g. The phosphotyrosine antibody-linked beads (Cell Signaling Technology) (250 μg in 40 μl) were washed with IAP buffer (1.4 ml of IAP buffer, mixed by invert five times) once and incubated with the cleared peptide solution for 30 min at 4 °C with gentle shaking. The phosphopeptide-bound antibody beads were washed twice with IAP buffer and twice with water at 4 °C. The phosphopeptides were eluted from the beads by incubation with 55 μl of 0.15% TFA at 20–25 °C for 10 min followed by second extraction with 45 μl of 0.15% TFA.
The eluted phosphopeptides were desalted using Stage-Tip protocol described previously (17). The peptide eluate was then dried at 25 °C in SpeedVac for mass spectrometry analysis.
LC-MS/MS Analysis of Tyrosine-phosphorylated Peptides
LC-MS/MS analysis of phosphopeptides enriched by IAP was carried out using a reversed phase liquid chromatography system interfaced with Fourier transform LTQ-Orbitrap Velos mass spectrometer on a “high-high” mode (both MS and MS/MS analyses were carried out in FT-MS mode) (Thermo Scientific). The reversed phase setup consisted of a trap column (2 cm × 75 μm, Magic C18 AQ material 5 μm, 100 Å) and an analytical column (10 cm × 75 μm, Magic C18 AQ material 5 μm, 100 Å). After concentration and desalting on the precolumn, the peptides were eluted and separated on the analytical column using a gradient increasing from 100% solvent A, 0% solvent B (Solvent A: 0.1% formic acid, solvent B: 100% acetonitrile, 0.1% formic acid) to 40% buffer A, 60% solvent B in 135 min. Each survey scan involved precursor scan from 350 to 1600 m/z at 60,000 resolution and data-dependent higher collision dissociation MS/MS scans for 10 most intense precursor ions at 7,500 resolution. Normalized collision energy was 35%, and monoisotopic precursor selection was enabled with isolation width of 1.9 m/z.
Data Analysis
The acquired mass spectra were processed and searched using MaxQuant (version 1.2) (18), Mascot (19), and Sequest (20) because these algorithms are complementary, which can broaden protein and site identification (21). Searches using the latter two were submitted via Proteome Discoverer (version 1.3; Thermo Scientific), which allowed merging search results and quantitating the SILAC pairs. It should be noted that although there was a large difference between Mascot and Proteome Discoverer for redundant peptides (∼10-fold), perhaps related to initial curation stringency, this difference becomes minor when comparing nonredundant peptides (<2-fold). We believe that the similarity in nonredundant peptides is the more important comparison. All of the MS/MS spectra were searched against the RefSeq mouse protein database (v. 40, containing 31,183 entries). For all searches, two missed cleavages were allowed; carbamidomethylation of cysteines was set as a fixed modification. N-terminal acetylation, deamidation of asparagine and glutamine, oxidation at methionine, phosphorylation at serine, threonine and tyrosine together with the heavy lysine and arginine were used as variable modifications.
Monoisotopic peptide tolerance was set to 10 p.p.m. for precursors and 0.05 mDa for MS/MS fragment ions. Peptides fulfilling a FDR of 1% were reported. Peptides with inconsistent ratios or unusual high ratios were manually checked. We established a threshold heavy/light ratio value of 1.2. Phosphopeptides showing an average (from the three biological replicates) SILAC ratio of 1.2 or above from Proteome Discoverer, MaxQuant, or both were considered up-regulated upon c-Src rescue. Four filtering criteria were used to obtain a high confidence data set of quantified phosphotyrosine peptides. If manual validation was satisfactory and showed a rounded SILAC ratio of 1.2 or above, the peptides were included on the list. A detailed flow chart of the process is included in supplemental Fig. 2.
Tyrosine Phosphoprotein Enrichment and Quantitative Analysis
SYF MEF cells stably transfected with R390A c-Src were cultured, isotopically labeled, and treated as described above. Immunoaffinity purification of tyrosine-phosphorylated proteins was carried out as described previously (3). The enriched tyrosine-phosphorylated proteins were subjected to in-gel digestion as described previously (3). The resulting tryptic peptides were analyzed by reversed phase LC-MS/MS using a quadrupole time of flight mass spectrometer (QSTAR Pulsar, MDS Sciex, Concord, Canada) coupled to an 1100 series CapLC (Agilent, Santa Clara, CA). The acquired data were queried against the RefSeq mouse protein database (v.26, containing 20311 entries) using MASCOT v.2.2.2 (Matrixscience, London, UK) and quantitated using MSQuant v1.4.3a39 (http://msquant.sourceforge.net). Mass tolerance of 1.1 Dalton was used for precursors and 0.1 Da for fragment ions. Fixed modification with carbamidomethylation of cysteines was set, whereas N-terminal acetylation, deamidation of asparagine and glutamine, oxidation at methionine, and phosphorylation at serine, threonine, and tyrosine together with heavy lysine and arginine were set as variable modifications. Quantitation was performed using MSQuant as described on http://msquant.sourceforge.net.
Network Modeling and Literature Curation
Phosphosite (http://phosphosite.org), the Human Protein Research Database (22) (http://hprd.org), and literature curation were used to differentiate previously unidentified and known substrates from the list of proteins showing increased phosphorylation upon c-Src rescue. Phosphosite was used to establish the protein type classification of the detected proteins. The Ingenuity pathway analysis software (Ingenuity® Systems, www.ingenuity.com) and literature curation were used to investigate previously observed connections between c-Src and the protein of study.
The Ingenuity pathway analysis software was used to perform ontology and canonical pathway enrichment analysis of the proteins associated with the found tyrosine phosphopeptides experiencing an increase in their tyrosine phosphorylation level upon c-Src rescue. The online web tool DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov) (23) and the Ingenuity pathway analysis software were used to obtain a representation of the role of c-Src on focal adhesion pathways. The list of proteins corresponding to the peptides identified as up-regulated upon c-Src rescue was uploaded in the DAVID tool, which incorporated the newly discovered proteins to the pathway. The pathway was completed using Ingenuity Systems. Both the known and the newly discovered potential c-Src phosphorylation sites were incorporated to the graph manually and validated with Phosphosite and the Human Protein Research Database.
Immunoblotting and Immunoprecipitations
After imidazole treatment, the cells were washed once with PBS, lysed for 30 min at 4 °C in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 1 mm Na3VO4, 1 mm NaF, 1 mm PMSF (phenyl methyl sulfonyl fluoride), and protease inhibitor cocktail tablets (Roche, 11873580001)), and centrifuged at 13,000 × g at 4 °C. The supernatants were collected for immunoblotting and immunoprecipitation.
For immunoblotting the supernatants were mixed with SDS loading buffer and boiled at 95 °C for 5 min, analyzed by SDS-PAGE. The proteins were electroblotted into PVDF membrane (Invitrogen). The membrane was probed with appropriate antibody, followed by incubation with the correspondent horseradish peroxidase-conjugated secondary antibody. Antibodies were used at the concentrations recommended in the manufacturer's instructions. The bands were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce).
For immunoprecipitation, the supernatants were precleared with protein A- or G-agarose beads and subsequently incubated one hour with the antibody at 4 °C. Antibodies were used at the recommended concentration in the manufacturer's instructions. The mixtures were treated with protein A- or G-agarose beads and then incubated at 4 °C overnight. The agarose beads were subsequently washed three times by radioimmune precipitation assay buffer, resuspended in SDS loading buffer with β-mercaptoethanol, and boiled. The eluates were analyzed by SDS-PAGE and Western blot analysis.
