Abstract
AIM: To establish if the juice of Moro, an anthocyanin-rich orange, may improve liver damage in mice with diet-induced obesity.
METHODS: Eight-week-old mice were fed a high-fat diet (HFD) and were administrated water or Moro juice for 12 wk. Liver morphology, gene expression of lipid transcription factors, and metabolic enzymes were assessed.
RESULTS: Mice fed HFD displayed increased body weight, insulin resistance and dyslipidemia. Moro juice administration limited body weight gain, enhanced insulin sensitivity, and decreased serum triglycerides and total cholesterol. Mice fed HFD showed liver steatosis associated with ballooning. Dietary Moro juice markedly improved liver steatosis by inducing the expression of peroxisome proliferator-activated receptor-α and its target gene acylCoA-oxidase, a key enzyme of lipid oxidation. Consistently, Moro juice consumption suppressed the expression of liver X receptor-α and its target gene fatty acid synthase, and restored liver glycerol-3-phosphate acyltransferase 1 activity.
CONCLUSION: Moro juice counteracts liver steatogenesis in mice with diet-induced obesity and thus may represent a promising dietary option for the prevention of fatty liver.
Keywords: Liver steatosis, Anthocyanins, Lipogenesis, Lipid oxidation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a chronic metabolic disorder with significant impact on cardiovascular and liver-related mortality[1,2]. NAFLD is closely associated with obesity, dyslipidemia, diabetes and the full spectrum of the metabolic syndrome[3,4], with insulin resistance as a common pathophysiological determinant[5]. Lifestyle, that is, dietary habits and physical activity, plays a pivot role in the pathogenesis of the metabolic syndrome[6]. Likewise, dietary factors have been shown to exert a major role in the development of fatty liver[7]. Recently, the consumption of foods and beverages containing fructose has been identified as a risk factor for NAFLD[8]. Nonetheless, despite their fructose content, fruits are also rich in different polyphenolic compounds that exert several beneficial effects on health, mainly by modulating expression of key enzymes involved in glucose sensitivity and lipid homeostasis[9].
Anthocyanins (ACNs) are water-soluble plant polyphenolic pigments that confer a typical blue, red or purple color, and are contained especially in berries, blood oranges and pigmented corn and potato[10]. ACNs have been attributed several putative therapeutic roles, including beneficial effects on obesity and related metabolic complications[11]. In this respect, Tsuda et al[12] firstly showed that ACN from purple corn prevented obesity, dyslipidemia and visceral fat inflammation in mice fed a high-fat diet (HFD). Recently, a further demonstration of the putative hepatoprotective properties of ACN has been provided by Liu et al[13], who observed that administration of protocatechuic acid, the major in vivo metabolite of the main ACN cyanidin-3-O-β-glucoside, reduced gene expression of lipogenic enzymes in the liver, thus leading to a decreased hepatic lipid accumulation. In vitro and in vivo experiments suggest that ACNs can modulate gene expression of several adipocytokines and regulate the pathways involved in lipogenesis and fat accumulation[12,14]. ACNs are contained also in blood oranges, a variety of sweet orange (Citrus sinensis) with an intense red pigmentation[15]. A recent study has demonstrated that blood orange consumption inhibits fat accumulation in mice[16] and may represent a promising dietary tool for the treatment of obesity.
In the current study, we aimed at clarifying whether consumption of the juice of Moro, a blood orange cultivated in Sicily, Italy, improved liver steatosis in mice fed HFD; a physiological model of NAFLD and metabolic syndrome.
