Abstract
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, progressive and disabling disorders. Over the last few decades, new therapeutic approaches have been introduced which have led not only to a reduction in the mortality rate but also offered the possibility of a favorable modification in the natural history of IBD. The identification of clinical, genetic and serological prognostic factors has permitted a better stratification of the disease, thus allowing the opportunity to indicate the most appropriate therapy. Early treatment with immunosuppressive drugs and biologics has offered the opportunity to change, at least in the short term, the course of the disease by reducing, in a subset of patients with IBD, hospitalization and the need for surgery. In this review, the crucial steps in the natural history of both UC and CD will be discussed, as well as the factors that may change their clinical course. The methodological requirements for high quality studies on the course and prognosis of IBD, the true impact of environmental and dietary factors on the clinical course of IBD, the clinical, serological and genetic predictors of the IBD course (in particular, which of these are relevant and appropriate for use in clinical practice), the impact of the various forms of medical treatment on the IBD complication rate, the role of surgery for IBD in the biologic era, the true magnitude of risk of colorectal cancer associated with IBD, as well as the mortality rate related to IBD will be stressed; all topics that are extensively discussed in separate reviews included in this issue of World Journal of Gastroenterology.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Natural history, Clinical course, Complications, Therapy, Surgery, Mortality
INTRODUCTION
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, progressive and relapsing inflammatory disorders of unknown etiology that may cause disability over time. Genetic, environmental and intestinal microbial factors have been reported to play a role in the etiology and pathogenesis of IBD[1]. IBD represents a life-long disorder that may occur at any time from early childhood to late adulthood, although over 80% of cases are currently diagnosed in the second or third decade of life. UC is characterized by inflammation and ulcerations in the large bowel mucosa and submucosa, whereas CD is a trans-mural inflammatory disorder that may involve various sites of the gastrointestinal tract: in 40%-70% of cases the terminal ileum. Approximately 50% of the patients with IBD present a slight evolutive disease with a low prevalence of relapses, hospitalizations, and complications[2,3]. Other patients have a more severe course and may develop complications that require surgery. In UC, the main complications include toxic megacolon, massive hemorrhage or colon perforation, while strictures and fistulas are uncommon. In CD, intestinal strictures, internal or perianal fistulas or abscesses are frequent, being reported in approximately one-third of patients.
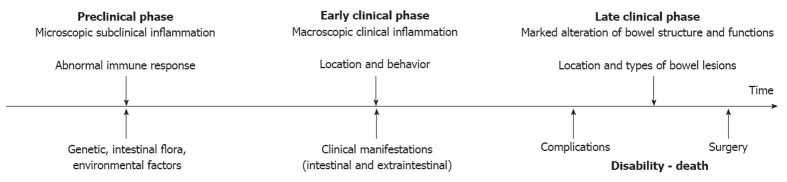
A better understanding of the natural history of this chronic disabling disorder provides valid opportunities: (1) by better defining etiological factors and pathology, it may allow the set up of new strategies of disease prevention; (2) by identifying the relevant clinical subsets in which disease prognosis can be stratified by initial clinical, serological or genetic features, it may be useful in the choice of the most appropriate management of these patients; and (3) by understanding the evolution and the time course of the disease, it may help to define the best end points for clinical trials designed to test drugs modifying disease course[4] (Figure 1).
Figure 1.
Longitudinal course of inflammatory bowel disease.
Questions regarding the natural history and prognosis of IBD are among the most prominent concerns both for patients and clinicians. Unfortunately, most data regarding the course of IBD are obtained from a limited number of cohorts and not all studies focusing on the course and prognosis are of high quality. Adequate knowledge of the methodological requirements of studies focused on prognosis is crucial for interpreting the results of observational studies and for applying these results in clinical practice. In this issue of World Journal of Gastroenterology (WJG), these methodological aspects have been critically discussed by Modesto et al[5]. In particular, they stressed the criteria for an excellent cohort study of prognosis: population-based, use of standard diagnostic criteria for UC and/or CD, start of follow-up already at inception, complete follow-up (80% or more), and use of survival methods to evaluate results.
The crucial steps in the natural history of IBD include the occurrence of lesions, the manifestation and severity of symptoms, the development of complications, the need for surgery, the disability and the mortality (Figure 2). In order to achieve a favorable modification in the disease course, an effective intervention must be carried out at the right time and with a specific therapeutic endpoint (Figure 1). The main outcomes considered include disease activity and relapse, corticosteroid therapy, hospitalizations, complications, surgery, post-operative recurrence, and mortality. More recently, mucosal healing (MH) has been included[6-8]. Early treatment is advisable, before the development of severe bowel damage and impaired functioning[6,8]. There is probably a difference between early disease and long-lasting disease, the control of the disease process being more difficult and unstable in the latter situation[6]. Immunological status of the patients may change over the course of the disease; a stable remission will usually be more difficult to obtain in long-lasting disease and the disease will be more treatment-dependent[6,9].
Figure 2.
