Abstract
AIM: To investigate the usefulness of endoscopic ultra-sound-guided fine needle aspiration (EUS-FNA) in the differentiation of autoimmune pancreatitis (AIP).
METHODS: We retrospectively reviewed 47 of 56 AIP patients who underwent EUS-FNA and met the Asian diagnostic criteria. On 47 EUS-FNA specimens, we evaluated the presence of adequate material and characteristic features of lymphoplasmacytic sclerosing pancreatitis (LPSP) and idiopathic duct-centric pancreatitis (IDCP) mentioned in the International Consensus Diagnostic Criteria and examined if these findings make a contribution to the differential diagnosis of type 1 and type 2 AIP. A disposable 22-gauge needle was used for EUS-FNA.
RESULTS: Adequate specimens including pancreatic tissue for differentiating AIP from cancer were obtained from 43 of 47 patients who underwent EUS-FNA. EUS-FNA was performed from the pancreatic head in 21 cases, which is known to be technically difficult when performed by core biopsy; there was no significant difference in the results compared with pancreatic body-tail. Nine of 47 patients met level 1 findings of LPSP and 5 patients met level 2 findings of LPSP. No one met level 1 findings of IDCP, but 3 patients met level 2 findings of IDCP. Of 10 seronegative cases, 2 cases were diagnosed with “definitive type 1 AIP”, and 3 cases were diagnosed with “probable type 2 AIP” when considering both the level 2 histological findings and response to steroids.
CONCLUSION: EUS-FNA is useful in the differentiation of type 1 and type 2 AIP, particularly in seronegative cases.
Keywords: Autoimmune pancreatitis, Endoscopic ultra-sound-guided fine needle aspiration, Idiopathic duct centric pancreatitis, Lymphoplasmacytic sclerosing pancreatitis, Pancreatic cancer
INTRODUCTION
Recently, the International Consensus Diagnostic Criteria (ICDC) for autoimmune pancreatitis (AIP) was proposed by Shimosegawa et al[1]. According to these criteria, AIP is classified into 2 types[2]. The histological substance of type 1 AIP is known as lymphoplasmacytic sclerosing pancreatitis (LPSP)[3-6], and type 2 AIP is characterized by a distinct histology called idiopathic duct centric pancreatitis (IDCP)[7-10]. Type 2 AIP patients are generally seronegative and lack other organ involvement (OOI) in contrast to type 1 AIP. However, the absence of serological abnormalities or lack of OOI in patients with AIP does not necessarily imply the diagnosis of type 2, as type 1 also can be seronegative and without OOI. Taking these findings into consideration, ICDC made separate diagnostic criteria for type 1 and type 2 AIP, and histological differentiation is becoming more important for diagnosing AIP.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is now widely accepted as a safe and effective modality for obtaining pancreatic tissue samples[11-14]. There are reports on the usefulness of EUS-FNA in the diagnosis of AIP[15,16] but only negative reports on the differentiation between LPSP and IDCP using specimens obtained by EUS-FNA[17,18]. The findings of expert panel deliberations at the AIP International 2009 Honolulu Meeting reached a uniform consensus that essential histological features of LPSP can only be obtained or evaluated in tissues with preserved architecture, i.e., either a surgical resection specimen or a core biopsy but not FNA[19]. However, a surgically resected specimen can only be obtained from a patient misdiagnosed with pancreatic cancer[20-22], and a core biopsy device may not function properly when used in the duodenum. We thus investigated the usefulness of EUS-FNA in the differentiation of type 1 and type 2 AIP using EUS-FNA with a 22-gauge needle.