MATERIALS AND METHODS
Animals and treatments
All procedures fulfilled the Italian Guidelines for the Use and Care of Laboratory Animals. Eight-week-old male C57BL6/J mice were purchased from Charles River Laboratories (Calco, Italy). Animals were maintained in a temperature- and light-controlled facility for 12 wk. Diets were obtained by Harlan Teklad (Madison, WI, United States). The standard diet (SD) provided 3.3 kcal/g with 60% carbohydrates, 23% proteins and 17% fat. The HFD provided 5.2 kcal/g with 60% fat, 20% proteins and 20% carbohydrates. Mice were distributed in three groups: group I included six mice fed SD and permitted ad libitum consumption of water (SD + water); group II comprised six mice fed HFD and permitted ad libitum consumption of water (HFD + water); group III comprised six mice fed HFD and permitted ad libitum consumption of Moro orange juice instead of water (HFD + Moro). Moro fruits were collected in the experimental farm of the Research Center for Citric Culture and Mediterranean Crops (Acireale, Italy). Fruits were immediately stored at 4 °C and squeezed a few days later; the juice obtained was pre-filtered and stored at -20 °C in aliquots of 0.5 L. Every 2 d, frozen juice aliquots were thawed, filtered and put in the bottle of each cage. Fruit juice analysis was performed as previously described[16]. Food and beverage consumption was recorded twice weekly; body weight was recorded weekly. After sacrifice by CO2 asphyxiation, blood and liver samples were obtained, processed and stored for further analysis.
Histopathology
Formalin-fixed paraffin-embedded liver sections were stained with haematoxylin-eosin and Masson’s trichrome, using standard procedures. Liver injury was blindly evaluated according to the NAFLD activity score.
Biochemical analysis
Serum glucose, total cholesterol, triglycerides and alanine aminotransferase (ALT) were measured using Reflotron Plus system from Roche Diagnostic (Milan, Italy). Liver triglycerides content was measured using a serum/tissue triglyceride colorimetric kit (Biovision, Mountain View, CA, United States). Insulin tolerance test (ITT) was performed on 5-h starved mice, and glycemia was measured immediately before and 15, 30 and 60 min after intraperitoneal injection of 0.4 U/kg recombinant human insulin. The total amount of ACN in Moro juice was determined as previously described[16]. Glycerol-3-phosphate acyltransferase 1 (GPAT1) activity was assayed as previously described [17].
RNA extraction and real-time polymerase chain reaction
Total RNA was extracted by homogenizing snap frozen liver samples in TRIzol reagent (Invitrogen, Milan, Italy). Quantitative real-time polymerase chain reaction (PCR) was performed in 7900HT Fast Real-Time PCR System Applied Biosystems (Applied Biosystems, Foster City, CA, United States), using the EXPRESS SYBR GreenER™ qPCR SuperMix with Premixed ROX (Invitrogen).
Reactions were performed in a 20 μL mixture containing cDNA, specific primers of each gene, and the SYBRR GreenER™ qPCR SuperMix. The specific PCR products were detected by the fluorescence of SYBR Green, the double-stranded DNA binding dye. The relative mRNA expression level was calculated by the threshold cycle (Ct) value of each PCR product and normalized with that of glyceraldehyde-3-phosphate dehydrogenase by using comparative 2ΔΔCt method. Primer sequences are shown in Table 1.
Table 1.
Primer sequences for real-time polymerase chain reaction
| Gene name | Forward | Reverse |
| PPAR-α | 5’-AGTCAAGGTGTGGCCCAAGGT-3’ | 5’- TGTCTATCGGACACTAGCGGAGGC-3’ |
| AOX | 5’-CTTGTTCGCGCAAGTGAGG-3’ | 5’-CAGGATCCGACTGTTTACC-3’ |
| LXR-α | 5’-TGCCATCAGCATCTTCTCTG-3’ | 5’-GGCTCACCAGCTTCATTAGC-3’ |
| FAS | 5’-AGCCCACGTCGTAGCAAACCA-3’ | 5’-GCAGGGGCTCTTGACGGCAG-3’ |
| HMG-CoA reductase | 5’-CCTGACACTGAACTGAAGCG-3’ | 5’-TCTTTCCAG AACACAGCACG-3’ |
| GAPDH | 5'-ACCACCATGGAGAAGGCCGG-3' | 5'-CTCAGTGTAGCCCAAGATGC-3' |
PPAR: Peroxisome proliferator-activated receptor; AOX: Acyl-CoA oxidase; LXR: Liver X receptor; FAS: Fatty acid synthase; HMG: Hydroxy methylglutaryl; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase .
Statistical analysis
Statistical analysis was performed by GraphPad Prism software (San Diego, CA, United States). Data are the results of three independent experiments. All results were expressed as mean ± SE. One-way analysis of variance with Bonferroni post-hoc analysis was used for parametric data. Kruskal-Wallis test was used for non-parametric data. P values < 0.05 were considered significant.
RESULTS
Moro juice improves dyslipidemia and enhances insulin sensitivity
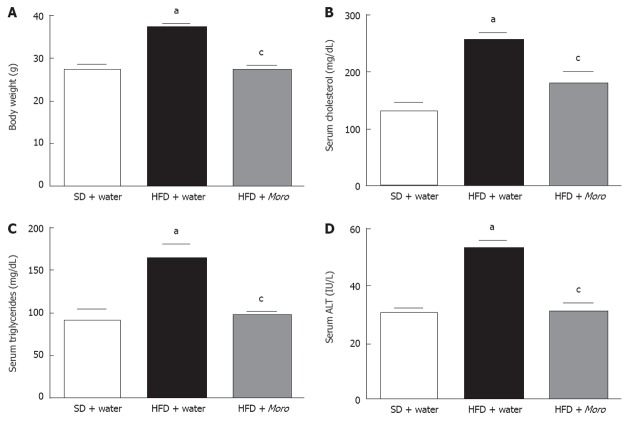
Moro juice had an ACN content of 85 mg/L. Food intake was identical among the three experimental groups (SD + water, 3.1 ± 0.5 g/d vs HFD + water, 3.0 ± 0.8 g/d vs HFD + Moro, 3.1 ± 0.7 g/d). The amount of Moro juice intake (4.1 ± 0.75 mL/d) did not differ from the amount of water intake in the control groups (4.0 ± 1.2 mL/d). All mice had similar body weight at the start of the experiment, and after 12 wk, mice fed HFD + water had higher body weight compared with SD mice (Figure 1A). Strikingly, mice fed HFD + Moro had the same body weight gain as mice fed SD. This effect on body weight gain occurred despite the fact that mice fed HFD + Moro received a 10% higher energy intake, due to the sugar content of the juice, as compared to mice fed HFD + water (Figure 1A). Obese mice had increased serum total cholesterol, triglycerides and ALT as compared to the SD group (Figure 1B-D). Moro juice decreased serum total cholesterol and triglycerides, and reduced serum ALT to the levels of lean controls (Figure 1B-D). Furthermore, orange juice consumption significantly enhanced insulin sensitivity, as demonstrated by the area under curve derived from ITT, which was 8685 ± 516 in mice fed HFD + water and was reduced to 7225 ± 718 in the HFD + Moro group (Figure 2).
Figure 1.
Effects of Moro juice on body weight and serum parameters. A: Body weight was markedly increased in mice fed high-fat diet (HFD) and was decreased to the levels of lean mice by Moro juice; B: Serum total cholesterol was significantly reduced in mice drinking Moro; C and D: Serum triglycerides (C) and alanine aminotransferase (ALT) (D) were restored to the levels of lean mice. aP < 0.05 vs standard diet (SD) + water; cP < 0.05 vs HFD + water.
Figure 2.
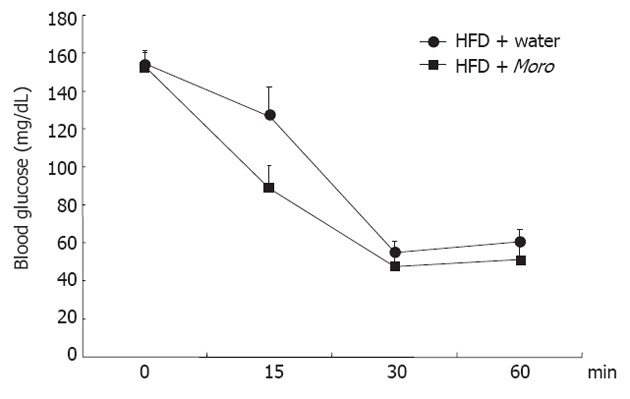
Effects of Moro juice on insulin sensitivity. Insulin tolerance test was performed with 0.4 U/kg recombinant human insulin in the two groups of mice fed high-fat diet (HFD); area under curve of blood glucose was 8685 ± 516 in mice fed HFD + water and was reduced to 7225 ± 718 in HFD + Moro (P < 0.05).
Moro juice induces liver peroxisome proliferator-activated receptor-α and inhibits liver lipogenesis
Liver sections of mice fed HFD + water showed moderate steatosis with a panacinar pattern (Figure 3B), mild lobular inflammation, and diffuse hepatocyte ballooning. In the HFD + Moro group, steatosis was almost absent (Figure 3C). Consistently, lobular inflammation and ballooning degeneration was less pronounced throughout the hepatic parenchyma. Fibrosis, assessed by Masson’s trichrome, was not found in any mice after 12 wk HFD (data not shown). Biochemical analysis confirmed that Moro juice induced a marked decrease of liver triglyceride content in mice fed HFD (Figure 3D).
Figure 3.
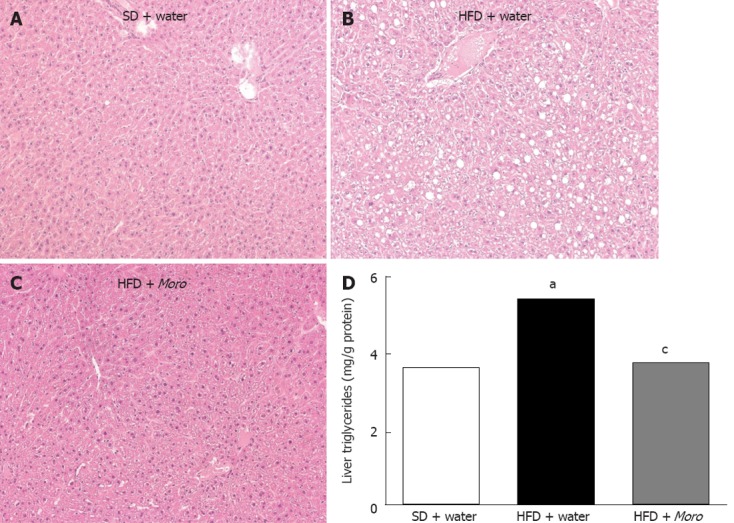
Effects of Moro juice on liver histology and liver triglycerides content. A: Hematoxylin-eosin-stained liver sections showing normal histology in lean mice; B: Moderate panacinar steatosis and hepatocyte ballooning in mice fed high-fat diet (HFD); C: Liver sections of mice fed HFD + Moro showing absence of steatosis and ballooning; D: Liver triglycerides content was significantly decreased in mice drinking orange juice. aP < 0.05 vs standard diet (SD) + water, cP < 0.05 vs HFD + water. Magnification: 10 ×.
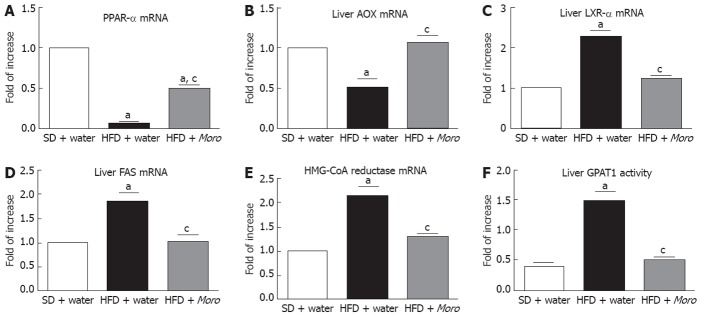
HFD was associated with impaired expression of key transcription factors and metabolic enzymes involved in lipid homeostasis. In particular, we found a decrease in the gene expression of peroxisome proliferator-activated receptor (PPAR)-α (Figure 4A) and acyl-CoA oxidase (AOX) (Figure 4B) and a significant increase in the expression of liver X receptor (LXR)-α (Figure 4C), fatty acid synthase (FAS) (Figure 4D) and hydroxy methylglutaryl (HMG)-CoA reductase (Figure 4E). Moro juice markedly decreased the mRNA levels of LXR-α, FAS and HMG-CoA reductase but augmented the mRNA levels of PPAR-α and AOX (Figure 4A-E). Consistent with gene expression findings, GPAT1 activity was restored to the levels of lean animals by Moro juice consumption (Figure 4F).
Figure 4.
Effects of Moro juice on liver lipid homeostasis. Gene expression of (A) peroxisome proliferator-activated receptor (PPAR)-α and (B) acyl-CoA oxidase (AOX) was significantly increased in mice fed high-fat diet (HFD) + Moro, whereas gene expression of (C) liver X receptor (LXR)-α, (D) fatty acid synthase (FAS) and (E) hydroxy methylglutaryl (HMG)-CoA reductase was markedly reduced by orange juice; Liver glycerol-3-phosphate acyltransferase 1 (GPAT1) was increased in mice fed HFD + water and was restored to control levels in mice drinking Moro juice (F). aP < 0.05 vs standard diet (SD) + water, cP < 0.05 vs HFD + water.
DISCUSSION
In this study, we explored the effect of the consumption of the juice of Moro, an ACN-rich orange cultivated in the Mediterranean region, on liver steatosis in mice with diet-induced obesity. In previous experiments in mice fed SD, we observed that C57BL6 mice and other strains tolerated the substitution of drinking water with orange juice as the only drinking source, had similar food intake, and did not show any behavioral abnormality. Here, we demonstrated that Moro juice drinking reversed most of the metabolic abnormalities exhibited by obese mice, including fatty liver.
Our results are in agreement with previous experimental data suggesting that ACNs from different fruits and vegetables are able to exert beneficial effects on several metabolic aspects related to obesity[11]. One common effect of ACN administration both in diet-induced and genetic models of obesity is the reduction of body weight and of visceral fat. In this respect, Kwon et al[18] showed that ACN extracted from black soybean led to improvement in dyslipidemia and a decrease in visceral adiposity in rats fed HFD. Similarly, extracts from tart cherries ameliorate dyslipidemia and decrease liver fat content in genetically insulin-resistant rats[19], as does cyanidin 3-glucoside in db/db mice[20].
As regards the molecular events underlying the effects of Moro on liver lipid metabolism, we demonstrated that a major mechanism is induction of PPAR-α. PPAR-α is a key transcription factor promoting lipolysis and lipid oxidation in different tissues[21]. Mice lacking PPAR-α develop obesity and liver steatosis[22]; similarly, the hepatic levels of PPAR-α are decreased in patients with NAFLD[23], and pharmacological agonists are able to improve liver steatosis[24]. Previous studies have suggested that PPAR-α induction is involved in the antisteatotic effect of extracts containing ACNs in different models of obesity[19,20]. Along with the promotion of lipid oxidation, Moro juice consumption induced the inhibition of lipogenesis. In this respect, a major mechanism for the antisteatotic effect is the reversion of LXR-α expression. LXR-α is a nuclear hormone receptor that promotes lipogenesis[25], which is increased in the liver of patients with NAFLD[26]. LXR-α stimulates lipogenesis through upregulation of enzymes of de novo lipogenesis such as FAS[25]. Suppression of LXR-α expression and activity exerts potent antisteatotic effects in the liver[27]; some flavonoids have been shown to inhibit LXR-α[28].