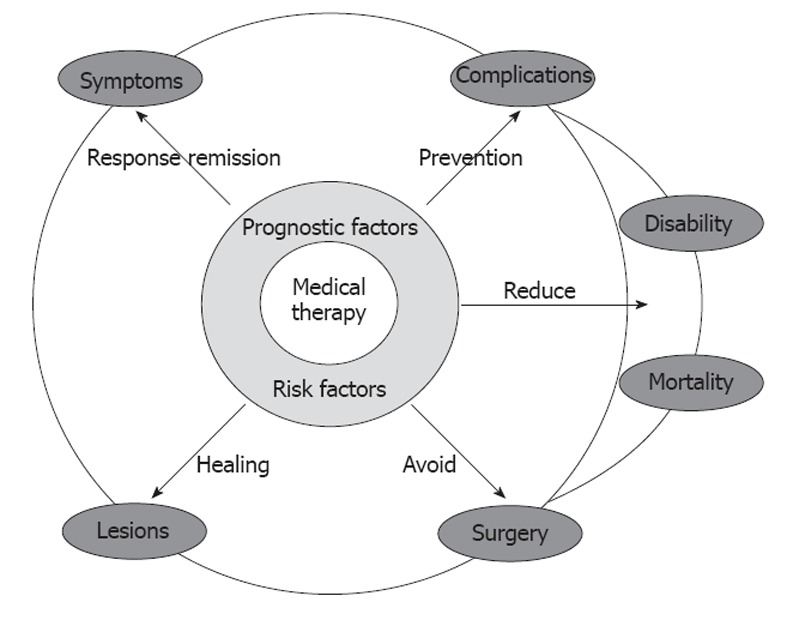
Potential effects of medical therapy on the natural history of inflammatory bowel disease.
RISK AND PROGNOSTIC FACTORS
As far as the approach to the treatment of IBD is concerned, it must take into consideration the potential impact of environmental factors (e.g., dietary factors, smoking, psychological stress, drugs and infections) on the clinical course. Clinical, serologic and genetic factors have been reported to be associated with a different clinical course, but their relevance in everyday clinical practice is still controversial[10].
Two reviews in this issue of WJG deal with prognostic factors in IBD[11,12].
In the first, Cabré et al[11] critically review the role of environmental and dietary factors on the clinical course of IBD. Smoking is the only well established risk and prognostic factor in IBD, with a different impact on CD and UC. In CD, there is consistent evidence that active smoking is a risk factor for post-operative disease recurrence[13,14] but the impact of smoking on the response to therapy is still controversial. In CD patients, the beneficial effect of giving up smoking can be observed; conversely, some patients with refractory UC may even benefit from taking up smoking again[13,15,16]. Based on these observations, therapeutic trials with transdermic nicotine have been performed but with inconclusive results.
The role of dietary habits and dietary manipulation on the clinical course of IBD is far from being well established; therefore, IBD patients are usually encouraged to follow a free diet. As far as concerns the possible therapeutic role of some dietary components, enteral nutrition appears to be effective in CD, particularly in the pediatric population and a low-fat diet seems to be particularly useful even in adult patients[17-20].
With regard to the relationship between psychological stress and IBD, a recent systematic review of 18 prospective studies examining stress as a risk factor for disease exacerbations showed a significant association, and coping behaviors appeared to modulate the effect of stress[21]. Furthermore, it has been reported that approximately 50% of IBD patients had experienced some type of stress; family stress was the most commonly reported form, followed by work or school and financial stress[22].
As far as drugs are concerned, nonsteroidal anti-inflammatory drugs (NSAIDs) are generally considered to potentially affect the IBD course. This concept is not supported by consistent evidence, although in a subset of susceptible patients, NSAID-induced IBD flares appear to occur early after NSAID administration[23]. Current evidence does not support the role of antibiotics and vaccines as a prognostic factor in IBD, albeit antibiotic use is included in the predisposing factors of IBD etiology[24].
Intestinal infections due to enteropathogens have been associated with IBD relapse and the response to therapy[25]. In particular, associated Clostridium difficile infection (which is more frequent in UC than CD) has been reported to have a negative effect on IBD outcome, and to lead to longer hospitalization time, as well as high rates of surgery and a high mortality rate[26]. The role of cytomegalovirus reactivation in the colon of patients with refractory colitis remains to be fully elucidated[27].
In the second paper, Beaugerie et al[12] review the role of clinical, serological and genetic predictors of the IBD course and discuss their potential role in clinical practice. This aspect appears to be particularly relevant: there is a need for predictors of a benign or unfavorable clinical course, in order to avoid over-treatment of patients who will experience a mild clinical course and under-treatment of those patients who will experience an aggressive and progressive disorder.
In CD, age under 40 years, perianal disease, ileal lesions and need of steroids at diagnosis have been consistently associated with an unfavorable medium- and long-term clinical course[28-31]. In the post-operative setting, smoking, history of previous resection and severity of early post-operative endoscopic recurrence are the strongest predictors of symptomatic recurrence. In UC, extensive colitis, high disease activity, younger age and female gender are associated with poor outcome in most population-based studies[32-34].
Genetic factors and luminal microbes, besides their role in triggering the IBD, may also be directly, or indirectly, involved in the clinical course of IBD[35,36].
Identifying genetic prognostic factors in IBD is very attractive as they are already present at the onset of the disease and remain stable over time, which is not the case for clinical and serologic parameters. However, despite the growing number of identified susceptibility loci in both CD and UC, only very few have been associated with disease outcomes. The development of genome-wide association scanning techniques has led to the discovery of more than 100 confirmed IBD loci[35-38]. Some of these loci, such as the Th 17 pathway genes (IL23R, IL12B, JAK2, STAT3), are shared between CD and UC, others are phenotype-specific (autophagy genes such as ATG16L1, IRGM and NOD2 for CD; epithelial barrier genes HNF4a, E-Cadherin, LAMB1 and IL-10 for UC). Variants of some of these genes would be excellent prognostic factors[39].
There is a growing body of evidence proving that in CD, the main NOD2/CARD15 variants are closely related to ileal disease, a stenosing phenotype, an earlier need for first surgery and a reduced post-operative disease-free interval[10]. All of these findings provide evidence that may encourage the clinical application of NOD2/CARD15 genotyping both as a marker of CD and as a prognostic factor of the need for early surgery due to stricturing and fibrostenosing disease[10].
