Abstract
Virotherapy of cancer exploits the potential of naturally occurring and engineered oncolytic viruses to selectively replicate in and cause cytotoxicity to tumor cells without affecting healthy normal cells. The tumor selectivity of Newcastle disease virus (NDV), a member of the family Paramyxoviridae, depends on the differential type I interferon (IFN) response. Further understanding of the key mechanisms and immune effector molecules involved will aid in augmenting the oncolytic properties of NDV. Here we report on the infection kinetics and innate immune responses to a recombinant LaSota strain of NDV (rLaSota eGFP) in human tumor and normal cells. We observed varying replicative fit and cytotoxicity of rLaSota eGFP depending on the tumor cell type, with severely restricted replication in normal cells. The absence of retinoic acid-inducible gene I (RIG-I), a cytosolic RNA sensor, determined sensitivity to NDV. Productive NDV infection with a moderate IFN-α induction in human multiple myeloma cells suggested a role for IFN-independent mechanisms or lack of type I IFN reinforcement by RIG-I. Proinflammatory cytokines and chemokines were altered differentially in infected normal and tumor cells. Our results suggest that tumor selectivity is dependent on variations in the cellular antiviral response to infection with NDV and RIG-I expression.
Introduction
Cancer remains the scourge of the modern world. In spite of extraordinary technological advances in prognosis and treatment of tumors, high numbers of patients still succumb to the disease, mainly due to treatment resistance and relapse. The latest answer is the integration of alternate treatment methods with conventional therapy (21). Oncolytic virotherapy, which exploits the potential of naturally occurring and genetically engineered viruses to selectively replicate in and cause cytotoxicity to tumor cells, is an attractive adjunct to conventional therapy, with Phase I/II and III clinical trials already in progress (17).
Newcastle disease virus (NDV) is an enveloped negative-sense RNA virus of the family Paramyxoviridae. Clinical trials in humans have shown promising results for certain strains of naturally occurring NDV in tumor therapy (26,34). NDV causes respiratory, neurologic, or enteric disease in birds, but is mostly asymptomatic in humans. Although proved to be safe in high doses in Phase I clinical trials in humans (22), current clinical trials are largely limited to low pathogenic, lentogenic pathotypes like MTH-68/H and HUJ (3,8).
NDV is a broad-spectrum oncolytic agent, but little is known about the effective dose and treatment schedule for different types of tumors. NDV has been reported to induce apoptosis in various tumor cells through intrinsic and extrinsic pathways (6). Further, engineering inherently oncolytic viruses such as NDV to target tumor cells at the cellular and/or molecular level is expected to result in enhanced effectiveness (2,5,33). Direct virus-induced cytolysis may not be the only factor that plays a role in antitumor efficacy. Understanding how the host immune system interacts with viruses to achieve antitumor immunity is important for effective tumor eradication. NDV has a long history as an immune stimulant, inducing a rapid type I interferon (IFN) response in infected and bystander cells (26). Increased knowledge of the key immune effector molecules produced by NDV-infected tumor cells, and the signaling pathways involved in initiating cytokine/chemokine production in tumor cells, will aid in the design of recombinant viruses with enhanced immunogenicity that can help recruit the body's own proinflammatory mechanisms for immune-mediated clearance of altered cells.
We hypothesized that the differential cytotoxicity of NDV to normal versus tumor cells is dependent on the innate immune response of the cell types against NDV. We therefore studied the kinetics of infection and the cellular responses to the lentogenic LaSota strain of NDV, which has been engineered to express enhanced green fluorescent protein (eGFP) in human tumor and normal cells of different tissues of origin. Our results suggest that tumor selectivity and cytotoxicity is dependent on variations in the RIG-I-dependent cellular antiviral response to infection with NDV.
Materials and Methods
Cells and virus
The U87MG human glioblastoma/astrocytoma and Vero cells (ATCC, Manasass, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/mL penicillin, and 0.1 μg/mL streptomycin (Invitrogen, Carlsbad, CA). MDA-MB231 adenocarcinoma and SVHUC-1 uroepithelial normal human cell lines (ATCC) were grown in DMEM/F12 Ham's media with supplements as above (Invitrogen). The RPMI-8226 myeloma cell line was originally obtained from ATCC, and was grown in RPMI-1640 medium (Invitrogen), with supplements as above. The lentogenic NDV LaSota strain was engineered to include an extra cistron-encoding eGFP inserted between the phosphoprotein (P) and matrix (M) gene sequences of the full-length infectious clone as previously described (6). The recombinant virus (rLaSota eGFP) was propagated in the allantoic fluid of 9-day-old embryonated specific-pathogen free chicken eggs, concentrated by ultracentrifugation, and purified on a 20–55% sucrose gradient in a Beckman Coulter Optima L-90K ultracentrifuge.
Fluorescent plaque assay
Fluorescent plaque assays were performed essentially as described elsewhere (24). Cells were seeded overnight (1×106/well) in a 6-well tissue culture-treated plate (Nunc, Roskilde, Denmark), and infected with 0.01 and 1 multiplicity of infection (MOI) of rLaSota eGFP in the presence of 1 μg/mL tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich, St. Louis, MO). Infected cell supernatants were collected at 24, 48, and 72 h post-infection. Serial 10-fold dilutions of infected cell supernatant were overlaid on Vero cell monolayers for 1 h at 37°C. Residual virus in the inoculum was removed and the cells were incubated in medium containing 1% methylcellulose and TPCK-treated trypsin for 120 h. Fluorescent plaques were quantified under the microscope using a UV light source.
