Abstract
Background
B-RafV600E is a frequent mutation in anaplastic thyroid cancers and is a novel therapeutic target. We hypothesized that PLX4720 (an inhibitor of B-RafV600E) and thyroidectomy would extend survival and decrease tumor burden in a mouse model.
Methods
Orthotopic anaplastic thyroid tumors were induced in SCID mice. Mice were treated with PLX4720 or vehicle after seven days of tumor growth, and thyroidectomy or sham surgery was performed at day 14. The neck space was re-explored and tumor volume was measured at day 35. Mice were sacrificed when they lost >25% of their initial weight.
Results
All five mice that received the vehicle developed cachexia, had invasive tumors (average 61 mm3), and were sacrificed by day 35. All six mice receiving PLX4720+sham had small tumors (average 1.3 mm3) and maintained their weight. 3/6 mice receiving PLX4720+thyroidectomy had no evidence of tumor at 35 days; the other three mice had small tumors (average 1.4 mm3) and showed no signs of metastatic disease. All mice treated with PLX4720 were alive and well-appearing at 50 days.
Conclusions
Thyroidectomy with neoadjuvant PLX4720 could be an effective therapeutic strategy for early anaplastic thyroid cancers that harbor the B-RafV600E mutation and are refractory to conventional therapeutic modalities.
Introduction
Anaplastic thyroid cancer is a rare, but deadly disease, and no effective systemic therapies are currently available. While the incidence of ATC is only 1–2 per million1, nearly everyone who is diagnosed with ATC will die from this disease with the exception of a few rare cases2. Patients with ATC typically present with a rapidly expanding neck mass, metastatic lymphadenopathy, and vocal cord paralysis because of invasion into the recurrent laryngeal nerve. Additionally, most patients have distant metastatic disease to the lungs and bone at the time of presentation; consequently, the median survival after diagnosis is only 6 months3.
At an early stage of the disease, surgery offers the best chance to cure ATC when the tumor is intra-thyroidal1. However, since ATC usually presents as a locally-advanced and unresectable mass, treatments are limited to chemotherapy, external beam radiation, and palliative care4. Therefore, new therapies are urgently needed that exploit recent advances in knowledge of the molecular transformations that drive these aggressive cancers.
The pathogenesis of anaplastic thyroid cancer still remains to be fully elucidated. However, the current prevailing model implicates a progressive de-differentiation from well-differentiated thyroid cancer (WDTC) subtypes. This view is supported by several lines of evidence. First, models of thyroid cancer have demonstrated progression to ATC from WDTC as a result of the loss of the p53 tumor suppressor gene5. Second, WDTC and ATC are known to coexist, and when papillary thyroid cancers (PTCs) and ATCs occur together, they generally share a B-RafV600E mutation in common6. This suggests that the acquisition of additional mutations drives the progression toward ATC. Third, ATCs more frequently occur in areas of endemic goiter/iodine deficiency in patients with incompletely treated WDTC (papillary or follicular)1,3,7.
In contrast to ATC, most well-differentiated thyroid cancers have excellent long term prognoses with standard therapy (thyroidectomy, radioiodine, and TSH-suppression). However, ATCs typically have de-differentiated and lost the ability to take up radioiodoine through the sodium iodine symporter, rendering this therapy ineffective. Additionally, some histologic variants of WDTC display more aggressive behavior (tall cell PTC, insular), and certain genetic mutations (Ras, B-Raf, Ret/PTC, PI3K) have been identified as the driving events in the pathogenesis of these well-differentiated thyroid cancers.
Of the numerous mutations in the mitogen activated protein kinase (MAPK) pathway, BRafV600E is the most common genetic alteration in papillary thyroid cancer and is associated with aggressive phenotypes, such as those with extrathyroidal extension and distant metastases8. B-RafV600E is a valine-for-glutamate substitution at position 600 of the B-Raf protein, and this mutation results in constitutive activation of the (MAPK) pathway. Since this mutation is present in 29–85% of PTCs8,9 and 25% of ATCs, B-RafV600E is a potential therapeutic target for these tumors.
PLX4720 is an orally-bioavailable novel selective inhibitor of B-RafV600E that inserts into the ATP-binding domain of B-RafV600E, which stabilizes the protein in an inactive conformation10. The full characterization and pharmacodynamics of PLX4720 have been previously described by Tsai et al10.This kinase inhibitor is selective for the mutant form of BRaf, making it uniquely suited to treat cancers that have B- B-RafV600E as the driving cellular catalyst for their growth and invasion. PLX4720 has demonstrated efficacy in melanoma models in vivo and in vitro10,11, and recent studies have shown efficacy against aggressive thyroid cancer cell lines in vitro12. In the present study, we demonstrate for the first time that neoadjuvant/adjuvant PLX4720 allows for a safe and effective resection of anaplastic thyroid carcinoma in an orthotopic mouse model.
Methods
PLX4720 preparation
PLX4720 was obtained from Plexxikon and dissolved in dimethyl sulfoxide (DMSO) to achieve a concentration of 120mg/ml. From this solution, daily aliquots were suspended in a 1% solution of carboxymethycellulose (Sigma) during the 21 day gavage administration of the drug.
Tumor cell preparation
The authenticated human anaplastic thyroid cancer cell line, 8505c with a known B-Raf V600E mutation, was cultured in 10 cm dishes and grown in RPMI medium with 10% FBS, penicillin, streptomycin, and amphotericin at 37 degrees C with 5% CO2 atmosphere. On the day of tumor implantation, the cells were trypsinized, gently centrifuged, and resuspended in serum-free RPMI to achieve a cell suspension concentration of 5×105 / 10 µL. The cells were kept on ice until implantation.
Tumor Implantation
The animal experiments were done in the animal facility at Massachusetts General Hospital (Boston, MA) in accordance with federal, local, and institutional guidelines. Seventeen severe combined immunodeficient (SCID) 6 week old female mice (Charles River Laboratories, Wilmington, MA) were anesthetized using 200 µl of a solution of 90 mg/ml ketamine and 10 mg/ml xylazine per mouse. The neck was shaved and prepared with betadine. The skin was incised, and the salivary glands were reflected superiorly. The central component of the neck was exposed and the strap muscles were bluntly dissected away from the right thyroid lobe. Once the right thyroid was exposed, 5×105 8505c cells in 10 µl of serum-free RPMI medium were injected into the thyroid using a Hamilton syringe (Fisher Scientific, USA) attached to a 27 gauge needle. The salivary glands were repositioned over the anterior neck, and the incision was closed with two or three interrupted 3-0 nylon sutures. The mice were placed under a warming lamp during recovery from anesthesia. We defined the day of tumor implantation as day 0 of this experiment.
Thyroidectomy
Mice have two anatomically separate lobes of the thyroid, and therefore one lobe could be completely resected without concern for bilateral recurrent laryngeal nerve injury or hypoparathyroidism. For the purpose of this experiment, ‘thyroidectomy’ refers to a right hemithyroidectomy and surrounding resection of orthotopic tumor.
After the initial implantation of tumor cells, mice were randomized into one of three groups: 1) Sham surgery + Vehicle (n=5); 2) Sham surgery + PLX4720 (n=6); or 3) Thyroidectomy + PLX4720 (n=6). After one week of tumor growth, animals were treated with either 30 mg/kg/day of PLX4720 by oral gavage (Plexxikon Inc., Berkeley, CA, USA) or vehicle control once daily for 21 days. At day 14 after tumor implantation, all mice underwent thyroidectomy or sham surgery.
In the thyroidectomy group, mice were anesthetized and prepped as previously described. The neck space was re-explored and each tumor was identified lying adjacent to the right aspect of the trachea extending as an outward growth from the right thyroid lobe. All 6 mice had evidence of small tumors prior to resection. The tumor and right thyroid were visualized with a 10× dissecting microscope and then were carefully dissected free from the trachea en bloc using microdissection scissors and fine-tipped forceps. Care was taken to avoid injury to the underlying trachea and the adjacent right carotid artery. The surgical specimens were fixed in 10% formalin and processed for histological analysis. Mice in the sham surgery group underwent the same preparation and dissection; however, no tissue was resected (n=11). The wounds of all mice were closed with 2–3 interrupted nylon sutures, triple antibiotic ointment was applied to the wound, and the mice were placed under a warming lamp for recovery.
Histological Analysis
Body-weight (grams) was measured by an electronic digital scale (Ohaus) on a weekly basis. When the mice lost 25% of their baseline weight or had evidence of tracheal compression symptoms, they were sacrificed. Mice not meeting these criteria by 50 days post-tumor implantation were sacrificed. The tumor, thyroid, trachea, esophagus, and surrounding tissues were removed en bloc; the lungs were removed separately. These tissues were fixed in 10% formalin, embedded in paraffin blocks, and sectioned at 5 mm cuts for hematoxylin and eosin (H&E) staining. Slides of the tumors were evaluated for invasion into the trachea, esophagus, and surrounding structures. Whole lung mount sections were obtained, and the metastases were counted per section of lung in each animal.
Tumor Volume Measurement
On day 35 after tumor implantation, all mice underwent surgical exploration and tumor measurement with electronic digital callipers. Tumor volume was calculated as (1/2) × length × width × height. On the last day of the experiment (day 50), all surviving mice (n=12) underwent a second tumor measurement and were sacrificed for tissue processing.
Proliferative Index
Nuclear immunostaining for Ki-67 was evaluated in tumor cells on formalin-fixed, paraffin-embedded tissue. Samples were evaluated after a three week period of either PLX4720 treatment (30 mg/kg/day by oral gavage) or vehicle to assess the in vivo proliferation activity. Three samples per treatment group were evaluated for KI-67 immunostaining positivity at 400x, and the percent of positive cells was determined and averaged from 4 areas of each tumor.
Statistical Analysis
Continuous variables were analysed using a two-tailed independent T-test using Microsoft Excel software. An alpha level of 0.05 was used as a measure of statistical significance. Data are reported at +/− standard error of the mean (SEM).
