Abstract
AIM: To use leptin-deficient (ob/ob) mice with demonstrated differences in steatosis levels to test a new diagnostic method using the acoustical structure quantification (ASQ) mode and the associated analytical parameter, “focal disturbance ratio” (FD-ratio).
METHODS: Nine ob/ob mice, at 5, 8, and 12 wk of age (n = 3 in each age group), were used as models for hepatic steatosis. Echo signals obtained from ultrasonography in the mice were analyzed by ASQ, which uses a statistical analysis of echo amplitude to estimate inhomogeneity in the diagnostic region. FD-ratio, as calculated from this analysis, was the focus of the present study. FD-ratio and fat droplet areas and sizes were compared between age groups.
RESULTS: No fibrosis or inflammation was observed in any of the groups. The fat droplet area significantly (P < 0.01) increased with age from 1.25% ± 0.28% at 5 wk to 31.07% ± 0.48% at 8 wk to 51.69% ± 3.19% at 12 wk. The median fat droplet size also significantly (P < 0.01) increased with age, from 1.33 (0.55-10.52) μm at 5 wk, 2.82 (0.61-44.13) μm at 8 wk and 6.34 (0.66-81.83) μm at 12 wk. The mean FD-ratio was 0.42 ± 0.11 at 5 wk, 0.11 ± 0.05 at 8 wk, and 0.03 ± 0.02 at 12 wk. The FD-ratio was significantly lower at 12 wk than at 5 wk and 8 wk (P < 0.01). A significant negative correlation was observed between the FD-ratio and either the fat droplet area (r = -0.7211, P = 0.0017) or fat droplet size (r = -0.9811, P = 0.0052).
CONCLUSION: This tool for statistical analysis of signals from ultrasonography using the FD-ratio can be used to accurately quantify fat in vivo in an animal model of hepatic steatosis, and may serve as a quantitative biomarker of hepatic steatosis.
Keywords: Non-alcoholic fatty liver disease, Quantitation of hepatic steatosis, Animal model, Focal disturbance ratio, Acoustic structure quantification, Ultrasonography
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a clinically important disease that occurs in subjects with underlying conditions such as obesity or insulin resistance, and is frequently accompanied by metabolic syndrome, including diabetes, hyperlipidemia and/or hypertension[1-5]. Non-alcoholic steatohepatitis (NASH) is the most extreme form of NAFLD, and is regarded as a major cause of cirrhosis of the liver of unknown cause[6-10]. Methods for early detection and assessment of NAFLD through quantitative measurement of steatosis are needed to achieve earlier intervention and avoid the progression to cirrhosis.
The gold standard for quantitative assessment of steatosis has been considered to be liver histology[11]. However, liver biopsy shows various limitations, such as potential sampling error, difficulties repeating the procedure because of ethical concerns, and complications including bleeding[12]. Non-invasive alternatives to liver biopsy thus need to be established.
Several methods have recently been established for non-invasively quantifying steatosis using imaging techniques. Non-invasive modalities such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) have been employed for the assessment of hepatic steatosis[13-15]. However, using these modalities for the repeated evaluation of NAFLD is difficult, because CT involves radiation exposure and MRI is expensive to perform. From these perspectives, ultrasonography represents an excellent examination modality that is minimally invasive, inexpensive, and can be performed repeatedly with no risk to the patient. Furthermore, ultrasonography is known to be highly sensitive for detecting fat accumulation in the liver[16]. However, the diagnostic performance of ultrasonography depends on the empirical and qualitative reading skills of the examiner, and quantitative methods to evaluate liver lipogenesis have yet to be established.
In recent years, Yamaguchi et al[17,18] have reported that diffuse pathological changes in the liver tissue can be quantitatively evaluated based on the statistical deviation of ultrasound signals compared to normal liver. Generally, ultrasonographic images of parenchymal organs such as the liver are designated as having a “speckle pattern” consisting of numerous fine echo spots. The speckle pattern is constructed by ultrasonic interference of scattered ultrasound waves generated by innumerable reflexive objects that are distributed closer than the ultrasonic wavelength[17-19]. Image analysis of speckle patterns has been used to identify tissue characteristics associated with chronic liver diseases, because the pattern changes according to the structural characteristics of the medium. One such analytical method, the probability density function (PDF) of the echo amplitude of a speckle pattern, has been reported to be approximated by a function called the Reyleigh distribution. Moreover, Toyoda et al[20] proposed the acoustical structure quantification (ASQ) method and reported the possibility of quantifying diffuse liver disease or monitoring regression/progression in cases of liver fibrosis and during treatment. However, no reports have yet described assessments of hepatic steatosis in NAFLD patients using this tool for the statistical analysis of ultrasonic signals.