Flow cytometry
Cells were infected with 1 and 10 MOI of rLaSota eGFP in the presence of TPCK-treated trypsin. Infected cells were harvested 24 h later (both floating and adherent cells were collected), and washed twice with ice-cold phosphate-buffered saline. Cells were then incubated for 15 min at room temperature in the dark with V450-conjugated annexin V antibody in annexin binding buffer according to the manufacturer's instructions (BD Biosciences, San Jose, CA). Immediately before acquisition, 1 μL of propidium iodide (1 mg/mL; Invitrogen) was added. The percentages of propidium iodide- and V450-positive cells were then quantified in a FACS Aria cytometer using FlowJo software version 9.2. The percentage of eGFP-positive cells was also quantified at 48 h post-infection (PI).
Cytotoxicity assay
Cells (30,000/well) were plated in a 96-well flat-bottom tissue culture-treated plate (Nunc) overnight, and infected with serial 10-fold dilutions of rLaSota eGFP (4 wells/treatment). Percentage cytotoxicity was determined at 72 h PI by WST-1 assay (Roche, Indianapolis, IN), as previously described (6). Percentage viability was normalized to 100% in uninfected cells. The 50% maximal effective concentration (EC50) of virus for each cell line was determined using GraphPad Prism version 5.0.
Real-time RT-PCR
Cells were infected with 1 MOI of rLaSota eGFP and harvested 24 h later in RLT buffer (Qiagen, Valencia, CA). Total RNA was isolated using the RNeasy kit and genomic DNA was removed using DNase1 (Qiagen). Then 1 μg of total RNA was reverse transcribed using random primers and the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). SYBR green real-time RT-PCR using specific primers was performed on a 7300 real-time system (Applied Biosystems), under the following cycling conditions: 95°C for 5 min; 40 cycles of 95°C for 15 sec and 60°C for 1 min, with melt curve data collection. Fold up- and downregulation values were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated using the 2−ΔΔCT formula.
Immunoblot
Cells were infected with 1 MOI of rLaSota eGFP, and at 24 and 48 h PI they were washed with ice-cold PBS and harvested in RIPA lysis buffer (Millipore, Billerica, MA) containing halt protease inhibitor cocktail (Pierce Biotechnology, Rockford, IL). The total protein content of the lysates was quantified with the Micro BCA assay (Pierce Biotechnology). Equal concentrations of protein were loaded on a 10% polyacrylamide gel (Bio-Rad, Hercules, CA), and transferred to a PVDF membrane using the iBlot gel transfer system (Invitrogen). Membranes were blocked in 5% skim milk and probed with antibodies to actin, STAT1, STAT2, and RIGI, and HRP-conjugated species-specific secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were visualized using the one-component TMB substrate (BioFX Laboratories, Owings Mills, MD).
Cytokine estimation
Cells (1×106/well) were infected with 1 MOI of rLaSota eGFP. Cytokine levels in the infected cell supernatants at 24 and 48 h PI were estimated by the human cytometric bead array (BD Biosciences), customized for the following cytokines and chemokines: IL-6, IL-8, IL-10, IL-12p70, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α. Supernatants were also assayed for IFN-α and IFN-β using the Verikine human IFN-α and IFN-β kits, according to the manufacturer's instructions (PBL Interferon Source, Piscataway, NJ). Sensitivity of the IFN-α assay was 12.5 pg/mL and 25 pg/mL for the IFN-β assay.
Statistical analysis
Differences in best-fit curves between normal and tumor cell lines were compared using the F test in GraphPad Prism version 5.0 for the cytotoxicity assay. All other experiments were analyzed by the Student's t-test, using JMP 9 software.