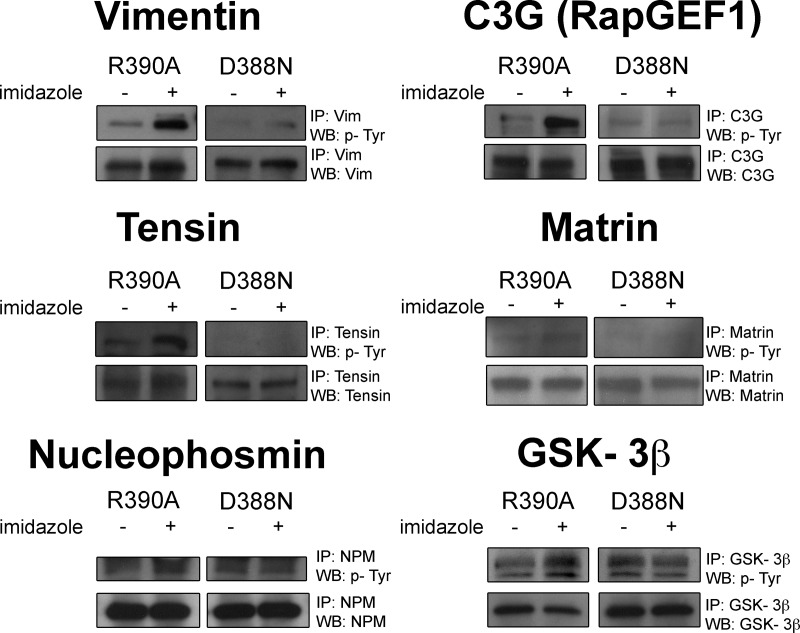
Antibodies were obtained as follows: phosphotyrosine, 4G10 from Upstate Biotechnology; FAK, 06-543 (FAK IP and WB) from Upstate Biotechnology; paxillin 05-417 (paxillin IP and WB) from Millipore; vimentin, ab8978 (vimentin IP) and ab58462 (vimentin WB) from Abcam, Inc.; C3G, sc869 (C3G IP and WB) from Santa Cruz Biotechnology; tensin, sc28542 (tensin IP and WB) from Santa Cruz Biotechnology; matrin, sc55724 (matrin IP and WB) from Santa Cruz Biotechnology; nucleophosmin, ab10530 (nucleophosmin IP and WB) from Abcam; Gsk3β, CST9315 (Gsk3β IP) from Cell Signaling Technology, sc81462 (Gsk3β WB) from Santa Cruz Biotechnology; antibody against C3G (C3G WB), sc-15359 from Santa Cruz Biotechnology; antibody against Tyr(P)-514 of C3G isoforms (Tyr(P)-514-C3G WB), sc-32621 from Santa Cruz Biotechnology; C3G IP/Crk WB, sc-15359 (C3G IP and WB) from Santa Cruz Biotechnology and 610035BD (Crk WB) from BD Transduction Laboratories; Crk (Tyr(P)-221) WB, ab76227 from Abcam; Tubulin WB, ab44928 from Abcam. The Western blot experiments were performed at least twice with representative results shown; although matrin phosphorylation was technically difficult to detect, the experiment when phosphorylation was observed (Fig. 4) provided modest evidence of mild chemical rescue effects (for more information on this point, please see Ferrando 2012). It should be noted that base-line values between rescuable and nonrescuable cells may not be the same because of subtle alterations in the cell passage number, growth conditions, or Western blot conditions (such as exposure time).
Fig. 4.
Validation of protein phosphorylation by immunoprecipitation-immunoblot analysis of R390A and D388N c-Src SYF cells after treatment with 10 mm for 5 min. Immunoprecipitation was carried out with commercially available antibodies corresponding to the protein of interest and immunoblotting was carried out with the 4G10 Tyr(P) antibody as well as protein-specific antibodies. Vimentin, C3G, tensin, matrin, nucleophosmin, and GSK-3β are some novel potential direct c-Src substrates that were validated by immunoprecipitation-immunoblot analysis.
Immunofluorescence and Confocal Microscopy
The cells were seeded and grown in 8-well glass microscopy slides (Lab-Tek). The cells were processed for immunofluorescence staining in the following way. After imidazole treatment (10 mm), the cells were washed once with ice-cold PBS. The cells were fixed with 4% formaldehyde (Tousimis Research Corporation) for 1 h at 4 °C followed by washing with 10 mm glycine in PBS. Permeabilization was performed with 0.2% (v/v) of Triton X-100 in PBS for 5 min at room temperature. After washing with PBS, the cells were incubated in blocking solution (BSA in PBS).
The primary antibodies used were rabbit polyclonal antibody against C3G (sc-13359 from Santa Cruz Biotechnology) and rabbit polyclonal antibody against Tyr(P)-514-C3G (sc-32621 from Santa Cruz). The secondary antibodies used were Alexa Fluor 555 goat anti-rabbit (A-21430 from Invitrogen) and Alexa Fluor 488 chicken anti-rabbit (A-21441 from Invitrogen), respectively. The slides were mounted with ProLong® Gold antifade reagent (with 4′,6′-diamino-2-phenylindole) from Invitrogen. The samples were acquired with a Zeiss meta confocal laser scanning microscope system, utilizing AIM software version 4.0. An argon laser exciting at 488 nm and a red HeNe at 561-nm wavelengths were used to obtain optical sections. Narrow band emission filters (nm) were utilized to eliminate channel cross-talk, and a 1.0 μm confocal aperture was used to obtain z-plane sections. The slides were imaged with 40× and 100× oil immersion Plan Apochromat objective lens (N.A. 1.4) through a Zeiss Axiovert inverted microscope. For quantification, at least 40 cells were counted for each condition and scored according to whether or not they had peripheral staining. The percentage of cells with a peripheral staining was calculated for each 40× window. Averages and standard errors are shown, and statistical analysis was done using a Student's t test.
FRET Microscopy
R390A and D388N c-Src SYF MEF cells were plated to glass-bottomed dishes (MatTech Corp.) and transiently transfected with 2 μg of Raichu-Rap1 (24) reporter using Lipofectamine (Invitrogen). The cells were starved for 16 h. After 15 h the cells were washed once, and the medium was replaced with Hanks' balanced salt solution. The dish was loaded onto the stage of an inverted Zeiss Axiovert 200M microscope controlled by METAFLUOR software (Universal Imaging, Downingtown, PA). Dual emission ratio imaging was performed using a 420DF20 excitation filter and a 450DRLP dichroic mirror and appropriate emission filters, 475DF40 for CFP and 535DF25 for YFP. The images were captured with a MicroMAX BFT512 CCD camera (Roper Scientific, Trenton, NJ). Base-line values were captured every 30 s for 2 min. The cells were treated with 10 mm imidazole at time 0 and imaged for an additional 15–20 min. The images were quantified using the Metafluor software. Regions of interest were selected, and the average intensities of the pixels in each region for each channel were quantified. FRET ratios were calculated by dividing the yellow emission over the cyan emission at each time point.
The raw data associated with this manuscript may be downloaded from ProteomeCommons.org Tranche, http://proteomecommons.org/tranche/, using the following hash code: 04W8a1fu+GSc7GMzdx0PJMaHuN+TEK8OpXcdzz07rrnT9h7USvqudUouF1vSinkQgXwrp+SA6WuF5IBCwMuF4oCtFDgAAAAAAAAJSg==.
RESULTS
c-Src Chemical Rescue
The chemical rescue of mutant v-Src provides precise temporal control of its intracellular kinase activity (3). To test whether we could observe similar results with cellular c-Src, we used SYF (Src-, Yes-, and Fyn-triple knockout) MEF cells stably transfected with R390A c-Src (R390A/SYF cells). The triple knockout reduces the background from endogenous c-Src, as well as two other members of the Src kinase family, Yes and Fyn (25). We observed that treatment of SYF MEF cells stably transfected with R390A c-Src using a range of 2.5–5 min and 2–10 mm imidazole induced a time-dependent, dose-dependent increase in range and intensity of tyrosine-phosphorylated protein bands as visualized by Western blot analysis of whole cell lysate with 4G10 anti-phosphotyrosine antibody (supplemental Fig. 3 and Fig. 1). The imidazole-insensitive inactive c-Src mutant D388N/SYF cells were used as a negative control because they express an inactive Src kinase at similar levels (3) and should be most similar to the untreated R390A/SYF cells. In prior studies (3), it was shown that D388N/SYF cells are unresponsive to imidazole. We confirmed here that D388N/SYF cells showed no such change in protein-tyrosine phosphorylation with 10 mm imidazole and 5 min treatment, compared with the imidazole-sensitive R390A/SYF c-Src mutant (Fig. 1B), suggesting that the imidazole effect was specific. The modest base-line Western blot differences (minus imidazole) between D388N/SYF and R390A/SYF cells are not consistent (3) and likely represent subtle differences in passage numbers and clonal populations. To further ensure that imidazole had no major effects on cellular tyrosine phosphorylation outside of chemical rescue under these conditions, we investigated its effect on SYF and wild-type MEF cells. As shown in supplemental Fig. 4, there were no appreciable changes in anti-Tyr(P) Western blots of cell lysates treated with or without imidazole.