MATERIALS AND METHODS
Patients
We retrospectively reviewed 47 patients who underwent EUS-FNA of 56 AIP patients who met the Asian Diagnostic Criteria[23] at our institute between July 2003 and July 2011. Forty-two men and 5 women with a mean age of 62.1 ± 13.6 years (range, 28-86 years) and a mean follow-up period of 839.8 ± 722.7 d (range, 19-2506 d) were included. The mean serum immunoglobulin G4 (IgG4) levels were 626.1 ± 1004.6 mg/dL (range of 4-5850 mg/dL), and 10 patients were seronegative. On 47 EUS-FNA specimens, we evaluated the presence of adequate material and characteristic features of LPSP [lymphoplasmacytic infiltration, storiform fibrosis, obliterative phlebitis, and abundant (> 10 cells per high-power field) IgG4-positive cells] and IDCP [granulocytic infiltration of the duct wall (GEL) or granulocytic acinar infiltrate] mentioned in the histological criteria for ICDC (Table 1). Adequate material indicated an adequate specimen including pancreatic tissue for differentiating AIP from cancer. EUS-FNA was performed from the pancreatic head in 21 cases, an approach that is known to be technically difficult when performed by core biopsy. The histological findings according to the locations of EUS-FNA were also evaluated. Using the results of EUS-FNA, we examined whether these findings make a contribution to the differential diagnosis of type 1 and type 2 AIP. Patients with jaundice or abdominal pain underwent steroid therapy with oral prednisolone (PSL). The initial dose of PSL was 30-40 mg/d, and it was tapered down to the maintenance dose (2.5-5 mg/d) within 12 wk. Relapse was defined as exacerbation of the pancreatic lesion or OOI morphology or emergence of new OOI. OOI include cholangitis[24] [proximal (hilar/intrahepatic) or proximal and distal bile stricture], siala-denitis, nephritis[25], inflammatory bowel disease (IBD), and retroperitoneal fibrosis.
Table 1.
Histological criteria for International Consensus Diagnostic Criteria
| Level 1 | Level 2 | |
| Type 1 AIP | ||
| Histology of the pancreas | LPSP (core biopsy/resection) | LPSP (core biopsy) |
| At least 3 of the following: | Any 2 of the following: | |
| (1) Periductal lymphoplasmacytic infiltrate without granulocytic infiltration | (1) Periductal lymphoplasmacytic infiltrate without granulocytic infiltration | |
| (2) Obliterative phlebitis | (2) Obliterative phlebitis | |
| (3) Storiform fibrosis | (3) Storiform fibrosis | |
| (4) Abundant (> 10 cells/HPF) IgG4-positive cells | (4) Abundant (> 10 cells/HPF) IgG4-positive cells | |
| Type 2 AIP | ||
| Histology of the pancreas (core biopsy/resection) | IDCP | |
| Both of the following: | Both of the following: | |
| (1) GEL with or without granulocytic acinar inflammation (2) Absent or scant (0-10 cells/HPF) IgG4-positive cells | (1) Granulocytic and lymphoplasmacytic acinar infiltrate (2) Absent or scant (0-10 cells/HPF) IgG4-positive cells |
AIP: Autoimmune pancreatitis; LPSP: Lymphoplasmacytic sclerosign pancreatitis; IDCP: Idiopathic duct-centric pancreatitis; GEL: Granulocytic infiltration of the duct wall; IgG4: Immunoglobulin G4; HPF: High power field.
EUS-FNA
After receiving written informed consent, the patients were submitted to conscious sedation with intravenous diazepam under appropriate cardiorespiratory monitoring. EUS-FNA was performed by expert endosonographers with experience of more than five thousand EUS cases. The apparatus used was a convex-type EUS, GF-UCT 240 (OLYMPUS Co., Ltd., Tokyo, Japan) and Prosound α10 (ALOKA Co., Ltd., Tokyo, Japan) with a frequency of 7.5 MHz. The needle used for EUS-FNA was a disposable 22-gauge needle (EZ shot; OLYMPUS Co., Ltd., Tokyo, Japan). After detailed evaluation of the pancreas with the B-mode and confirmation that no vessels were present in the puncture route in the color Doppler mode, EUS-FNA was performed from the stomach to puncture the pancreatic body or tail and from the duodenum to puncture the pancreatic head.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, United States). The χ2 test and Fisher’s exact test were used to compare categorical parameters between the groups. Continuous parameters were presented as the mean ± SD and/or median (range), and Student’s t test was used. A P value of less than 0.05 was considered statistically significant.
