Abstract
Soft tissue sarcomas (STS) are a rare, heterogeneous group of solid tumors in need of improved therapeutic options. First-line chemotherapy is considered the current standard of care for patients with advanced, symptomatic STS, but the median survival is only 8 to 12 months. Efforts to increase response rates by using combination or dose-dense regimens have largely failed to improve patient outcomes. However, increasing evidence supports the use of specific treatments for certain histological subtypes of STS, and novel therapies, including tyrosine kinase and mammalian target of rapamycin inhibitors, are currently under active investigation. In addition, novel treatment approaches (such as maintenance therapy) designed to prolong the duration of response to chemotherapy and delay disease progression are being explored. This article provides an overview of current systemic therapies for patients with advanced STS and discusses ongoing efforts designed to improve patient outcomes through the use of novel therapeutic agents and treatment strategies. Cancer 2011;. © 2011 American Cancer Society.
Soft tissue sarcomas (STS) are a rare, heterogeneous group of solid tumors in need of improved therapeutic options. This article provides an overview of current systemic therapies for patients with advanced STS and discusses ongoing efforts designed to improve patient outcomes through the use of novel therapeutic agents and treatment strategies.
Keywords: soft tissue sarcoma, novel therapies, mammalian target of rapamycin (mTOR) inhibitors, tyrosine kinase inhibitors, maintenance therapy
INTRODUCTION
Soft tissue sarcomas (STS) are a heterogeneous group of solid tumors of mesenchymal origin that account for < 1% of all adult and 15% of pediatric malignancies in the United States. These tumors can arise anywhere in the body, but develop predominantly in the limbs, limb girdle, and abdominal cavity.1 Overall, an estimated 10,520 new STS cases are expected to be diagnosed in 2010 in the United States, and 3920 individuals will die of these tumors.2 The overall 5-year survival rate for patients with STS is approximately 50% to 60% in adults and 75% in children, depending on tumor grade, size, depth, site, and histological subtype.3-5
Surgical resection, with the goal of achieving an appropriately negative margin, is the primary modality of treatment and is considered definitive for patients with localized low-grade tumors.6 Adjuvant radiation with or without chemotherapy is indicated for patients with high-grade STS [stage II-III; American Joint Committee on Cancer, Cancer Staging Manual, Seventh Edition (2010)]. Alternatively, these modalities may be delivered preoperatively to reduce tumor size or improve resectability, particularly in potentially resectable cases or when there are concerns for adverse functional outcomes after surgery.6 Patients with stage IV disease are characterized as having regional lymph node involvement or distant metastases, regardless of tumor size or depth.1 Patients with advanced local disease or metastatic disease have a particularly poor prognosis, with a median overall survival (OS) of only 8 to 12 months.7 However, outcome varies depending on the histologic subtype (eg, metastatic disease may persist for ≥ 10 years in patients with alveolar soft part sarcoma [ASPS]).7-10
The treatment of stage IV disease depends on the extent of metastases. The lung is the most common site of metastasis in patients with STS of the extremities, whereas extrapulmonary metastases usually develop as a later manifestation of widely disseminated disease.9 Liver metastases are also frequently encountered in patients with STS in the abdominal cavity.9 Patients with limited metastases confined to a single organ or regional lymph node may be considered for resection whenever possible and subsequently managed in the same manner as those with high-grade disease.6, 11 Patients with disseminated metastases, however, should be considered for palliative systemic therapy.11, 12 This article provides an overview of the current approach to systemic therapy in patients with unresectable and metastatic STS (excluding gastrointestinal stromal tumors [GIST]), highlights novel therapies, and introduces new treatment paradigms that may offer the potential to improve outcomes.
Current Approach to the Treatment of Patients With Advanced STS
Current guidelines from the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) consider single-agent or combination regimens as options for the first-line treatment of patients with advanced unresectable STS.6, 11 Chemotherapy may be continued until disease progression, although clinical efficacy decreases over time and cumulative toxicity may become problematic as additional cycles are administered (eg, higher cumulative doses of doxorubicin cause cardiomyopathy and an associated mortality risk).13, 14 The guidelines also support a “watchful waiting” approach for asymptomatic patients diagnosed with advanced disease, especially those patients with long disease-free intervals and minimal disease burden and patients who have achieved best response with first-line chemotherapy.6, 12 The lack of evidence supporting the continuation of chemotherapy until disease progression, the limitations of the watchful waiting approach (including potential disease progression and associated patient anxiety), and the poor OS of patients with advanced STS underscore the need for new therapies and treatment approaches.
In first-line chemotherapy, efforts were made to improve the objective response rate (ORR), either by increasing the dose intensity or administering active agents in combination, with the goal of improving patient survival.15 However, although some improvement was achieved, it did not translate into a survival benefit. A pooled analysis of 8 randomized clinical trials involving 2281 patients with STS demonstrated a slight trend favoring doxorubicin-based combination therapy for ORR (odds ratio [OR], 0.79; 95% confidence interval [95% CI], 0.60-1.05 [P = .10]); these results, as well as OS data (OR, 0.84; 95% CI, 0.67-1.06 [P = .13]), did not reach statistical significance compared with single-agent doxorubicin.16 Nausea, vomiting, and myelosuppression were consistently more severe with the combination regimens.
The European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group retrospectively evaluated factors important in predicting response and survival among 2185 patients with advanced STS who received a first-line anthracycline-containing regimen.8 For the entire cohort, the ORR was 26% and the median OS was 51 weeks. Although the absence of liver metastases and younger age of the patients were found to be independently associated with both response and survival, high histopathological grade was associated with response to chemotherapy, whereas low histopathological grade was associated with survival, suggesting that the ORR may not be sufficient for determining the potential clinical benefit of new agents for the treatment of STS.
Single-agent regimens
Single-agent chemotherapy with doxorubicin, ifosfamide, or dacarbazine and combination regimens with or without an anthracycline backbone have been widely used to treat patients with disseminated metastatic STS (Table 1).15-29 Doxorubicin is the single most active agent in the treatment of metastatic STS, producing ORRs of 16% to 27% in clinical trials.16, 17 Although the response to doxorubicin may depend on dose intensity, this needs to be balanced against the greater toxicity associated with higher doses (eg, cardiotoxicity).30
Table 1.
