Abstract
Many morphogenetic movements during development require the formation of transient intermediates called rosettes. Within rosettes, cells are polarized with apical ends constricted towards the rosette center and nuclei basally displaced. Whereas the polarity and cytoskeletal machinery establishing these structures has been extensively studied, the extracellular cues and intracellular signaling cascades that promote their formation are not well understood. We examined how extracellular Fibroblast growth factor (Fgf) signals regulate rosette formation in the zebrafish posterior lateral line primordium (pLLp), a group of ∼100 cells that migrates along the trunk during embryonic development to form the lateral line mechanosensory system. During migration, the pLLp deposits rosettes from the trailing edge, while cells are polarized and incorporated into nascent rosettes in the leading region. Fgf signaling was previously shown to be crucial for rosette formation in the pLLp. We demonstrate that activation of Fgf receptor (Fgfr) induces intracellular Ras-MAPK, which is required for apical constriction and rosette formation in the pLLp. Inhibiting Fgfr-Ras-MAPK leads to loss of apically localized Rho-associated kinase (Rock) 2a, which results in failed actomyosin cytoskeleton activation. Using mosaic analyses, we show that a cell-autonomous Ras-MAPK signal is required for apical constriction and Rock2a localization. We propose a model whereby activated Fgfr signals through Ras-MAPK to induce apical localization of Rock2a in a cell-autonomous manner, activating the actomyosin network to promote apical constriction and rosette formation in the pLLp. This mechanism presents a novel cellular strategy for driving cell shape changes.
Keywords: Fgfr-Ras-MAPK, Rho-kinase, Apical constriction, Lateral line primordium
INTRODUCTION
In many species, apical constriction drives cell shape changes that are important for diverse developmental processes including gastrulation and neural tube closure (Sawyer et al., 2010). This cellular behavior is typically dependent on contraction of an actomyosin network composed of non-muscle myosin (Myosin II) and an apical meshwork of filamentous actin (F-actin). To form this actomyosin network, an apical domain is established through Par protein activity (Suzuki and Ohno, 2006) and the microtubule organizer γ-tubulin is apically localized. Subsequently, cytoskeletal components including F-actin, Myosin II and adherens junctions accumulate apically. Finally, the molecular motor Myosin II is activated by phosphorylation of Myosin regulatory light chain (MRLC), relieving auto-inhibition and promoting constriction (reviewed by Sawyer et al., 2010; Somlyo and Somlyo, 2003). Activation of MRLC is crucial for myosin activity and is mediated by multiple kinases, including Rho-associated kinase (Rock) (Vicente-Manzanares et al., 2009). Whereas the cytoskeletal network driving constriction is fairly well understood, how extracellular cues regulate this process during development is largely unknown. Here, we examine how extracellular signals regulate apical constriction during rosette formation in the zebrafish posterior lateral line primordium (pLLp).
The pLLp is a group of ∼100 cells that migrates along the developing embryonic trunk between 22 and 48 hours postfertilization (hpf). The pLLp is organized into rosettes (Ghysen and Dambly-Chaudière, 2007), within which cells are apically constricted, with basally displaced nuclei (Lecaudey et al., 2008). During migration, the pLLp deposits mature rosettes from the trailing edge that give rise to mechanosensory neuromasts. Concurrently, new cells are generated, polarized and assembled into nascent rosettes in the leading edge (Nechiporuk and Raible, 2008). We and others have previously demonstrated that rosette renewal in the pLLp is dependent on Fibroblast growth factor (Fgf) signaling (Lecaudey et al., 2008; Nechiporuk and Raible, 2008). However, how extracellular Fgf is interpreted intracellularly to control cell shape is not understood.
Fgfs are secreted molecules that promote diverse cellular processes including survival and fate specification. Upon binding to transmembrane receptors (Fgfrs), Fgfs activate multiple intracellular cascades, including the Ras-MAPK (ERK) pathway (Tsang and Dawid, 2004). Ras-MAPK signaling mediates multiple processes ranging from transcription to cytoskeletal remodeling (Tsang and Dawid, 2004). However, whether Ras-MAPK mediates Fgf signaling and cell shape changes in the pLLp is unknown.
We show that the Ras-MAPK pathway is activated in the pLLp in an Fgf-dependent manner, and that Fgfr-Ras-MAPK signaling is required cell-autonomously for apical constriction and apical positioning of Rock, two necessary processes for rosette formation. This previously unrecognized mechanism is crucial for proper pLLp development and might reveal a novel cellular strategy for driving cell shape changes.
MATERIALS AND METHODS
Fish strains, heat shock, pharmacological treatments and live imaging
Adult zebrafish were maintained under standard conditions (Westerfield, 1995). The pLLp was visualized using Tg(–8.0claudinB:lynEGFP)zf106 (Haas and Gilmour, 2006), referred to here as claudinB:EGFP. Ras signaling was conditionally inhibited using Tg(hsp70l:dnHRAS,cryaa:EGFP)pd7 (also called hsp70:dn-Ras) embryos (Lee et al., 2009) heat shocked at 28 hpf for 40 minutes at 38°C, unless otherwise indicated. Inhibitors were diluted in embryo medium containing 1% DMSO and used at the following concentrations unless indicated otherwise: 100 μM SU5402 (Calbiochem), 7 μM PD0325901 (Stemgent) and 50 μM Rockout (Calbiochem). Live imaging was performed as described (Nechiporuk and Raible, 2008).
Plasmids and injections
par3-EGFP plasmid (von Trotha et al., 2006) was modified to express a Par3-TagRFP fusion. par3-TagRFP mRNA was synthesized using the mMessage mMachine Kit (Life Technologies) and microinjected at 500 pg/embryo.
Immunolabeling and in situ hybridization
Immunolabeling followed established protocols (Ungos et al., 2003), with the following exceptions: embryos stained with anti-Rock2a and anti-pMAPK were fixed in Glyo-Fixx (Thermo Scientific) and embryos stained with anti-pMRLC were fixed in Bouin’s fixative (Ortiz-Hidalgo, 1992). Antibodies used: rabbit anti-GFP (1:1000; Invitrogen), mouse anti-GFP (1:1000; Roche), rabbit anti-pMAPK (1:50; Cell Signaling), rabbit anti-Rock2a (1:50; Anaspec), rabbit anti-pMRLC (1:20; Cell Signaling), rabbit anti-γ-tubulin (1:1000; Sigma), rabbit anti-Myosin IIA (1:500; Sigma); and Alexa Fluor 568 phalloidin (1:500; Invitrogen). In situ hybridization was performed as described (Andermann et al., 2002). Digoxygenin-labeled antisense RNA probes were generated for fgfr1 (Scholpp et al., 2004), pea3 (Raible and Brand, 2001) and fgf10a (Grandel et al., 2000). Fluorescently labeled embryos were imaged using an FV1000 confocal microscope (Olympus). Images were processed in ImageJ (Abramoff, 2004); brightness and contrast were adjusted in Adobe Photoshop.
Cell shape and fluorescence intensity analysis
Three-dimensional reconstructions were generated from images of claudinB:EGFP pLLp using Imaris software (Bitplane). Apical constriction indexes (ACIs) were generated from manually collected measurements. Measurements were taken from the surface of each cell oriented toward the midline. Apical width measurements were made 1 μm below the apical surface. Height measurements represent a straight line between the apical and basal surfaces. