Abstract
Limited data are available regarding the role of bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBB) as diagnostic tools in pulmonary Langerhans’ Cell Histiocytosis (LCH) and lymphangioleiomyomatosis (LAM).
The aim of this study was to review our experience regarding the value of these two techniques in the diagnosis of these cystic lung diseases. Records of 452 patients with the presumptive diagnosis of interstitial lung disease were reviewed; 67 had a clinical-radiological diagnosis of either LCH (n=27) or LAM (n= 40). Of 16 patients with LCH who underwent BAL, four specimens (25%) contained cells which had positive immunoreactivity for CD1a. Of three patients with negative BAL fluid who had TBB, only one had a positive tissue diagnosis. Ten LCH patients were diagnosed by surgical lung biopsy of which five had negative BAL fluid. The remaining 12 patients were diagnosed by clinical and radiologic features. Standard examination of BAL fluid was of no diagnostic value in LAM. TBB was performed in seven patients and was diagnostic in six, not resulting in complications. All 13 patients who underwent surgical lung biopsies had a positive histopathologic diagnosis The remaining 21 patients were diagnosed by clinical and radiologic features. We suggest that BAL may assist in the diagnosis of LCH whereas TBB may be useful in the diagnosis of LAM, thus avoiding the need for surgical biopsy.
Keywords: Interstitial lung diseases, fiberoptic bronchoscopy
Introduction
Interstitial lung diseases constitute a heterogeneous group of pulmonary parenchymal diseases that are defined by radiologic, physiologic and histopathologic features 1. With the advent of computerized tomography (CT), the radiologic characteristics of these conditions have been refined, and by defining the nature of the individual lesions (e.g., nodules, cysts, etc) a differential diagnosis may be established. Still, in many instances, surgical lung biopsy is necessary to make a diagnosis. Cystic lung diseases are a subgroup of interstitial lung diseases that are characterized by radiological and pathological evidence of a cystic pattern. At advanced stages of asbestosis, cystic fibrosis, collagen vascular diseases, usual interstitial pneumonia (UIP), bronchiectasis, lymphangioleiomyomatosis (LAM), Langerhans Cell Histiocytosis (LCH), Sjogrens syndrome, and Birt Hogg Dubé syndrome, cysts may be found throughout the lung parenchyma 1–3. In the absence of features of extra-pulmonary disease, diagnosis may require clinical, radiologic and physiologic data and ultimately, a lung biopsy 2–3. Since cystic diseases show characteristic lesions on CT scans and may have similar clinical presentation and functional impairment, a histopathologic confirmation of the diagnosis may be necessary. However, since cystic lung diseases are relatively rare, few data are available about the role of bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBB) as diagnostic tools 4. We report here our experience with BAL, TBB, and surgical lung biopsy in 67 patients with diffuse cystic lung diseases.
Methods
We retrospectively evaluated the records of 452 patients referred to the Pulmonary Service of San Giuseppe Hospital between 1997 and 2008 with a diagnosis of interstitial lung disease. San Giuseppe Hospital is a tertiary care facility and referral center in Milan, for undiagnosed interstitial lung diseases. The retrospective review was approved by the San Giuseppe Hospital Institutional Review Board (Comitato Etico degli Ospedali di Milano e Sacra Famiglia di ERBA, approval number 55/09), which has a Federal-Wide Assurance (FWA) with the U.S. Office of Human Research Protections, and the NHLBI Institutional Review Board (approval number 95-H-0186). Among the 452 patients, we found 67 patients with cystic lung disease; 27 had clinical-radiological diagnosis of LCH and 40 had a presumptive diagnosis of LAM. Patients with other cystic lung diseases (e.g., cystic fibrosis, Sjogren’s syndrome, etc) were referred to the appropriate subspecialty services. The diagnosis of LCH was based on the presence of nodular and cystic lesions on CT scan predominantly involving the middle and upper lobes, history of smoking or extrapulmonary involvement 5. A definite diagnosis of LAM was based on the presence of lung cysts and angiomyolipoma, tuberous sclerosis complex, or lymphatic involvement (e.g., chylous effusions) 3. The diagnosis of probable LAM was made in the presence of a history of pneumothorax, airflow obstruction and/or impairment of diffusion capacity, and the presence of thin-walled round-shaped well-defined cysts on the CT scan in the absence of extrapulmonary disease 3.