RESULTS
EUS-FNA
The number of FNA passes ranged from 1 to 4 with a mean of 2.00 ± 0.48. One pass included approximately 15 to 20 back-and-forth movements in the target lesions. Adequate sample material was obtained from 43 of 47 patients who underwent EUS-FNA as well as 17 of 21 cases from the pancreatic head and all 26 cases from the body and tail. Sixteen of 47 EUS-FNA specimens showed lymphoplasmacytic infiltration, and 34 showed storiform fibrosis, but obliterative phlebitis could not be detected in any of the cases. Abundant IgG4-positive plasmacyte infiltration was shown in 10 of 28 patients who underwent immunostaining. Although GEL was not detected in any of the cases, three cases showed granulocytic acinar infiltrate. No significance was seen in the results of EUS-FNA between those performed at the pancreatic head and those obtained at the body-tail. There were no complications from EUS-FNA (Table 2).
Table 2.
Results of endoscopic ultrasound-guided fine needle aspiration specimen n (%)
| Pancreatic head (n = 21) | Pancreatic body-tail (n = 26) | Total (n = 47) | P value | |
| Average number of FNA passes | 2.00 ± 0.43 | 2.04 ± 0.514 | 2.02 ± 0.48 | 0.78 |
| (1-3) | (1-4) | (1-4) | ||
| Adequate sample material | 17 (80.9) | 26 (100) | 43 (91.4) | 0.07 |
| Lymphoplasmacytic infiltration | 6 (28.6) | 10 (38.4) | 16 (34.0) | 0.68 |
| Storiform fibrosis | 12 (57.1) | 22 (84.6) | 34 (72.3) | 0.07 |
| Obliterative phlebitis | 0 (0) | 0 (0) | 0 (0) | 1 |
| Abundant IgG4-positive plasmacyte infiltration | 3/10 (30) | 7/18 (38.8) | 10/28 (35.7) | 1 |
| Granulocytic infiltration of duct wall | 0 (0) | 0 (0) | 0 (0) | 1 |
| Granulocytic acinar infiltrate | 1 (4) | 2 (7.7) | 3 (6.3) | 1 |
| Complications | 0 (0) | 0 (0) | 0 (0) | 1 |
IgG4: Immunoglobulin G4; FNA: Fine needle aspiration.
On comparing the histological results of EUS-FNA against ICDC (Figure 1), 9 of 47 patients met level 1 findings of LPSP (Figure 2), and 5 patients met level 2 findings of LPSP. Two of 5 patients who met level 2 findings of LPSP were seronegative and without OOI and were finally diagnosed with “definitive type 1 AIP” after considering both the level 2 histological findings and response to steroids (Table 3). No one met level 1 findings of IDCP (GEL), but 3 patients met level 2 findings of IDCP. All 3 patients were relatively young, seronegative, and had no OOI, including IBD. They were diagnosed with “probable type 2 AIP” (Figure 3) after considering the level 2 histological findings and response to steroids. They have shown improvement without relapse on radiological findings following steroid therapy thus far (Table 4).
Figure 1.
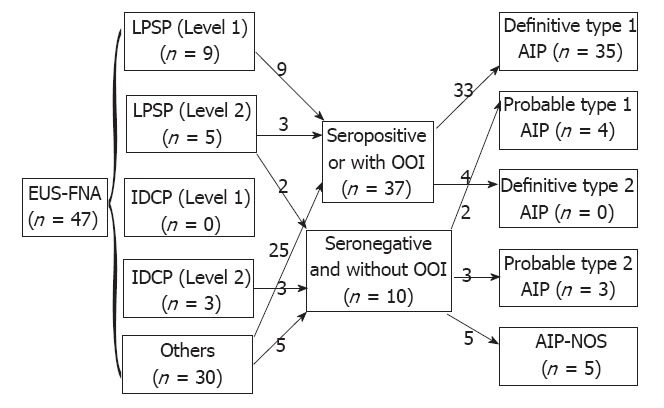
Comparison of endoscopic ultrasound-guided fine needle aspiration with International Consensus Diagnostic Criteria. EUS-FNA: Endoscopic ultra-sound-guided fine needle aspiration; LPSP: Lymphoplasmacytic sclerosing pancreatitis; IDCP: Idiopathic duct-centric pancreatitis; OOI: Other organ involvement; AIP: Autoimmune pancreatitis; NOS: Not otherwise specified.