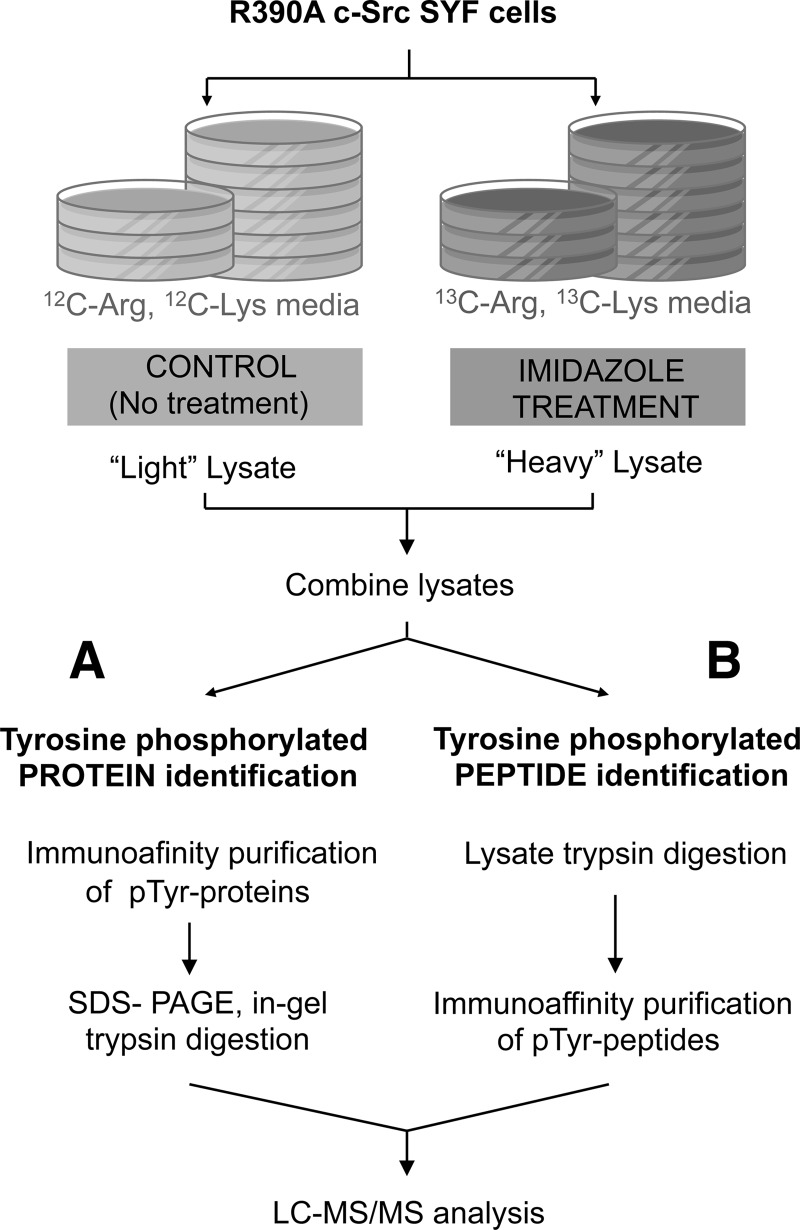
After a series of optimization experiments, we selected 10 mm imidazole and 5 min exposure as the optimal conditions for c-Src rescue in our R390A/SYF cells. Because our goal was to identify the c-Src-induced phosphoproteome, we combined c-Src chemical rescue with mass spectrometry and SILAC (Fig. 2) (26). R390A c-Src-expressing SYF cells were labeled with either 13C6 Arg/13C6 Lys (heavy) or 12C6 Arg/12C6 Lys (light). Heavy cells were treated with 10 mm imidazole for 5 min, and light cells were left untreated (control) to distinguish the c-Src induced tyrosine phosphorylation events from the background of steady-state tyrosine-phosphorylated proteins in the cell.
Fig. 2.
Scheme of the experimental design of SILAC-based quantitative phosphoproteomic approach. SYF MEF cells stably transfected with R390A c-Src were divided into two populations that were grown in light or heavy medium. The population of cells cultured in heavy medium was treated with 10 mm imidazole for 5 min, and the one cultured in light medium was left untreated. The cell lysates were combined in equal proportions. A, the protein mixture was incubated with anti-phosphotyrosine antibodies for enrichment of tyrosine-phosphorylated proteins. The enriched fraction was subjected to in-gel trypsinization. B, the protein mixture was first digested with trypsin and then incubated with anti-phosphotyrosine antibodies for enrichment of tyrosine-phosphorylated peptides. In both cases, the enriched samples were analyzed by LC-MS/MS.
Phosphoprotein Identification and Quantitation
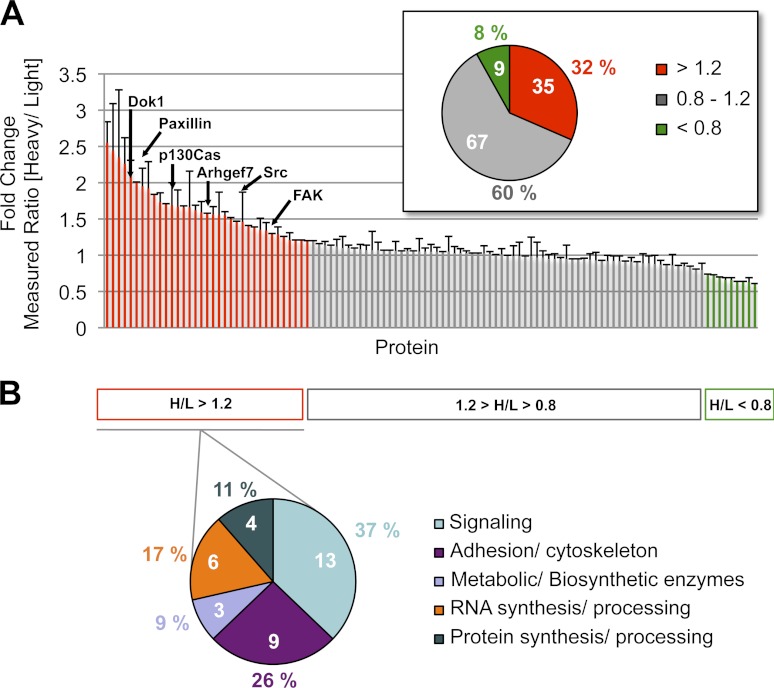
As an initial approach to test the combined advantages of chemical rescue and a proteomics analysis, protein enrichment was performed by immunoprecipitation with anti-phosphotyrosine antibody (Fig. 2A). The phosphotyrosine proteins were analyzed by tandem mass spectrometry. The complete list of identified proteins is shown in supplemental Table 1, and a summary of fold change in phosphorylation upon c-Src chemical rescue for all proteins is included in Fig. 3A. It can be surmised that the 35 proteins showing SILAC ratios greater than or equal to 1.2 are targets of c-Src-mediated tyrosine phosphorylation and in fact, at least six of these are previously documented c-Src substrates. Although it is tempting to infer that larger SILAC ratios suggest greater confidence in undergoing c-Src-mediated phosphorylation, this is not necessarily the case. For example, the well established c-Src substrate focal adhesion kinase (FAK) showed a modest ratio of 1.2. The proteins with a SILAC ratio of 1.2 or above were classified into five protein type classes (Fig. 3B) according to the Phosphosite bioinformatics resource (www.phosphosite.org) (27). Interestingly, the most represented cellular functions were signaling pathways (37%) with proteins such as nucleophosmin, G3BP-2, Map kinase, C3G, and cullin 5 and adhesion and cytoskeleton pathways (26%) with vimentin, tensin 1, matrin 3, plectin 1, fibronectin 1, and myosin X. A subset of the 29 potentially novel c-Src targets have been functionally linked to c-Src signaling pathways such as vimentin (28) and nucleophosmin (29), although the experiments here show unique evidence that they are closely temporally linked to c-Src kinase action.
Fig. 3.
Distribution of identified and quantified phosphoproteins. A, summary of fold change in phosphorylation upon c-Src chemical rescue for all proteins in R390A c-Src SYF MEF cells. The known Src protein substrates are indicated. Proteins with a heavy to light (H/L) ratio > 1.2 (red) increased their tyrosine phosphorylation level, and those with an H/L ratio < 0.8 (green) decreased their tyrosine phosphorylation level. Proteins that showed no significant change in their tyrosine phosphorylation level (0.8 < H/L ratio < 1.2) are indicated in gray. The pie chart indicates the distribution of proteins with either up-regulated (H/L >1.2), constant (0.8 < H/L < 1.2), or down-regulated (H/L <0.8) tyrosine phosphorylation levels. B, functional distribution of identified proteins with increased tyrosine phosphorylation level upon c-Src rescue.