For each group of patients, we analyzed data for those who underwent bronchoscopy with BAL and/or TBB, video-assisted thoracoscopic surgery (VATS) or open lung biopsy. Cytology and cell count and differential were performed in all specimens. The presence of 5% or greater cells with positive immunoreactivity for cluster of differentiation 1a (CD1a) was considered strongly suggestive of LCH. In most cases, cytofluorimetric studies for cluster of CD1a were also performed. Monoclonal antibodies directed against CD1a or the intracellular S100 protein were used to determine immunoreactivity on lung tissue specimens. Electron microscopy was employed to detect Birbeck granules. When LAM was suspected, histological evaluation of specimens from TBB, VATS or open lung biopsy included immunohistochemical reactivity with the monoclonal antibody HMB45. Lung biopsy tissue specimens were evaluated by pathologists with experience in interstitial lung diseases.
Results
Langerhans Cell Histiocytosis
Twenty-seven patients (12 men; mean age at diagnosis 35 ± 3 years; 22 smokers, 5 ex-smokers) received a clinical-radiological diagnosis of LCH. Among these, seven had multisystem disease: three had bone involvement, three had pituitary gland disease, and one had skin involvement (Table 1). Two subjects had respiratory failure and pulmonary hypertension. The mean percent-predicted FEV1 and DLCO were respectively, 84.4±4.1 and 67.6±4.2.
Table 1.
Demographic and clinical data in 27 LCH patients*
| Patient | Sex | Smoking history | Age | Extrapulmonary manifestations | Procedure performed |
|---|---|---|---|---|---|
| 1 | F | ES | 34 | VATS | |
| 2 | F | S | 58 | FBS, BAL | |
| 3 | M | S | 65 | FBS, BAL | |
| 4 | M | S | 36 | Diabetes insipidus | FBS, BAL |
| 5 | M | ES | 49 | ||
| 6 | F | S | 35 | Bone | FBS, BAL |
| 7 | F | S | 46 | FBS, BAL, TBB, VATS | |
| 8 | M | S | 24 | Skin | Thoracotomy, skin biopsy |
| 9 | M | S | 35 | FBS, BAL, VATS | |
| 10 | M | S | 25 | FBS, BAL | |
| 11 | F | S | 24 | FBS, BAL, TBB, VATS | |
| 12 | M | S | 19 | FBS, BAL | |
| 13 | F | S | 28 | FBS, BAL | |
| 14 | M | S | 47 | FBS, BAL | |
| 15 | F | S | 36 | ||
| 16 | M | ES | 78 | ||
| 17 | M | S | 23 | Bone | Bone biopsy |
| 18 | F | S | 33 | VATS | |
| 19 | F | S | 25 | FBS, BAL, TBB | |
| 20 | F | S | 23 | Thoracotomy | |
| 21 | F | ES | 51 | FBS, BAL | |
| 22 | M | S | 17 | Diabetes insipidus, hypogonadism | FBS, BAL, VATS |
| 23 | F | S | 44 | VATS | |
| 24 | M | ES | 31 | Bone | Bone biopsy |
| 25 | F | S | 17 | Thoracotomy | |
| 26 | F | S | 30 | Diabetes insipidus | FBS, BAL |
| 27 | F | S | 14 | FBS, BAL |
Abbreviations: M = male; F = female; S = smoker; ES = ex smoker; TBB = transbronchial lung biopsy; VATS = video assisted thoracoscopy; BAL = bronchoalveolar lavage
Diagnostic procedures
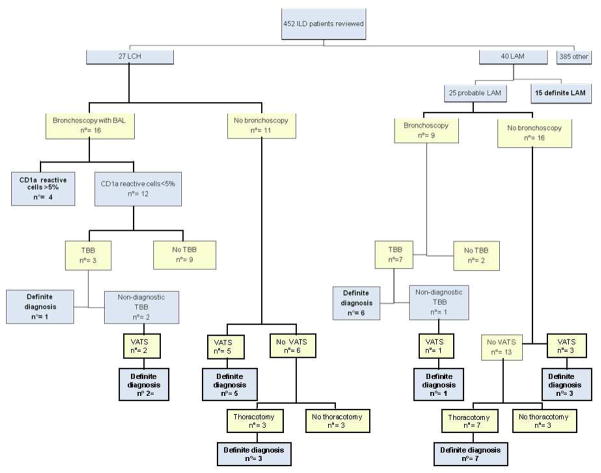