Figure 2.
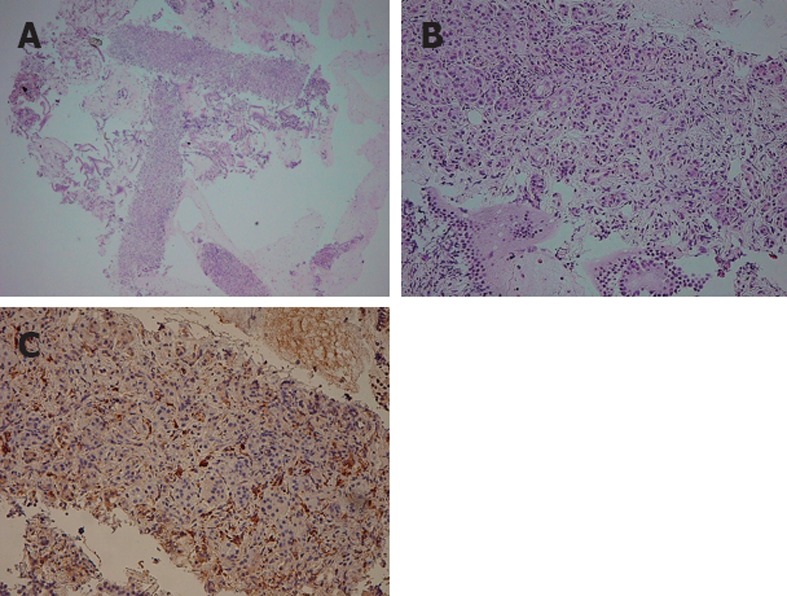
Endoscopic ultrasound-guided fine needle aspiration specimen of "definitive type 1 autoimmune pancreatitis”. A, B: Hematoxylin and eosin staining of a resected pancreas specimen obtained by endoscopic ultra-sound-guided fine needle aspiration shows replacement of the acinar structure by lymphoplasmacytic infiltration and fibrosis; C: Numerous plasma cells show positive immunoreactivity for immunoglobulin G4.
Table 3.
Patients with level 2 histological findings of lymphoplasmacytic sclerosing pancreatitis
| Case | Sex | Age, yr | IgG4 (mg/dL) | Location | Response to steroid | OOI | Diagnosis |
| 1 | Male | 74 | 263 | Diffuse | (+) | Nephritis | Definitive type 1 AIP |
| 2 | Female | 71 | 364 | Diffuse | (+) | Cholangitis | Definitive type 1 AIP |
| 3 | Male | 54 | 230 | Focal | (+) | Sialadenitis | Definitive type 1 AIP |
| 4 | Male | 47 | 104 | Focal | (+) | None | Definitive type 1 AIP |
| 5 | Male | 57 | 46 | Focal | (+) | None | Definitive type 1 AIP |
IgG4: Immunoglobulin G4; OOI: Other organ involvement; AIP: Autoimmune pancreatitis.
Figure 3.