Confirmation of c-Src Targets by Immunoprecipitation-Western Blot Analysis
We thus selected a set of identified proteins to confirm chemical rescue-induced tyrosine phosphorylation using an immunoprecipitation-Western blot approach in c-Src R390A/SYF cells. The D388N/SYF cells serve as negative controls. As described previously, paxillin and FAK are well established c-Src substrates and therefore served as positive controls, which showed imidazole-enhanced signal intensity when blotting with 4G10 anti-phosphotyrosine antibody after immunoprecipitation (supplemental Fig. 5).
We showed that C3G, vimentin, tensin 1, matrin 3, nucleophosmin, and Gsk-3β could be efficiently immunoprecipitated from SYF cells (Fig. 4). Analysis of these immunoprecipitated proteins with anti-phosphotyrosine antibody showed an increase in the phosphorylation of each of these proteins from R390A/SYF but not D388N/SYF cells when treated with 10 mm imidazole for 5 min. The loading controls were performed by immunoprecipitation with a specific protein antibody followed by blotting with an antibody against the same protein. These data suggest that tyrosine phosphorylation of each of these proteins depends on c-Src kinase activity, with a close temporal connection to c-Src rescue.
Phosphosite Mapping by Chemical Rescue of Mutant c-Src
Given the success in our preliminary attempt to combine c-Src chemical rescue and phosphoproteomics, we decided to perform an extensive phosphopeptide mapping and quantitation of phosphorylation changes in the cell upon c-Src rescue. The protocol described above for quantitative analysis of protein-tyrosine phosphorylation was modified such that trypsin proteolysis of extracted proteins was performed prior to affinity-based enrichment with anti-phosphotyrosine antibody (Fig. 2B). In addition, three biological replicates and two technical replicates were performed (supplemental Fig. 1) and analyzed using high resolution, high accuracy mass spectrometry (30). Mascot and Sequest led us to the identification of an initial list of redundant tyrosine phosphopeptides (supplemental Table A). That translated into a list of 335 nonredundant peptide sequences (corresponding to 205 proteins). In parallel, the quantitative proteomics software MaxQuant led us to the identification of an initial list of redundant tyrosine phosphopeptide spectrum matches (supplemental Table B) that translated into a list of 216 nonredundant tyrosine phosphopeptides (corresponding to 147 proteins). We assembled a proteome comprising 382 nonredundant peptide sequences, corresponding to 334 tyrosine phosphorylation sites on 213 proteins (supplemental Table 2).
We set a threshold value of 1.2-fold for SILAC ratio (heavy versus light ≥ 1.2) to filter for tyrosine-containing peptides with increased phosphorylation level upon c-Src rescue. A high confidence data set of quantified phosphotyrosine peptides was obtained through four filtering criteria described under “Experimental Procedures.” After manual validation we obtained a list of 97 nonredundant peptide sequences, corresponding to 85 tyrosine phosphorylation sites (corresponding to 52 proteins), as up-regulated in response to chemical rescue of c-Src, suggesting that they may be c-Src targets. A complete list of the mapped proteins with sites showing an increased phosphorylation level upon c-Src chemical rescue is provided in supplemental Table 3.
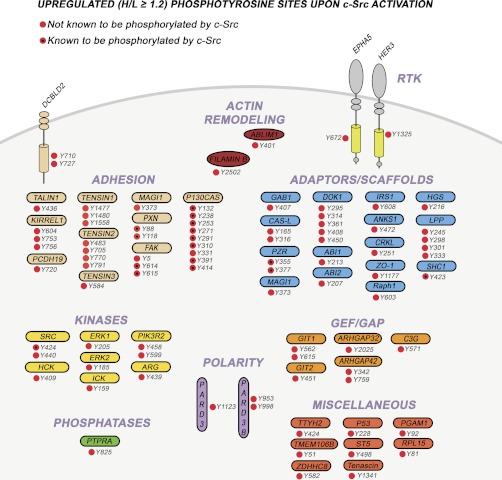
Several of these sites were identified in proteins that also showed an increased phosphorylation level at the protein level (such as C3G, tensin 1, Pard3, and Lpp), adding further confidence to their significance. The proteins containing the tyrosine residues that showed an increased phosphorylation level upon c-Src rescue can be classified into several categories (Fig. 5) including actin modeling, adaptor/scaffold, adhesion, guanine exchange factors/GTPase-activating proteins, nonreceptor protein kinases, phosphatases, cell polarity, and receptor tyrosine kinases and a group of proteins with diverse functionalities.
Fig. 5.
Overview of identified phosphopeptides with up-regulated tyrosine phosphorylation upon c-Src rescue in R390A c-Src SYF MEF cells. All the detected up-regulated phosphopeptides are shown whether they are novel (red) or known (red and black dot) c-Src substrate sites. As shown, the corresponding proteins span a wide variety of cellular functions, such as polarity, actin remodeling, adaptors, kinases, phosphatases, and guanine exchange factors/GTPase activating proteins and are modulated by tyrosine phosphorylation following c-Src activation.
We analyzed the c-Src up-regulated phosphoproteome with the Ingenuity Pathways Knowledge Software (Ingenuity® Systems, http://www.ingenuity.com) and looked for enrichment of both canonical pathways (Fig. 6A) and ontology (Fig. 6B). Not surprisingly, two of the most enriched pathways are the integrin and the focal adhesion signaling pathways. The cellular movement category appears to be the functionality that is especially impacted upon c-Src activation. Both integrin-mediated focal adhesion formation and adherens junction formation and focal adhesion disassembly (turnover) mediate cellular motility and are involved in cancer invasiveness and progression (1). A large number of the identified sites are involved in cell adhesion, particularly in focal adhesion regulation, where c-Src plays key role (31, 32). In this context, several phosphosites have been found in potential novel c-Src substrates. Also, novel sites in well established c-Src substrates have shown an increased tyrosine phosphorylation level (Fig. 7). Although the tyrosine phosphoproteome provided here is not likely to include the entire c-Src phosphoproteome, it is the first snapshot of the tyrosine phosphorylation events that take place immediately (5 min) after c-Src specific activation and the first one that has been obtained through high resolution, high accuracy mass spectrometry.
Fig. 6.
Ingenuity pathway analysis of the c-Src up-regulated phosphoproteome. A, enrichment plots of canonical pathways (x axis) built on the list of 52 c-Src up-regulated phosphoproteins. The right y axis (line) represents the percentage enrichment (ratio between the number of c-Src up-regulated proteins and the total number of proteins included in the pathway). The left y axis (bars) represents the significance of the enrichment, which was calculated using the Benjiamini-Hochberg multiple testing correction, as −log10 of p values. From the complete list of pathways obtained from the list of the c-Src up-regulated phosphoproteins by Ingenuity Systems, the ones with a value of −log(BH-P) ≥ 6 are represented. B, enrichment plots of ontology (x axis) built on the list of 52 c-Src up-regulated phosphoproteins. The y axis represents the significance of the enrichment, which was calculated using the Benjiamini-Hochberg multiple testing correction, as log10 of p values.
Fig. 7.
Role of c-Src in focal adhesion signaling. The signaling pathways involved in focal adhesion activation by integrins, and the resulting downstream signaling events are represented. The biological functions resulting from focal adhesions are also summarized. For each of the proteins involved, the sites that are known substrates of c-Src are indicated. The modulation of phosphotyrosine sites that were identified in our experiment is indicated as significantly increased (black), not modulated (gray), or decreased (white). Among the identified sites, some are known c-Src substrates (circles with X inside).