Sixteen patients underwent BAL. Cytology studies examining the morphology of cells stained with H&E were performed in all patients. All 16 patients had immunocytochemistry studies with CD1a. Ten of the 16 had also had cytofluorimetric studies for cluster of CD1a. Ten patients had positive immunocytochemistry and cytofluorimetric studies for CD1a. but only four had greater than 5% CD1a-reactive cells in their BAL fluid samples (25%). Electron microscopy studies to search for Birbeck granules were done in only one patient and the results were negative. No discrepancy was found between conventional cytology and cytofluorimetry. BAL cells reactive with anti-CD1a antibodies were found in a patient by both conventional cytology and cytofluorimetric examination. In the remaining three patients with anti-CD1a-reactive cells on cytological evaluation of BAL fluid, cytofluorimetric studies were not performed. TBB was performed in three patients with negative BAL fluid analysis; a diagnosis of LCH was established only in one case. One patient suffered a pneumothorax. Ten of the 27 patients underwent surgical lung biopsy (7 VATS and 3 open lung biopsy). All surgical lung biopsies were diagnostic (Table 2, Figure 1). The relationship between CT pattern patterns and results of BAL, TBB and surgical lung biopsy are shown in Table 3. Among the four patients with CDa1- positive cells in BAL fluid, one had a cystic pattern on CT scan and the others had both cysts and nodules.
Table 2.
Diagnostic yield of invasive diagnostic tests in 27 patients with clinical-radiological diagnosis of Langerhans cell histiocytosis*
| Performed | Diagnostic | Percent | |
|---|---|---|---|
| BAL | 16 | 4 | 25 |
| TBB | 3 | 1 | 33 |
| VATS | 7 | 7 | 100 |
| Thoracotomy | 3 | 3 | 100 |
| Bone biopsy | 2 | 2 | 100 |
Abbreviations: see Table 1.
Figure 1.
Illustrative diagram showing the outcome of diagnostic methods employed in 27 patients with LCH and 25 patients with probable LAM. Abbreviations: LCH= Langerhans’ cell histiocytosis; LAM= lymphangioleiomyomatosis; TBB = transbronchial lung biopsy; VATS = video assisted thoracoscopy; BAL = bronchoalveolar lavage
Table 3.
Relationship between computed tomography pattern and bronchoalveolar lavage, transbronchial biopsy and video-assisted thoracoscopic surgery findings in 27 patients with clinical-radiological diagnosis of Langerhans cell histiocytosis
| CT pattern | Positive BAL | Positive TBB | Positive VATS |
|---|---|---|---|
| Cystic lesions | 1/6 | 1/1 | 2/2 |
| Nodules | 0/2 | 0 | 1/1 |
| Nodules and cysts | 3/8 | 0/2 | 4/4 |
For abbreviations see Table 1.
Lymphangioleiomyomatosis
Forty patients (all women; mean age at diagnosis 36 ± 2 years, 32 non smokers, 7 ex-smokers, one smoker) received a diagnosis of LAM. All patients showed characteristic lung CT scans in association with the following clinical findings: six had TSC and angiomyolipomas; three had chylous effusions and a diagnostic lymph-node biopsy; five had renal angiomyolipomas. One patient had typical functional impairment and a lymphangioleiomyoma. Eleven patients had a history of recurrent or bilateral pneumothorax; nine subjects presented with dyspnea and another patient presented with cough and hemoptysis. The mean percent-predicted FEV1 and DLCO was respectively, 61.5±4.1 and 50.4±4.2. Other characteristics of the patients are shown in Table 4. On the basis of clinical findings, extrapulmonary manifestations and CT scans, 15 patients were diagnosed with definite LAM and 25 patients with probable LAM. Seventeen patients with probable LAM underwent additional diagnostic tests. The criteria for diagnosis of definite or probable LAM is presented in Table 4.
Table 4.