Certain genetic factors appear to influence the response to medical treatments. Polymorphisms in multi drug resistant-1, migration inhibitory factor, tumor necrosis factor (TNF) and apoptosis genes have been associated with a higher risk of treatment failure (steroids, cyclosporine, infliximab) in CD and UC[40-45].
Antibodies directed against microbial peptides represent good serological markers that could help in the prediction of the clinical course of IBD[10,46]. Patients with a stronger immune response to microbial peptides are associated with early disease onset of CD, fibrostenosing and penetrating disease, and need for early small bowel surgery[10,46]. In pediatric CD patients, baseline anti-Saccharomyces cerevisiae antibody (ASCA) reactivity is associated with earlier complications, relapsing disease and need for additional surgery[10,46]. The frequency of disease complications increases with reactivity to increasing numbers of microbial antigens (ASCA, anti-I2, anti-OmpC, and anti-CBir1). High levels of perinuclear anti-neutrophil antibody are associated with the risk of subsequent chronic pouchitis in UC patients undergoing ileal pouch-anal anastomosis[47].
Overall, these data suggest that serological markers of microbial peptides may be useful predictors of IBD complications. In future, genetic and serological markers will be associated with clinical findings to obtain more reliable and useful predicting tools.
INTESTINAL LESIONS, CLINICAL MANIFESTATIONS AND DISEASE PROGRESSION
Progression of intestinal lesions may range from weeks to decades; however, it can be slowed down, stopped, or reversed spontaneously or by means of medical therapy[2,3] (Figure 2). Superficial mucosal lesions are most prone to heal, whereas deep ulcers or transmural fissures may heal with more difficulty; fibrotic strictures are usually definitive. IBD becomes symptomatic when lesions are extensive or distal, associated with a systemic inflammatory response, or when associated with local complications such as dilatation (toxic megacolon), massive hemorrhage, strictures, perforation (abscesses and fistulas) and cancer[2,3]. Colorectal lesions usually present more and early symptoms, whereas small bowel lesions may remain latent for several years[2,3]. The disease course is generally characterized by a sequence of flares and remission of varying duration, while approximately one-fifth of these patients undergo a chronic, active, continuous disease course. Abdominal pain, abnormal bowel functions and rectal bleeding are the patients’ main complaints that significantly alter their quality of life. Since UC is a pathological disorder that affects the mucosa and submucosa, while CD is a transmural inflammation, the anatomic evolution of the lesions and the disease progression are different, and will therefore be considered separately.
UC involves the rectum and colon and extends proximally in a continuous fashion. Upon presentation, lesions are limited to the rectum (proctitis) in 30%-35% of patients, to the splenic flexure (left-sided colitis) in 30%-45% and to the cecum (pancolitis) in 20%-25%[2,3]. During the course of the disease, after 20 years, the rate of pancolitis may increase reaching 50% of cases. Pancolitis may be associated with inflammation of the terminal ileum (“back wash ileitis”); children do not always have rectal lesions. Mucosal lesions are usually diffuse and superficial, and deep ulcerations are present only in patients with severe disease. Perianal disease may develop in rare cases of UC. Diagnosis may change from UC to CD in 5%-10% of adult patients and in 15%-40% of pediatric patients[2,3,32,48]. UC appears to be particularly severe in younger patients (especially in children), with a higher frequency of flares that do not respond to medical treatment. Severity of flares and their response to therapy vary and are difficult to predict. Disease activity tends to decrease over time, with 40%-50% of patients in prolonged remission and about 30% with active disease. Clinical remission is usually associated with MH[8,49]. Extra-intestinal manifestations are observed in one-third of UC patients[50].
In CD, the lesions can involve any segment of the digestive tract, from the mouth to the anus, but mainly affect the distal ileum and the colon. At the time of diagnosis, approximately 40% of patients present with ileo-colonic disease, about 30% have isolated ileal disease, and another 30% have a pure colonic disease[2,3]. Approximately 5%-15% of patients have associated upper gastrointestinal lesions and 20%-30% present perianal lesions[2,3,51,52]. The localization of the lesions changes only minimally over time, with only 10%-15% of patients presenting a change in lesion localization 10 years after diagnosis[53-55].
Although the location remains relatively stable, the clinical behavior of CD shows a dynamic evolution with striking changes over the course of the disease[53-55]. During the first few years of CD, the non-penetrating/non-stricturing (inflammatory) form predominates, whereas most patients develop complications during follow-up and are then classified as having a penetrating or a stricturing disease. These two forms may co-exist in the same patient, since internal fistulae may complicate long-standing intestinal stenosis. Disease evolution is related to lesion localization, the development of complications (abscess, fistula, stricture) being more frequent and rapid when the small bowel is involved, whereas when the disease is localised in the colon, it may remain inflammatory and uncomplicated for many years. There is no relationship between symptoms and progression of the intestinal lesions, since strictures and fistulae may develop for several years with only mild symptoms or, in some cases, without any symptoms at all[3]. Approximately 50% of CD patients have only a slight evolutive disease and, therefore, overtreatment should be avoided[28-30,56]. The remaining patients present a more aggressive and evolutive disease with high rates of relapse, complications, hospitalization and surgery, all conditions that considerably affect the patients’ everyday life and long-term projects. For these patients, sustained control of disease activity and progression is clearly warranted. Taken together, these data obviously indicate the need for strategies aimed at interrupting or delaying the natural evolution of this pathological condition. Current treatment options (antibiotics, steroids, immunosuppressive drugs, biological therapies) may relieve the inflammatory symptoms, but do not improve fibrostenotic obstruction[57-63]. The results of medical treatment aimed at stricturing or penetrating CD are poor, since 64% of these patients ultimately require surgery within one year[64]. This situation should be taken into consideration (and discussed with the patient) when planning medical treatment. It is likely that progression to a stricturing or penetrating disease phenotype is an end-stage sequel of CD associated with either irreversible fibrosis or severe inflammation that will not abate despite optimal medical therapy introduced at too late a stage[63].