Options for First-Line Chemotherapy in Patients With Advanced STS
| Treatment | Response Rate | Median OS, Months | Study |
|---|---|---|---|
| Single-agent regimen | |||
| Doxorubicin (60-75 mg/m2 every 3 wk) | 16%-27% | 7.7-12.0 | Bramwell 200016 |
| Lorigan 200721 | |||
| Epirubicin (75 mg/m2) | 18% | 4.0 | Mouridsen 198718 |
| Ifosfamide (5 g/m2 over 24 h every 3 wk) | 10%-25% | 12.0 | van Oosterom 200219 |
| High-dose ifosfamidea | 25%-38% | 10.2-18.5 | van Oosterom 200219 |
| Buesa 199820 | |||
| Temozolomide | |||
| (Oral bid × 5 d every 4 wk)b | 8% | 13.2 | Talbot 200315 |
| (Oral every d × 6 wk; then 3 wk off treatment)b | 16% | 8.1 | Garcia del Muro 200522 |
| Dacarbazine (1.2 g/m2 every 3 wk) | 18% | NR | Buesa 199129 |
| Combination regimens | |||
| Doxorubicin (50 mg/m2) + ifosfamide (5 g/m2) every 3 wk | 21%-28% | 13.8-14.0 | Santoro 199524 |
| Le Cesne 200028 | |||
| Doxorubicin (60 mg/m2) + ifosfamide (7.5 g/m2 over 2 d) every 3 wk | 34% | ∼11.5 | Edmonson 199323 |
| Doxorubicin (60 mg/m2) + dacarbazinec | 17%-30% | 8.0-12.0 | Borden 198717 |
| Antman 199325 | |||
| Mesna, doxorubicin, ifosfamide, and dacarbazine (MAID)d | 32% | 13.0 | Antman 199325 |
| Gemcitabine (900 mg/m2 on d 1 and 8) + docetaxel (100 mg/m2) on d 8 every 3 wk | 16% | 17.9 | Maki 200726 |
| Gemcitabine (800 mg/m2) + vinorelbine (25 mg/m2) on d 1 and 15 every 4 wk | 13% | 75% (12-mo OS) | Dileo 200727 |
Abbreviations: bid, twice daily; NR, not reported; OS, overall survival; STS, soft tissue sarcoma.
High-dose ifosfamide regimens included 9 g/m2 over 3 days every 3 weeks or 14 g/m2 over 6 days every 4 weeks.
Temozolomide was administered orally at a loading dose of 200 g/m2, then every 12 hours at 90 mg/m2 for 4.5 days every 4 weeks, or it was administered at doses of 75 mg/m2 or 100 mg/m2 once daily for 6 weeks followed by a 3-week treatment break before the next cycle.
Dacarbazine was administered intravenously every 3 weeks at a dose of 250 mg/m2/day every 5 days or 1000 mg/m2/day every 4 days.
MAID was comprised of doxorubicin at a dose of 60 mg/m2 and dacarbazine at a dose of 1000 mg/m2 infused continuously over 4 days plus ifosfamide at a dose of 7.5 g/m2 and mesna at a dose of 10 g/m2 infused continuously over 3 or 4 days. The ifosfamide dose was subsequently reduced to 6 g/m2 due to unacceptable myelosuppression after 154 of 374 patients had been accrued.
Epirubicin and the liposomal anthracyclines were developed to improve the safety profile of doxorubicin. Epirubicin tended to be slightly less efficacious than doxorubicin (ORR, 18% vs 25%; P = .33), but produced less hematological toxicity and less nausea and vomiting.18 Improved ORRs were reported with higher doses of epirubicin at the expense of greater toxicity.31 However, in a cohort of 334 patients with advanced STS, 2 different schedules of high-dose epirubicin failed to improve the ORR or OS when compared with a standard dose of doxorubicin (75 mg/m2), and any toxicity advantage was lost.32 Pegylated liposomal doxorubicin appeared to be as effective as standard-dose doxorubicin in a randomized trial of patients with advanced STS (N = 94).33 However, in this study, both agents produced low ORRs (10% and 9%, respectively), but had differing toxicity profiles. In other phase 2 trials, ORRs with pegylated liposomal doxorubicin ranged from 0% to 10%, although approximately one-third of the patients achieved stable disease (SD).15, 34, 35
Standard-dose ifosfamide is active in the first-line treatment of patients with advanced STS (ORRs of 10%-25%).19, 36 High-dose ifosfamide (HDI) regimens produced ORRs as high as 38%, but were associated with higher hematologic and nonhematologic toxicities than the standard dose.19, 20, 37 The EORTC Soft Tissue and Bone Sarcoma Group compared 2 investigational HDI schedules versus standard-dose doxorubicin in a phase 3 trial of patients with advanced STS (N = 326).21 No differences in ORR, progression-free survival (PFS), or OS were observed, but myelosuppression occurred more frequently with HDI. Higher doses may be effective in patients who develop disease progression or recurrence after doxorubicin pretreatment and/or first-line standard-dose ifosfamide.38 In a phase 2 study of patients whose disease had progressed after pretreatment, HDI produced responses in 33% of patients and SD in 22%. It is interesting to note that 24% of patients with disease refractory to standard-dose ifosfamide achieved partial responses (PR); the median duration of response was 8 months and the median OS was 12 months. However, HDI was associated with dose-limiting neutropenia, as well as neurotoxicity and renal toxicity. In a subsequent EORTC multicenter phase 2 trial, HDI administered with adequate mesna protection appeared to be somewhat less effective.39
Dacarbazine has been available for more than 3 decades. In a pooled analysis of published and unpublished data, the ORR of single-agent dacarbazine was 18%.40 In a phase 2 trial of patients with metastatic STS (N = 11), temozolomide, an oral prodrug of dacarbazine, produced an ORR of 8%.15 The ORR rate improved to 16% when temozolomide was administered once daily for 6 weeks followed by a 3-week break from treatment in a patient population with pretreated STS.22
Combination chemotherapy regimens
Although combination regimens involving anthracyclines, ifosfamide, and dacarbazine were developed to increase ORRs and improve patient outcomes, studies with these regimens were largely unsuccessful at improving outcomes, often increasing the toxicity burden. The Eastern Cooperative Oncology Group (ECOG) conducted a phase 3 trial comparing the combination of ifosfamide and doxorubicin with single-agent doxorubicin in patients with metastatic STS (N = 178).23 Although the combination produced a significantly higher ORR (34% vs 20%; P = .03), it was associated with greater myelosuppression and did not significantly prolong OS. This study also included a third treatment arm with mitomycin, doxorubicin, and cisplatin, which produced a higher ORR than doxorubicin without increasing myelotoxicity; again, survival was not improved. In an EORTC phase 3 trial, the combination of ifosfamide and doxorubicin did not significantly improve the ORR (28% vs 23%), median response duration (44 weeks vs 46 weeks), or median OS (55 weeks vs 52 weeks) compared with single-agent doxorubicin, but did increase hematologic toxicity.24 EORTC-62012 is an ongoing phase 3 trial comparing the combination of doxorubicin and ifosfamide (with pegfilgrastim support) versus doxorubicin alone.