Age, mode of presentation, extrapulmonary manifestations and procedures performed in 40 patients with lymphangioleiomyomatosis*
| Smoking | Clinical manifestations | FEV1 (%) | DLCO (%) | Extrapulmonary manifestations | Diagnosis | Diagnostic procedures | |
|---|---|---|---|---|---|---|---|
| 1 | NS | Pneumothorax | 19 | 22 | - | Probable | Thoracotomy |
| 2 | NS | Dyspnea | 51 | 81 | - | Probable | Thoracotomy |
| 3 | NS | Pneumothorax | 70 | 37 | - | Probable | - |
| 4 | NS | Pneumothorax | 56 | 36 | - | Probable | BAL, TBB |
| 5 | NS | Pneumothorax | 96 | 66 | - | Probable | - |
| 6 | NS | Dyspnea | 96 | 64 | Lymphangioleiomyoma | Definite | Abdominal biopsy |
| 7 | NS | Dyspnea | 49 | 17 | Angiomyolipoma, TSC | Definite | - |
| 8 | ES | Pneumothorax | 29 | 37 | - | Probable | - |
| 9 | NS | Dyspnea, pneumothorax | 43 | 31 | Angiomyolipoma | Definite | - |
| 10 | NS | Pneumothorax | - | - | - | Probable | Thoracotomy |
| 11 | ES | Dyspnea | 76 | 14 | - | Probable | - |
| 12 | NS | Dyspnea | 53 | - | Probable | - | |
| 13 | NS | Dyspnea | 38 | 55 | Angiomyolipoma | Definite | - |
| 14 | NS | Pneumothorax | 29 | 37 | Angiomyolipoma | Definite | - |
| 15 | ES | Dyspnea | 62 | 29 | - | Probable | BAL, TBB |
| 16 | NS | Dyspnea | 55 | 10 | - | Probable | BAL, TBB |
| 17 | S | Pneumothorax | 104 | 85 | - | Probable | - |
| 18 | NS | Pneumothorax | - | - | Angiomyolipoma | Definite | - |
| 19 | NS | Pneumothorax, dyspnea | 54 | 61 | Angiomyolipoma, TSC | Definite | - |
| 20 | NS | Cough | 78 | 64 | Angiomyolipoma, TSC | Definite | - |
| 21 | NS | - | 85 | 83 | Angiomyolipoma, TSC | Definite | - |
| 22 | NS | Chylous effusion | 75 | 60 | Lymphangioleiomyomas | Definite | Lymph-node biopsy |
| 23 | NS | Pneumothorax | - | - | - | Probable | Thoracotomy |
| 24 | NS | Pneumothorax | 16 | 20 | Angiomyolipoma, TSC | Definite | Thoracotomy |
| 25 | ES | Hemoptysis, cough | 70 | 24 | - | Probable | Thoracotomy |
| 26 | NS | Pneumothorax | - | - | Lymphangioleiomyomas, angiomyolipoma, TSC | Definite | Lymph-node biopsy |
| 27 | NS | Pneumothorax | 90 | 95 | Angiomyolipoma | Probable | VATS |
| 28 | ES | Pneumothorax | 90 | 76 | Angiomyolipoma found after biopsy | Probable | VATS |
| 29 | NS | Dyspnea | 71 | - | - | Probable | BAL, TBB |
| 30 | NS | Pneumothorax | 48 | 59 | Angiomyolipoma, found after biopsy | Probable | Thoracotomy |
| 31 | NS | Dyspnea | 80 | - | - | Probable | BAL |
| 32 | NS | Dyspnea | 33 | 60 | - | Probable | BAL, TBB, VATS |
| 33 | ES | Pneumothorax | 87 | 56 | - | Probable | BAL, TBB |
| 34 | NS | Dyspnea, chylous effusion | 24 | 26 | Lymphangioleiomyomas | Definite | Lymph-node biopsy |
| 35 | NS | Pneumothorax | 92 | 65 | Angiomyolipoma, found after biopsy | Probable | VATS |
| 36 | NS | Pneumothorax | 71 | 60 | - | Probable | Thoracotomy |
| 37 | NS | Chylous effusion | 69 | 76 | Lymphangioleiomyomas, angiomyolipoma | Definite | Lymph-node biopsy |
| 38 | NS | Pneumothorax | 95 | 81 | Angiomyolipoma | Definite | VATS |
| 39 | ES | Cough, dyspnea | 31 | 26 | - | Probable | BAL |
| 40 | NS | Pneumothorax | 32 | 13 | - | Probable | BAL, TBB |
FEV1 and DLCO are shown as percent-predicted of the normal values. Abbreviations: NS = non-smoker, S = smoker, ES = ex-smoker. TBB = transbronchial lung biopsy. VATS = video-assisted thoracoscopic surgery. In the absence of extra-pulmonary LAM, a probable diagnosis of LAM was made based on a characteristic history (recurrent pneumothorax, dyspnea, chylous effusions) in the presence of thin-wall lung cysts on high resolution CT scans.