Results
Recombinant LaSota eGFP replicates differentially in normal and tumor cell lines
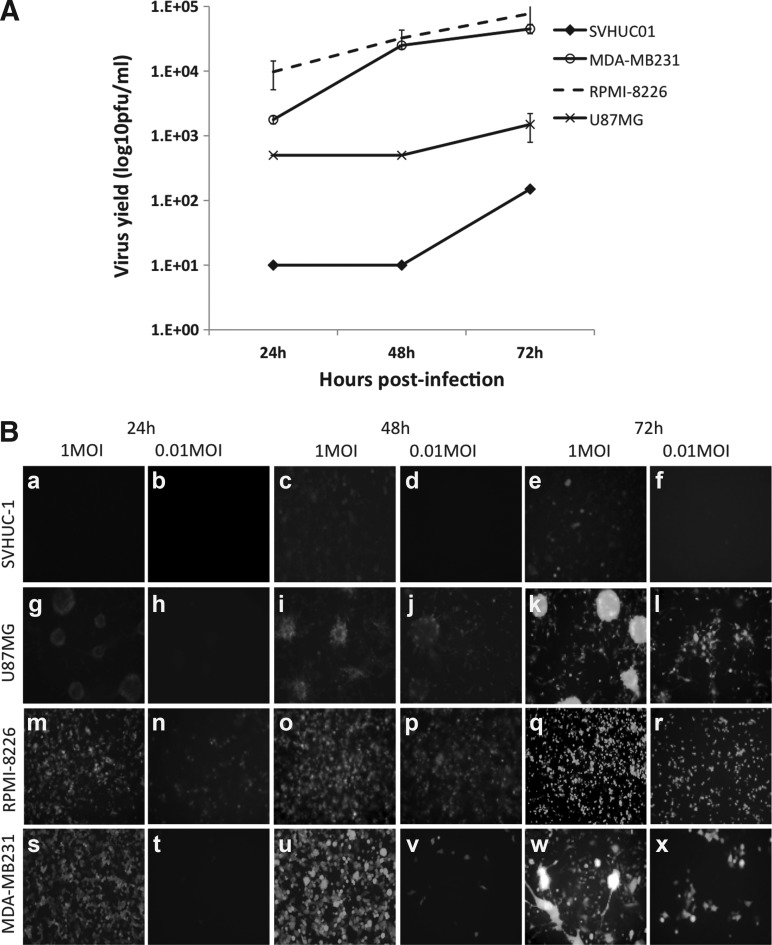
Time course of infection with 0.01 MOI of rLaSota eGFP in human tumor cell lines of different tissue-specific origins and a normal uroepithelial cell line demonstrated a high permissiveness of the RPMI-8226 lymphoblast cell line, with virus yields peaking at 72 h PI (7×105 pfu/mL; Fig. 1A and B). Virus titers observed in virus-infected RPMI-8226 cells were significantly higher than those observed in normal SVHUC-1 cells at all time points tested (p<0.05). High virus yield was also obtained in MDA-MB231 breast adenocarcinoma cells, while the U87MG astroglioma cells showed intermediate virus replication, which was significantly lower than that observed for RPMI-8226 infected cells at 48 and 72 h PI (p<0.05). In the normal human uroepithelial SVHUC-1 cells, the replication of NDV was severely restricted, with very low virus yield (1×102 pfu/mL) at 72 h PI (Fig. 1A and B).
FIG. 1.
Replication kinetics and cytotoxicity of rLaSota eGFP in normal human and tumor cell lines. (A) SVHUC-1 and MDA-MB231, RPMI-8226, U87MG tumor cells were infected with NDV at 0.01 MOI. The supernatant from infected cells at 24, 48, and 72 h were analyzed for virus yield by a fluorescent plaque assay on Vero cells. The plaques were enumerated and virus yield expressed as pfu/mL±SD for each time point. (B) Time course of infection of normal human and tumor cell lines. SVHUC-1 (a–f), U87MG (g–l), RPMI-8226 (m–r), and MDA-MB231 (s–x) cell lines infected with 1 MOI and 0.01 MOI rLaSota eGFP (magnification 10×).
NDV infects normal human cells at a higher MOI without cell death
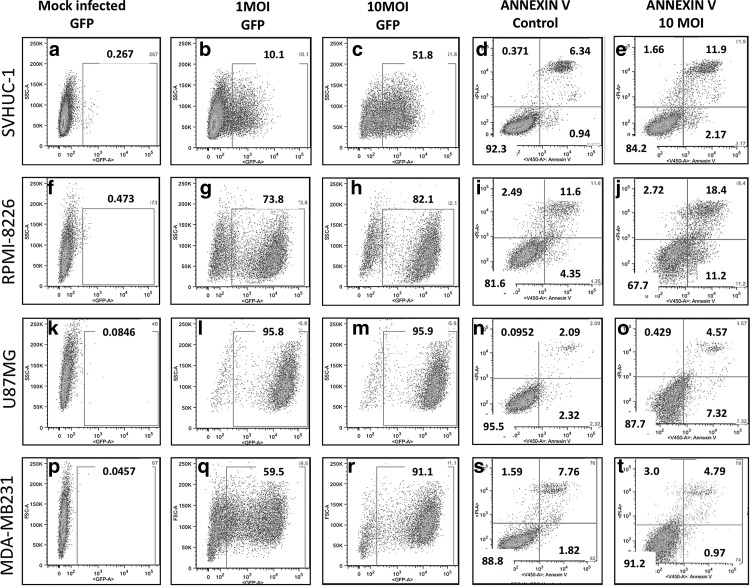
As NDV infection with a low MOI is restricted in normal cells, we questioned whether normal cells could be infected at a higher MOI. To confirm this, we infected normal and tumor cell lines with 1 and 10 MOI of rLaSota eGFP, and analyzed them for eGFP-positive cells by flow cytometry (Fig. 2). Only 10% of 1-MOI-infected normal SVHUC-1 cells were found to be eGFP positive at 48 h PI, while 51% were eGFP positive in the 10-MOI-infected group (Fig. 2). Tumor cell lines were found to be 59–95% eGFP positive at 1 MOI, and 82–95% eGFP positive at 10 MOI with rLaSota eGFP (Fig. 2).
FIG. 2.