Results
Weight loss and clinical exam
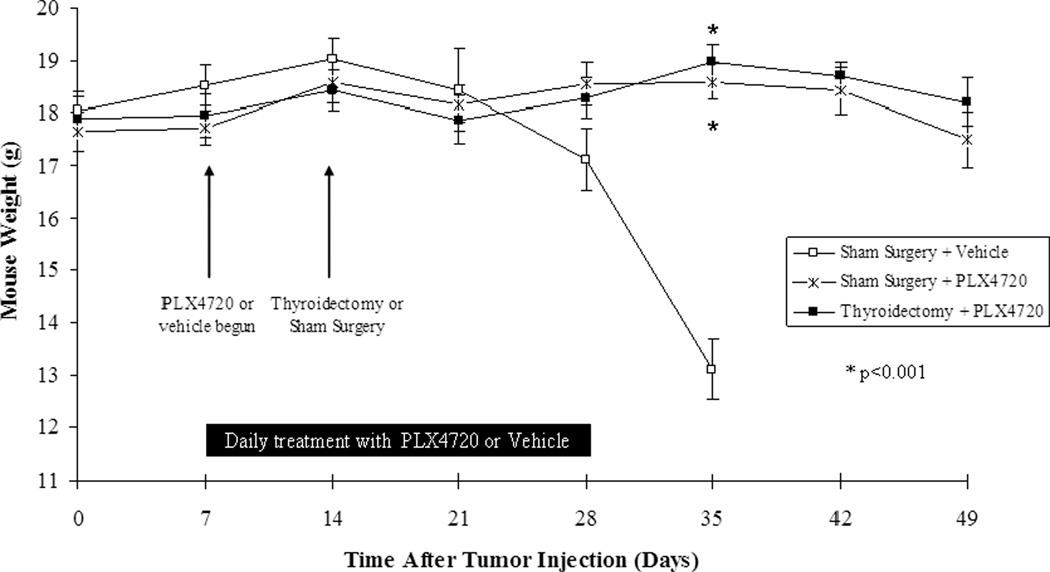
Mice in the Sham surgery+vehicle group began to lose weight by day 28 after tumor implantation, developed progressive cachexia, and all met criteria to be sacrificed by day 35 as shown in figure 1 (average weight loss: 27% from baseline, n=5). However, mice in both groups treated with PLX4720 had maintained their baseline weight significantly greater than the mice treated with vehicle (Sham+PLX4720: average 1% weight loss from baseline, p<0.001 and Thyroidectomy+PLX4720: average 2% weight gain from baseline, p<0.001 compared to vehicle). Additionally, all PLX4720-treated mice showed no evidence of systemic illness (cachexia, piloerection) throughout the 50-day course of this experiment. There was no significant difference in weight between both groups treated with PLX4720 (p=0.3).
Figure 1. Mouse weight.
Mice were treated with PLX4720 or vehicle at 7 days post tumor implantation. At day 14, thyroidectomy or sham surgery was performed. Sham Surgery+vehicle-treated mice began to lose weight by 28 days and were cachectic by day 35. Mice in both groups treated with PLX4720 maintained their weight and showed no signs of cachexia.
Tumor Volume
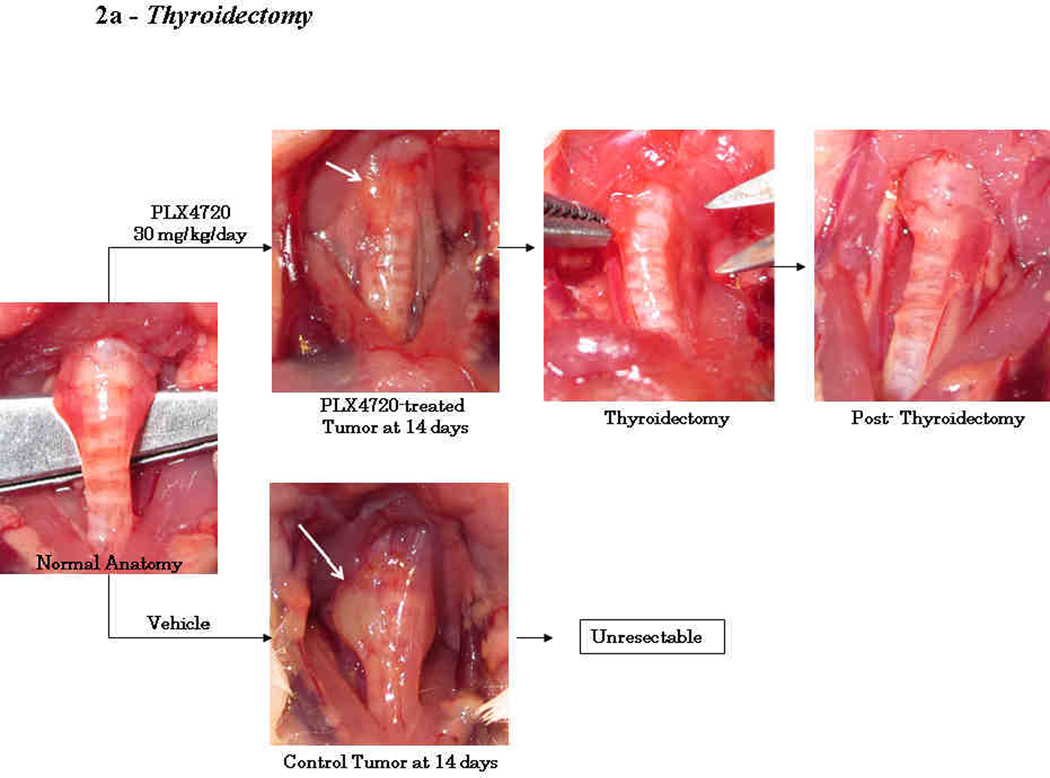
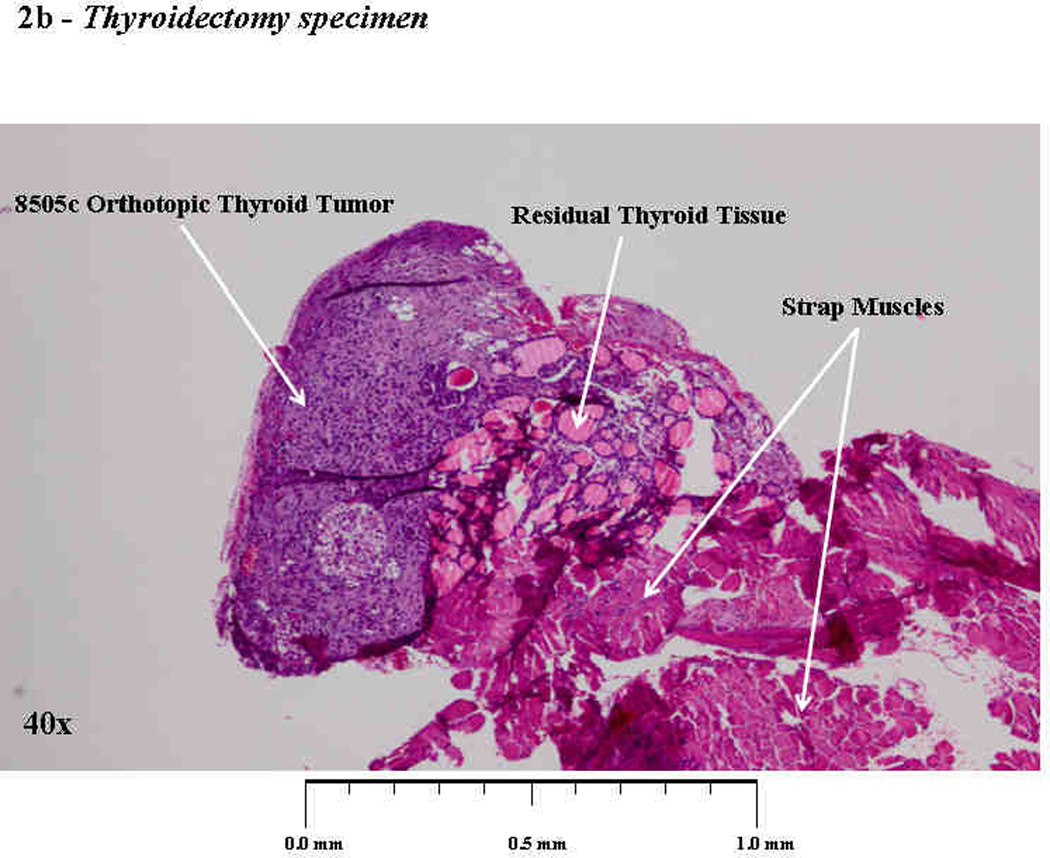
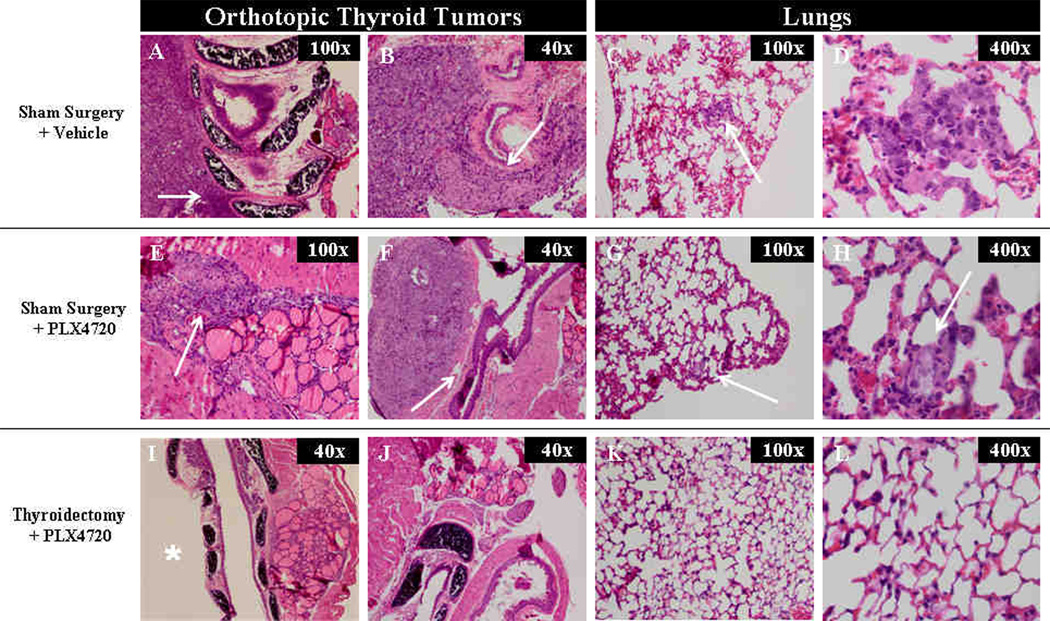