The present study aimed to validate a quantitative imaging technique used to detect and measure steatosis with statistical information from ultrasound echo signals with the focal disturbance ratio (FD-ratio) as a parameter in leptin-deficient (ob/ob) mice, a pure NAFLD model.
MATERIALS AND METHODS
This study was a collaborative effort between Iwate Medical University and Toshiba Medical Systems. However, no direct financial support was received from Toshiba Medical Systems for this study.
Animals
The animal research protocols for this prospective study were approved by our institutional research animal resource center. Nine 5 wk old (at the start of the study) male ob/ob mice were purchased from Charles River Laboratories (Yokohama, Japan) and maintained on conventional food and water throughout the experiment. These mice were divided into 3 groups (n = 3 each) that underwent the experiment described below at 5, 8 and 12 wk old, respectively.
General anesthesia was induced in mice by intraperitoneal administration of 40-50 mg/kg pentobarbital sodium (Ovation Pharmaceutical, Deerfield, IL), and underwent laparotomic ultrasonography, prior to having the liver extracted for histological examination.
Ultrasonographic imaging
An AplioXG ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) was combined with a 12 MHz linear transducer (PLT-1204BT) for ultrasonographic investigations in this study. The scan mode was harmonic B-mode imaging (T: 6.0/R: 12.0 MHz). Display depth and transmit focus were fixed at 15 mm and 7.5 mm, respectively, and cross-sectional images of the hepatic parenchyma were recorded digitally with raw data, consisting substantially of the linear amplitudes without any cosmetic image processing. Raw data were uploaded to a personal computer in the DICOM format. FD-ratio was determined using ASQ software (details described in the next section). A region of interest (ROI) was set to a fixed depth of 2.5 mm to the liver surface (Figure 1). FD-ratio was measured 10 times in succession, and the mean value (after excluding outliers) was used as the final result. In parallel, mean echo intensity of the hepatic parenchyma was measured using Image-J image analysis software (NIH, United States).
Figure 1.

Acoustic structure quantification imaging. In parametric imaging, the intensity distribution can be visualized and displayed on a split screen by color coding. The large-region of interest was set at a fixed depth of 2.5 mm from the liver surface.
Analytical method
The principles of the ASQ method[20] are as follows. When echo signals are generated from very small, dense scatters located beyond the limit of spatial resolution, the pattern of the ultrasound image is constructed based on the interference of the sound waves (speckle noise). In that case, the PDF of the echo amplitude can be approximated using the Rayleigh distribution function[21]. In normal liver parenchyma, such statistical results are not described by the Rayleigh distribution due to the existence of structures such as vessel walls. Results for livers with either nodules or fibrosis, i.e., liver cirrhosis, are even less similar to the Rayleigh distribution. We hypothesized that in the case of progression of fat drops, these scatters would generate wave interference or mask the original small structures, which would change the PDF to more closely resemble a Rayleigh distribution.
Once the examiner sets a comprehensive ROI (hereinafter referred to as a large-ROI) on the image, several hundred small ROIs (small-ROIs hereinafter) are automatically set therein to calculate the PDF (Figure 2A). The essential parameter, Cm2, in the analysis is defined by the equation:
Figure 2.
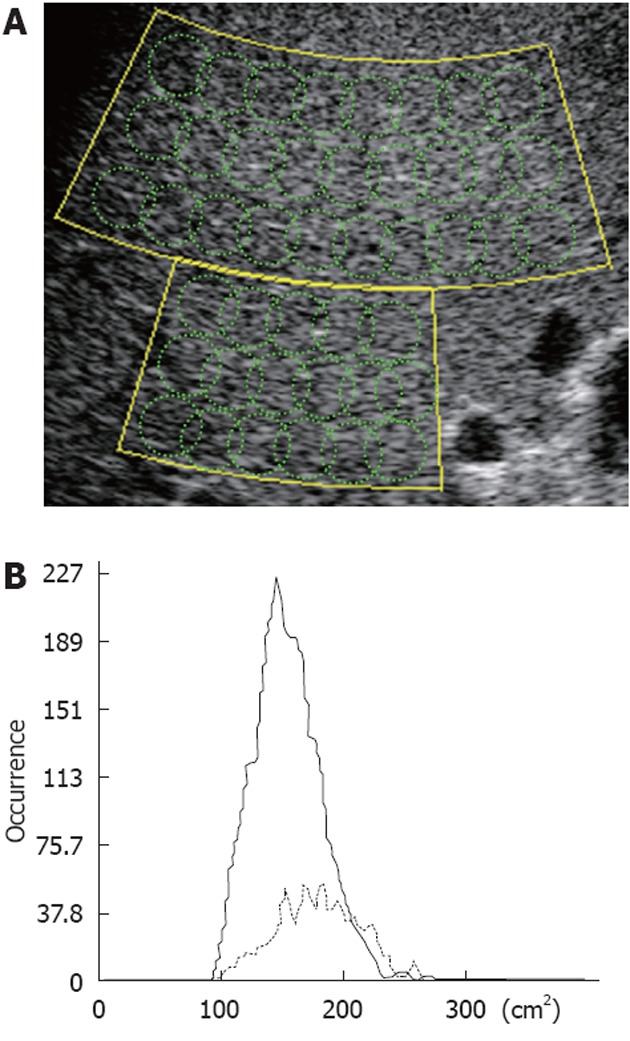
Schematic of region of interests used for statistical analysis of the radio frequency signal with the acoustic structure quantification method, and the Cm2-histogram. A: The set large-region of interest (ROI) actually consists of several hundred small ROIs used to calculate multiple Cm2 (intensity or amplitude) values; B: Results are shown as the occurrence in the Cm2 histogram.
where μ and σ2 are the average and variance of the echo amplitude in a small-ROI, respectively. The σR2 (μ) is a variant if the Rayleigh distribution is estimated from the measured average. Multiple results for small-ROIs in a large-ROI are displayed as an occurrence histogram of C2 (real line in Figure 2B). If samples consist of speckle noise, the C2 histogram will gather to 100 with narrow variance, while structural information will make the average value larger and variance wider.
FD-ratio is calculated in the following manner. First, Cm2 is defined as:
where σm is the variance calculated from limited samples less than μ + 4σ. If the ratio C2/Cm2 is larger than the threshold α, the result of Cm2 is eliminated from the histogram (real line), but added to the alternative histogram (dotted line). The FD-ratio is the ratio of the area under the curve (AUC) for these two histograms: RFD = [AUC (real)]/[AUC (dotted)].
Here, the threshold α was set to 1.2, so RFD = 0 when the samples show a Rayleigh distribution for the PDF, and has a positive value in the presence of tiny structural changes.
The ASQ software also has an imaging function that reconstructs the 2-dimensional color map of the C2 measured for each position (parametric image).
Histopathological analysis
Histopathological examinations were performed by an experienced pathologist certified by the Japanese Society of Pathology. Images from liver biopsy samples were recorded using the JPEG format. Using Image-J software, the percentage fat area was calculated from the ratio between total fat tissue area and total specimen area, and the mean value was used to denote the fat droplet area. In addition, again using Image-J software, the maximum diameter of fat droplets in 8 and 12 wk old mice was measured, and median values were used to denote the size of fat droplets. The fat droplet area and size obtained in this manner were compared with the FD-ratio.
Statistical analysis
Values are shown as mean ± SD, or median (range) according to the distribution of values. Stat View software (version 5.0; SAS Institute, Cary, NC, United States) was used for all statistical analysis. The Spearman rank-order correlation test was used to study correlations between two variables, with a significant correlation considered to exist for values of P < 0.05, and for correlation coefficient r ≥ 0.40. The Tukey-Kramer method was used for multiple comparison tests, and values of P < 0.05 were considered to indicate a significant difference.