The combination of doxorubicin and dacarbazine has a response rate that is comparable to or better than single-agent doxorubicin (Table 1).16, 17, 21, 25 An intensified regimen comprised of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) significantly increased the ORR (32% vs 17%; P < .005) and prolonged time to disease progression (TTP) (6 months vs 4 months; P < .02) compared with the combination of doxorubicin and dacarbazine in a phase 3 trial.25 However, MAID did not improve OS and was associated with greater myelosuppression.
Several other drugs, including gemcitabine, docetaxel, and vinorelbine, have been evaluated in combination regimens in phase 2 trials, largely involving patients with disease that is resistant to front-line chemotherapy. Compared with gemcitabine alone, the gemcitabine and docetaxel combination produced a higher ORR (16% vs 8%) and longer median OS (17.9 months vs 11.5 months; P = .03), albeit with greater toxicity, in patients with metastatic STS (N = 122).26 However, a phase 2, retrospective, pooled analysis of patients with leiomyosarcoma (N = 121) who were treated with the combination of gemcitabine and docetaxel in the second-line (n = 84) setting demonstrated no significant improvement in the ORR or median PFS relative to single-agent gemcitabine.41 A gemcitabine and vinorelbine combination produced clinical benefit (25%) in a single-arm study and was associated with a 12-month OS rate of 75%.27
Several studies have explored dose-escalated doxorubicin and ifosfamide combination regimens with colony-stimulating factor (CSF) support. The EORTC compared standard and intensified doses of doxorubicin in combination with ifosfamide in a phase 3 trial of patients with advanced STS (N = 314).28 Granulocyte-macrophage–CSF (GM–CSF) was administered to patients receiving the intensified dose. The intensified regimen significantly prolonged the median TTP compared with the standard-dose regimen (29 weeks vs 19 weeks), but did not improve OS. Similarly, an intensified MAID regimen with granulocyte (G-CSF) support, in which the doses of doxorubicin, ifosfamide, and dacarbazine were 20% to 33% higher than those in the standard MAID regimen, failed to improve the ORR, PFS, or OS in a recent phase 3 trial.42 High-dose chemotherapy followed by peripheral blood stem cell transplantation did not improve the outcomes of patients who responded to first-line MAID.43 Because these regimens do not significantly improve patient outcomes, better treatment options are still needed.
Treatment Considerations for Certain Sarcoma Subtypes
For many clinical trials evaluating chemotherapeutic agents, patients with advanced STS have been grouped together, regardless of tumor histology. However, growing evidence suggests that for patients with certain histological subtypes, specific agents besides doxorubicin may be preferred options.
Angiosarcoma
Current NCCN guidelines recommend paclitaxel and bevacizumab as treatment options for patients with angiosarcoma, a rare STS subtype with an aggressive clinical course.6 In a phase 2 trial (N = 26), bevacizumab produced a 12% PR rate in patients with angiosarcoma and epithelioid hemangioendothelioma.44 Paclitaxel has exhibited low activity in nonselected sarcoma populations, but favorable responses in patients with angiosarcomas of the scalp.45, 46 Paclitaxel is highly active in angiosarcomas, with an ORR of 62% and a median TTP of 7.6 months.47 In a phase 2 trial examining patients with advanced angiosarcomas (N = 27), paclitaxel demonstrated a 19% PR rate after 2 cycles, a median TTP of 4 months, and a median OS of 8 months, but no difference in PFS was noted between chemotherapy-treated and chemotherapy-naive patients.48
Other nonapproved treatment options for angiosarcomas include multikinase inhibitors (eg, sorafenib and sunitinib) and liposomal doxorubicin. In phase 2 trials, sorafenib produced a 14% PR rate and a median OS of 14.3 months in an angiosarcoma trial (N = 37),49 whereas sunitinib did not produce a response rate in patients with angiosarcoma (n = 2) or fibrous tumor/hemangiopericytoma (n = 3).50 In another phase 2 trial, pegylated liposomal doxorubicin demonstrated similar efficacy and reduced myelosuppression compared with doxorubicin in patients with advanced STS.33 Other results suggest that liposomal doxorubicin might be considered as a palliative therapy in patients with cutaneous angiosarcoma.51
Leiomyosarcoma
Leiomyosarcomas are generally believed to be insensitive to conventional chemotherapy. A phase 2 trial has shown that the combination of gemcitabine and docetaxel has some activity in leiomyosarcomas.52 Patients with unresectable leiomyosarcomas (N = 34), mostly of the uterine type, received the combination of gemcitabine and docetaxel; 16 of the patients were treated after developing disease progression while receiving doxorubicin-based therapy. This regimen produced a 53% response rate and a median TTP of 5.6 months. The same regimen was evaluated as second-line treatment for patients with advanced uterine leiomyosarcomas; the ORR (27%) was lower than in the first trial, but an additional 50% of the patients had SD for a median of 5.4 months and the 24-week PFS rate was 52%.53 Although a recent pooled analysis suggests the combination of gemcitabine and docetaxel has only limited benefit over gemcitabine alone,41 other reports have demonstrated the activity of the gemcitabine and docetaxel combination in leiomyosarcomas and also suggest that this regimen is active in other sarcoma subtypes.26, 54
Synovial sarcoma
In synovial sarcomas, both the size and location of the primary tumor are independent factors that govern disease-free survival after surgical resection of localized disease.55, 56 For adults with high-risk synovial sarcomas, neoadjuvant/adjuvant ifosfamide-based therapy has been associated with improved disease-specific survival.55 In a phase 3 ECOG trial, the combination of ifosfamide and doxorubicin produced a significantly higher ORR compared with single-agent doxorubicin (88% vs 20%; P = .02) in a small subgroup of patients with advanced synovial sarcoma (N = 20),23 whereas a PR rate of 42% and a median OS of 11 months were observed in a follow-up study.57 In a retrospective analysis of patients with advanced STS (N = 1337) who received first-line ifosfamide-based therapy, synovial sarcoma histology was identified as an independent, favorable prognostic factor for both treatment response and OS.58