NDV infects and induces cell death differentially in normal human and tumor cells. Percentage GFP-positive SVHUC-1 (a–c), RPMI-8226 (f–h), U87MG (k–m), and MDA-MB231 (p–r) cells infected with 10 MOI and 1 MOI of rLaSota eGFP at 48 h PI. Percentage annexin V-positive mock- and NDV-infected (1 MOI) SVHUC-1 (d and e), RPMI-8226 (i and j), U87MG (n and o), and MDA-MB231 (s and t), early apoptotic cells at 24 h PI. Propidium iodide staining identified dead or late apoptotic/necrotic cells.
To determine whether a high MOI in normal cells would induce cell death, we stained virus-infected cells at 24 h PI (10 MOI) with annexin V antibody and propidium iodide. Only a small fraction of normal cells were found to be apoptotic/necrotic (Fig. 2), while 14% of RPMI-8226 infected cells were apoptotic/necrotic (Fig. 2). Surprisingly, only 5% of the U87MG cells were apoptotic/necrotic (Fig. 2), while MDA-MB231 cells did not undergo cell death at 24 h PI, though these cells were almost entirely eGFP positive (Fig. 2), suggesting delayed apoptosis.
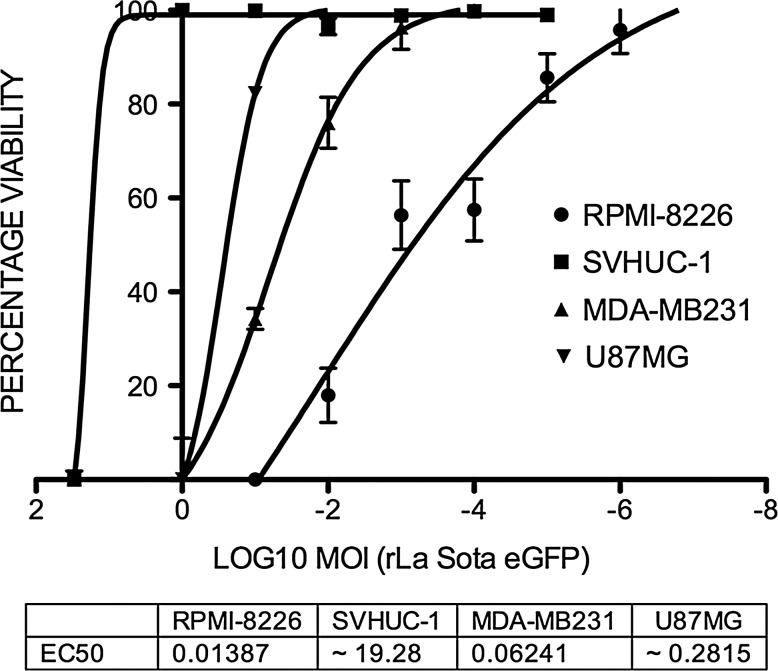
To further confirm that a higher MOI would not induce significant cell death in normal human cells, we performed WST-1 cytotoxicity assays with different MOIs in normal and tumor cell lines at 72 h PI. Very high cytotoxicity was observed in virus-infected RPMI-8226 cells, with an EC50 of 0.01387 MOI, and MDA-MB231 cells, with an EC50 of 0.06241, correlating with the higher virus yields obtained with a low MOI (Fig. 3). The EC50 of U87MG infected tumor cells was 0.2815 MOI, consistent with the moderate virus yields seen at low MOI of infection. The EC50 for the SVHUC-1 cell line was 19.28 MOI, suggesting that normal human cells could only be infected by rLaSota eGFP at a very high MOI, and that these cells severely restrict virus replication and undergo minimal cell death.
FIG. 3.
Dose-response curves to determine cytotoxicity of rLaSota eGFP in normal (SVHUC-1) and tumor (U87MG, RPMI-8226, and MDA-MB231) cell lines. Cells were infected with serial 10-fold dilutions of each virus and subjected to WST-1 assay after 48 h. The EC50 for each cell line was calculated using the non-linear curve-fitting analysis of percentage viability against log10 MOI.
Recombinant NDV is replication restricted in type I IFN-producing tumor cell lines
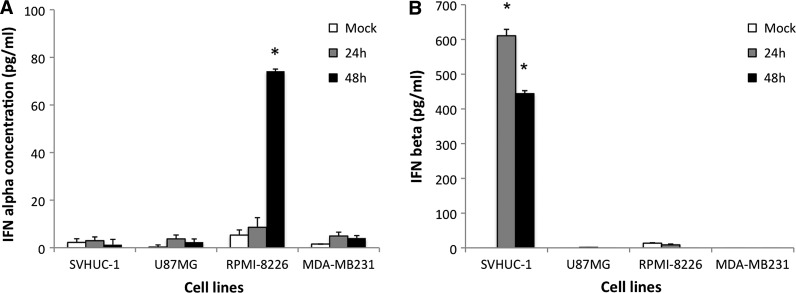
Selective infectivity of tumor cells by NDV has been linked to a lack of type I IFN production and more recently, a failure to respond to type I IFN in tumor cells (5,7,13). We examined type I IFN production by normal and tumor cell lines following 1 MOI of rNDV LaSota eGFP infection. Normal SVHUC-1 cells produced IFN-β (610 pg/mL) at early time points PI (24 h), which was sustained at 48 h, but no IFN-α was induced (Fig. 4A and B), and virus replication was severely restricted in these cells. The tumor cell line RPMI-8226 did not produce any IFN-β, and had low levels of IFN-α only at 48 h PI (74 pg/mL), and these cells were extremely permissive to NDV infection (Fig. 4A and B). IFN-α production by the plasmacytoma RPMI-8226 cells was consistent with this cytokine being produced mainly by leukocytes. The levels of IFN-α observed were also much lower than IFN-β levels, as observed with SVHUC-1, thus probably indicating a threshold concentration of type I IFN required to initiate an effective antiviral response (13). The other two tumor cell lines tested did not produce IFN-α/β, and also supported virus replication well.