The Role of c-Src in C3G-mediated Mechanism of Rap1 Activation
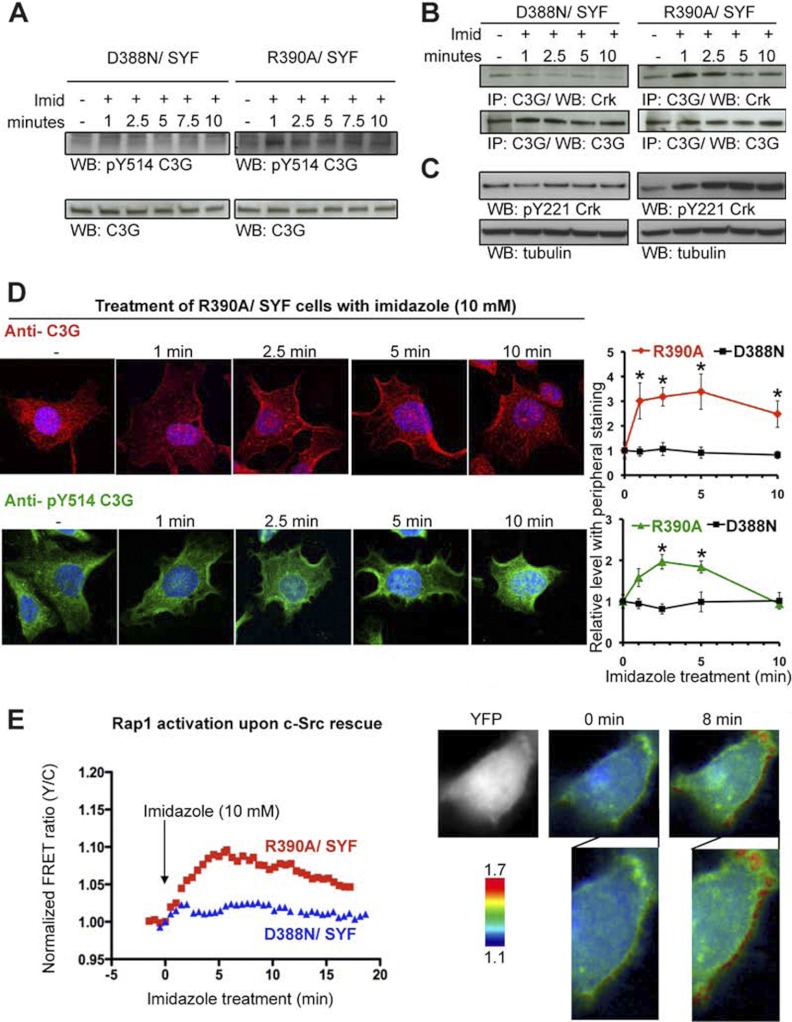
We next explored the potential of chemical rescue as a tool to explore the biological significance of the phosphorylation of one of the detected proteins, C3G, in response to c-Src chemical rescue. C3G was identified in both proteomic approaches (phosphoprotein enrichment and phosphopeptide enrichment) and was validated through immunoprecipitation and Western blot as mentioned above. Furthermore, C3G has shown to play a role in focal adhesion signaling (33) as can be shown in Fig. 7. C3G is a guanine nucleotide exchange factor for the small Ras-related G-proteins Rap1, Rap2, and R-Ras (34–37). It is known that C3G is phosphorylated on Tyr-514, and this post-translational modification has been shown to be critical for the activation of C3G (38). Previous studies have suggested possible roles of Hck, Jak2, and c-Src kinases in Tyr-514 phosphorylation (38–40). In this study, we have observed that imidazole treatment of R390A/SYF cells but not D388N/SYF cells led to an increase in Tyr-514 phosphorylation of C3G ISOFORM as observed by Western blot with an anti-Tyr(P)-514 antibody (Fig. 8A). This increase was observed within 1 min of imidazole exposure, supporting the possibility that C3G is a direct substrate of c-Src. Additional tyrosine phosphorylation sites may account for the large overall increase in C3G overall tyrosine phosphorylation level (Fig. 4), such as the newly identified site, Tyr-571, which shows an approximately 3-fold SILAC ratio upon c-Src rescue.
Fig. 8.
Spatiotemporal dissection of the activation mechanism for the identified c-Src substrate, C3G. A, Western blot using anti-Tyr(P)-514-C3G antibody showed an increase in the phosphorylation level of tyrosine 514 in C3G ISOFORM after 1 min upon c-Src rescue in R390A c-Src SYF cells but not in D388N c-Src SYF cells. Western blot using an antibody against C3G shows equal loading along the different time points in both R390A c-Src SYF and D388N c-Src SYF cells. Imid, imidazole. B, co-immunoprecipitation using C3G immunoprecipitation and Crk blotting showed an increase in C3G-Crk binding after 1 min upon imidazole treatment, which was sustained until 2.5 min and decreased at 5 min in R390A c-Src SYF cells but not in D388N c-Src SYF cells. C, Western blot using anti-Tyr(P)-221 in Crk showed a gradual increase in phosphorylation upon c-Src rescue, reaching the highest level of phosphorylation at minute 5 in R390A c-Src SYF cells but not in D388N c-Src SYF cells. The maximum level of phosphorylation was reached at the same point at which C3G and Crk showed a decreased binding. D, R390A c-Src/SYF cells were treated with 10 mm imidazole and fixed at various time points. Localization of both C3G and Tyr(P)-514-C3G was detected by staining with C3G (rhodamine) and Tyr(P)-514-C3G (FITC), and the nuclei were stained by 4′,6′-diamino-2-phenylindole. Images were acquired by confocal microscopy. Representative images at 100× magnification are shown. Cells (n>40) were categorized according to phenotype (cytosolic versus peripheral) and quantified. Quantitative analysis of C3G and Tyr(P)-514-C3G cellular localization was measured as a function of imidazole treatment time. The images were captured by confocal microscopy at 40× magnification as the ones shown in supplemental Fig. 6. Six representative images (at least 40 cells) were quantified for each condition. Each cell was scored for whether it had peripheral staining. The fraction of cells with peripheral staining was calculated for each image, and the averages were calculated by combining the six images for each condition. Each time point was normalized to time 0 and standard errors are shown. Statistical significance (p < 0.05) compared with time 0 was calculated using a Student's t test and is indicated with an asterisk. E, Rap1 activation upon c-Src rescue in R390A c-Src SYF cells (red) and D388N c-Src SYF cells (blue). The FRET-based GTPase sensor, Raichu-Rap1 showed a 10% increase in FRET response (Rap1 activation indicator) along the cell membrane after 8 min of treatment with imidazole to rescue c-Src activity.
In addition to C3G phosphorylation, it has been shown that C3G activation occurs through membrane recruitment (39, 42–44). Redistribution of C3G within the cell, probably to sites where Rap1 is located, occurs through binding of C3G proline-rich regions to the SH3 domain of the adaptor protein Crk, whose SH2 domains bind to phosphorylated sites resulting from activated receptors (40, 41, 45). To further dissect this signaling process, we explored the potential changes in protein-protein interactions between C3G and Crk as a result of c-Src chemical rescue. After imidazole treatment of R390A/SYF cells, C3G protein was pulled down, and co-immunoprecipitated Crk was detected by Western blot (Fig. 8B). We observed that increased Crk was pulled down by C3G immunoprecipitation only 1 min after rescue of R390A/SYF but not D388N/SYF cells. The increased binding of C3G and Crk was maintained until 5 min, when it experienced a decrease. We could therefore conclude that c-Src activation leads to an immediate increase in binding between C3G and Crk.
To gain a better understanding of the dynamics of the Crk-C3G interaction, we explored the possible cause of the observed decrease in binding after 5 min upon c-Src rescue. Crk is negatively regulated when it is phosphorylated on Tyr-221, which occurs as a result of various types of stimulation (46–48). We examined the phosphorylation level of the Tyr-221 site with a phosphospecific antibody after chemical rescue at the same time points used to observe C3G-Crk interaction (Fig. 8C). Indeed, phosphorylation of Crk on Tyr-221 reaches a peak at 5 min, the same time as when C3G-Crk dissociation is first observed.
To further examine the connections between c-Src and C3G, we explored the subcellular localization of C3G using immunofluorescence and confocal microscopy after chemical rescue. As expected, C3G was mainly cytoplasmic at baseline. However, upon imidazole treatment, a greater concentration was observed at the cell periphery in R390A c-Src expressing SYF cells (Fig. 8D), but this was not observed in D388N c-Src-expressing SYF cells (supplemental Fig. 6). This was detected 1 min after imidazole treatment and persisted until 5 min, after which it showed a decrease (Fig. 8D and supplemental Fig. 6). Cells with peripheral staining showed both membrane and subcortical localization. Moreover, localization of Tyr(P)-514-C3G before and after c-Src rescue showed a similar trend as C3G, with a peak in peripheral localization of Tyr(P)-514-C3G at 2.5 min (Fig. 8D and supplemental Fig. 6).
Rap1 is a small G-protein of the Ras family that antagonizes Ras by sequestering their common target, Raf1, in its inactive form (49–51). It has also been suggested to play an important role in integrin-mediated cell adhesion (52). Thus we wanted to examine the effects of c-Src-mediated activation of C3G on Rap1. We employed the cell-based reporter Raichu-Rap1 (24), which is designed to undergo a FRET change upon Rap1 activation. As shown in Fig. 8E, imidazole treatment of R390A/SYF cells but not D388N/SYF cells indeed stimulates Rap1 within 5 min primarily at the cell periphery. C3G activation of Rap1 appears therefore to be controlled by c-Src activation. In this way we were able to further elucidate the mechanism of C3G activation following c-Src chemical rescue in a precise spatiotemporal manner that could have been very difficult to obtain with traditionally used methods for the study of signal transduction.