The DNA-binding cytotoxic agent trabectedin (ecteinascidin-743) and the multikinase inhibitor pazopanib may also have antitumor activity in synovial sarcoma. In a retrospective analysis of patients (N = 39) with advanced/metastatic disease who were treated with trabectedin, 18% achieved a PR, 5% had minor responses, and 28% experienced SD.59 In a phase 2 study of pazopanib in patients (N = 37) with recurrent/refractory advanced STS, 13% of patients with synovial sarcoma experienced a PR, with 49% remaining free of disease progression at 12 weeks.60
Novel Therapeutics
Cytotoxic agents
Trabectedin is approved in Europe and 25 other countries (excluding the United States) for the treatment of patients with advanced STS after failure of an anthracycline and ifosfamide and in patients who are not suitable candidates for these agents.6, 61-63 In an EORTC phase 2 trial of 99 patients, a PR rate of 8% and an SD rate of 26% (of > 6 months) were observed in patients treated with trabectedin who failed previous chemotherapy regimens.64 The median PFS was 105 days and the median OS was 9.2 months. Trabectedin was generally well tolerated, with neutropenia and asymptomatic transaminase elevations reported to be the main toxicities. Comparable results were reported in other phase 2 trials involving more heavily pretreated patients.65, 66 Trabectedin has also shown promising activity in a retrospective analysis of pretreated patients (N = 51) with advanced myxoid liposarcomas (ORR of 51% and a median PFS of 14 months).67 Currently, trabectedin is being evaluated against dacarbazine in a randomized, multicenter, phase 3 trial in patients with advanced pretreated L-sarcoma (liposarcoma or leiomyosarcoma).68
TH-302, a hypoxia-activated cytotoxic prodrug, was administered in combination with doxorubicin and produced a 25% PR rate in a phase 1/2 trial of patients with advanced or metastatic STS (N = 20).69 Palifosfamide, the active moiety of the chemotherapy prodrug ifosfamide, in combination with doxorubicin, was found to double the PFS over doxorubicin alone (7.8 months vs 4.4 months) in a phase 2 trial of patients with unresectable or metastatic STS.70 An ongoing phase 3 trial is examining this combination as a front-line treatment for patients with STS.71
Tyrosine kinase inhibitors
To our knowledge, no tyrosine kinase inhibitors (TKIs) have been approved to date for the treatment of sarcoma other than GIST. Pazopanib, which targets vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), has shown promising activity in patients with advanced STS.60 Compared with historical controls receiving second-line chemotherapy, pazopanib prolonged PFS and OS in patients with STS (including leiomyosarcomas and synovial sarcomas). Pazopanib was generally well tolerated; the most common grade 3/4 toxicities were hypertension, fatigue, and hyperbilirubinemia. Based on these findings, the EORTC initiated the Pazopanib Explored in Soft Tissue Sarcoma a Phase 3 (PALETTE) trial to compare pazopanib with placebo in patients (N = 369) whose disease had progressed during or after at least 1 anthracycline-containing regimen. Preliminary results have indicated that although treatment with pazopanib did not improve OS, the median PFS was significantly prolonged by 13 weeks.72
Other multikinase inhibitors have been tested in patients with advanced STS, generally with limited success reported to date. In a phase 2 trial (N = 48), sunitinib produced a PR in a patient with a desmoplastic round cell tumor and SD lasting ≥ 16 weeks in 10 additional patients, including 2 of 3 patients with solitary fibrous tumors. Patients who underwent positron emission tomography/computed tomography scanning (n = 21) demonstrated evidence of a metabolic PR or SD.50 Sorafenib has exhibited single-agent activity against angiosarcomas, but showed minimal activity against other histological subtypes.49 Skin toxicity was the dose-limiting side effect. Sorafenib has also demonstrated activity in desmoid tumors, which are fibroblastic neoplasms that arise from musculoaponeurotic stromal elements.73 Imatinib, a KIT/PDGFR/BCR-ABL TKI that is used as first-line therapy for patients with unresectable/metastatic GIST, was evaluated in 10 histological sarcoma subtypes, producing responses in 4 of 185 patients (2%).74 When SD (> 4 months) was included, the clinical benefit rate (CBR) was 15% overall, with the highest CBR noted in patients with liposarcomas and leiomyosarcomas.
ARQ 197, a selective inhibitor of the c-Met receptor tyrosine kinase, is indicated for a rare group of tumors associated with the microphthalmia transcription factor family, including clear cell sarcoma (CCS) and ASPS.75, 76 In a phase 2 trial of patients with CCS (n = 7), ASPS (n = 17), and translocation-associated renal cell carcinoma, treatment with ARQ 197 demonstrated a 14% PR rate in patients with CCS and a SD rate of 29% and 59%, respectively, in patients with CCS and ASPS. Treatment with the fibroblast growth factor receptor and VEGFR inhibitor brivanib in patients with advanced STS has been shown to prolong PFS (from week 12) in comparison with placebo (2.8 months vs 1.4 months) in basic fibroblast growth factor (FGF2)-positive patients.77 Cediranib, another VEGFR inhibitor, has also demonstrated promising preliminary results; in 1 study (N = 7), 4 PRs and 1 case of SD78 were observed and in another study (N = 36), a 43% PR rate was noted in evaluable patients (n = 28).79 Axitinib, a small-molecule TKI with multiple targets, is currently being investigated in patients with advanced/metastatic STS.80
Insulin-like growth factor 1 inhibitors
Several anti–insulin-like growth factor 1 monoclonal antibodies are being investigated in patients with advanced sarcoma, including figitumumab and cixutumumab (IMC-A12). Figitumumab has been evaluated in a phase 1 study in patients with sarcoma and Ewing sarcoma; among patients with STS (N = 9), an SD rate of 56% and a 44% progressive disease rate were observed.81 Figitumumab has also been investigated in combination with everolimus in a phase 1 study of patients with advanced sarcoma and other advanced tumors (N = 21); of 18 evaluable patients, 1 achieved a PR and 83% experienced SD.82 In a phase 2 trial (N = 113), initial results with single-agent cixutumumab demonstrated the highest CBR (57%) in patients with adipocytic sarcoma (n = 37).83 Currently, cixutumumab is being investigated in combination with temsirolimus in patients with metastatic sarcomas.84
