ABSTRACT
Peritoneal metastasis is the most frequent and life-threatening type of metastasis in patients with advanced gastric cancer. Despite recent advances in chemotherapeutic agents, any treatment, if administered only via the intravenous (IV) route, cannot satisfactorily control peritoneal metastasis in gastric cancer. Although intraperitoneal (IP) chemotherapy has been proposed as a treatment option, the clinical efficacy of IP chemotherapy for peritoneal lesions has not been examined in gastrointestinal cancer. One hundred patients with gastric cancer received combination chemotherapy of S-1 plus IV (50 mg/m2) and IP (20 mg/m2) paclitaxel (PTX) via a subcutaneously implanted peritoneal access port. S-1 was administered at 80 mg/m2 per day for 14 consecutive days, followed by 7 days' rest. Radical gastrectomy was performed in a salvage setting when macroscopic curative resection was made feasible by the downstaging achieved by the combined chemotherapy. The median survival time (MST) of the patient sample was 23.6 months, with a 1-year survival of 80%. Combination chemotherapy of S-1 plus IV and IP PTX is well tolerated and very effective in patients with gastric cancer and peritoneal metastasis. Systemic chemotherapy combined with repeated IP administration of paclitaxel is a promising strategy for peritoneal carcinomatosis in gastrointestinal cancer.
Recently, systemic chemotherapy for unresectable or recurrent gastric cancer has progressed steadily, and 5-FU-based or cisplatin-based regimens are generally accepted as the standard worldwide.1,2 However, regarding the efficacy of the treatment against peritoneal metastasis, only a few phase II studies have been reported, and no large-scale trials have been performed, presumably because of the difficulty of evaluating the response for diffusely disseminated and unmeasuarable peritoneal lesions. Sequential MTX/5-FU therapy has been reported to be effective for treating gastric cancer patients with malignant ascites,3 with a 1-year survival rate of 16.2% in 37 patients. Another study has shown the efficacy of modified FOLFOX-4, with a 1-year survival of 27.2% in 48 patients with gastric cancer with malignant ascites.4 According to the low survival rates in these phase II studies, neither of these regimens is acceptable as a standard protocol.
Paclitaxel (PTX) is an effective drug for various types of cancer. It inhibits cell division by accelerating the polymerization of microtubule proteins, followed by stabilization and excessive microtubule formation.5 In unresectable or recurrent gastric cancer, including cases with malignant ascites, PTX showed good response rates.6–8 Intraperitoneal (IP) administration of PTX was developed to enhance antitumor activity against peritoneal metastasis by maintaining high concentrations of the drug in the peritoneal cavity over a long period, and its clinical effectiveness has been verified by convincing clinical trials in ovarian cancer with peritoneal metastasis.9,10 The regimen including IP PTX was shown to prolong survival in a recent phase III study11 and was approved as the recommended regimen of the NCI. On the other hand, no clinical trial has been performed to determine the effects of PTX on peritoneal metastasis due to gastric cancer, which is biologically more malignant, although several individual cases with dramatic results have been reported.4,12
These facts strongly inspired us to explore the use of IP PTX in chemotherapy for the treatment of peritoneal metastasis in gastric cancer, and thus we developed a new regimen to add weekly IP PTX to an established regimen of systemic S-1 and IV PTX. First, we examined the safety of the regimen and determined 20 mg/m2 as the recommended dose (RD) of IP PTX in a phase I dose-escalation study.13 We then performed a phase II study of weekly IV and IP PTX combined with S-1, to evaluate efficacy and tolerability in gastric cancer patients with peritoneal metastasis.14 In this study, we report the results in 100 cases of peritoneal dissemination due to gastric cancer treated with this method.
PATIENTS AND METHODS
From 2004 through 2009, macroscopic peritoneal metastasis was confirmed by laparotomy or investigative laparoscopy in 100 patients with gastric cancer (80 with primary tumors, 10 after palliative gastrectomy, and 10 with peritoneal recurrence). Subcutaneous peritoneal access ports were implanted in the patients, and a combination chemotherapy of S-1 plus IV and IP PTX was administered. The patients received 50 mg/m2 IV and 20 mg/m2 IP on days 1 and 8, respectively. S-1 was administered at 80 mg/m2 per day for 14 consecutive days, followed by 7 days' rest. The response was evaluated by CT image for measurable lesions and additionally by serum tumor marker or peritoneal lavage cytology for unmeasurable lesions. In cases with a good response, we performed second-look laparoscopy and then radical gastrectomy in a salvage setting when macroscopic curative resection was made feasible after downstaging by the combined chemotherapy.