DISCUSSION
Previous studies have focused on proteomic analysis on oncogenic viral v-Src (3) or constitutively active Src mutants (4, 5). In addition, some of these studies have examined tyrosine phosphorylation patterns several hours or days after Src is expressed, allowing chronic changes in phosphoproteins to be ascertained. Such analyses are well suited to identification of possible biomarkers in v-Src expressing tumors, which appear to be very rare, but likely reflect many secondary changes in cellular protein levels relating to the well established effects of Src on growth and gene expression. A recent study focused on the kinase action of cellular c-Src in response to treatment with SU6656 inhibitors and posterior stimulation with platelet-derived growth factor (7). Proteomic analysis after the acute activation of tyrosine kinases by ligands such as growth factors have been particularly informative because they have revealed how a cascade of signaling is initiated in a temporal fashion but give rise to indirect information about nonreceptor kinases.
To identify temporally direct substrates of the proto-oncogene c-Src, it would be ideal to use a method to rapidly and specifically switch on c-Src activity in the cell. In contrast to receptor tyrosine kinases, most nonreceptor tyrosine kinases cannot be readily controlled in this fashion. However, the chemical rescue of the R390A c-Src mutant provides a strategy that perfectly fits this purpose because it provides a rapid, specific, and reversible mechanism of c-Src activation by the small molecule imidazole, allowing the detection of the immediate effects of the restored c-Src activity in cells (3). Prior in vitro studies on the tyrosine kinases Csk, Src, and Abl have indicated that the imidazole concentration used here (<30 mm) has no effect (<10%) on wild-type kinase activity (3, 12, 14). Furthermore, previous experiments on chemical rescue of R/A Csk and Src with imidazole in cell culture have revealed that this chemical rescue approach recapitulates, without apparent toxic effects, expected tyrosine kinase activities involved in stress fiber formation, vascular tube formation, cell migration and transformation, and guanylyl cyclase modulation (3, 12–14, 53, 54). Extensive Western blot analyses from an earlier report (3) and contained in the current study show no evidence of cellular tyrosine phosphorylation mediated by imidazole in control cell lines. Although we cannot completely rule out that at the level of mass spectrometric analysis some imidazole effects may be nonspecific, we believe that the accumulated evidence suggests that the chemical rescue approach is a robust technique for phosphoproteomics.
Combining the power of chemical rescue with proteomics led us to the identification of 29 potential new c-Src protein substrates and 85 tyrosine phosphorylation sites (corresponding to 52 proteins), which showed an increased phosphorylation level upon c-Src rescue. It is reassuring that several of the putative protein substrates of c-Src that we have uncovered in this work have been linked to Src in prior studies. We note that 16 of the proteins found here using c-Src rescue were found among the 140 putative Src kinase targets identified in a combined list (supplemental Table 4) of the four prior mass spectrometry phosphoproteomics studies (3–5, 7) reported previously. The lack of greater overlap is likely due in part to the divergent experimental approaches. It must be cautioned that even chemical rescue cannot unequivocally assign a protein as a direct kinase substrate of c-Src because the changes in phosphorylation we see can result from secondary effects, which can happen within the time frame used in our observations (5 min). Indeed, the class of proteins that show a decrease in tyrosine phosphorylation likely reflects examples of a secondary phenomenon where a protein-tyrosine phosphatase is activated by phosphorylation. For example, protein-tyrosine phosphatases SHP1 and SHP2 are known to be activated by tyrosine phosphorylation, and such a mechanism could contribute to specific dephosphorylation events (55, 56). Nevertheless, to define the characteristics of signaling networks, it is helpful to elucidate the temporal connection among phosphoprotein changes, even if the protein interactions are indirect.
Activation of c-Src by growth factors or activating mutations leads to an increased cellular proliferation, invasion and motility, and decreased cell-cell (adherens junctions) and cell-matrix adhesion (focal adhesions) (1). A significant number of the phosphopeptides detected in our chemical rescue analysis are involved in cell adhesion and, in particular, in focal adhesion pathways (Fig. 7). c-Src is known to play a role in the regulation and disassembly of focal adhesions, which link the extracellular matrix to the actin cytoskeleton (57) and are also involved in signaling pathways that regulate proliferation and gene transcription (58). Our work here provides a number of possible novel connections in this regard. A network map that includes the role of c-Src in focal adhesions and highlights the novel proteins and phosphopeptides identified in our study is shown in Fig. 7.
Focal adhesions form when integrin connects the extracellular matrix to a complex of proteins that acts as a link between integrins and the actin cytoskeleton. Several tyrosine sites from cytoskeletal proteins that are part of this supramolecular structure and have not previously been described as c-Src substrates experienced an increase in their phosphorylation level include talin (Tyr-436), filamin B (Tyr-2502), tensin 1 (Tyr-1477, Tyr-1480, and Tyr-1558), and tensin2 (Tyr-483, Tyr-705, Tyr-770, and Tyr-791). Tensin 3 is a recently discovered c-Src target (59), but the site found in this study, Tyr-584, has never been shown to be phosphorylated by c-Src.
The activation of RhoA is one of the key events of focal adhesion assembly (60). c-Src blocks downstream signaling by RhoA through activation of p190 RhoGAP, leading to focal adhesion disruption and increased motility (61). Two other RhoGAPs are known to be phosphorylated by c-Src: RhoGAP3 (Tyr-21 known (62)) and RhoGAP5 (63) (Tyr-1091 and Tyr-1109 known). Interestingly, we detected two other RhoGAPs that have never been described as c-Src substrates: RhoGAP42 (Tyr-342 unknown and Tyr-759 unknown) and RhoGAP32 (Tyr-2025 unknown).
It is known that c-Src phosphorylates and activates FAK at sites Tyr-614 and Tyr-615, promoting maximal FAK catalytic activity (64). Interestingly, we detected a new FAK phosphorylation site, Tyr-5, which underwent a c-Src-mediated increase in phosphorylation and has never been identified as a c-Src substrate. Similarly, p130Cas is a well known substrate of active FAK-Src. Several known sites and a new phosphorylation site, Tyr-331, showed an increased phosphorylation level upon c-Src rescue.
The Rap1 guanine exchange factor (C3G) was also detected in our proteomic studies and plays a role in focal adhesion signaling as indicated in Fig. 7. The temporal connection between c-Src and C3G identified here allow us to propose a molecular mechanistic pathway for Rap1 control. It has been shown that Crk is constitutively associated with C3G (65). We show that rapid activation of c-Src enhances interaction between C3G and Crk by 1 min. Such regulation of this complex may account for reported responses after the stimulation of cells by insulin, integrin engagement, and other natural triggers (66–68). Presumably, the adaptor protein Crk drives C3G to the cell periphery, where we detected it within minutes after c-Src rescue. Increased phosphorylation of C3G on Tyr-514 is known to facilitate C3G activity to catalyze GTP exchange on Rap1 (38), consistent with our live cell reporter assay, which showed a maximum level of activity at 5 min. Interestingly, the binding between C3G and Crk shows a decrease at this time, possibly initiating retrograde signaling in this pathway (66).
We showed that the level of phosphorylation of Crk on Tyr-221 increases upon c-Src activation. Interestingly, the observed decrease in Crk-C3G binding occurs when phosphorylation of Crk on Tyr-221 reaches a maximum level. Subsequently, Rap1 activation appears to be indirectly modulated downward in a fashion that is correlated with Crk phosphorylation on residue Tyr-221. Phosphorylation of Crk on Tyr-221 and the consequent dissociation of the C3G-Crk complex at 5 min might contribute to the decrease in Rap1 activity that follows. Abl kinase has been identified as Crk upstream kinase on Tyr-221 (47). In this regard, our phosphopeptide mapping revealed that site Tyr-393/Tyr-439 of Abl/Arg, which has additive effects on Abl activation (69) and has not been described previously as a c-Src substrate, increased phosphorylation level significantly 5 min after c-Src chemical rescue. It is also noteworthy that rescue induced an ∼3-fold increase in phosphorylation of a new site, Tyr-571 (supplemental Fig. 7), on C3G that may also modulate the ability of C3G to activate Rap1. However, we caution that the exact timing of each event: C3G phosphorylation, C3G-Crk binding, C3G translocation to the membrane, and Rap1 activation, cannot be revealed with certainty because of the varying techniques and limited temporal resolution associated with these methods. This may account for the slight delay in Rap1 activity decrease relative to Crk-C3G binding disruption and C3G phosphorylation.