Mammalian target of rapamycin inhibitors
The rationale for mammalian target of rapamycin (mTOR) inhibitors in patients with advanced STS has been reviewed.85, 86 In a case study, treatment with sirolimus (rapamycin, the prototype mTOR inhibitor) and cyclophosphamide produced a PR in a patient with metastatic myxoid chondrosarcoma, a slow-growing sarcoma that responds poorly to chemotherapy and radiotherapy.87 Treatment with sirolimus in patients (n = 3) with perivascular epithelioid cell tumors also resulted in radiographic responses as well as molecular tuberous sclerosis complex (TSC) and mTOR complex changes.88 In a phase 2 trial, treatment with everolimus in patients (N = 61) with recurrent or refractory sarcoma produced a complete response (CR), PR, or SD (after 4 months) in 13% of patients with STS or bone sarcoma and 27% of patients with GIST.89 Common adverse events (AEs) included mucositis, fatigue, and elevation of liver transaminases. In combination with imatinib, everolimus has demonstrated activity in a phase 1/2 study of imatinib-resistant GIST.90 Everolimus also reduces tumor volume by regulating gene products of the TSC in subependymal giant cell astrocytomas.91 Temsirolimus was investigated in a phase 2 trial of patients (N = 40) with advanced sarcoma, but only 5% of evaluable patients (those with undifferentiated fibrosarcoma and uterine leiomyosarcoma) achieved a PR.92 Tolerability was poor, with 43% of evaluable patients experiencing an AE of grade 3 or higher possibly related to the drug. Ridaforolimus has demonstrated antitumor activity in 2 phase 1 trials that included patients with advanced STS.93, 94 In a subsequent phase 2 trial of patients (N = 212) with advanced STS or bone sarcomas, the majority of whom were previously treated, treatment with ridaforolimus resulted in a 29% CBR and a median OS of 40 weeks.95 The most common AEs were fatigue, stomatitis, hypertriglyceridemia, anemia, rash, and nausea. In a phase 1 trial, an oral formulation of ridaforolimus produced a 23% CBR in the subgroup of patients with advanced sarcomas.96
Other agents
In phase 1 and 2 trials, the Akt inhibitor perifosine has been evaluated in patients with advanced STS. One study failed to meet the primary objective of a 40% PFS rate at 6 months,97 whereas in another study, no objective responses were observed and 27% of patients experienced SD.98 In contrast, a retrospective analysis (N = 60) demonstrated a 5% PR rate in evaluable patients (45% of patients with SD for ≥ 4 months),99 suggesting that perifosine may not be particularly effective in producing tumor responses but may have some role in disease stabilization. The mitotic inhibitor eribulin was examined in an EORTC phase 2 trial of patients with STS stratified by subtype and met prespecified statistical boundaries for the primary endpoint of PFS at 12 weeks for both leiomyosarcoma and adipocytic sarcoma.100 Eribulin is currently being evaluated in a phase 3 clinical trial for patients with advanced, refractory STS.101 ABT-510, a peptide that mimics the antiangiogenic activity of thrombospondin-1, was tested in a phase 2 trial in patients (N = 88) with locally advanced or metastatic STS. Approximately 50% of patients achieved SD, and only 1 objective response occurred.102 Rexin-G (Epeius Biotechnologies, San Marino, Calif), a gene therapy vector bearing a cytocidal dominant negative cyclin D1 construct, was investigated in a phase 1/2 study of patients with STS and bone sarcomas (N = 20); 65% of patients achieved SD according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria.103 Histone deacetylase (HDAC) inhibitors have also shown some promise in treating sarcoma. A phase 1/2 trial of patients with metastatic sarcoma is currently examining the safety and tolerability of the HDAC inhibitor valproic acid in combination with bevacizumab, gemcitabine, and docetaxel.104
Maintenance Therapy as a New Strategy for the Treatment of Sarcoma
Maintenance therapy has been developed as part of treatment paradigms to prolong response duration and delay disease progression in responsive patients or patients with SD after a defined number of chemotherapy cycles (Fig. .1). Given the inherent toxicity associated with first-line chemotherapy and the need to continue maintenance therapy for prolonged periods of time, agents used in maintenance therapy should be well tolerated. Some chemotherapeutic agents, as well as targeted therapies with cytostatic properties and documented tolerability, have been found to be effective as maintenance therapy in patients with non–small cell lung cancer and ovarian cancer.105-107 However, some studies have not demonstrated a benefit of maintenance therapy.108
Figure 1.
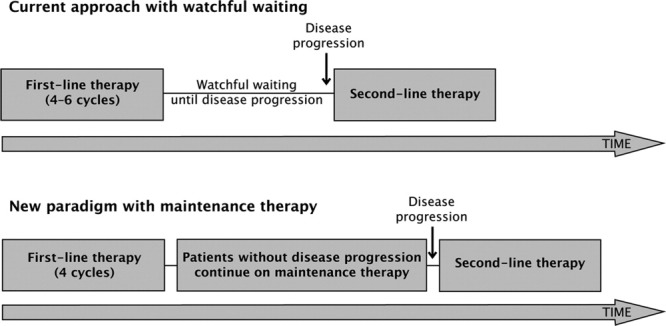
Elements of “watchful waiting” versus maintenance treatment are shown. Watchful waiting indicates first-line chemotherapy followed by monitoring until disease progression; maintenance treatment, first-line chemotherapy followed by maintenance therapy until disease progression.
The Sarcoma Multi-center Clinical Evaluation of the Efficacy of Ridaforolimus (SUCCEED) trial, one of the largest studies of patients with metastatic STS or bone sarcoma published to date, is a pivotal phase 3 trial that evaluated maintenance therapy with oral ridaforolimus in patients (N = 711) who achieved a favorable response (CR, PR, or SD) after receiving a minimum of 4 cycles of chemotherapy (Fig. .2). Preliminary data have demonstrated a significant increase in PFS (ridaforolimus vs placebo), with a 21% improvement in the median PFS (17.7 weeks vs 14.6 weeks; hazard ratio [HR], 0.72 [P = .0001]) and a nonstatistically significant trend toward an OS benefit (21.4 months vs 19.2 months; HR, 0.88 [P = .2256]).109 The incidence of stomatitis and other AEs was higher in patients receiving ridaforolimus, and the overall safety profile was considered to be similar to that of other mTOR inhibitors. Further studies will help confirm the benefit of maintenance therapy with mTOR inhibitors in patients with advanced STS.
Figure 2.
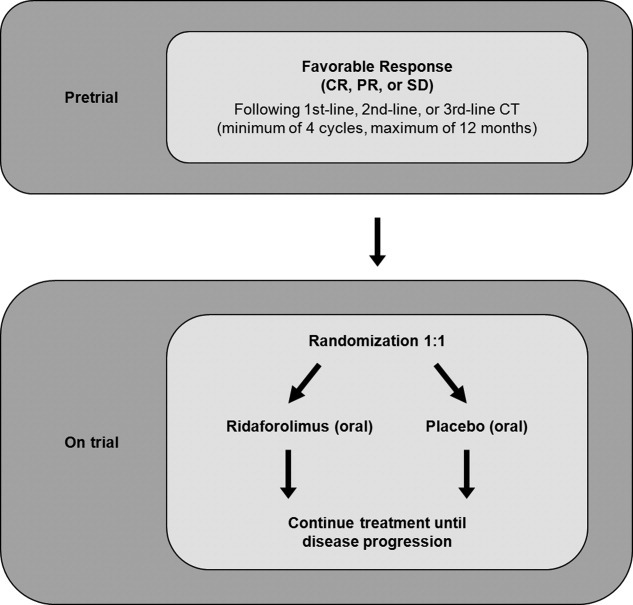
The Sarcoma Multi-center Clinical Evaluation of the Efficacy of Ridaforolimus (SUCCEED) study scheme is shown. CR indicates complete response; PR, partial response; SD, stable disease; CT, chemotherapy.