IMPACT OF MEDICAL THERAPY
The advancements in knowledge of IBD over the past few years have modified the treatment goals. While, in the past, the aim of medical treatment was an improvement in IBD symptoms, the current objective is to achieve a deep remission, defined, both in UC and CD, as clinical remission [Mayo score for UC activity < 2 and Crohn's disease activity index (CDAI) < 150] with MH (Mayo endoscopic score for UC < 1 and simple endoscopic score for CD < 2) and cessation of steroid administration[6-8,65-67] (Figure 2). Therefore, treatment should modify the course of the disease by avoiding the disabling condition and irreversible tissue damage. The treatment strategy in IBD should, therefore, be tailored according to the risk that each patient runs in developing a disabling disease. In this issue of WJG, the review by Reenaers et al[68] focuses on the impact of treatment on the natural history of IBD.
Clinical and endoscopic remission is the best result that one can hope to reach and every effort should be made to maintain this condition for as long as possible. Healing of the mucosa, therefore, appears to be an obvious endpoint of treatment. MH can be considered appropriate for UC which is a disease of the mucosa, whereas the term intestinal healing would be more correct for CD which is a transmural disease[67]. Complete assessment of intestinal healing in CD can be obtained only by using both endoscopy and cross-sectional imaging techniques (magnetic resonance, computed tomography, ultrasonography). In CD, it is not uncommon to find healed mucosa covering symptomatic intestinal stenotic segments. Another crucial point is the timing of endoscopic evaluation which was seen to differ (range: 4-52 wk) in studies evaluating the MH[7,8,67]. Many studies have been performed investigating the relationships between clinical symptoms and intestinal lesions but, to date, MH has been evaluated as the primary clinical end point in a single randomized clinical trial[69]. Efficacy of adalimumab (anti-TNFα antibody) for induction and maintenance of MH in 135 adults with moderate-to-severe ileocolonic CD was evaluated in this trial[69]. Twenty-seven percent of patients receiving adalimumab had MH at week 12 (the primary end point) vs 13% given placebo (P = 0.056). At week 52, rates of MH were 24% and 0%, respectively (P < 0.001). Clinical remission rates (CDAI < 150) were 52% for adalimumab and 28% for placebo at week 12 (P = 0.006) and 28% and 3%, respectively, at week 52 (P < 0.001). Despite the lack of data to confirm an impact of MH induced by anti-TNFs on outcomes in CD, the issue of potential benefits is widely debated. Furthermore, scientific evidence that MH may change the natural course of IBD needs to be proved in long-term studies[6-8,65-67].
The current medical armamentarium for treating moderate-severe IBD consists of corticosteroids, immunosuppressants and biologics (anti-TNFα antibodies), but for most of these medications it is unclear whether treating patients more aggressively will actually slow down disease progression[67,70,71].
In UC, the percentage of patients achieving MH appears to be of the same order for treatment with immunosuppressants (53%-68%)[8,72,73] and biologics (30%-71%)[8,74-76]; albeit, it is obtained more rapidly with the latter agents. Complete restoration of the mucosal architecture may be achieved when acute UC is of short duration and a prompt response to medical therapy has been reached.
In CD, anti-TNFα antibody therapy has been reported, during 54 wk trials, to reduce the need for CD-related hospitalization and surgery[77-80]; however, the duration of these effects is unknown. Although biological therapies have shown disease-modifying characteristics in other pathological conditions, more data are necessary before it can be confirmed whether they can influence the long-term natural history of CD[65,66,81]. There is no doubt that these agents work best when introduced early in the course of the disease, when they could reasonably be expected to change the course of CD. The fact is that they are not: the median duration of disease when this practice is adopted is almost 10 years. It is these patients, who are not frequently found in clinical practice but represent the majority, upon whom attention is focused: new therapeutic agents may add even more delay and ultimately be associated with a higher burden of disease.
It is interesting to note that the overall percentage of CD patients achieving MH with anti-TNFα antibodies (29%-73%)[8,67,77,82-84] is of the same order as that reported with immunosuppressants (35%-73%)[8,67,85-89]. Efficacy of infliximab monotherapy, azathioprine monotherapy, and the two drugs combined in adults with moderate-to-severe CD was evaluated by the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC study)[83]. At week 26, MH had occurred in 43.9% of patients receiving combination therapy, as compared with 30.1% of patients receiving infliximab (P = 0.06) and 16.5% of patients receiving azathioprine (P < 0.001 for the comparison with combination therapy and P = 0.02 for the comparison with infliximab). In a recent prospective comparative study on CD, the MH rate achieved with azathioprine (50%) was not statistically different from that obtained with infliximab (60%)[90]. On the other hand, another recent study showed that only non-complicated inflammatory CD behavior and long-term anti-TNF treatment were associated with a lower risk of the need for surgery, whereas azathioprine only slightly reduced this risk[91].
A crucial point is the timing of commencement of early treatment. In clinical practice, early CD is usually considered as a newly diagnosed case, and this does not always correspond to onset of the early purely inflammatory form of the disease. Approximately 50% of patients already present a stricturing or penetrating disease at the time of diagnosis[55], thus indicating a late disease which is more resistant to treatment both with immunosuppressants and biological agents.
SURGERY
Surgery plays an important role in the management of IBD. Up to 75% of CD patients will require an operation at some point in the course of the disease[92], and, although surgery is not curative, it appears to be the most efficacious treatment in inducing prolonged remission[93]. Therefore, surgery should not be dismissed as the end of the road after all medical options have failed, but should be considered a valid part of the overall management strategy[63]. Nevertheless, in the biological era, avoiding surgery is becoming an emerging and interesting therapeutic endpoint. Considering that biological agents are claimed to induce MH in a large percentage of cases, a substantial reduction in the need for surgery would be expected. Although data from RCTs and observational studies[94] suggest that biological use may reduce the need for surgery in the short term, the real impact of biologics on the lifetime risk of surgery remains to be established. Recent data from population-based cohorts have shown that in the pre-biologic era, the rate of surgery ranged between 27% and 61% within 5 years after diagnosis, and, in the era of anti-TNFα, ranged between 25% and 33%[95], thus suggesting that the need for surgery also remains high in the era of biologics. Moreover, an analysis of secular trends of hospitalization and surgery rates in the United States, from 1990 to 2003, showed stable rates of bowel resection surgery for CD despite advances in treatment[96]. In this issue of WJG, de Buck van Overstraeten et al[97] discuss the need for surgery in randomized trials as well as the need for surgery in population studies (the real world).
In UC, the cumulative probability of colectomy within 10 years after diagnosis appears to be lower than previously reported[98], but with considerable geographical variations (up to 25% in Denmark and 3.9% in Southern Europe, thus reflecting the different policies in approaching surgery)[99]. In severe UC, colectomy is a life-saving intervention in patients refractory to intravenous steroids; prompt surgery, when necessary, is probably the major determinant of the improved outcome of severe UC in the past 30-40 years[100,101]. During the last 20 years, medical rescue therapy with cyclosporine, and, more recently, with infliximab and tacrolimus, has received growing interest on account of its high efficacy in avoiding colectomy, in the short term, in severe steroid-refractory UC. However, the overall impact of rescue therapies on the outcome of severe UC remains to be defined: the short-term colectomy rate has remained stable over the last 30 years, despite the introduction of cyclosporine[102] and the long-term efficacy of infliximab remains to be defined. In this issue of WJG, Dayan et al[103] discuss, in detail, the role of surgery in severe UC in the era of medical rescue therapy.
RISK OF COLORECTAL CANCER
There is general consensus that the risk of colorectal cancer (CRC) is increased in IBD. Duration and extent of colitis, persistent inflammatory activity, family history of sporadic CRC and concomitant primary sclerosing cholangitis are well established risk factors. Although CRC risk has been studied more extensively in UC[104], recent data suggest that CD carries a similar risk[105]. However, the exact magnitude of the risk is controversial. In a meta-analysis published in 2001 which included 116 studies, a cumulative probability of CRC in UC of 2% by 10 years, 8% by 20 years, and 18% by 30 years has been reported[104]. These results are in contrast with data from population-based studies from Scandinavia and the United States which report a 30-year cumulative probability of CRC in UC as low as approximately 2% with an overall risk of CRC among UC patients similar to that expected in the general population[106,107].
Although geographical variations in the risk of developing CRC can play a role, differences in the methodology of individual studies (population-based vs referral center based) and different clinical approaches in the management of patients and follow-up (cumulative proctocolectomy rate, maintenance treatment with aminosalicylates, close follow-up evaluation of patients and surveillance programs) can explain the high variability in the risk. The exact definition of the risk appears to be crucial when planning “reducing-risk strategies”, for example, an endoscopic surveillance program, chemoprevention or both. In fact, the cost-effectiveness of any strategy aimed at reducing the risk of CRC is affected primarily by the baseline risk. Currently there is no strong evidence to support a chemoprophylactic role for 5-aminosalicylic acid, as well as for other drugs used in the treatment of IBD[108-110].
An exhaustive discussion of the molecular biology and all the potential risk factors of IBD-associated CRC is reported by Dyson et al[111] in this issue of WJG.
MORTALITY
Mortality is the most relevant clinical endpoint in studies focusing on the natural history of a chronic disease. In UC, mortality has continuously decreased over the last 50 years. This time trend probably results from improved medical and surgical management. Data from population-based studies suggest that the overall mortality in UC is not different from that of the background population. However, subgroups of patients, particularly those with extensive disease in the first few years after diagnosis, may be at greater risk of dying[112]. Conversely, CD is associated with a small, but nevertheless significantly increased, risk of death compared to the general population[113].
Although a slight decrease in the standardized mortality ratios has been observed over the last 30 years, this decrease is not statistically significant. This would appear to suggest that the overall prognosis of CD has not really changed despite the improvement in medical and surgical management over the last 30 years.
It will be interesting to see the trend in mortality due to CD in the near future. Preliminary data suggest that in-hospital mortality for CD is reduced in centers with a very large number of admissions for IBD[114], thus suggesting that specialist care could improve outcomes. Besides the reduction in mortality related to the disease, we will, in the near future, also be facing more severe side-effects, including mortality, related to the more aggressive medical treatment.
CONCLUSION
Onset of IBD usually occurs in young adulthood and lasts throughout the patient’s life. Despite the enormous progress that has been made in the understanding of these pathological conditions, the etiology remains unknown and no definite cure is yet available[1-3]. The incidence of IBD is increasing worldwide, including also developing countries. UC and CD both have a negative effect on the quality of life and the capacity for work, and, furthermore, increase disability[115]. Disease progression and prognosis have greatly benefited from the use of steroids introduced in the 1950s, immunosuppressants in the 1970s and biological agents in the 1990s. Although these treatments appear to be effective in the management of disease activity in the majority of patients, and to improve the quality of life, it is still not clear whether they are able to modify the natural history of IBD[3,65-67]. There is evidence that new approaches aimed at optimizing immunosuppressants and biological agents by using them as early as possible could prevent disease progression and have a positive effect on the natural history of IBD.