On the day of thyroidectomy or sham surgery (day 14), all 17 mice were confirmed to have tumors of the right thyroid lobe. All 5 mice treated with the vehicle had firm, fixed tumors that were invading into the trachea, esophagus, and/or carotid artery (Fig 2a). Since these untreated tumors were unresectable by day 14, no Thyroidectomy+Sham group was attempted in this experiment. Conversely, mice given neoadjuvant PLX4720 (n=12) were found to have small tumors (approximately 1–2 mm3) that were mobile, well confined masses and only minimally adherent to the surrounding strap muscles (Fig. 2a). Six of these mice underwent resection of the mass and right thyroid lobe; these specimens were processed for histology to confirm an adequate resection (Fig. 2b).
Figure 2. Thyroidectomy.
At 14 days, tumors treated with neoadjuvant PLX4720 were small (1–2 mm3), well-circumscribed, mobile, and were resected with the right thyroid lobe. Permanent section of the surgical specimen in figure 2b shows a completely resected orthotopic tumor and right thyroid lobe. Conversely, tumors treated with vehicle were larger (20–25 mm3), invasive into the trachea, and unresectable (2a).
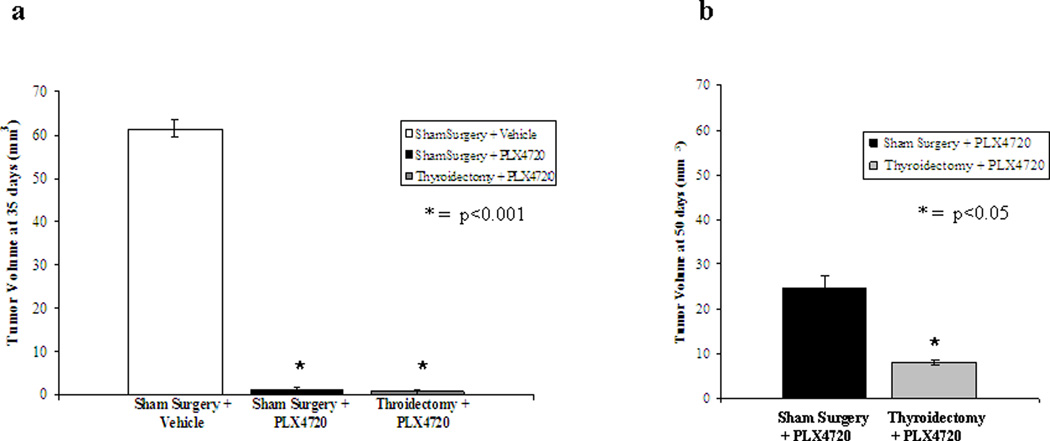