Conclusions
The current standard of care for patients with unresectable advanced STS includes first-line treatment with single-agent doxorubicin or a doxorubicin-based combination chemotherapy regimen. However, patients in this setting have a poor prognosis. Efforts to increase ORRs through the use of combination or dose-intense regimens have had little impact on patient outcome. Several recent advances offer promise for the treatment of patients with advanced STS. First, increasing evidence suggests that patients with certain STS histologies may benefit from specific treatments, such as paclitaxel or sorafenib in those with angiosarcomas and ifosfamide in patients with synovial sarcomas. Second, several targeted drugs have demonstrated clinical benefit in patients with advanced STS, and ongoing efforts are exploring how to best deploy such agents in conjunction with existing treatment options. Finally, drawing on clinical experience in treating other malignancies, maintenance therapy is being evaluated as a new treatment paradigm for patients with advanced disease. These data suggest that the future is bright for improving treatment options and outcomes for patients with advanced STS.
FUNDING SUPPORT
Editorial assistance was funded by Merck & Co., Inc. Dr. Riedel was responsible for all content and editorial decisions and received no compensation for the development of the article.
CONFLICT OF INTEREST DISCLOSURES
Dr. Riedel has received clinical research support from Novartis Pharmaceuticals, ARIAD Pharmaceuticals, and Ziopharm Oncology and an honorarium from ARIAD Pharmaceuticals. He is also a consultant for Merck & Co. Inc, and has served on speakers bureaus for both Novartis and Pfizer, Inc.
REFERENCES
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Soft Tissue Including Heart. 2010. Available at: http://seer.cancer.gov/statfacts/html/soft.html. Accessed August 12.
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Soft Tissue Sarcoma. V1. 2011. 2011. Available at http://www.nccn.org. Accessed May 11,
- 7.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003:CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens–a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin RS, Pisters PW, Helman LJ, Bramwell V, Rubin EH, O'Sullivan B. In: Sarcomas of soft tissue Abeloff's Clinical Oncology, Chapter 97. 4th ed. Abeloff M, Armitage J, Niederhuber J, Kastan M, McKenna W, editors. Philadelphia: Churchill Livingston; 2008. [Google Scholar]
- 10.Anderson ME, Hornicek FJ, Gebhardt MC, Raskin KA, Mankin HJ. Alveolar soft part sarcoma: a rare and enigmatic entity. Clin Orthop Relat Res. 2005;438:144–148. doi: 10.1097/01.blo.0000180049.50832.4a. [DOI] [PubMed] [Google Scholar]
- 11.Casali PG, Jost L, Sleijfer S, Verweij J, Blay JY. ESMO Guidelines Working Group. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(suppl 2):ii89–ii93. doi: 10.1093/annonc/mdn101. [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Blay JY. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v198–v203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 13.Halpern JL, Gilbert J, Holt GE, Schwartz HS. Chemotherapy for soft tissue sarcoma. Curr Opin Orthop. 2007;18:604–610. [Google Scholar]
- 14.Longhi A, Ferrari S, Bacci G, Specchia S. Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anticancer Drugs. 2007;18:737–744. doi: 10.1097/CAD.0b013e32803d36fe. [DOI] [PubMed] [Google Scholar]
- 15.Talbot SM, Keohan ML, Hesdorffer M, et al. A phase II trial of temozolomide in patients with unresectable or metastatic soft tissue sarcoma. Cancer. 2003;98:1942–1946. doi: 10.1002/cncr.11730. [DOI] [PubMed] [Google Scholar]
- 16.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: a meta-analysis and clinical practice guideline. Sarcoma. 2000;4:103–112. doi: 10.1080/13577140020008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 18.Mouridsen HT, Bastholt L, Somers R, et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas. A randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol. 1987;23:1477–1483. doi: 10.1016/0277-5379(87)90089-7. [DOI] [PubMed] [Google Scholar]
- 19.van Oosterom AT, Mouridsen HT, Nielsen OS, et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 2002;38:2397–2406. doi: 10.1016/s0959-8049(02)00491-4. [DOI] [PubMed] [Google Scholar]
- 20.Buesa JM, Lopez-Pousa A, Martin J, et al. Phase II trial of first-line high-dose ifosfamide in advanced soft tissue sarcomas of the adult: a study of the Spanish Group for Research on Sarcomas (GEIS) Ann Oncol. 1998;9:871–876. doi: 10.1023/a:1008474802882. [DOI] [PubMed] [Google Scholar]
- 21.Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–3150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 22.Garcia del Muro X, Lopez-Pousa A, Martin J, et al. A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: a study by the Spanish Group for Research on Sarcomas. Cancer. 2005;104:1706–1712. doi: 10.1002/cncr.21384. [DOI] [PubMed] [Google Scholar]
- 23.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 25.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285. doi: 10.1200/JCO.1993.11.7.1276. [DOI] [PubMed] [Google Scholar]
- 26.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 27.Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer. 2007;109:1863–1869. doi: 10.1002/cncr.22609. [DOI] [PubMed] [Google Scholar]
- 28.Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 29.Buesa JM, Mouridsen HT, van Oosterom AT, et al. High-dose DTIC in advanced soft-tissue sarcomas in the adult. A phase II study of the E.O.R.T.C. Soft Tissue and Bone Sarcoma Group. Ann Oncol. 1991;2:307–309. doi: 10.1093/oxfordjournals.annonc.a057942. [DOI] [PubMed] [Google Scholar]
- 30.O'Bryan RM, Baker LH, Gottlieb JE, et al. Dose response evaluation of adriamycin in human neoplasia. Cancer. 1977;39:1940–1948. doi: 10.1002/1097-0142(197705)39:5<1940::aid-cncr2820390505>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Lopez M, Vici P, Di Lauro L, Carpano S. Increasing single epirubicin doses in advanced soft tissue sarcomas. J Clin Oncol. 2002;20:1329–1334. doi: 10.1200/JCO.2002.20.5.1329. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen OS, Dombernowsky P, Mouridsen H, et al. High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer. 1998;78:1634–1639. doi: 10.1038/bjc.1998.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37:870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 34.Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Invest. 2003;21:167–176. doi: 10.1081/cnv-120016412. [DOI] [PubMed] [Google Scholar]