RESULTS
Comparison of histological findings and liver echogenicity
No fibrosis or inflammation was observed in any groups. Large fat droplets were mainly observed at 12 wk (Figure 3). Fat droplet area increased significantly (P < 0.01 each) with age from 1.25% ± 0.28% at 5 wk to 31.07% ± 0.48% at 8 wk, and to 51.69% ± 3.19% at 12 wk. Median fat droplet size also increased significantly (P < 0.01 each) with age, from 1.33 μm (range: 0.55-10.52 μm) at 5 wk to 2.82 μm (range: 0.61-44.13 μm) at 8 wk and 6.34 μm (range: 0.66-81.83 μm) at 12 wk (Figure 4). Mean Gray values in each group were 65.31 ± 22.52 at 5 wk, 65.95 ± 19.41 at 8 wk and 91.32 ± 21.83 at 12 wk (Figure 5). Although no differences were observed between the 5 and 8 wk old groups, mean gray value was significantly elevated in the 12 wk old group, with an increase observed in the brightness of the hepatic parenchyma.
Figure 3.
Representative histological findings, B-mode images and acoustic structure quantification-mode images for each group. No fibrosis or inflammation was observed in any groups. Large fat droplets were mainly observed at 12 wk old. In parametric imaging, red and green existed together at 5 wk, and red decreased and green was more abundant at 12 wk. ASQ: Acoustical structure quantification.
Figure 4.
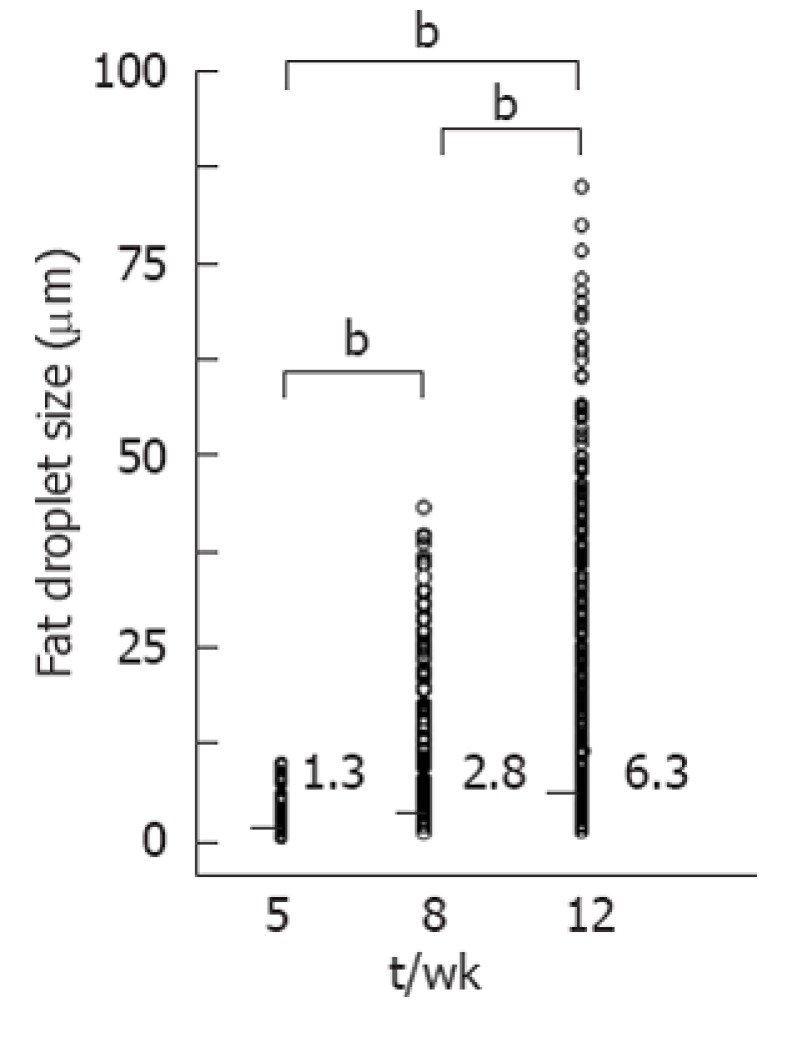
Comparison of fat droplet size. Median fat droplet size increased significantly (bP < 0.01) with age, from 1.33 μm (range: 0.55-10.52 μm) at 5 wk, to 2.82 μm (range: 0.61-44.13 μm) at 8 wk and 6.34 μm (range: 0.66-81.83 μm) at 12 wk.