Diagnostic procedures
Bronchoscopy with BAL was performed in nine of 25 patients with probable LAM of which seven underwent TBB. In no instance were BAL fluid findings helpful for the diagnosis of LAM. In six cases, TBB confirmed the diagnosis (85.7%). No complications were observed after the TBB. Eleven patients with probable LAM underwent surgical lung biopsy: four VATS (one patient with a non-diagnostic TBB) and seven open lung biopsies. One VATS and one open lung biopsy were performed after pneumothorax in two patients with definite LAM. Four VATS biopsies confirmed the diagnosis of LAM; all open lung biopsies were diagnostic (Table 5, Figure 1).
Table 5.
Method of diagnosis in 40 patients with LAM
| Diagnostic method | Diagnostic | Total | Percentage |
|---|---|---|---|
| Clinical-radiologic | 17 | 40 | 42.5 |
| BAL | 0 | 9 | 0 |
| TBB | 6 | 7 | 85.7 |
| VATS | 4 | 5 | 80 |
| Open lung biopsy | 8 | 8 | 100 |
| Extra-pulmonary biopsy | 5 | 5 | 100 |
For abbreviations see Table 1.
Discussion
In a review of 452 patients with interstitial lung disease who were referred to our tertiary care hospital we found 67 with cystic lung disease, 27 with LCH and 40 with LAM. The prevalence of these two conditions among all cases of interstitial lung disease is high, suggesting that because San Giuseppe Hospital is a referral center for patients with uncommon lung diseases, our population sample may be biased. Twenty seven patients had LCH, a smoking–related lung disease that is among a spectrum of disorders characterized by proliferation and infiltration of organs by Langerhans’ cells 5. Several organ systems may be involved in LCH, including lungs, bone, skin, pituitary gland, liver, lymph nodes, and thyroid gland 5. LCH is common in young adults, with a peak incidence between 20 and 40 years of age 5,6. Adult LCH may represent a subset of histiocytic disorders, characterized by polyclonal expansion of Langerhans cells in the lung, possibly induced by antigens in cigarette smoke 7,8. Histologically, the pulmonary lesions begin as a proliferation of Langerhans cells along the small airways 9,10. These cells are of monocyte-macrophage lineage and distinguished from dendritic cells by their characteristic penta-laminar, plate-like, cytoplasmic organelles (Birbeck granules) seen by electron microscopy. Cells exhibit strong surface expression of the CD1a antigen 10,11 and are also reactive with anti-S-100 antibodies 11.
Pathological findings vary with the stage of the disease. In the early stages, numerous Langerhans cells accumulate in areas adjacent to terminal or respiratory bronchioles. These cells appear to invade the bronchiole, destroying the bronchiolar wall in an eccentric fashion, and forming nodules. The nodules contain inflammatory cells, including Langerhans’cells, and eosinophils. These cellular nodules progress to fibrotic nodules that often have a stellate configuration and may lack Langerhans’ cells entirely. Central cavitation of the nodules can sometimes be traced to ectatic, destroyed small airways. In addition, traction emphysema of alveoli adjacent to the stellate scars and peribronchiolar fibrosis are commonly observed 9. The chest radiograph is abnormal in most cases showing micronodular or reticulonodular and interstitial infiltration, with a predominance of middle- and upper-lobe involvement 12,13. The most common radiological findings on CT scans are nodularity with irregularly shaped cystic changes involving predominantly the middle and upper lobes 13.