On day 35, we found vehicle-treated mice to have large tumors (average 61mm3) that grew circumferentially around the trachea and invaded surrounding structures. All 6 mice in the Sham Surgery+PLX4720 group had small, well-defined tumors (average 1.3 mm3, p<0.001 compared to vehicle) that extended as an outgrowth from the normal right thyroid lobe. There was no gross evidence of invasion of the trachea, esophagus, or carotid artery. Three of six mice treated with thyroidectomy showed no evidence of tumor on re-exploration at 35 days, and the other three mice had small masses in the thyroid bed similar in size and appearance to the shamtreated group (average 1.4 mm3, p<0.001 compared to vehicle), figure 3a.
Figure 3. Tumor volume at 35 and 50 days.
(a) At 35 days, tumor volume was measured in vivo in all mice. Sham surgery+vehicle mice (n=5) had large tumors (61 mm3) that were invasive and grew circumferentially around the trachea. Sham surgery+PLX4720 treated mice (n=6) all had small tumors (1.3 mm3) that were only minimally adherent to the trachea. Three mice in the Thyroidectomy+PLX4720 group had no evidence of recurrent tumor in the neck on exploration and three had small tumors (average 1.4 mm3) that were minimally adherent to the trachea. (b) At 50 days, only PLX4720-treated mice were alive. All Sham Surgery+PLX4720 mice had medium sized tumors (25 mm3). Thyroidectomy+PLX4720 mice had small tumors (ave. 8 mm3) and 2/6 mice had no evidence of recurrent tumor.