RESULTS
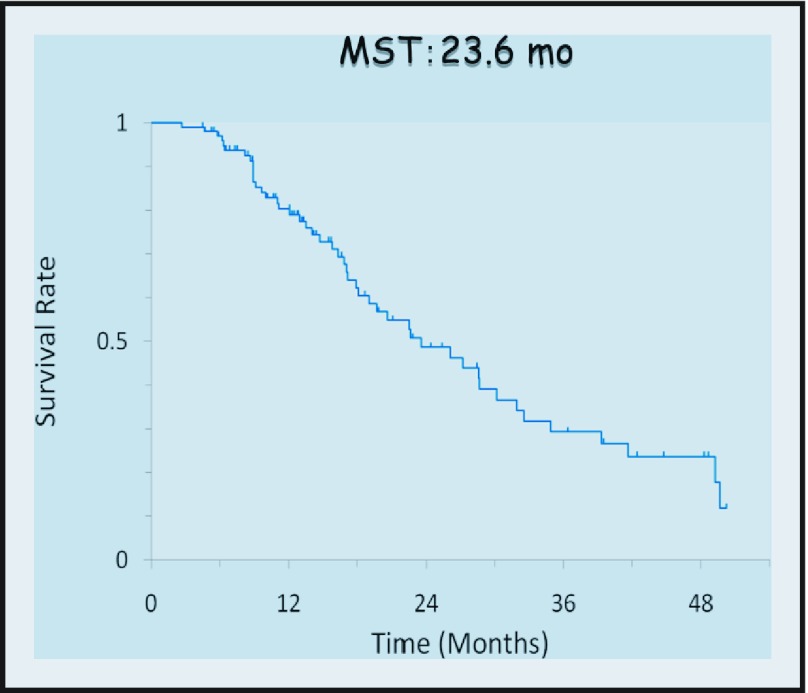
The median number of courses of chemotherapy was eight (range, 1–48). The MST of 100 patients was 23.6 months with a 1-year survival of 80% (Figure 1). As shown in Figure 2, survival among the 71 patients with apparent malignant ascites was worse than that of the 29 patients with no ascitic fluid (MST; 19.0 months vs. 39.3 months). However, ascites disappeared completely in some cases (Figure 3). Similarly, the MST of 10 patients in whom metastasis was confined to the adjacent peritoneum (P1) was 49.6 months, which was significantly better than that in the other 90 patients, in whom metastasis was observed in the distant peritoneum (19.7 months; n = 90). Partial response was obtained in 17 (59%) of 29 cases with measureable lesions, and peritoneal cytology was negative in 86%.
Figure 1.
Overall survival of all (n = 100) patients with peritoneal metastasis due to gastric cancer.
Figure 2.
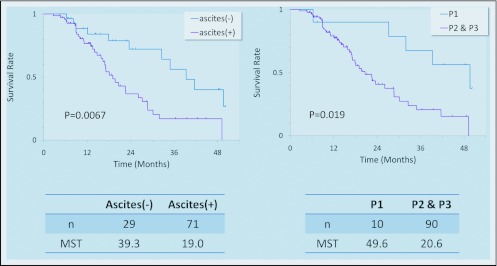
Overall survival of patients with or without malignant ascites and different extents of peritoneal dissemination. Ascites was detected in 71 patients by CT scan. The severity of dissemination was classified by Japanese criteria. P1: metastasis confined to the peritoneum adjacent to the stomach. P2–P3: metastasis detected all over the abdominal cavity.
Figure 3.
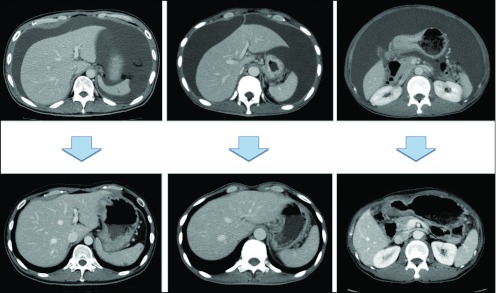
CT images before and after combination chemotherapy in three cases.
In some cases, the peritoneal nodules were confirmed to be significantly reduced in size and appearance by second-look laparoscopy (Figure 4). In those cases, gastrectomy was considered and performed in 52 patients. Grade 3/4 neutropenia, leukopenia, and anemia were observed in 36%, 20%, and 8% of the cases, respectively, while nonhematologic toxicities, including abdominal pain, were rare.