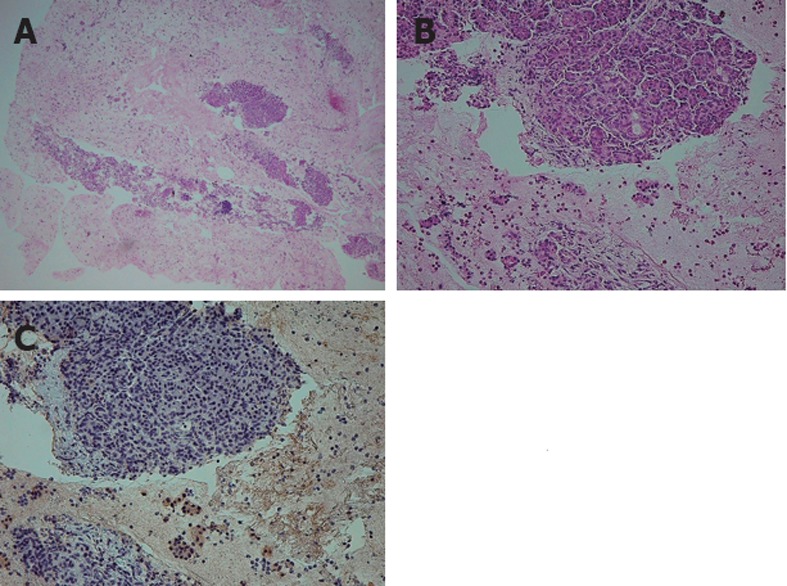
Endoscopic ultrasound-guided fine needle aspiration specimen of "probable type 2 autoimmune pancreatitis”. A, B: Hematoxylin and eosin staining of a resected pancreas specimen obtained by endoscopic ultra-sound-guided fine needle aspiration shows the infiltration of neutrophils in addition to lymphocyte infiltration and fibrosis; C: Immunostaining for immunoglobulin G4 is negative.
Table 4.
Patients with level 2 histological findings of idiopathic duct-centric pancreatitis
| Case | Sex | Age, yr | IgG4 (mg/dL) | Location | Response to steroid | OOI | Follow-up, d | Relapse | Diagnosis |
| 1 | Male | 28 | 69 | Diffuse | (+) | (-) | 973 | (-) | Probable type 2 AIP |
| 2 | Fe-male | 31 | 43 | Diffuse | (+) | (-) | 425 | (-) | Probable type 2 AIP |
| 3 | Male | 30 | 23 | Focal | (+) | (-) | 120 | (-) | Probable type 2 AIP |
IgG4: Immunoglobulin G4; OOI: Other organ involvement; AIP: Autoimmune pancreatitis.
DISCUSSION
EUS-FNA is an established and widely used technique to evaluate pancreatic masses. The diagnostic accuracy of EUS-FNA for pancreatic cancer is reported to be between 60% and 90%[26-28], but conclusive diagnosis of AIP is often difficult due to the small size of specimens obtained by FNA. Recently, there have been several reports on the usefulness of EUS-guided tru-cut biopsy (EUS-TCB) for the diagnosis of AIP[29-31]. Tru-cut biopsy needles have been developed to acquire samples while preserving tissue architecture, thus allowing histological examination[32,33]. Previous reports describe the safety and the technical feasibility of performing EUS-TCB from a transgastric approach. However, the TCB device may not function properly when used in the second portion of the duodenum, and there is also some difficulty when using the TCB device from the duodenal bulb and along the greater curvature of the antrum[29,34]. Moreover, because a 19-gauge needle is used for EUS-TCB, the risk of bleeding is higher compared with EUS-FNA using a 22-gauge needle, indicating that reexamination of safety is required. We previously reported[35,36] the feasibility of EUS-FNA using a 22-gauge needle for the histological evaluation of gastrointestinal submucosal tumors, and we believe that this method can also be applied to pancreatic lesions. In our study, adequate material for differentiating cancer from AIP was obtained in 43 of 47 cases (91.4%), and no significant difference in EUS-FNA results was seen between those obtained from the pancreatic head and body-tail. Nine of 47 patients (19.1%) met 3 of 4 characteristic features of LPSP and were diagnosed with “definitive type 1 AIP” based on histological findings alone. Detailed analysis of 8 patients who showed level 2 histological findings of type 1 or type 2 AIP revealed that 3 patients with level 2 findings of type 1 were seropositive and/or with OOI and could be diagnosed with “definitive type 1 AIP” without histological findings, but the other 5 patients were seronegative and without OOI and diagnosed with “definitive type 1 AIP” or “probable type 2 AIP” based on combination of the level 2 histological findings and the response to steroid treatment. Therefore, out of 10 seronegative cases, 2 cases were diagnosed with “definitive type 1 AIP”, and 3 cases were diagnosed with “probable type 2 AIP” using the histological findings of EUS-FNA. As mentioned earlier, type 1 AIP often can be diagnosed without histology, but it is difficult to differentiate type 1 and type 2 AIP when results are seronegative and without OOI. We believe histological evaluation of EUS-FNA is rather important in such cases.