FIG. 4.
Induction of type I IFN in normal and tumor cells by NDV. (A) IFN-α and (B) IFN-β production in normal (SVHUC-1) and tumor (U87MG, RPMI-8226, and MDA-MB231) cell lines at 24 and 48 h PI in vitro. Sample concentrations were derived by extrapolation using serial dilutions of standards. Sensitivity of the IFN-α assay is 12.5 pg/mL and 25 pg/mL for the IFN-β assay (*p<0.0002 for IFN-α and p<0.0001 for IFN-β assay).
Recombinant NDV-infected tumor cells upregulate antiviral signaling mediators
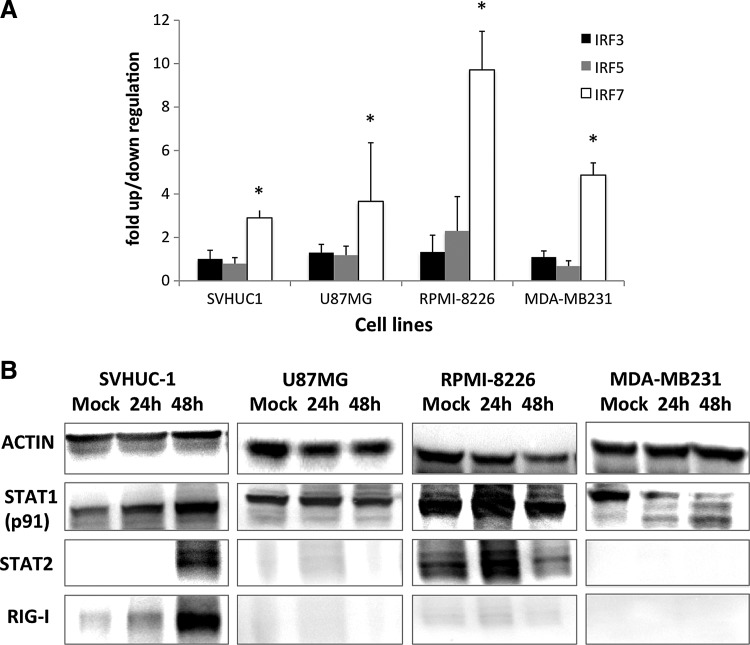
To determine whether these tumor cell types lack functional IFN induction and signaling mediators, we examined the upregulation of interferon regulatory factors (IRFs), and signal transducers and activators of transcription (STAT), after NDV infection. Significant upregulation of IRF7 in rLaSota eGFP-infected normal and tumor cell lines was detected by real time RT-PCR at 24 h PI (Fig. 5A). Upregulation of IRF3 and IRF5 were not significant in any of the cell lines tested.
FIG. 5.
Type I IFN signaling in NDV-infected cells. (A) Fold upregulation in mRNA expression of IRF3, IRF5, and IRF7 in normal and tumor cell lines at 24 h PI with rNDV LaSota eGFP. Expression levels in mock- and virus-infected cells were normalized to levels of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase. (B) Mock- and NDV-infected SVHUC-1, U87MG, RPMI-8226, and MDA-MB231 cellular lysates probed for actin and STAT1, STAT2, and RIG-I proteins. Cell lysates at 24 h and 48 h PI were analyzed.
Estimation of type I IFN-inducible STAT1 (p91) and STAT2 proteins revealed increased production of STAT1 and STAT2 in SVHUC-1 and RPMI-8226 cell lines upon infection with NDV (Fig. 5B). Levels of STAT1 did not vary between mock and infected U87MG cell lysates, while STAT1 degradation in MDA-MB231 cells was observed as early as 24 h PI. STAT2 was not detected in U87MG and MDA-MB231 cells at any time point. This surprising observation could be related to defects in type I IFN signaling in these cells. A deletion in type I IFN genes has previously been observed for U87MG cells (25).
RIG-I was induced as early as 24 h PI, and very high expression was found at 48 h PI, only in the normal SVHUC-1 cell line, but not in any of the tumor cell lines tested (Fig. 5B). The related cytoplasmic dsRNA sensor MDA-5 was not detected in any of the cell lines tested (data not shown).