ACKNOWLEDGMENTS
Authors are grateful to Mrs Marian Shields for help in editing the manuscript.
Footnotes
Peer reviewers: Bruno Bonaz, Professor, Department of Gastroenterology and Liver Diseases, Clinique Universitaire d’Hépato-Gastroentérologie, CHU de Grenoble, BP217, 38043 Grenoble Cedex 09, France; Dr. Alan Colm Moss, MD, Department of Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Bidmc, Boston, MA 02215, United States
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
References
- 1.Schirbel A, Fiocchi C. Inflammatory bowel disease: Established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11:266–276. doi: 10.1111/j.1751-2980.2010.00449.x. [DOI] [PubMed] [Google Scholar]
- 2.Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. 2009;11:481–487. doi: 10.1007/s11894-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 5.Modesto I, Perricone G, Orlando A, Cottone M. Methodological requirements for high quality studies on the course and prognosis of inflammatory bowel disease. World J Gastroenterol. 2012;18:3800–3805. doi: 10.3748/wjg.v18.i29.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–147. doi: 10.1136/gut.2009.187120. [DOI] [PubMed] [Google Scholar]
- 7.Vatn MH. Mucosal healing: impact on the natural course or therapeutic strategies. Dig Dis. 2009;27:470–475. doi: 10.1159/000233285. [DOI] [PubMed] [Google Scholar]
- 8.Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 9.Kugathasan S, Saubermann LJ, Smith L, Kou D, Itoh J, Binion DG, Levine AD, Blumberg RS, Fiocchi C. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut. 2007;56:1696–1705. doi: 10.1136/gut.2006.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder F, Lawrance IC, Leite A, Sans M. Predictors of fibrostenotic Crohn’s disease. Inflamm Bowel Dis. 2011;17:2000–2007. doi: 10.1002/ibd.21627. [DOI] [PubMed] [Google Scholar]
- 11.Cabré E, Domènech E. Environmental and dietary factors in IBD: What is their real impact on the clinical course? World J Gastroenterol. 2012;18:3814–3822. doi: 10.3748/wjg.v18.i29.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaugerie L, Sokol H. Clinical, serological and genetic predictors of IBD course: Which of them are relevant and usable in clinical practice? World J Gastroenterol. 2012;18:3806–3813. doi: 10.3748/wjg.v18.i29.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 14.Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213–1221. doi: 10.1007/s00384-008-0542-9. [DOI] [PubMed] [Google Scholar]
- 15.Johnson GJ, Cosnes J, Mansfield JC. Review article: smoking cessation as primary therapy to modify the course of Crohn’s disease. Aliment Pharmacol Ther. 2005;21:921–931. doi: 10.1111/j.1365-2036.2005.02424.x. [DOI] [PubMed] [Google Scholar]
- 16.Bastida G, Beltrán B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol. 2011;17:2740–2747. doi: 10.3748/wjg.v17.i22.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn‘s disease in children. J Pediatr Gastroenterol Nutr. 2000;31:8–15. doi: 10.1097/00005176-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther. 2007;26:795–806. doi: 10.1111/j.1365-2036.2007.03431.x. [DOI] [PubMed] [Google Scholar]
- 19.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn‘s disease. Cochrane Database Syst Rev. 2007:CD000542. doi: 10.1002/14651858.CD000542.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Enteral nutrition for the maintenance of remission in Crohn’s disease: a systematic review. Eur J Gastroenterol Hepatol. 2010;22:1–8. doi: 10.1097/MEG.0b013e32832c788c. [DOI] [PubMed] [Google Scholar]
- 21.Cámara RJ, Ziegler R, Begré S, Schoepfer AM, von Känel R. The role of psychological stress in inflammatory bowel disease: quality assessment of methods of 18 prospective studies and suggestions for future research. Digestion. 2009;80:129–139. doi: 10.1159/000226087. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Blanchard A, Walker JR, Graff LA, Miller N, Bernstein CN. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2011;9:769–775. doi: 10.1016/j.cgh.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 24.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn‘s disease and ulcerative colitis. Am J Gastroenterol. 2011;106:2133–2142. doi: 10.1038/ajg.2011.304. [DOI] [PubMed] [Google Scholar]
- 25.Mylonaki M, Langmead L, Pantes A, Johnson F, Rampton DS. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16:775–778. doi: 10.1097/01.meg.0000131040.38607.09. [DOI] [PubMed] [Google Scholar]
- 26.Sinh P, Barrett TA, Yun L. Clostridium difficile Infection and Inflammatory Bowel Disease: A Review. Gastroenterol Res Pract. 2011;2011:136064. doi: 10.1155/2011/136064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayre K, Warren BF, Jeffery K, Travis SP. The role of CMV in steroid-resistant ulcerative colitis: A systematic review. J Crohns Colitis. 2009;3:141–148. doi: 10.1016/j.crohns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Wolters FL, Russel MG, Sijbrandij J, Ambergen T, Odes S, Riis L, Langholz E, Politi P, Qasim A, Koutroubakis I, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124–1130. doi: 10.1136/gut.2005.084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol. 2008;43:948–954. doi: 10.1080/00365520801957149. [DOI] [PubMed] [Google Scholar]
- 32.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 33.Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692–1701. doi: 10.1111/j.1572-0241.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 34.Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvan-carova M, Bernklev T, Henriksen M, Sauar J, Vatn MH, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44:431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 35.Latella G, Fiocchi C, Caprili R. News from the “5th International Meeting on Inflammatory Bowel Diseases” CAPRI 2010. J Crohns Colitis. 2010;4:690–702. doi: 10.1016/j.crohns.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagacé C, Li C, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henckaerts L, Van Steen K, Verstreken I, Cleynen I, Franke A, Schreiber S, Rutgeerts P, Vermeire S. Genetic risk profiling and prediction of disease course in Crohn’s disease patients. Clin Gastroenterol Hepatol. 2009;7:972–980.e2. doi: 10.1016/j.cgh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Cucchiara S, Latiano A, Palmieri O, Canani RB, D’Incà R, Guariso G, Vieni G, De Venuto D, Riegler G, De’Angelis GL, et al. Polymorphisms of tumor necrosis factor-alpha but not MDR1 influence response to medical therapy in pediatric-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:171–179. doi: 10.1097/MPG.0b013e31802c41f3. [DOI] [PubMed] [Google Scholar]