In conclusion, EUS-FNA is useful in diagnosing AIP even when performed from the pancreatic head and may also provide complementary histological information to distinguish type 1 and type 2 AIP, particularly in seronegative cases.
COMMENTS
Background
Recently, the International Consensus Diagnostic Criteria (ICDC) for autoimmune pancreatitis (AIP) was proposed. ICDC made separate diagnostic criteria for type 1 and type 2 AIP, and histological differentiation is becoming more important for diagnosing AIP. There have been reports on the usefulness of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in the diagnosis of AIP but only negative reports on the differentiation between type 1 and type 2 AIP using specimens obtained by EUS-FNA.
Research frontiers
In the area of AIP, the research hotspot is how to obtain sufficient materials from AIP patients and differentiate type 1 and type 2 AIP correctly.
Innovations and breakthroughs
Adequate specimens including pancreatic tissue for differentiating AIP from cancer were obtained from 43 of 47 patients who underwent EUS-FNA. EUS-FNA was performed from the pancreatic head in 21 cases, which is known to be technically difficult when performed by core biopsy; there was no significant difference in the results compared with pancreatic body-tail. Of 10 seronegative cases, 2 cases were diagnosed with “definitive type 1 AIP,” and 3 cases were diagnosed with “probable type 2 AIP” when considering both the level 2 histological findings and response to steroids.
Applications
The study results suggested that EUS-FNA (instead of core biopsy) was useful in diagnosing AIP even when performed from the pancreatic head and may also provide complementary histological information to distinguish type 1 and type 2 AIP, particularly in seronegative cases.
Terminology
Type 1 and type 2 AIP: The histological substance of type 1 AIP is known as lymphoplasmacytic sclerosing pancreatitis, and type 2 AIP is characterized by a distinct histology called idiopathic duct centric pancreatitis. Type 2 AIP patients are generally seronegative and lack other organ involvement (OOI) in contrast to type 1 AIP. However, the absence of serological abnormalities or lack of OOI in patients with AIP does not necessarily imply the diagnosis of type 2, as type 1 also can be seronegative and without OOI.
Peer review
The authors reported the usefulness of EUS-FNA in the diagnosis of type 1 and type 2 AIP and also stressed the importance of this method for the differential diagnosis between AIP and pancreatic cancer especially in the cases with negative results of serology and absence of other organ involvement. The content is clear and the discussion is straightforward. This paper is useful for understanding the ICDC and the classification of type 1 and 2 AIP.