At the end of the experiment (day 50), only the mice receiving PLX4720 were surviving, and these tumors were measured again to evaluate the tumor reactivation. At 50 days, Sham + PLX4720 tumors had an average volume of 25 mm3 +/− 8.2 mm3 compared with Thyroidectomy + PLX4720 tumors that had an average volume of only 8 mm3 +/− 3.6 mm3 (p<0.05), figure 3b. Two of the six mice in the thyroidectomy group had no evidence of recurrent tumor.
Histology and Lung Metastases
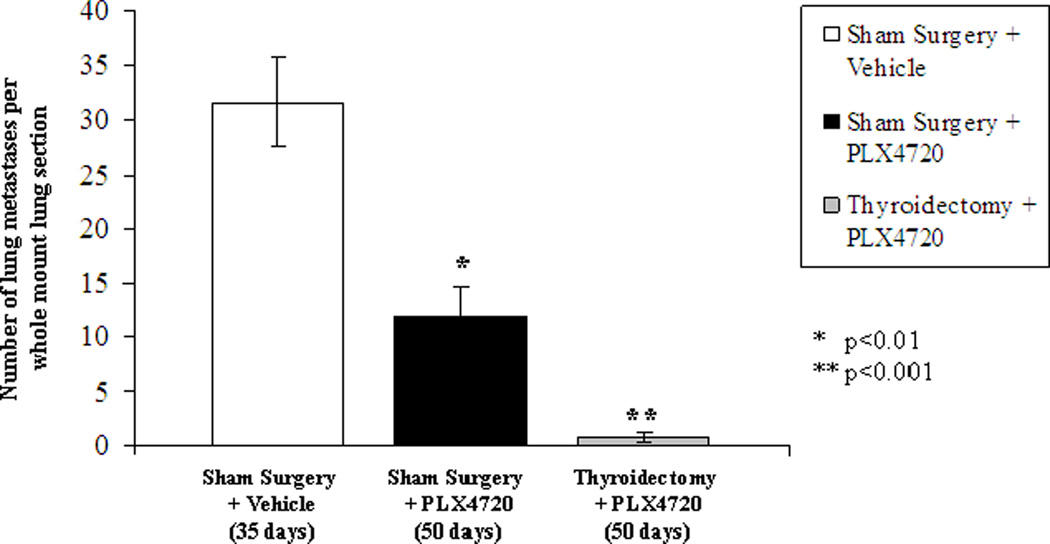
All mice in the Sham Surgery+Vehicle group had high grade anaplastic thyroid cancers with invasion into the trachea (Fig 4A) and esophagus (Fig 4B). Additionally, this group of mice had large and numerous lung metastases (average 32/lung section at 35 days) with evidence of active mitosis within these foci. Mice receiving sham surgery and PLX4720 had small tumors (4E) that did not invade the trachea or esophagus (4F), but were confined masses with minimal invasion of the surrounding muscle tissue. There were significantly fewer lung metastases in Sham Surgery+PLX4720-treated mice compared to controls as shown in figure 5 (11.8 metastases /lung section +/−2.8 at 50 days, p<0.01). In addition to being fewer in number, the metastases were smaller in size (4H). Two mice in the Thyroidectomy+PLX4720 group showed no evidence of tumor in the neck (4I, 4J). One mouse had a microscopic focus of tumor near the resection bed, and the other three mice had small tumors that were similar to the Sham+PLX4720 group. Four of six mice receiving thyroidectomy and PLX4720 showed no evidence of lung metastases (4K, 4L) and the other two mice had an average of 2 metastases / lung section at 50 days (p<0.001 compared to controls) as shown in figure 5.
Figure 4. Histology.
Sham surgery + vehicle (control) mice had histologic evidence of tracheal (a) and esophageal (b) invasion. There were numerous large lung metastases (c, d). Sham surgery+PLX4720 treated mice had small tumors with minimal evidence of invasion (e,f). Lung metastases in this group were smaller (g,h) compared to controls. 2 mice in the Thyroidectomy+PLX4720 group had no evidence of tumor (I*, J), and four/six mice had no evidence of lung metastases (k,l).
Figure 5. Lung Metastases.