Figure 5.

Comparison of liver echogenicity. Mean gray values in each group were 65.31 ± 22.52 at 5 wk, 65.95 ± 19.41 at 8 wk, and 91.32 ± 21.83 at 12 wk. Although no difference was apparent between the 5 and 8 wk old groups, mean gray value was significantly elevated in the 12 wk old group, with increased brightness of the hepatic parenchyma. bP < 0.01. NS: Not significant.
Relationship between FD-ratio and fat droplet area or size
Mean FD-ratio was 0.42 ± 0.11 at 5 wk, 0.11 ± 0.05 at 8 wk, and 0.03 ± 0.02 at 12 wk. The FD-ratio was significantly lower at 12 wk than at 5 or 8 wk (P < 0.01 each). In parametric imaging, red and green existed together at 5 wk, and the red had decreased and green was more abundant at 12 wk (Figure 3). A significant negative correlation was observed between FD-ratio and both fat droplet area (r = -0.7211, P = 0.0017) (Figure 6A) and fat droplet size (r = -0.9811, P = 0.0052) (Figure 6B).
Figure 6.

Relationship between “focal disturbance ratio” and fat droplet area or size of fat droplets. A: Significant negative correlations were observed between focal disturbance ratio (FD-ratio) and either fat droplet area (r = -0.7211, P = 0.0017); B: Fat droplet size (r = -0.9811, P = 0.0052).
DISCUSSION
The results of this study demonstrated a close correlation between FD-ratio and the degree of histologically evaluated fat accumulation in the liver. These data suggest that ASQ analysis of liver ultrasonography and its representative parameter, FD-ratio, may offer a reliable new clinical modality for the assessment of liver steatosis.
With regard to the ultrasonographic diagnosis of fatty liver disease, Joseph et al[16] advocated the bright liver pattern, and attempts to quantify the hepatosplenic contrast or hepatorenal contrast were subsequently proposed[22-24]. Although findings of a bright liver pattern and hepatorenal contrast are widely accepted as sensitive, reliable findings for the presence of fatty liver disease, no quantitative method to evaluate the severity of hepatic fat accumulation has previously been established. The difficulties in ultrasonographic diagnosis may originate from the fact that image information obtained by conventional ultrasonography lacks an objective or quantitative nature, unlike the X-ray absorbance in CT. In contrast, ASQ analysis generate objective data and provide a quantitative assessment of liver histology with respect to fat accumulation. Taking into account the non-invasive and inexpensive nature of ultrasonography, ASQ analysis could become a mainstay in the diagnosis of fatty liver disease and evaluations of disease severity and response to treatment. In addition, these studies of tissue characterization using ASQ analysis could lead to the development of methods for the quantitative diagnosis of other diffuse liver diseases, including fibrosis or inflammation, thus decreasing the need for liver biopsies.
With regard to the ASQ method, procedures are undertaken to analyze the RF signals for each of a large number of small-ROIs set up within a large ROI, for the purpose of improving analytical precision[17-19]. The assumption is that in small-ROIs with a high degree of deviation from the Rayleigh distribution, the strength of the signals contained therein would be non-homogeneous. We also assumed the presence of two kinds of inhomogeneous samples, i.e., diffuse inhomogeneity and focal inhomogeneity, and by placing our focus on the small-ROIs with a high degree of deviation from the Rayleigh distribution resulting from a focally inhomogeneous structure, we established FD-ratio as a parameter.
Conversely, a bright liver pattern and vascular blurring are observed in fatty livers, due to reflection and scattering of the ultrasound waves and physical pressure on small blood vessels by ballooning hepatocytes induced by the fat droplets. We predicted that, due to the large number of fat droplets assembled densely (and thereby enveloping structures such as small blood vessels and bile ducts), the brightness of the hepatic parenchyma would be increased, and hepatic vein walls would become blurred, thus resulting in homogenization of the signal strength in each small-ROI and a decrease in the number of focally inhomogeneous small-ROIs. As a result, we believed that, as the area and diameter of fat droplets increased, the FD-ratio would decrease. Interestingly, no significant difference was seen in the brightness of hepatic parenchyma between 5 and 8 wk old mice, while FD-ratios were lowest in 8 wk old mice. We can therefore infer that a tool for the statistical analysis of ultrasonic signals may detect, beyond a qualitatively oriented reading ability, small changes in the speckle pattern caused by steatosis.