- 41.Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- 42.Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004;5:530–539. doi: 10.1038/sj.gene.6364123. [DOI] [PubMed] [Google Scholar]
- 43.Daniel F, Loriot MA, Seksik P, Cosnes J, Gornet JM, Lémann M, Fein F, Vernier-Massouille G, De Vos M, Boureille A, et al. Multidrug resistance gene-1 polymorphisms and resistance to cyclosporine A in patients with steroid resistant ulcerative colitis. Inflamm Bowel Dis. 2007;13:19–23. doi: 10.1002/ibd.20046. [DOI] [PubMed] [Google Scholar]
- 44.Hlavaty T, Pierik M, Henckaerts L, Ferrante M, Joossens S, van Schuerbeek N, Noman M, Rutgeerts P, Vermeire S. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn’s disease. Aliment Pharmacol Ther. 2005;22:613–626. doi: 10.1111/j.1365-2036.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 45.Dubinsky MC, Mei L, Friedman M, Dhere T, Haritunians T, Hakonarson H, Kim C, Glessner J, Targan SR, McGovern DP, et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–1366. doi: 10.1002/ibd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubinsky M. What is the role of serological markers in IBD? Pediatric and adult data. Dig Dis. 2009;27:259–268. doi: 10.1159/000228559. [DOI] [PubMed] [Google Scholar]
- 47.Fleshner PR, Vasiliauskas EA, Kam LY, Fleshner NE, Gaiennie J, Abreu-Martin MT, Targan SR. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. doi: 10.1136/gut.49.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner D, Walsh CM, Benchimol EI, Mann EH, Thomas KE, Chow C, McLernon RA, Walters TD, Swales J, Steinhart AH, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008;57:331–338. doi: 10.1136/gut.2007.136481. [DOI] [PubMed] [Google Scholar]
- 49.Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 50.Vavricka SR, Brun L, Ballabeni P, Pittet V, Prinz Vavricka BM, Zeitz J, Rogler G, Schoepfer AM. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 51.Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 53.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Papi C, Festa V, Fagnani C, Stazi A, Antonelli G, Moretti A, Koch M, Capurso L. Evolution of clinical behaviour in Crohn’s disease: predictive factors of penetrating complications. Dig Liver Dis. 2005;37:247–253. doi: 10.1016/j.dld.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995;30:699–706. doi: 10.3109/00365529509096316. [DOI] [PubMed] [Google Scholar]
- 57.Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63–101. doi: 10.1016/j.crohns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 60.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55–60. doi: 10.1097/00054725-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Spinelli A, Correale C, Szabo H, Montorsi M. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2010;11:242–248. doi: 10.2174/138945010790309984. [DOI] [PubMed] [Google Scholar]
- 63.Latella G, Caprilli R, Travis S. In favour of early surgery in Crohn’s disease: a hypothesis to be tested. J Crohns Colitis. 2011;5:1–4. doi: 10.1016/j.crohns.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Samimi R, Flasar MH, Kavic S, Tracy K, Cross RK. Outcome of medical treatment of stricturing and penetrating Crohn’s disease: a retrospective study. Inflamm Bowel Dis. 2010;16:1187–1194. doi: 10.1002/ibd.21160. [DOI] [PubMed] [Google Scholar]
- 65.Vermeire S, van Assche G, Rutgeerts P. Review article: Altering the natural history of Crohn’s disease--evidence for and against current therapies. Aliment Pharmacol Ther. 2007;25:3–12. doi: 10.1111/j.1365-2036.2006.03134.x. [DOI] [PubMed] [Google Scholar]
- 66.Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2010;7:79–85. doi: 10.1038/nrgastro.2009.220. [DOI] [PubMed] [Google Scholar]
- 67.Caprilli R, Latella G, Frieri G. Treatment of inflammatory bowel diseases: to heal the wound or to heal the sick? J Crohns Colitis. 2012;6:621–625. doi: 10.1016/j.crohns.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Reenaers C, Belaiche J, Louis E. Impact of medical therapies on IBD complication rate. World J Gastroenterol. 2012;18:3823–3827. doi: 10.3748/wjg.v18.i29.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111.e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 70.Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, Kane SV, Sandborn WJ, Ullman TA, Moayyedi P. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106 Suppl 1:S2–25; quiz S26. doi: 10.1038/ajg.2011.58. [DOI] [PubMed] [Google Scholar]
- 71.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 72.Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, Rivera M, Paoluzi P. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 73.Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 75.Barreiro M, Lorenzo A, Mera J, Dominguez-Munoz E. Prospective, open pilot study for evaluating the clinical efficacy and mucosal healing rate of Infliximab in steroid-dependent ulcerative colitis. Gastroenterology. 2008;134 Suppl 1:A667. [Google Scholar]