In the appropriate clinical setting, the presence of typical findings on CT scan is often sufficient to establish the diagnosis of LCH. A nodular and cystic pattern predominating in the upper half of the lung fields in a young smoker makes the diagnosis almost certain and may obviate the need for a lung biopsy 13–15. The presence CD1a-stained cells in BAL fluid renders the diagnosis of LCH very likely 16. However, there is no gold standard for the diagnosis of LCH. The presence of a history of smoking, bilateral pulmonary interstitial infiltrates and nodular and cystic lesions that spare the costophrenic angles is suggestive of LCH. To establish a firm diagnosis, especially in those patients with atypical roentgenographic presentation, BAL or lung biopsy should be considered 5. In our study, using a threshold of 5% anti-CD1a-reactive cells to increase the specificity of the test in the BAL fluid 17,18, immunohistochemical confirmation of LCH was obtained in four of sixteen patients (25%) who underwent BAL. In the remaining 12 patients with a suggestive radiological pattern, BAL fluid was not diagnostic, although it was useful to exclude infectious lung diseases. Five patients with a negative BAL were found to have LCH after lung biopsy.
Although Auerswald et al. 16 and Chollet et al. 19 reported good sensitivity of immunocytochemistry against CD1a in BAL fluid as a diagnostic test 16,19,20, our data are more in accordance with recent evidence indicating a low sensitivity of this approach 21. Examination of the BAL fluid may be of value in patients with atypical clinical and/or radiological presentation when it can be used to exclude other interstitial lung diseases with more typical lavage findings (e.g., sarcoidosis) and pulmonary infections, such as cavitary forms of Pneumocystis Jiroveci pneumonia or mycobacterial infections. BAL fluid samples collected from six patients showing cystic lesions alone on CT were diagnostic in only one case, reflecting the absence of an active inflammatory process. Low frequency of Langerhans cells in BAL fluid of patients with a nodular or cystic-nodular CT pattern may be explained by the presence of fibrotic nodules where Langerhans cells are less abundant or absent, as occurs in advanced disease. Three patients with LCH had TBB and in only one was the diagnosis established, which is consistent with the reported poor sensitivity of this technique in LCH, ranging from 10 to 40 percent 11,22. The patchy nature of the disease with a focal distribution of the lesions, as well as the smaller number of active nodules in advanced disease and the small amounts of tissue obtained by TBB may account for the low diagnostic yield. However, because of the small number of patients who had TBB we are unable to draw any conclusions regarding the safety and usefulness of TBB in the diagnosis of LCH. The value of TBB in LCH remains to be determined.
Our data confirm that surgical lung biopsy is the best diagnostic test in LCH patients in whom the diagnosis can not be made by clinical-radiologic methods or BAL.
LAM is a multisystem disease characterized by proliferation of abnormal smooth muscle-like cells (LAM cells), leading to the formation of thin-walled cysts in the lungs, fluid-filled cystic structures (i.e., lymphangioleiomyomas) in the axial lymphatics, and abdominal tumors (e.g. renal angiomyolipomas) 3,23. LAM occurs in a sporadic form and in about 30% of woman with tuberous sclerosis complex (TSC) 23. LAM cells are reactive with HMB-45, a monoclonal antibody recognizing gp100, a protein found in melanocytes and melanoma cell lines 3,23. Lung cysts are characteristically round-shaped and have thin, regular walls, ranging from barely perceptible to several millimeter in diameter, and typically appear scattered throughout the lung without any lobar predominance 24–26.
The gold standard for the diagnosis of LAM is a biopsy of lung, angiomyolipomas or lymphatics 3,23. Nevertheless, not all patients with a suspicion of LAM require tissue biopsy; in an appropriate clinical and functional setting, the presence of round or oval, thin-walled cysts scattered throughout the lungs on lung CT scan, in a female makes the diagnosis of LAM very likely. Current data suggest that a definite diagnosis of LAM requires manifestations other than cystic lung disease 3. In our population, 25 of the 40 patients had a diagnosis of probable LAM. Nine patients had a subsequent bronchoscopy with BAL and in 7 of those cases a TBB was performed. The frequency of a diagnostic TBB was high, confirming the usefulness of this test in LAM. Poletti al.27 had already shown that LAM may be diagnosed by transbronchial biopsy 28,29. However, in most cases, the diagnostic features of LAM were recognized in retrospect after review of the subsequent open lung biopsy specimen 30,31.
Our results show that TBB may be of greater usefulness in LAM than in LCH. The more uniform distribution of histological lesions in LAM compared to LCH possibly accounts for this difference. The absence of complications after TBB among LAM patients suggests the relative safety of the procedure. BAL is a useful diagnostic method in LCH, because of its safety and because it may preclude the need for more invasive tests. The poor sensitivity and risk of pneumothorax do not support the use of TBB in LCH.