Besides the effects on lipid homeostasis, the beneficial impact of Moro on insulin sensitivity is also noteworthy. Insulin resistance is the metabolic hallmark of patients with NAFLD[29]. Studies using the hyperinsulinemic-euglycemic clamp coupled with infusion tracers have demonstrated that hepatic fat content is directly related to insulin resistance in the liver, skeletal muscle, and adipose tissue of obese subjects[5]. In contrast, recent findings have demonstrated that liver triglyceride content, not visceral adipose tissue volume, predicts the impairment of insulin sensitivity and of very low density lipoprotein secretion in patients with obesity[30,31]. Although mice drinking Moro juice remained hyperglycemic, probably because of the sugar content, the ITT unequivocally revealed that Moro juice exerted insulin-sensitizing activity. An insulin-sensitizing effect has been demonstrated for ACN-rich extracts from bilberry in genetically obese mice[32], and for dietary blueberry in mice fed HFD[33].
In conclusion, in this study, we demonstrated that the juice of Moro exerts metabolic hepatoprotective effects due to changes in the expression of several enzymes involved in lipid homeostasis. Thus, the dietary administration of this food may be effective in preventing liver steatosis, and may be considered as a nutritional approach for the prevention of NAFLD. Clinical trials are now warranted.
COMMENTS
Background
Nonalcoholic fatty liver disease (NAFLD) is a chronic metabolic disorder with significant impact on cardiovascular and liver-related mortality. Lifestyle, that is, dietary habits and physical activity, plays a pivot role in the pathogenesis of NAFLD.
Research frontiers
Anthocyanins (ACNs) are water-soluble plant polyphenolic pigments that confer a typical blue, red or purple color, and are contained especially in berries, blood oranges, and pigmented corn and potato. ACNs have been attributed several putative therapeutic roles, including beneficial effects on obesity and related metabolic complications. Diet enriched with ACNs may provide a useful tool to counteract liver steatogenesis.
Innovations and breakthroughs
ACNs are contained in blood oranges, a variety of sweet orange (Citrus sinensis) with an intense red pigmentation. A recent study has demonstrated that blood orange consumption inhibits fat accumulation in mice. Furthermore, the administration of protocatechuic acid, the major in vivo metabolite of ACN, reduces the activity of lipogenic enzymes in the liver, thus leading to decreased hepatic lipid accumulation. The data in this article demonstrated for the first time that Moro juice counteracted liver steatogenesis in mice with diet-induced obesity, through modulation of enzymes involved in lipogenesis and lipid oxidation. Thus, Moro juice consumption may represent a promising dietary option for prevention of fatty liver.
Terminology
ACNs are water-soluble pigments that belong to the family of flavonoids. They are contained in berries, blood oranges, and pigmented corn and potato. They are able to modulate lipid and glucose metabolic pathways in humans.
Peer review
It is interesting to readers and may be effective dietary supplements for NAFLD. The manuscript described an interesting finding about the protective effect of this orange juice on the development of fatty liver induced by a high-fat diet in mice.