The number of lung metastases per whole mount section of lung tissue was evaluated on H&E staining. Sham surgery+vehicle (control) mice had numerous metastases in all mice (ave. 32/ lung section at 35 days). Sham surgery+PLX4720 mice had significantly fewer metastases compared to controls (Ave. 12/ lung section at 50 days, p<0.01). Thyroidectomy+PLX4720 mice had the fewest metastases compared to controls (Ave. = 1/section at 50 days, p<001), which includes 4 mice with no evidence of metastatic disease
KI-67 Proliferative Index
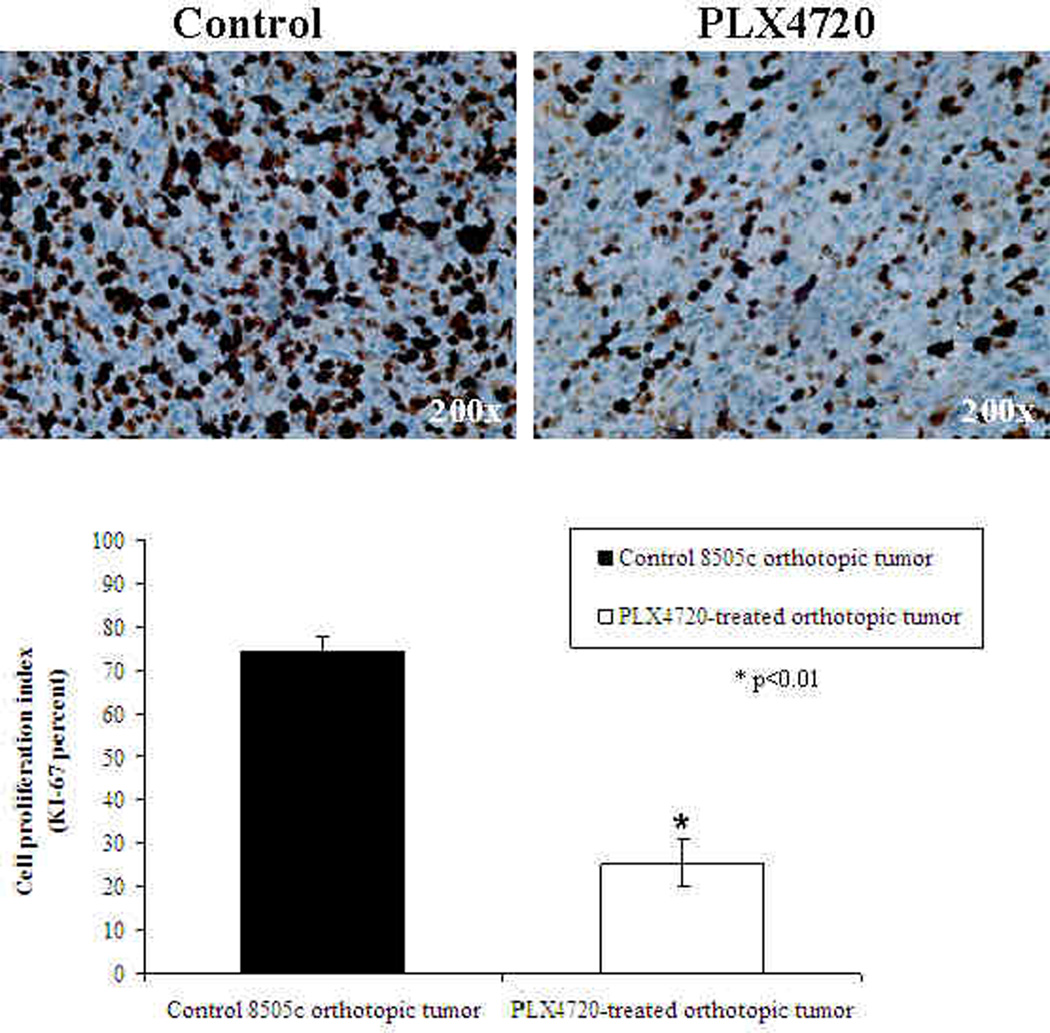
8505c orthotopic tumors from control mice had a strong KI-67 immunostaining (average 74% +/− 3.5%) as shown in Figure 6. This finding of mitotically active tumor cells is consistent with the large and invasive tumors that were found on histology. In contrast, PLX4720-treated tumors had significantly reduced proliferation by KI-67 immunostaining (average 25% +/− 5.4% p<0.01) after three weeks of treatment compared to the controls.
Figure 6. KI-67 Proliferation Index.
8505c orthotopic tumors from control mice had a strong KI-67 immunostaining (average= 74% +/− 3.5%). PLX4720-treated tumors, however, had significantly reduced proliferation (average = 25% +/− 5.4% p<0.01) after three weeks of treatment.
Discussion
Here we describe for the first time the use of PLX4720, a selective B-RafV600E inhibitor, as neoadjuvant therapy prior to thyroidectomy for anaplastic thyroid cancer in SCID mice. This model of anaplastic thyroid cancer13 recapitulates its human counterpart with rapid growth of a tracheal mass and invasion of surrounding structures, multiple lung metastases, and inevitable death. It is therefore well-suited to test novel therapeutics and treatment modalities against this uniformly fatal disease. The anaplastic thyroid cancer cell line, 8505c, has a hemizygous B-RafV600E mutation that leads to constitutive activation of the MAPK pathway. This hyper-activation of the MAPK pathway leads to increased cellular proliferation and invasion of surrounding structures in this model.