Acknowledgments
Authors roles in the manuscript: Drs Harari, Torre and Cassandro performed the studies, collected and analyzed the data, and wrote the initial draft of the manuscript. Dr. Taveira-DaSilva and Dr. Moss, revised and rewrote the manuscript. The authors attest the integrity of this work.
Supported in part by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute
Footnotes
The authors have no financial or other conflicts of interest to disclose. The manuscript has been seen and approved by all authors who attest to the integrity of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sergio Harari, Email: sharari@ilpolmone.it.
Olga Torre, Email: Olga.Torre@libero.it.
Roberto Cassandro, Email: rcassandro@hotmail.com.
Angelo M. Taveira-DaSilva, Email: dasilvaa@nhlbi.nih.gov.
Joel Moss, Email: mossj@nhlbi.nih.gov.
References
- 1.Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82:976–986. doi: 10.4065/82.8.976. [DOI] [PubMed] [Google Scholar]
- 2.Harari S, Paciocco G. An integrated clinical approach to diffuse cystic lung diseases. Sarcoidosis Vasc Diffuse Lung Dis. 2005 Dec;22(Suppl 1):S31–9. [PubMed] [Google Scholar]
- 3.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, Bonetti F, Kingswood C Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 4.Torre O, Harari S. The diagnosis of cystic lung diseases: a role for bronchoalveolar lavage and transbronchial biopsy? Respir Med. 2010;104 (Suppl 1):S81–5. doi: 10.1016/j.rmed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Vassallo R, Ryu JH. Pulmonary Langerhans’ cell Histiocytosis. Clin Chest Med. 2004;25:561–571. doi: 10.1016/j.ccm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langherhans’-cell Histiocytosis in adults. N Engl J Med. 2002;346:484–490. doi: 10.1056/NEJMoa012087. [DOI] [PubMed] [Google Scholar]
- 7.Yousem SA, Colby TV, Chen YY, Chen WG, Weiss LM. Pulmonary Langerhans Cell Histiocytosis: Molecular Analysis of Clonality. Am J Surg Pathol. 2001;25:630–636. doi: 10.1097/00000478-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Bernstrand C, Cederlund K, Ashtrom L, Henter JI. Smoking Preceded Pulmonary Involvement in Adults with Langerhans Cell Histiocytosis Diagnosed in Childhood. Acta Paediatr. 2000;89:1389–1392. doi: 10.1080/080352500300002642. [DOI] [PubMed] [Google Scholar]
- 9.Colby TV, Lombard C. Histiocytosis X in the Lung. Hum Pathol. 1983;14:847–856. doi: 10.1016/s0046-8177(83)80160-9. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Borok Z, Roum JH, Zhang J, Feuerstein I, Ferrans VJ, Crystal RG. Pulmonary Langerhans cell granulomatosis (Histiocytosis X): a clinicopathologic study of 48 cases. Am J Surg Pathol. 1993;17:971–986. doi: 10.1097/00000478-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Soler P, Kambouchner M, Valeyre D, Hance AJ. Pulmonary Langerhans’ cell granulomatosis (Histiocytosis X) Annu Rev Med. 1992;43:105–115. doi: 10.1146/annurev.me.43.020192.000541. [DOI] [PubMed] [Google Scholar]
- 12.Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax. 1982;37:104–109. doi: 10.1136/thx.37.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brauner MW, Grenier P, Mouelhi MM, Mompoint D, Lenoir S. Pulmonary histiocytosis X: evaluation with high-resolution CT. Radiology. 1989;172:255–258. doi: 10.1148/radiology.172.1.2787036. [DOI] [PubMed] [Google Scholar]
- 14.Bonelli FS, Hartman TE, Swenson SJ, et al. Accuracy of high resolution CT in diagnosing lung diseases. Am J Roentgenol. 1998;170:1507–1512. doi: 10.2214/ajr.170.6.9609163. [DOI] [PubMed] [Google Scholar]
- 15.Brauner MW, Grenier P, Tijani K, Battesti JP, Valeyre D. Pulmonary Langerhans cell histiocytosis: evolution of lesions on CT scans. Radiology. 1997;204:497–502. doi: 10.1148/radiology.204.2.9240543. [DOI] [PubMed] [Google Scholar]