Footnotes
Peer reviewers: Dr. Nagarajan Perumal, Compliance veteri-narian, Center for life science, IACUC Office, National Uni-versity of Singapore, Singapore 117456, Singapore; Carlos J Pirola, PhD, FAHA, Medical Research Institute A Lanari, Combatientes de Malvinas 3150, Buenos Aires-1427, Argentina
S- Editor Cheng JX L- Editor Kerr C E- Editor Li JY
References
- 1.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamone F, Galvano F, Li Volti G. Treating fatty liver for the prevention of cardiovascular diseases. Hepatology. 2010;52:1174–1175. doi: 10.1002/hep.23741. [DOI] [PubMed] [Google Scholar]
- 5.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown T, Avenell A, Edmunds LD, Moore H, Whittaker V, Avery L, Summerbell C. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev. 2009;10:627–638. doi: 10.1111/j.1467-789X.2009.00641.x. [DOI] [PubMed] [Google Scholar]
- 7.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711–717. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bladé C, Arola L, Salvadó MJ. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. 2010;54:37–59. doi: 10.1002/mnfr.200900476. [DOI] [PubMed] [Google Scholar]
- 10.Galvano F, La Fauci L, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S, Battistini NC, Tavazzi B, Galvano G. Cyanidins: metabolism and biological properties. J Nutr Biochem. 2004;15:2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Galvano F, La Fauci L, Vitaglione P, Fogliano V, Vanella L, Felgines C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann Ist Super Sanita. 2007;43:382–393. [PubMed] [Google Scholar]
- 12.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 13.Liu WH, Lin CC, Wang ZH, Mong MC, Yin MC. Effects of protocatechuic acid on trans fat induced hepatic steatosis in mice. J Agric Food Chem. 2010;58:10247–10252. doi: 10.1021/jf102379n. [DOI] [PubMed] [Google Scholar]
- 14.Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59:2663–2671. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 15.Fiore A, La Fauci L, Cervellati R, Guerra MC, Speroni E, Costa S, Galvano G, De Lorenzo A, Bacchelli V, Fogliano V, et al. Antioxidant activity of pasteurized and sterilized commercial red orange juices. Mol Nutr Food Res. 2005;49:1129–1135. doi: 10.1002/mnfr.200500139. [DOI] [PubMed] [Google Scholar]
- 16.Titta L, Trinei M, Stendardo M, Berniakovich I, Petroni K, Tonelli C, Riso P, Porrini M, Minucci S, Pelicci PG, et al. Blood orange juice inhibits fat accumulation in mice. Int J Obes (Lond) 2010;34:578–588. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Li D, Ling W, Feng X, Xia M. Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ. J Lipid Res. 2011;52:908–922. doi: 10.1194/jlr.M013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon SH, Ahn IS, Kim SO, Kong CS, Chung HY, Do MS, Park KY. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J Med Food. 2007;10:552–556. doi: 10.1089/jmf.2006.147. [DOI] [PubMed] [Google Scholar]
- 19.Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. 2008;11:252–259. doi: 10.1089/jmf.2007.658. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, Xia M, Zou T, Ling W, Zhong R, Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem. 2012;23:349–360. doi: 10.1016/j.jnutbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801:299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 23.Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, Korn O, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–1086. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, et al. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23:102–109. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 25.Faulds MH, Zhao C, Dahlman-Wright K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Curr Opin Pharmacol. 2010;10:692–697. doi: 10.1016/j.coph.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Lima-Cabello E, García-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillón J, Lozano-Rodríguez T, Fernández-Bermejo M, Olcoz JL, González-Gallego J, García-Monzón C, Sánchez-Campos S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond) 2011;120:239–250. doi: 10.1042/CS20100387. [DOI] [PubMed] [Google Scholar]
- 27.Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid Redox Signal. 2011;15:2135–2146. doi: 10.1089/ars.2010.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YW, Kim YM, Yang YM, Kim TH, Hwang SJ, Lee JR, Kim SC, Kim SG. Inhibition of SREBP-1c-mediated hepatic steatosis and oxidative stress by sauchinone, an AMPK-activating lignan in Saururus chinensis. Free Radic Biol Med. 2010;48:567–578. doi: 10.1016/j.freeradbiomed.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 30.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamone F, Bugianesi E. Nonalcoholic fatty liver disease: the hepatic trigger of the metabolic syndrome. J Hepatol. 2010;53:1146–1147. doi: 10.1016/j.jhep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140:527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 33.DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]