By inhibiting the B-RafV600E mutant protein with PLX4720, we found that thyroidectomy became a technically-feasible operation whereas controls were unresectable. It is remarkable that this potent targeted therapy was able to suppress anaplastic thyroid tumor growth enough to make surgical removal of the thyroid possible after only 1 week of neoadjuvant PLX4720 treatment. It is also notable that all PLX4720-treated mice survived until day 50, whereas all control mice were cachectic and died by day 35 (a 40% extension of lifespan with PLX4720). Additionally, the dramatically decreased tumor progression in PLX4720-treated animals illustrates the efficacy of this targeted therapy (97% decreased tumor size compared to controls). Despite this inhibition of tumor progression, we did observe reactivation of growth in tumors in the PLX4720-treated groups after the drug was stopped. This suggests that PLX4720 has the ability to inhibit tumor growth, but that it must be continued for its effects to be lasting. The potential for this tumor (8505c) to evolve resistance around B-RafV600E inhibition after prolonged therapy has not been evaluated but should be tested, particularly for patients with metastatic disease or those who cannot undergo thyroidectomy.
In our study, thyroidectomy added an additional benefit to PLX4720 alone, as we found an average tumor volume of only 8mm3 at 50 days in the thyroidectomy+PLX4720 group compared to 25mm3 in the Sham+PLX4720 group. In this model, two of six mice that underwent thyroidectomy had no evidence of disease in the thyroid bed or lungs at 50 days. It is unclear if the other four mice treated with thyroidectomy had an incompletely resected tumor or whether there was spillage into adjacent perithyroidal tissues during tumor induction. In either case, it is likely that PLX4720 would have had an even more dramatic impact on tumor burden and survival if it were continued beyond three weeks of therapy. While this study focused on the treatment of early anaplastic thyroid cancers, the feasibility of treating late-stage/metastatic thyroid cancers remains an area of ongoing investigation and will provide further insights into this drug’s potential for human clinical trails.
An important consideration for the application of this model toward human disease is that only 25% of human anaplastic thyroid cancers harbor B-Raf mutations. Recent in vitro evidence suggests that selective B-Raf inhibitors, such as PLX4720, may actually activate the MAPK pathway in melanoma and colon cancer cell lines with wt B-Raf and Ras mutations in vitro14. PLX4720 can stabilize the heterodimerization of B-Raf to C-Raf, and this interaction can lead to MEK phosphorylation and progression of the MAPK pathway14–16. In vitro testing of thyroid cancer cell lines with wt B-Raf and RET/PTC mutations in our laboratory confirm this finding; however, wt B-Raf thyroid cancer cells do not gain the ability to migrate or invade with PLX4720 treatment (data not shown). This suggests that PLX4720 would not cause selection of wt B-Raf clones within a tumor with a heterogeneous gentotype, although this specific question has not yet been tested. Therefore, it is imperative that patients are appropriately stratified according to the genetic profile of their disease prior to anti-B-Raf therapy until this important question can be answered.
Targeted therapies such as PLX4720 hold great potential for treating anaplastic or aggressive papillary thyroid cancers that harbor a B-RafV600E mutation in appropriately selected patients. It is possible that neoadjuvant treatment of an unresectable anaplastic thyroid cancer with PLX4720 could inhibit growth and induce tumor regression to a point that would allow a safe and effective tumor resection. By implementing treatments that exploit the signature molecular events that drive the progression of these aggressive cancers, we will likely expand the spectrum of disease for which surgery is a curative treatment.
Acknowledgements
This work was supported by the NIH T32 grant #5T32 DK007754-10. We are grateful to Dr. Gideon Bollag, Dr. Paul Lin, and Plexxikon for providing PLX4720 and technical assistance for its administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinology & Metabolism Clinics of North America. 2008;37(2):525–538. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Pichardo-Lowden A, Durvesh S, Douglas S, Todd W, Bruno M, Goldenberg D. Anaplastic thyroid carcinoma in a young woman: a rare case of survival. Thyroid. 2009;19(7):775–779. doi: 10.1089/thy.2009.0025. [DOI] [PubMed] [Google Scholar]
- 3.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Annals of Surgical Oncology. 2006;13(4):453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Pasieka JL. Anaplastic thyroid cancer. Current Opinion in Oncology. 2003;15(1):78–83. doi: 10.1097/00001622-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Fagin JA. Molecular genetics of human thyroid neoplasms. Annual Review of Medicine. 1994;45:45–52. doi: 10.1146/annurev.med.45.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Modern Pathology. 2004;17(11):1359–1363. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66(2):321–330. doi: 10.1002/1097-0142(19900715)66:2<321::aid-cncr2820660221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Nucera C, Goldfarb M, Hodin R, Parangi S. Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E) Biochimica et Biophysica Acta. 2009;1795(2):152–161. doi: 10.1016/j.bbcan.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 10.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell & Melanoma Research. 2008;21(5):534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, et al. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. Journal of Clinical Endocrinology & Metabolism. 95(1):450–455. doi: 10.1210/jc.2009-0373. [DOI] [PubMed] [Google Scholar]
- 13.Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 2009;19(10):1077–1084. doi: 10.1089/thy.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 15.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]