Figure 4.
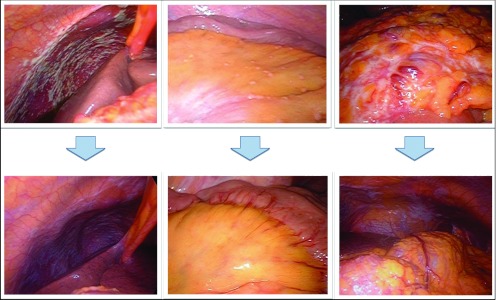
Laparoscopic images before and after combination chemotherapy in three cases.
DISCUSSION
Peritoneal dissemination due to gastric cancer is a grave disease with a short survival time, and effective treatment strategies are urgently needed. IP administration of anticancer drugs is an effective method of treating peritoneal metastasis, as it brings high concentrations of drugs into direct contact with the target cancer lesions in the peritoneal cavity. It has been reported that IP administration of mitomycin or cisplatin yields no successful therapeutic effect against peritoneal metastasis of gastric cancer, because of immediate absorption through the peritoneum.15 In contrast, because of its large molecular size and fat solubility, PTX is known to be absorbed slowly through the lymphatic system after IP administration. Thus, IP PTX was expected to demonstrate high efficacy against peritoneal metastasis, because its antitumor effect has been shown to depend on its concentration and exposure time in experiments in vitro.16
In our series of 100 cases, we found good response of peritoneal lesions to the combination of IV and IP administration of PTX combined with oral S-1 and a significant prolongation of survival. In particular, salvage gastrectomy was performed in 52 patients who showed significant response to this combination chemotherapy, and the median survival of those patients was around 3 years. Since most of the patients were categorized in relatively severe cases of peritoneal metastasis, the survival was much better than that of patients in recent reports.17,18 Moreover, toxicity was relatively low, even after the protocol had continued for months, and port-related trouble was rare. From our experience, we conclude that systemic chemotherapy combined with repeated IP administration of paclitaxel is a promising strategy for peritoneal carcinomatosis in gastrointestinal cancer.
REFERENCES
- 1. Ajani JA: Standard chemotherapy for gastric carcinoma: is it a myth? J Clin Oncol 18:4001–4003, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Ohtsu A, Yoshida S, Saijo N: Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol 24:2188–2196, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ohtsu A, Shimada Y, Shirao K, et al. : Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 21:54–59, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Kodera Y, Ito Y, Ito S, et al. : Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology 54:960–963, 2007 [PubMed] [Google Scholar]
- 5. Rowinsky EK, Donehower RC, Jones RJ, Tucker RW: Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res 48:4093–4100, 1988 [PubMed] [Google Scholar]
- 6. Ohtsu A, Boku N, Tamura F, et al. : An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol 21:416–419, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Yamada Y, Shirao K, Ohtsu A, et al. : Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol 12:1133–1137, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Hironaka S, Zenda S, Boku N, Fukutomi A, Yoshino T, Onozawa Y: Weekly paclitaxel as second-line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 9:14–18, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Francis P, Rowinsky E, Schneider J, Hakes T, Hoskins W, Markman M: Phase I feasibility and pharmacologic study of weekly intraperitoneal paclitaxel: a Gynecologic Oncology Group pilot study. J Clin Oncol 13:2961–2967, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC: Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group study. J Clin Oncol 16:2620–2624, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong DK, Bundy B, Wenzel L, et al. : Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Tamura S, Miki H, Nakata K, et al. : Intraperitoneal administration of paclitaxel and oral S-1 for a patient with peritoneal dissemination and hydronephrosis due to advanced gastric cancer. Gastric Cancer 10:251–255, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Ishigami H, Kitayama J, Otani K, et al. : Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology 76:311–314, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Ishigami H, Kitayama J, Kaisaki S, et al. : Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 21:67–70 [DOI] [PubMed] [Google Scholar]
- 15. Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R: Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol 12:970–974, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Chang YF, Li LL, Wu CW, et al. : Paclitaxel-induced apoptosis in human gastric carcinoma cell lines. Cancer 77:14–18, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Imazawa M, Kojima T, Boku N, et al. : Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer 12:153–157, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Glehen O, Gilly FN, Arvieux C, et al. : Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370–2377 [DOI] [PubMed] [Google Scholar]