- 35.Chidiac T, Budd GT, Pelley R, et al. Phase II trial of liposomal doxorubicin (Doxil) in advanced soft tissue sarcomas. Invest New Drugs. 2000;18:253–259. doi: 10.1023/a:1006429907449. [DOI] [PubMed] [Google Scholar]
- 36.Bramwell VH, Mouridsen HT, Santoro A, et al. Cyclophosphamide versus ifosfamide: a randomized phase II trial in adult soft-tissue sarcomas. The European Organization for Research and Treatment of Cancer [EORTC] Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 1993;31(suppl 2):S180–S184. [PubMed] [Google Scholar]
- 37.Antman KH, Montella D, Rosenbaum C, Schwen M. Phase II trial of ifosfamide with mesna in previously treated metastatic sarcoma. Cancer Treat Rep. 1985;69:499–504. [PubMed] [Google Scholar]
- 38.Le Cesne A, Antoine E, Spielmann M, et al. High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol. 1995;13:1600–1608. doi: 10.1200/JCO.1995.13.7.1600. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen OS, Judson I, van Hoesel Q, et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2000;36:61–67. doi: 10.1016/s0959-8049(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb JA, Benjamin RS, Baker LH, et al. Role of DTIC (NSC-45388) in the chemotherapy of sarcomas. Cancer Treat Rep. 1976;60:199–203. [PubMed] [Google Scholar]
- 41.Duffaud F, Pautier P, Nguyen BB, et al. A pooled analysis of the final results of the 2 randomized phase II studies comparing gemcitabine (G) vs gemcitabine + docetaxel (G + D) in patients (pts) with metastatic/relapsed leiomyosarcoma (LMS) [abstract] Ann Oncol. 2010:21. suppl 8 Abstract 13450. [Google Scholar]
- 42.Fayette J, Penel N, Chevreau C, et al. Phase III trial of standard versus dose-intensified doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line treatment of metastatic and locally advanced soft tissue sarcoma. Invest New Drugs. 2009;27:482–489. doi: 10.1007/s10637-008-9217-1. [DOI] [PubMed] [Google Scholar]
- 43.Binh NN, Chevreau C, Penel N, et al. Consolidation with high-dose chemotherapy for responding patients to standard chemotherapy in advanced, metastatic soft tissue sarcoma (STS): a randomized trial from FNCLCC French Sarcoma Group [abstract] J Clin Oncol. 2009;27(15 suppl) Abstract 10505. [Google Scholar]
- 44.Agulnik M, Okuno SH, Von Mehren M, et al. An open-label multicenter phase II study of bevacizumab for the treatment of angiosarcoma [abstract] J Clin Oncol. 2009;27(15 suppl) doi: 10.1093/annonc/mds237. Abstract 10533. [DOI] [PubMed] [Google Scholar]
- 45.Casper ES, Waltzman RJ, Schwartz GK, et al. Phase II trial of paclitaxel in patients with soft-tissue sarcoma. Cancer Invest. 1998;16:442–446. doi: 10.3109/07357909809011697. [DOI] [PubMed] [Google Scholar]
- 46.Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361–366. doi: 10.1002/cncr.21140. [DOI] [PubMed] [Google Scholar]
- 47.Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433–2436. doi: 10.1016/j.ejca.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 48.Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 49.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27:3154–3160. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wollina U, Hansel G, Schonlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964–968. doi: 10.1111/j.1468-3083.2010.03905.x. [DOI] [PubMed] [Google Scholar]
- 52.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 53.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer. 2006;119:706–711. doi: 10.1002/ijc.21867. [DOI] [PubMed] [Google Scholar]
- 55.Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246:105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer SA. Synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edmonson JH, Ryan LM, Falkson CI, Hicks DG, Blum RH. Phase II Study of Ifosfamide+Doxorubicin in Patients With Advanced Synovial Sarcomas (E1793): A Trial of the Eastern Cooperative Oncology Group. Sarcoma. 2003;7:9–11. doi: 10.1080/1357714031000114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer. 2010;46:72–83. doi: 10.1016/j.ejca.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 59.Dileo P, Sanfilippo R, Grosso F, et al. Trabectedin (T) in advanced, pretreated synovial sarcomas (SS): a retrospective analysis of 39 patients (pts) from 3 European institutions [abstract] J Clin Oncol. 2010;28(suppl 15) Abstract 10030. [Google Scholar]
- 60.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 61.European Medical Agency. Summary of Product Characteristics (Trabectedin) 2010. Available at: http://www.ema.europa.eu/humandocs/PDFs/EPAR/yondelis/emea-combined-h773en.pdf. Accessed February 11,
- 62.Rinehart KL. Antitumor compounds from tunicates. Med Res Rev. 2000;20:1–27. doi: 10.1002/(sici)1098-1128(200001)20:1<1::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 63.PharMar SA. Yondelis in the News, March 10, 2010. 2011. Available at: http://www.yondelis.com/professionals/news/yondelis_receives_5_new_approvals.html. Accessed March 15,
- 64.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 65.Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 67.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 68.Study of Trabectedin or Dacarbazine for the Treatment of Patients With Advanced L-sarcoma. 2011. ClinicalTrials.gov. NCT01343277. Available at: http://clinicaltrials.gov/ct2/show/NCT01343277?term=NCT01343277&rank=1. Accessed June 7,
- 69.Cranmer LD, Chawla SP, Rushing DA, et al. Phase I/II study of TH-302 combined with doxorubicin in soft tissue sarcoma. J Clin Oncol. 2010;28(suppl 15) Abstract 10036. [Google Scholar]
- 70.Verschraegen CF, Chawla SP, Mita MM, et al. A phase II, randomized, controlled trial of palifosfamide plus doxorubicin versus doxorubicin in patients with soft tissue sarcoma (PICASSO) J Clin Oncol. 2011;28(suppl 15) Abstract 1004. [Google Scholar]
- 71.Study of Palifosfamide-tris in Combination With Doxorubicin in Patients With Front-Line Metastatic Soft Tissue Sarcoma (PICASSO III) [abstract] ClinicalTrials.gov. NCT01168791. Available at: http://clinicaltrials.gov/ct2/show/NCT01168791?term=NCT01168791&rank=1. Accessed June 7, 2011.