- 16.Auerswald U, Barth J, Magnussen H. Value of CD-1-positive cells in bronchoalveolar lavage fluid for the diagnosis of pulmonary histiocytosis X. Lung. 1991;169:305–309. doi: 10.1007/BF02714167. [DOI] [PubMed] [Google Scholar]
- 17.Tazi A, Soler P, Hance AJ. Adult pulmonary Langerhans’ cell histiocytosis. Thorax. 2000;55:405–416. doi: 10.1136/thorax.55.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casolaro MA, Bernaudin JF, Saltini C, Ferrans VJ, Crystal RG. Accumulation of Langerhans’ cells on the epithelial surface of the lower respiratory tract in normal subjects in association with cigarette smoking. Am Rev Respir Dis. 1988;137:406–411. doi: 10.1164/ajrccm/137.2.406. [DOI] [PubMed] [Google Scholar]
- 19.Chollet S, Soler P, Dournovo P, Richard MS, Ferrans VJ, Basset F. Diagnosis of pulmonary histiocytosis X by immunodetection of Langerhans cells in bronchoalveolar lavage fluid. Am J Pathol. 1984;115:225–232. [PMC free article] [PubMed] [Google Scholar]
- 20.Xaubet A, Agusti C, Picado C, Gueréquiz S, Martos JA, Carrión M, Agustí-Vidal A. Bronchoalveolar lavage analysis with anti-T6 monoclonal antibody in the evaluation of diffuse lung diseases. Respiration. 1989;56:161–166. doi: 10.1159/000195796. [DOI] [PubMed] [Google Scholar]
- 21.Tazi A. Adult pulmonary Langerhans’ cell histiocytosis. Eur Res J. 2006;27:1272–1285. doi: 10.1183/09031936.06.00024004. [DOI] [PubMed] [Google Scholar]
- 22.Housini I, Tomashefski JF, Jr, Cohen A, Crass J, Kleinerman J. Transbronchial biopsy in patients with pulmonary eosinophilic granuloma: comparison with findings on open lung biopsy. Arch Pathol Lab Med. 1994;118:523–530. [PubMed] [Google Scholar]
- 23.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 24.Merchant RN, Pearson MG, Rankin RN, Morgan WK. Computerized tomography in the diagnosis of lymphangioleiomyomatosis. Am Rev Respir Dis. 1985;131:295–297. doi: 10.1164/arrd.1985.131.2.295. [DOI] [PubMed] [Google Scholar]
- 25.Avila NA, Chen CC, Chu SC, Wu M, Jones EC, Neumann RD, Moss J. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000;214:441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 26.Avila NA, Kelly JA, Dwyer AJ, Johnson DL, Jones EC, Moss J. Lymphangioleiomyomatosis: correlation of qualitative and quantitative thin-section CT with pulmonary function tests and assessment of dependence on pleurodesis. Radiology. 2002;223:189–197. doi: 10.1148/radiol.2231010315. [DOI] [PubMed] [Google Scholar]
- 27.Poletti V, Patelli M, Poggi S, Bertanti T, Spiga L, Ferracini R. Transbronchial lung biopsy and bronchoalveolar lavage in diagnosis of diffuse infiltrative lung diseases. Respiration. 1988;54 (Suppl 1):66–72. doi: 10.1159/000195479. [DOI] [PubMed] [Google Scholar]
- 28.Bonetti F, Chiodera PL, Pea M, Martignoni G, Bosi F, Zamboni G, Mariuzzi GM. Transbronchial biopsy in lynphangiomyomatosis of the lung: HMB-45 for diagnosis. Am J Surg Pathol. 1993;17:1092–1102. doi: 10.1097/00000478-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Guinee DG, Jr, Feuerstein I, Koss MN, Travis WD. Pulmonary Lymphangioleiomyomatosis: diagnosis based on result of transbronchial biopsy and immunohistochemical studies and correlation with high-resolution computed tomography findings. Arch Pathol Lab Med. 1994;118:846–849. [PubMed] [Google Scholar]
- 30.Carrington CB, Cugell DW, Gaensler EA, Marks A, Redding RA, Schaaf JT, Tomasian A. Lymphangioleiomyomatosis. Physiologic-pathologic-radiologic correlations. Am Rev Respir Dis. 1977;116:977–995. doi: 10.1164/arrd.1977.116.6.977. [DOI] [PubMed] [Google Scholar]
- 31.Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990;323:1254–60. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]