In summary, the chemical rescue approach has allowed a kinetic description of c-Src signaling events that would otherwise have been difficult to obtain. In principle, this technique can be applied to other nonreceptor protein-tyrosine kinases to provide a more complete portrait of tyrosine phosphorylation networks in cell signaling.
Acknowledgments
We thank Dr. Michiyuki Matsuda for providing us with the Raichu-Rap1 FRET Reporter. We thank Barbara Smith and Michael Delannoy from the Microscope Facility at the Johns Hopkins School of Medicine for their assistance. We thank Bob Cole and Bob O'Meally from the Mass Spectrometry and Proteomics Facility for help.
Footnotes
* This work was supported in part by the National Institutes of Health and a Fulbright Graduate Scholarship from the International Institute of Education-Fulbright Spain, Government of Spain (to I. M. F.).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- SILAC
- stable isotope labeling of amino acids in cell culture
- FAK
- focal adhesion kinase
- SYF MEF
- Src-, Yes-, and Fyn-knock out mouse embryonic fibroblast
- IAP
- immunoaffinity purification
- H/L
- heavy/light
- IP
- immunoprecipitation
- WB
- Western blotting.
REFERENCES
- 1. Yeatman T. J. (2004) A renaissance for Src. Nat. Rev. Cancer 4, 470–480 [DOI] [PubMed] [Google Scholar]
- 2. Shah K., Shokat K. M. (2002) A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signalling pathway. Chem. Biol. 9, 35–47 [DOI] [PubMed] [Google Scholar]
- 3. Qiao Y., Molina H., Pandey A., Zhang J., Cole P. A. (2006) Chemical rescue of a mutant enzyme in living cells. Science 311, 1293–1297 [DOI] [PubMed] [Google Scholar]
- 4. Amanchy R., Zhong J., Molina H., Chaerkady R., Iwahori A., Kalume D. E., Grønborg M., Joore J., Cope L., Pandey A. (2008) Identification of c-Src tyrosine kinase substrates using mass spectrometry and peptide microarrays. J. Proteome Res. 7, 3900–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo W., Slebos R. J., Hill S., Li M., Brábek J., Amanchy R., Chaerkady R., Pandey A., Ham A. J., Hanks S. K. (2008) Global impact of oncogenic Src on a phosphotyrosine proteome. J. Proteome Res. 7, 3447–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Irby R. B., Mao W., Coppola D., Kang J., Loubeau J. M., Trudeau W., Karl R., Fujita D. J., Jove R., Yeatman T. J. (1999) Activating SRC mutation in a subset of advanced human colon cancers. Nat. Genet. 21, 187–190 [DOI] [PubMed] [Google Scholar]
- 7. Amanchy R., Zhong J., Hong R., Kim J. H., Gucek M., Cole R. N., Molina H., Pandey A. (2009) Identification of c-Src tyrosine kinase substrates in platelet-derived growth factor receptor signaling. Mol. Oncol. 3, 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bishop A. C., Ubersax J. A., Petsch D. T., Matheos D. P., Gray N. S., Blethrow J., Shimizu E., Tsien J. Z., Schultz P. G., Rose M. D., Wood J. L., Morgan D. O., Shokat K. M. (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 [DOI] [PubMed] [Google Scholar]
- 9. Pratt M. R., Schwartz E. C., Muir T. W. (2007) Small-molecule-mediated rescue of protein function by an inducible proteolytic shunt. Proc. Natl. Acad. Sci. U.S.A. 104, 11209–11214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karginov A. V., Ding F., Kota P., Dokholyan N. V., Hahn K. M. (2010) Engineered allosteric activation of kinases in living cells. Nat. Biotechnol. 28, 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dephoure N., Howson R. W., Blethrow J. D., Shokat K. M., O'Shea E. K. (2005) Combining chemical genetics and proteomics to identify protein kinase substrates. Proc. Natl. Acad. Sci. U.S.A. 102, 17940–17945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams D. M., Wang D., Cole P. A. (2000) Chemical rescue of a mutant protein-tyrosine kinase. J. Biol. Chem. 275, 38127–38130 [DOI] [PubMed] [Google Scholar]
- 13. Lowry W. E., Huang J., Ma Y. C., Ali S., Wang D., Williams D. M., Okada M., Cole P. A., Huang X. Y. (2002) Csk, a critical link of G protein signals to actin cytoskeletal reorganization. Dev. Cell 2, 733–744 [DOI] [PubMed] [Google Scholar]
- 14. Muratore K. E., Seeliger M. A., Wang Z., Fomina D., Neiswinger J., Havranek J. J., Baker D., Kuriyan J., Cole P. A. (2009) Comparative analysis of mutant tyrosine kinase chemical rescue. Biochemistry 48, 3378–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. (2005) Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 [DOI] [PubMed] [Google Scholar]
- 16. Harsha H. C., Molina H., Pandey A. (2008) Quantitative proteomics using stable isotope labeling with amino acids in cell culture. Nat. Protoc. 3, 505–516 [DOI] [PubMed] [Google Scholar]
- 17. Rappsilber J., Mann M., Ishihama Y. (2007) Protocol for micro-purification, pre-fractionation and storage of peptides using StageTips. Nat. Protoc. 2, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 18. Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., Mann M. (2011) Andromeda: a peptide search Enghien integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 19. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 20. Eng J. K., Mccormack A. L., Yates J. R. (1994) An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 21. Balgley B. M., Laudeman T., Yang L., Song T., Lee C. S. (2007) Comparative evaluation of tandem MS search algorithms using a target-decoy search strategy. Mol. Cell. Proteomics 6, 1599–1608 [DOI] [PubMed] [Google Scholar]
- 22. Peri S., Navarro J. D., Amanchy R., Kristiansen T. Z., Jonnalagadda C. K., Surendranath V., Niranjan V., Muthusamy B., Gandhi T. K., Gronborg M., Ibarrola N., Deshpande N., Shanker K., Shivashankar H. N., Rashmi B. P., Ramya M. A., Zhao Z., Chandrika K. N., Padma N., Harsha H. C., Yatish A. J., Kavitha M. P., Menezes M., Choudhury D. R., Suresh S., Ghosh N., Saravana R., Chandran S., Krishna S., Joy M., Anand S. K., Madavan V., Joseph A., Wong G. W., Schiemann W. P., Constantinescu S. N., Huang L., Khosravi-Far R., Steen H., Tewari M., Ghaffari S., Blobe G. C., Dang C. V., Garcia J. G., Pevsner J., Jensen O. N., Roepstorff P., Deshpande K. S., Chinnaiyan A. M., Hamosh A., Chakravarti A., Pandey A. (2003) Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 13, 2363–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dennis G., Jr., Sherman B. T., Hosack D. A., Yang J., Gao W., Lane H. C., Lempicki R. A. (2003) DAVID: Database for Annotation, Visualization and Integrated Discovery. Genome Biol. 4, P3. [PubMed] [Google Scholar]
- 24. Mochizuki N., Yamashita S., Kurokawa K., Ohba Y., Nagai T., Miyawaki A., Matsuda M. (2001) Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065–1068 [DOI] [PubMed] [Google Scholar]
- 25. Klinghoffer R. A., Sachsenmaier C., Cooper J. A., Soriano P. (1999) Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ong S. E., Mann M. (2006) A practical recipe for stable isotope labeling by aminoacids in cell culture (SILAC). Nat. Protoc. 1, 2650–2660 [DOI] [PubMed] [Google Scholar]
- 27. Hornbeck P. V., Chabra I., Kornhauser J. M., Skrzypek E., Zhang B. (2004) PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 4, 1551–1561 [DOI] [PubMed] [Google Scholar]
- 28. Wei J., Xu G., Wu M., Zhang Y., Li Q., Liu P., Zhu T., Song A., Zhao L., Han Z., Chen G., Wang S., Meng L., Zhou J., Lu Y., Wang S., Ma D. (2008) Overexpression of vimentin contributes to prostate cancer invasion and metastasis via src regulation. Anticancer Res. 28, 327–334 [PubMed] [Google Scholar]
- 29. Cussac D., Greenland C., Roche S., Bai R. Y., Duyster J., Morris S. W., Delsol G., Allouche M., Payrastre B. (2004) Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-Src to mediate its mitogenicity. Blood 103, 1464–1471 [DOI] [PubMed] [Google Scholar]
- 30. Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 31. Carragher N. O., Westhoff M. A., Fincham V. J., Schaller M. D., Frame M. C. (2003) A novel role for FAK as a protease-targeting adaptor protein: Regulation by p42 ERK and Src. Curr. Biol. 13, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 32. Hynes R. O. (1992) Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 [DOI] [PubMed] [Google Scholar]