Recombinant NDV infection induces effector cytokines and chemokines involved in immune cell recruitment
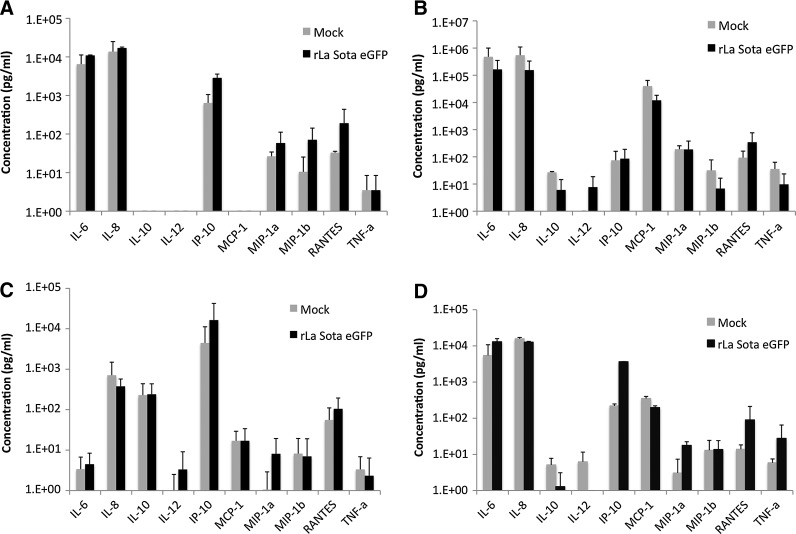
RIG-I-like helicases (RLH) signal through a common adaptor molecule, IFN-β promoter stimulator (IPS)-1. IPS-1 (also known as MAVS) initiates the phosphorylation of IRF3 and IRF7 and activation of NF-κB transcription factors, and the subsequent production of type I IFN and inflammatory cytokines (15,20,32). As RIG-I expression was absent in all of the tumor cell types tested and was found only in normal cells, we sought to analyze the expression of key cytokines and chemokines in mock- and rLaSota eGFP-infected normal and tumor cells (Fig. 6). Infection of normal SVHUC-1 cells with rLaSota eGFP resulted in increased production of IL-6, IL-8, IP-10, MIP-1α, MIP-1β, and RANTES. IL-10, IL-12, and MCP-1 were absent in SVHUC-1 infected cells, while they were produced at variable levels in all tumor cells tested (Fig. 6A).
FIG. 6.
Innate immune response to NDV in normal human and tumor cell lines. Production of cytokines and chemokines in mock- and NDV-infected (1 MOI) (A) SVHUC-1, (B) U87MG, (C) RPMI-8226, and (D) MDA-MB231 cells, were examined at 24 h PI by the cytometric bead assay. The levels were determined relative to serial dilutions of multiple cytokine and chemokine standards.
Tumor cells release a multitude of chemokines, cytokines, and growth factors into the tumor microenvironment, which affect their growth, angiogenesis, and recruitment of immune effector cells and stromal cells. NDV infection of tumor cell lines did not result in the upregulation of IL-6, IL-8, MIP-1α, and MIP-1β expression compared to constitutively expressed levels (Fig. 6B, C, and D). However, increases in the levels of IP-10 and RANTES in NDV-infected cells was consistent in all the cell lines tested, both tumorigenic and normal.
Discussion
We found that tumor-mediated mechanisms of immune subversion can be altered by the introduction of oncolytic viruses. Understanding and exploiting the conditions that result in antitumor efficacy may help to maximize oncolytic viral therapy. With safety issues related to therapy with more pathogenic oncolytic viruses, and select agent restriction on the use of moderately and highly pathogenic strains of NDV, the understanding of the innate immune mechanisms elicited by lentogenic NDV strains is imperative for moving them to the clinic. Our results agreed with our earlier reports of selective replication of NDV in tumor cell lines (5,6), but also revealed varying replicative fit depending on the type of tumor cell.
Similarly to others (7,13), we noticed that permissiveness of a cell to NDV replication is determined by a functional IFN antiviral system. Previous studies have also shown productive NDV infection in non-small cell lung cancer and melanoma cells capable of a robust type I IFN response (16,18). However, pre-treatment with IFN-β inhibited NDV growth and replication in these cells, suggesting that the ability to respond to type I IFN determines resistance to NDV (18). At higher MOI, even normal SVHUC-1 cells were infected by NDV. This is expected, as type I IFN only limits the spread of infection (13), and not the ability of normal cells to be infected by virus, since most cells with sialic acid receptors can be infected by NDV. Further, the high percentage of eGFP-positive normal cells may also indicate the functional activity of the IFN-antagonistic V protein of NDV, which is known to limit type I IFN induction and signaling, and can possibly overwhelm antiviral defenses at high MOI (1). Though earlier studies have reported functional V protein activity only in the natural host chicken, results from our laboratory indicated that V protein function extends to mammalian cell lines (1,5). But this is highly unlikely, as the spread of NDV is restricted, and there was only a limited productive viral infection in normal cells despite a high MOI.
Further, despite a very high MOI with >50% eGFP-positive cells, NDV-infected SVHUC-1 cells underwent very minimal cell death, suggesting that NDV cytotoxicity is cell type-dependent. U87MG and MDA-MB231 cells were apoptosis resistant at 24 h PI, despite >60% eGFP-positive cells and productive NDV replication with 1 MOI, reinforcing the view that NDV-induced apoptosis is not dependent on virus replication (31,35). U87MG cells have also been found to be apoptosis-resistant in other studies, by mechanisms associated with alterations in elements of the extrinsic pathway (25). Recent reports have shown selectivity of NDV infection to apoptosis-resistant tumor cell lines, with cell lysis being attributed to the mechanism of delayed apoptosis (18). Our results also suggest that the apoptosis resistance observed in certain tumor cells at early time points could be overcome later by overwhelming virus replication.