- 76.Afif W, Leighton JA, Hanauer SB, Loftus EV, Faubion WA, Pardi DS, Tremaine WJ, Kane SV, Bruining DH, Cohen RD, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009;15:1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- 77.Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Feagan BG, Panaccione R, Sandborn WJ, D’Haens GR, Schreiber S, Rutgeerts PJ, Loftus EV, Lomax KG, Yu AP, Wu EQ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 79.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 80.Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128:862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 81.Cosnes J. Can we modulate the clinical course of inflammatory bowel diseases by our current treatment strategies? Dig Dis. 2009;27:516–521. doi: 10.1159/000233291. [DOI] [PubMed] [Google Scholar]
- 82.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 83.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 84.van Assche G, Vermeire S, Rutgeerts P. Mucosal healing and anti TNFs in IBD. Curr Drug Targets. 2010;11:227–233. doi: 10.2174/138945010790309902. [DOI] [PubMed] [Google Scholar]
- 85.Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 86.Sandborn WJ, Van O EC, Zins BJ, Tremaine WJ, Mays DC, Lipsky JJ. An intravenous loading dose of azathioprine decreases the time to response in patients with Crohn’s disease. Gastroenterology. 1995;109:1808–1817. doi: 10.1016/0016-5085(95)90747-5. [DOI] [PubMed] [Google Scholar]
- 87.D’Haens G, Geboes K, Ponette E, Penninckx F, Rutgeerts P. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn’s disease. Gastroenterology. 1997;112:1475–1481. doi: 10.1016/s0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 88.Mantzaris GJ, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, Polyzou P. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15:375–382. doi: 10.1002/ibd.20777. [DOI] [PubMed] [Google Scholar]
- 89.Mañosa M, Naves JE, Leal C, Cabré E, Moreno V, Lorenzo-Zuñiga V, Boix J, Domènech E. Does methotrexate induce mucosal healing in Crohn’s disease? Inflamm Bowel Dis. 2010;16:377–378. doi: 10.1002/ibd.21015. [DOI] [PubMed] [Google Scholar]
- 90.Laharie D, Reffet A, Belleannée G, Chabrun E, Subtil C, Razaire S, Capdepont M, de Lédinghen V. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther. 2011;33:714–721. doi: 10.1111/j.1365-2036.2010.04569.x. [DOI] [PubMed] [Google Scholar]
- 91.Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930–936. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 92.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, Harmsen WS, Zinsmeister AR. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. doi: 10.1016/s0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 94.Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 95.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 96.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 97.de Buck van Overstraeten A, Wolthuis AM, D’Hoore A. Surgery for Crohn’s disease in the era of biologicals: A reduced need or delayed verdict? World J Gastroenterol. 2012;18:3828–3832. doi: 10.3748/wjg.v18.i29.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selby W. The natural history of ulcerative colitis. Baillieres Clin Gastroenterol. 1997;11:53–64. doi: 10.1016/s0950-3528(97)90053-1. [DOI] [PubMed] [Google Scholar]
- 99.Hoie O, Wolters FL, Riis L, Bernklev T, Aamodt G, Clofent J, Tsianos E, Beltrami M, Odes S, Munkholm P, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology. 2007;132:507–515. doi: 10.1053/j.gastro.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Hyde GM, Jewell DP. Review article: the management of severe ulcerative colitis. Aliment Pharmacol Ther. 1997;11:419–424. doi: 10.1046/j.1365-2036.1997.00187.x. [DOI] [PubMed] [Google Scholar]
- 101.Caprilli R, Viscido A, Latella G. Current management of severe ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:92–101. doi: 10.1038/ncpgasthep0687. [DOI] [PubMed] [Google Scholar]
- 102.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–110. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 103.Dayan B, Turner D. Role of surgery in severe ulcerative colitis in the era of medical rescue therapy. World J Gastroenterol. 2012;18:3833–3838. doi: 10.3748/wjg.v18.i29.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 106.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 107.Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039–1046. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 108.Rubin DT, Cruz-Correa MR, Gasche C, Jass JR, Lichtenstein GR, Montgomery EA, Riddell RH, Rutter MD, Ullman TA, Velayos FS, et al. Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: a clinical review and update. Inflamm Bowel Dis. 2008;14:265–274. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 109.Ullman T, Croog V, Harpaz N, Hossain S, Kornbluth A, Bodian C, Itzkowitz S. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol. 2008;6:1225–1230; quiz 1177. doi: 10.1016/j.cgh.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 110.Lyakhovich A, Gasche C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther. 2010;31:202–209. doi: 10.1111/j.1365-2036.2009.04195.x. [DOI] [PubMed] [Google Scholar]
- 111.Dyson J, Rutter M. Colorectal cancer in IBD: What is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jess T, Gamborg M, Munkholm P, Sørensen TI. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am J Gastroenterol. 2007;102:609–617. doi: 10.1111/j.1572-0241.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 113.Canavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn’s disease. Aliment Pharmacol Ther. 2007;25:861–870. doi: 10.1111/j.1365-2036.2007.03276.x. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen GC, Steinhart AH. Nationwide patterns of hospitalizations to centers with high volume of admissions for inflammatory bowel disease and their impact on mortality. Inflamm Bowel Dis. 2008;14:1688–1694. doi: 10.1002/ibd.20526. [DOI] [PubMed] [Google Scholar]
- 115.Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, Kostanjsek N, Stucki G, Colombel JF. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241–247. doi: 10.1136/gutjnl-2011-300049. [DOI] [PMC free article] [PubMed] [Google Scholar]