- 33. Voss A. K., Gruss P., Thomas T. (2003) The guanine nucleotide exchange factor C3G is necessary for the formation of focal adhesions and vascular maturation. Development 130, 355–367 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka S., Morishita T., Hashimoto Y., Hattori S., Nakamura S., Shibuya M., Matuoka K., Takenawa T., Kurata T., Nagashima K. (1994) C3G, a guanine nucleotide releasing protein expressed ubiquitously binds to SH3 domain of CRK and GRB2/ASH proteins. Proc. Natl. Acad. Sci. U.S.A. 91, 3443–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knudsen B. S., Feller S. M., Hanafusa H. (1994) Four proline rich sequences of the guanine nucleotide exchange factor C3G bind with unique specificity to the first SH3 domain of Crk. J. Biol. Chem. 269, 32781–32787 [PubMed] [Google Scholar]
- 36. Gotoh T., Hattori S., Nakamura S., Kitayama H., Noda M., Takai Y., Kaibuchi K., Matsui H., Hatase O., Takahashi H. (1995) Identification of Rap1 as a target for the Crk SH3 domain-binding GNRF-C3G. Mol. Cell. Biol. 15, 6746–6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohba Y., Mochizuki N., Matsuo K., Yamashita S., Nakaya M., Hashimoto Y., Hamaguchi M., Kurata T., Nagashima K., Matsuda M. (2000) Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol. Cell. Biol. 20, 6074–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ichiba T., Hashimoto Y., Nakaya M., Kuraishi Y., Tanaka S., Kurata T., Mochizuki N., Matsuda M. (1999) Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J. Biol. Chem. 274, 14376–14381 [DOI] [PubMed] [Google Scholar]
- 39. Radha V., Rajanna A., Swarup G. (2004) Phosphorylated guanine nucleotide Exchange factor C3G, induced by pervanadate and Src family kinases localizes to the Golgi and subcortical actin cytoskeleton. BMC Cell Biol. 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuda M., Tanaka S., Nagata S., Kojima A., Kurata T., Shibuya M. (1992) Two species of human CRK cDNA encode proteins with distinct biological activities. Mol. Cell. Biol. 12, 3482–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xing L., Ge C., Zeltser R., Maskevitch G., Mayer B. J., Alexandropoulos K. (2000) c-Src signaling induced by the adapters Sin and Cas is mediated by Rap1 GTPase. Mol. Cell. Biol. 20, 7363–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiyokawa E., Mochizuki N., Kurata T., Matsuda M. (1997) Role of Crk oncogene product in physiologic signaling. Crit. Rev. Oncog. 8, 329–342 [DOI] [PubMed] [Google Scholar]
- 43. Zhu T., Goh E. L., LeRoith D., Lobie P. E. (1998) Growth hormone stimulates the formation of a multiprotein signaling complex involving p130(Cas) and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J. Biol. Chem. 273, 33864–33875 [DOI] [PubMed] [Google Scholar]
- 44. Ling L., Zhu T., Lobie P. E. (2003) Src-CrkII-C3G-dependent activation of Rap1 switches growth hormone-stimulated p44/42 MAP kinase and JNK/SAPK activities. J. Biol. Chem. 278, 27301–27311 [DOI] [PubMed] [Google Scholar]
- 45. Knudsen B. S., Zheng J., Feller S. M., Mayer J. P., Burrell S. K., Cowburn D., Hanafusa H. (1995) Affinity and specifity requirements for the first Src homology 3 domain of the Crk proteins. EMBO J. 14, 2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feller S. M., Knudsen B., Hanafusa H. (1994) c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 13, 2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hashimoto Y., Katayama H., Kiyokawa E., Ota S., Kurata T., Gotoh N., Otsuka N., Shibata M., Matsuda M. (1998) Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor. J. Biol. Chem. 273, 17186–17191 [DOI] [PubMed] [Google Scholar]
- 48. Rosen M. K., Yamazaki T., Gish G. D., Kay C. M., Pawson T., Kay L. E. (1995) Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature 374, 477–479 [DOI] [PubMed] [Google Scholar]
- 49. Bos J. L. (1998) All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 17, 6776–6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. (1989) A Ras-related gene with transformation suppressor activity. Cell 56, 77–84 [DOI] [PubMed] [Google Scholar]
- 51. Cook S. J., Rubinfeld B., Albert I., McCormick F. (1993) RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12, 3475–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bos J. L., de Rooij J., Reedquist K. A. (2001) Rap1 signalling: Adhering to new models. Nat. Rev. Mol. Cell Biol. 2, 369–377 [DOI] [PubMed] [Google Scholar]
- 53. Madhusoodanan K. S., Guo D., McGarrigle D. K., Maack T., Huang X. Y. (2006) Csk mediates G-protein-coupled lysophosphatidic acid receptor-induced inhibition of membrane-bound guanylyl cyclase activity. Biochemistry 45, 3396–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Im E., Kazlauskas A. (2007) Src family kinases promote vessel stability by antagonizing the Rho/ROCK pathway. J. Biol. Chem. 282, 29122–29129 [DOI] [PubMed] [Google Scholar]
- 55. Lu W., Gong D., Bar-Sagi D., Cole P. A. (2001) Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell 8, 759–769 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Z., Shen K., Lu W., Cole P. A. (2003) The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J. Biol. Chem. 278, 4668–4674 [DOI] [PubMed] [Google Scholar]
- 57. Sastry S. K., Burridge K. (2000) Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261, 25–36 [DOI] [PubMed] [Google Scholar]
- 58. Jamora C., Fuchs E. (2002) Intercellular adhesion, signalling and the cytoskeleton. Nature Cell Biol. 4, E101–E108 [DOI] [PubMed] [Google Scholar]
- 59. Qian X., Li G., Vass W. C., Papageorge A., Walker R. C., Asnaghi L., Steinbach P. J., Tosato G., Hunter K., Lowy D. R. (2009) The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell 16, 246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fujisawa K., Madaule P., Ishizaki T., Watanabe G., Bito H., Saito Y., Hall A., Narumiya S. (1998) Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J. Biol. Chem. 273, 18943–18949 [DOI] [PubMed] [Google Scholar]
- 61. Chang J. H., Gill S., Settleman J., Parsons S. J. (1995) c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 130, 355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kai M., Yasuda S., Imai S., Kanoh H., Sakane F. (2007) Tyrosine phosphorylation of β2-chimaerin by Src-family kinase negatively regulates its Rac-specific GAP activity. Biochim. Biophys. Acta 1773, 1407–1415 [DOI] [PubMed] [Google Scholar]
- 63. Zrihan-Licht S., Fu Y., Settleman J., Schinkmann K., Shaw L., Keydar I., Avraham S., Avraham H. (2000) RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene 19, 1318–1328 [DOI] [PubMed] [Google Scholar]
- 64. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 65. Zhu T., Goh E. L., Lobie P. E. (1998) Growth hormone stimulates the tyrosine phosphorylation and association of p125 focal adhesion kinase (FAK) with JAK2. Fak is not required for stat-mediated transcription. J. Biol. Chem. 273, 10682–10689 [DOI] [PubMed] [Google Scholar]
- 66. Okada S., Pessin J. E. (1997) Insulin and epidermal growth factor stimulate a conformational change in Rap1 and dissociation of the CrkII-C3G complex. J. Biol. Chem. 272, 28179–28182 [DOI] [PubMed] [Google Scholar]
- 67. Okada S., Matsuda M., Anafi M., Pawson T., Pessin J. E. (1998) Insulin regulates the dynamic balance between Ras and Rap1 signaling by coordinating the assembly states of the Grb2-SOS and CrkII-C3G complexes. EMBO J. 17, 2554–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Buensuceso C. S., O'Toole T. E. (2000) The association of CRKII with C3G can be regulated by integrins and defines a novel means to regulate the mitogen-activated protein kinase. J. Biol. Chem. 275, 13118–13125 [DOI] [PubMed] [Google Scholar]
- 69. Tanis K. Q., Veach D., Duewel H. S., Bornmann W. G., Koleske A. J. (2003) Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol. Cell. Biol. 23, 3884–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ferrando I. M. (2012) c-Src Chemical Rescue: Contribution to the c-Src Phosphoproteome and the Elucidation of the Mechanism of Activation of C3G. Ph.D. thesis, The Sheridan Libraries, Johns Hopkins University, Baltimore, MD: (in press, http://jhir.library.jhu.edu/handle/1774.2/36134) [Google Scholar]