In conclusion, a novel tool for the statistical analysis of ultrasonic signals using FD-ratio as a parameter would be useful for the quantitative evaluation of liver steatosis. FD-ratio can therefore be used as a non-invasive biological marker for the early detection and quantitative evaluation of hepatic steatosis.
ACKNOWLEDGMENTS
We wish to thank Ms. Yuriko Mikami for her invaluable technical assistance with ultrasonography.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease. The gold standard for quantitative assessment of steatosis has been considered to be liver histology. However, liver biopsy shows various limitations and repeating the procedure is difficult because of ethical concerns. Non-invasive alternatives to liver biopsy are thus needed.
Research frontiers
Non-invasive modalities such as ultrasonography, computed tomography, and magnetic resonance imaging have been employed for the assessment of hepatic steatosis. Researchers have recently reported that diffuse pathological changes in liver tissue can be quantitatively evaluated as the statistical deviation of ultrasound signals compared to normal liver. However, no reports have described the assessment of hepatic steatosis in NAFLD patients using this tool for the statistical analysis of ultrasonic signals.
Innovations and breakthroughs
This study validated a quantitative imaging technique used to detect and measure hepatic steatosis with statistical information from ultrasound echo signals using focal disturbance ratio (FD-ratio) as a parameter in an animal model.
Applications
FD-ratio can be used as a non-invasive biological marker for the early detection and quantitative evaluation of hepatic steatosis.
Peer review
The authors present some pilot data from their research on the diagnostic utility of a new ultrasound technique in an animal model of fatty liver.
Footnotes
Peer reviewer: Ilker Tasci, MD, Associate Professor, Department of Internal Medicine, Gulhane School of Medicine, 06018 Ankara, Turkey
S- Editor Gou SX L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 2.Harrison SA, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2004;8:861–79, ix. doi: 10.1016/j.cld.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861–867. doi: 10.1038/ajg.2009.67. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 7.Cuadrado A, Orive A, García-Suárez C, Domínguez A, Fernández-Escalante JC, Crespo J, Pons-Romero F. Non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma. Obes Surg. 2005;15:442–446. doi: 10.1381/0960892053576596. [DOI] [PubMed] [Google Scholar]
- 8.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 10.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 13.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 16.Joseph AE, Dewbury KC, McGuire PG. Ultrasound in the detection of chronic liver disease (the “bright liver”) Br J Radiol. 1979;52:184–188. doi: 10.1259/0007-1285-52-615-184. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Hachiya H, Kamiyama N, Moriyasu F. Examination of the spatial correlation of statistics information in the ultrasonic echo from diseased liver. Jpn J Appl Phys. 2002;41:3585–3589. [Google Scholar]
- 18.Yamaguchi T, Hachiya H, Kamiyama N, Ikeda K, Moriyasu F. Estimation of characteristic of echo envelope using RF echo signal from the liver. Jpn J Appl Phys. 2001;40:3900–3904. [Google Scholar]
- 19.Kamiyama N, Yamaguchi T, Hachiya H. Tissue characterization using statistical information from ultrasound echo signals. Med Imag Tech. 2003;21:112–116. [Google Scholar]
- 20.Toyoda H, Kumada T, Kamiyama N, Shiraki K, Takase K, Yamaguchi T, Hachiya H. B-mode ultrasound with algorithm based on statistical analysis of signals: evaluation of liver fibrosis in patients with chronic hepatitis C. AJR Am J Roentgenol. 2009;193:1037–1043. doi: 10.2214/AJR.07.4047. [DOI] [PubMed] [Google Scholar]
- 21.Burckhardt CB. Speckle in ultrasound B-mode scans. IEEE Transactions on Sonics and Ultrasonics. 1978;25:1–6. [Google Scholar]
- 22.Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25–29. doi: 10.1002/(SICI)1097-0096(199601)24:1<25::AID-JCU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Lupsor M, Badea R. Imaging diagnosis and quantification of hepatic steatosis: is it an accepted alternative to needle biopsy? Rom J Gastroenterol. 2005;14:419–425. [PubMed] [Google Scholar]
- 24.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]