Footnotes
Supported by The Research Committee of Intractable Pancreatic Diseases provided by the Ministry of Health, Labour, and Welfare of Japan
Peer reviewer: Tooru Shimosegawa, Professor, Gastroenter-ology, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY
References
- 1.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 2.Maire F, Le Baleur Y, Rebours V, Vullierme MP, Couvelard A, Voitot H, Sauvanet A, Hentic O, Lévy P, Ruszniewski P, et al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol. 2011;106:151–156. doi: 10.1038/ajg.2010.314. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809–814. doi: 10.1097/MPA.0b013e3182258a15. [DOI] [PubMed] [Google Scholar]
- 5.Zen Y, Bogdanos DP, Kawa S. Type 1 autoimmune pancreatitis. Orphanet J Rare Dis. 2011;6:82. doi: 10.1186/1750-1172-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016; quiz 934. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010;45:787–793. doi: 10.1007/s00535-010-0265-x. [DOI] [PubMed] [Google Scholar]
- 9.Kamisawa T, Okamoto A. Prognosis of autoimmune pancreatitis. J Gastroenterol. 2007;42 Suppl 18:59–62. doi: 10.1007/s00535-007-2052-x. [DOI] [PubMed] [Google Scholar]
- 10.Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis. Gut. 2009;58:1680–1689. doi: 10.1136/gut.2008.155853. [DOI] [PubMed] [Google Scholar]
- 11.Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013–1023. doi: 10.1007/s00535-005-1717-6. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–292. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer. 2002;96:174–180. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande V, Mino-Kenudson M, Brugge WR, Pitman MB, Fernandez-del Castillo C, Warshaw AL, Lauwers GY. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464–1471. doi: 10.1097/01.pas.0000173656.49557.48. [DOI] [PubMed] [Google Scholar]
- 16.Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Pantazopoulou A, Tsiotos G. EUS-FNA contribution in the identification of autoimmune pancreatitis: a case report. JOP. 2007;8:598–604. [PubMed] [Google Scholar]
- 17.Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869–874. doi: 10.1016/j.dld.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 19.Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549–554. doi: 10.1097/MPA.0b013e3181e4d9e5. [DOI] [PubMed] [Google Scholar]
- 20.Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF, Conlon K. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129–137; discussion 137-139. doi: 10.1016/s1091-255x(02)00148-8. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 22.de Castro SM, de Nes LC, Nio CY, Velseboer DC, ten Kate FJ, Busch OR, van Gulik TM, Gouma DJ. Incidence and characteristics of chronic and lymphoplasmacytic sclerosing pancreatitis in patients scheduled to undergo a pancreatoduodenectomy. HPB (Oxford) 2010;12:15–21. doi: 10.1111/j.1477-2574.2009.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–408. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 24.Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yagioka H, Yashima Y, Sasaki T, Kogure H, Togawa O, et al. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010;71:85–90. doi: 10.1016/j.gie.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, Yamanaka N, Inoue D, Yamamoto M, Takahashi H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615–626. doi: 10.1007/s10157-011-0521-2. [DOI] [PubMed] [Google Scholar]
- 26.Savides TJ, Donohue M, Hunt G, Al-Haddad M, Aslanian H, Ben-Menachem T, Chen VK, Coyle W, Deutsch J, DeWitt J, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277–282. doi: 10.1016/j.gie.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243–250. doi: 10.1016/s0016-5107(97)70266-9. [DOI] [PubMed] [Google Scholar]
- 28.Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–464. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 29.Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467–472. doi: 10.1016/s0016-5107(04)02802-0. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742–750. doi: 10.1007/s00535-009-0062-6. [DOI] [PubMed] [Google Scholar]
- 31.Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163–166. doi: 10.1159/000104240. [DOI] [PubMed] [Google Scholar]
- 32.Levy MJ, Smyrk TC, Takahashi N, Zhang L, Chari ST. Idiopathic duct-centric pancreatitis: disease description and endoscopic ultrasonography-guided trucut biopsy diagnosis. Pancreatology. 2011;11:76–80. doi: 10.1159/000324189. [DOI] [PubMed] [Google Scholar]
- 33.Wiersema MJ, Levy MJ, Harewood GC, Vazquez-Sequeiros E, Jondal ML, Wiersema LM. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–278. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 34.Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, Kawai T, Moriyasu F. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–366. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 35.Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–755. doi: 10.1055/s-2007-1001416. [DOI] [PubMed] [Google Scholar]
- 36.Ando N, Niwa Y, Ohmiya N, Ito B, Sasaki Y, Goto H. Simultaneous multiple early cancers of esophagus and stomach treated by endoscopic mucosal resection. Endoscopy. 2002;34:667–669. doi: 10.1055/s-2002-33240. [DOI] [PubMed] [Google Scholar]