- 72.Van Der Graaf W, Blay J, Chawla SP, et al. PALETTE: a randomized, double-blind, phase III trial of pazopanib versus placebo in patients (pts) with soft-tissue sarcoma (STS) whose disease has progressed during or following prior chemotherapy—an EORTC STBSG Global Network Study (EORTC 62072) [abstract] J Clin Oncol. 2011:29. doi: 10.1002/cncr.29426. Abstract LBA10002. [DOI] [PubMed] [Google Scholar]
- 73.Gounder MM, Lefkowitz RA, Keohan ML, et al. Activity of sorafenib against desmoid tumor/deep fibromatoses. Clin Cancer Res. 2011;17:4082–4090. doi: 10.1158/1078-0432.CCR-10-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chugh R, Wathen JK, Maki RG, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. 2009;27:3148–3153. doi: 10.1200/JCO.2008.20.5054. [DOI] [PubMed] [Google Scholar]
- 75.Goldberg J, Demetri GD, Choy E, et al. Preliminary results from a phase II study of ARQ 197 in patients with microphthalmia transcription factor family (MiT)-associated tumors [abstract] J Clin Oncol. 2009;27(suppl 15) Abstract 10502. [Google Scholar]
- 76.Garcia A, Rosen L, Cunningham CC, et al. Phase 1 study of ARQ 197, a selective inhibitor of the c-Met RTK in patients with metastatic solid tumors reaches recommended phase 2 dose [abstract] J Clin Oncol. 2007;25(suppl 15) Abstract 3525. [Google Scholar]
- 77.Schwartz GK, Maki RG, Ratain MJ, et al. Brivanib (BMS-582664) in advanced soft-tissue sarcoma (STS): biomarker and subset results of a phase II randomized discontinuation trial [abstract] J Clin Oncol. 2011:29. Abstract 10000. [Google Scholar]
- 78.Gardner K, Judson I, Leahy M, et al. Activity of cediranib, a highly potent and selective VEGF signaling inhibitor, in alveolar soft part sarcoma [abstract] J Clin Oncol. 2009;27(suppl 15) Abstract 10523. [Google Scholar]
- 79.Kummar S, Strassberger A, Monks A, et al. An evaluation of cediranib as a new agent for alveolar soft part sarcoma (ASPS) [abstract] J Clin Oncol. 2011:29. Abstract 10001. [Google Scholar]
- 80.A Study of Axitinib in Patients With Advanced Angiosarcoma and Other Soft Tissue Sarcomas (Axi-STS) ClinicalTrials.gov. NCT01140737. Available at: http://clinicaltrials.gov/ct2/show/NCT01140737?term=NCT01140737&rank=1. Accessed May 12, 2011.
- 81.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2010;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 83.Schoffski P, Adkins D, Blay J, et al. Phase II trial of anti-IGF-IR antibody cixutumumab in patients with advanced or metastatic soft-tissue sarcoma and Ewing family of tumors [abstract] J Clin Oncol. 2011:29. doi: 10.1016/j.ejca.2013.06.010. Abstract 10004. [DOI] [PubMed] [Google Scholar]
- 84.Temsirolimus and Cixutumumab in Treating Patients With Locally Advanced, Metastatic, or Recurrent Soft Tissue Sarcoma or Bone Sarcoma. ClinicalTrials.gov. NCT01016015. Available at: http://clinicaltrials.gov/ct2/show/NCT01016015. Accessed May 11, 2011.
- 85.Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12:1007–1018. doi: 10.1634/theoncologist.12-8-1007. [DOI] [PubMed] [Google Scholar]
- 86.MacKenzie AR, von Mehren M. Mechanisms of mammalian target of rapamycin inhibition in sarcoma: present and future. Expert Rev Anticancer Ther. 2007;7:1145–1154. doi: 10.1586/14737140.7.8.1145. [DOI] [PubMed] [Google Scholar]
- 87.Merimsky O, Bernstein-Molho R, Sagi-Eisenberg R. Targeting the mammalian target of rapamycin in myxoid chondrosarcoma. Anticancer Drugs. 2008;19:1019–1021. doi: 10.1097/CAD.0b013e328312c0e5. [DOI] [PubMed] [Google Scholar]
- 88.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richter S, Pink D, Hohenberger P, et al. Multicenter, triple-arm, single-stage, phase II trial to determine the efficacy and safety of everolimus (RAD001) in patients with refractory bone or soft tissue sarcomas including GIST [abstract] J Clin Oncol. 2010;28(suppl 15) Abstract 10038. [Google Scholar]
- 90.Schoffski P, Reichardt P, Blay JY, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol. 2010;21:1990–1998. doi: 10.1093/annonc/mdq076. [DOI] [PubMed] [Google Scholar]
- 91.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 92.Okuno S, Bailey H, Mahoney MR, et al. A phase 2 study of temsirolimus (CCI-779) in patients with soft tissue sarcomas: a study of the mayo phase 2 consortium (P2C) Cancer. 2011;117:3468–3475. doi: 10.1002/cncr.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hartford CM, Desai AA, Janisch L, et al. A phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin Cancer Res. 2009;15:1428–1434. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 95.Chawla SP, Staddon AP, Baker LH, et al. A phase 2 study of the mTOR inhibitor ridaforolimus (AP23573; MK-8669) in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. In press. [Google Scholar]
- 96.Mita MM, Britten CD, Poplin E, et al. Deforolimus trial 106- A Phase I trial evaluating 7 regimens of oral Deforolimus (AP23573, MK-8669) [abstract] J Clin Oncol. 2008;26(Supple 15) Abstract 3509. [Google Scholar]
- 97.Bailey HH, Mahoney MR, Ettinger DS, et al. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- 98.Knowling M, Blackstein M, Tozer R, et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- 99.Birch R, Chawla S, Nemunaitis J, et al. Perifosine (P) as an active agent in the treatment of patients with advanced sarcoma [abstract] J Clin Oncol. 2007;25(suppl 18) Abstract 10059. [Google Scholar]
- 100.Schoffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate (E7389) in patients with soft tissue sarcoma (STS): phase II studies of the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC 62052) [abstract] J Clin Oncol. 2010;28(suppl 15) Abstract 10031. [Google Scholar]
- 101.Phase 3 Study to Compare the Efficacy and Safety of Eribulin With Dacarbazine in Subjects With Soft Tissue Sarcoma. ClinicalTrials.gov. NCT01327885. Available at: http://clinicaltrials.gov/ct2/show/NCT01327885?term=NCT01327885&rank=1. Accessed May 12, 2011.
- 102.Baker LH, Rowinsky EK, Mendelson D, et al. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J Clin Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 103.Chawla SP, Chua VS, Fernandez L, et al. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther. 2009;17:1651–1657. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bevacizumab, Chemotherapy and Valproic Acid in Advanced Sarcomas. ClinicalTrials.gov. NCT01106872. Available at: http://clinicaltrials.gov/ct2/show/NCT01106872?term=NCT01106872&rank=1. Accessed May 12, 2011.
- 105.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 106.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 107.Markman M, Liu PY, Wilczynski S, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 108.Mannel RS, Brady MF, Kohn EC, et al. A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2011;122:89–94. doi: 10.1016/j.ygyno.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chawla SP, Blay J, Ray-Coquard IL, et al. Results of the phase III, placebo-controlled trial (SUCCEED) evaluating the mTOR inhibitor ridaforolimus (R) as maintenance therapy in advanced sarcoma patients (pts) following clinical benefit from prior standard cytotoxic chemotherapy (CT) [abstract] J Clin Oncol. 2011:29. Abstract 10005. [Google Scholar]