In RPMI-8226 cells, NDV replication was high despite IFN-α production at low levels. A strong correlation between IRF7 expression and type I IFN-mediated antiviral responses has previously been demonstrated (1,29). IRF7 has a preferential ability to activate IFN-α promoters (11), and we correspondingly observed the highest upregulation of IRF7 in RPMI-8226 cells, which produced IFN-α in response to NDV infection. When we examined the ability of RPMI-8226 cells to induce antiviral signaling pathways, the STAT-1-mediated IFN feed-forward mechanism was found to be functional. Analysis of mRNA levels of interferon-stimulated genes (ISGs) like IRF1, ISG-15, 6–16, and 2′5′-A, showed only basal levels of expression (data not shown). Despite the induction of STAT1 and STAT2 at early time points in NDV-infected RPMI-8226 cells, the ISGs were not upregulated, suggesting possible defects in downstream signaling events. An earlier induction of IFN-α than IFN-β has been shown to limit West Nile virus (4) and NDV infection (28,30).
It is well known that tumor cells have acquired defects in many facets of antiviral signaling. The tumor cell lines tested in this study lacked RIG-I expression. RIG-I senses ssRNA having a 5′ triphosphate, short dsRNA, and certain RNA viruses, including vesicular stomatitis virus and paramyxoviruses such as NDV (12). Attenuation of RIG-I induction could be one of the means by which NDV infects and spreads in tumor cells, in addition to other mechanisms. Type I IFN feed-forward mechanisms, either through RIG-I, or by virus replication-independent recognition by TLR-7, have been shown to be important for NDV replication in plasmacytoid dendritic cells (14). The expression of RIG-I has further been shown to determine susceptibility or resistance to NDV (29). An early production of type I IFN rather than high maximal levels at later time points has been suggested to be important for resistance to infection by NDV in mouse macrophages (30). It is possible that in RPMI-8226 cells, NDV double-stranded RNA intermediates could have been recognized by intracellular TLRs, but the absence of type I IFN signaling reinforcement by RIG-I permitted virus replication. Interestingly, in U87MG and MDA-MB231 cells, the failure to induce type I IFN signaling through RIG-I appears to determine selectivity. As we did not see any type I IFN induction in these virus-infected cells, it is quite possible that the TLR signaling was also defective in these cell types, allowing NDV replication.
RIG-I has been shown to activate NF-κB transcription factors and the subsequent production of type I IFN and inflammatory cytokines (20,32). In normal cells with a functional RIG-I helicase, a proinflammatory cytokine response was seen, consistent with an absence of the regulatory cytokine IL-10. Expression of this proinflammatory milieu of cytokines/chemokines indicates that immune effector cells such as neutrophils, NK cells, T cells, macrophages, and dendritic cells (DCs) can be recruited to the site of NDV infection (19). However, expression levels were not significantly high, concurring with reports of the LaSota strain being a poor activator of proinflammatory responses (6). NDV has earlier been reported to have pleiotropic immune stimulatory properties, though effector cytokine levels varied with the strain of NDV used, with more virulent strains resulting in increased effector molecule responses (23).
Earlier studies have linked NDV infection with increased IFN-γ, IL-6, IL-8, IL-1β, and iNOS in chickens (23). Reovirus-infected melanoma cells secreted eotaxin, IP-10, RANTES, IL-8, MIP-1α, MIP-1β, and IFN-β, effectors similar to those observed in this study (27). IL-6 serves to enhance vascular permeability and stimulates inflammatory cell recruitment to the affected site, whereas IL-8 is a recognized factor in the chemotaxis of neutrophils. IP-10 and RANTES both play major roles in the galvanization of T cells and NK cells into the effector site, while MCP-1, MIP-1α, and MIP-1β recruit macrophages and DCs to the tumor site. Thus we observed the induction of a predominantly proinflammatory environment in lentogenic NDV-infected normal cells, and the chemokines RANTES and IP-10 in normal and tumor cells. The absence of a proinflammatory response in tumor cells could be due to the absence of RIG-I expression in these cells.
Engineering of lentogenic NDV strains to express chemotactic factors that induce immune effector cells to enhance therapy is a major and feasible approach. Preliminary studies with engineered NDV expressing IL-2 and GM-CSF genes have shown promising results in the induction of host antiviral immunity (9,10).
Differentially-regulated IFN induction and response determines the outcome of NDV infection in normal and tumor cell lines. Besides, the amount and kinetics of the type I IFN response also appears to impart permissiveness to NDV. However, this may not be the only mechanism for tumor cell selectivity to infection. Our results suggest that reinforcement of type I IFN responses mediated by the RNA helicase RIG-I appears to be important for resistance to infection with NDV. Our data demonstrate that individual tumor cell responses to oncolytic viral therapy can vary with the cell type. This cell type-specific response may be of importance because it allows custom tailoring of recombinant NDV to manipulate antitumor immunity in addition to direct viral oncolysis. Further insights could help direct future approaches and offer enhanced therapy, leading to improved clinical outcomes.
Acknowledgments
The authors would like to thank Ms. Melissa Makris, Virginia-Maryland Regional College of Veterinary Medicine, for flow cytometry, and the Institute of Critical Technology and Applied Science, Virginia Tech, for supporting part of the work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alamares JG. Elankumaran S. Samal SK. Iorio RM. The interferon antagonistic activities of the V proteins from two strains of Newcastle disease virus correlate with their known virulence properties. Virus Res. 2010;147:153–157. doi: 10.1016/j.virusres.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altomonte J. Marozin S. Schmid RM. Ebert O. Engineered Newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mole Therapy. 2010;18:275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csatary LK. Gosztonyi G. Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neuro-oncol. 2004;67:83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 4.Daffis S. Samuel MA. Suthar MS, et al. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elankumaran S. Chavan V. Qiao D, et al. Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy. J Virol. 2010;84:3835–3844. doi: 10.1128/JVI.01553-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elankumaran S. Rockemann D. Samal SK. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J Virol. 2006;80:7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiola C. Peeters B. Fournier P, et al. Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int J Cancer. 2006;119:328–338. doi: 10.1002/ijc.21821. [DOI] [PubMed] [Google Scholar]
- 8.Freeman AI. Zakay-Rones Z. Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Molec Therapy. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Janke M. Peeters B. de Leeuw O, et al. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Therapy. 2007;14:1639–1649. doi: 10.1038/sj.gt.3303026. [DOI] [PubMed] [Google Scholar]
- 10.Janke M. Peeters B. Zhao H, et al. Activation of human T cells by a tumor vaccine infected with recombinant Newcastle disease virus producing IL-2. Int J Oncol. 2008;33:823–832. [PubMed] [Google Scholar]
- 11.Kawai T. Sato S. Ishii KJ, et al. Interferon-a induction through toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nature Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 12.Kato H. Takeuchi O. Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy S. Takimoto T. Scroggs RA. Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80:5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumagai Y. Kumar H. Koyama S, et al. Cutting Edge: TLR-Dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-α production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 15.Kumar H. Kawai T. Kato H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exper Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazar I. Yaacov B. Shiloach T, et al. The oncolytic activity of Newcastle disease virus NDV-HUJ on chemoresistant primary melanoma cells is dependent on the proapoptotic activity of the inhibitor of apoptosis protein livin. J Virol. 2010;84:639–646. doi: 10.1128/JVI.00401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TC. Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 18.Mansour M. Palese P. Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011;85:6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik JD. Twelves CJ. Selby PJ, et al. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res. 2011;17:4214–4224. doi: 10.1158/1078-0432.CCR-10-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakhaei P. Genin P. Civas A. Hiscott J. RIG-I-like receptors: sensing, responding to RNA virus infection. Seminars in immunology. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Ottolino-Perry K. Diallo JS. Lichty BD, et al. Intelligent design: combination therapy with oncolytic viruses. Molec Therapy. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecora AL. Rizvi N. Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Rue CA. Susta L. Cornax I, et al. Virulent Newcastle disease virus elicits a strong innate immune response in chickens. J Gen Virol. 2011;92:931–939. doi: 10.1099/vir.0.025486-0. [DOI] [PubMed] [Google Scholar]
- 24.Sen Sharma K. Moorkanat G. Qiao D, et al. Efficient fluorescence-based imaging methods for quantitating infectivity and oncolytic efficacy of Newcastle disease virus. J Virol Methods. 2010;163:390–397. doi: 10.1016/j.jviromet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Sgorbissa A. Tomasella A. Potu H, et al. Type I IFNs signaling and apoptosis resistance in glioblastoma cells. Apoptosis. 2011;16:1229–1244. doi: 10.1007/s10495-011-0639-4. [DOI] [PubMed] [Google Scholar]
- 26.Sinkovics JG. Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 27.Steele L. Errington F. Prestwich R, et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-kappaB mediated and supports innate and adaptive anti-tumour immune priming. Molec Cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washburn B. Schirrmacher V. Human tumor cell infection by Newcastle disease virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 2002;21:85–93. doi: 10.3892/ijo.21.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Wilden H. Fournier P. Zawatzky R. Schirrmacher V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle disease virus. Int J Oncol. 2009;34:971–982. doi: 10.3892/ijo_00000223. [DOI] [PubMed] [Google Scholar]
- 30.Wilden H. Schirrmacher V. Fournier P. Important role of interferon regulatory factor (IRF)-3 in the interferon response of mouse macrophages upon infection by Newcastle disease virus. Int J Oncol. 2011;39:493–504. doi: 10.3892/ijo.2011.1033. [DOI] [PubMed] [Google Scholar]
- 31.Yang S. Liu W. Cui H, et al. In vitro induction of apoptosis in tumor cells by inactivated NDV and IAV. Cancer Biother Radiopharmaceuticals. 2007;22:200–205. doi: 10.1089/cbr.2007.337. [DOI] [PubMed] [Google Scholar]
- 32.Yoneyama M. Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 33.Zamarin D. Martinez-Sobrido L. Kelly K, et al. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Molec Therapy. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemp FJ. Corredor JC. Lun X, et al. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21:103–117. doi: 10.1016/j.cytogfr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Zeng J. Fournier P. Schirrmacher V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297:19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]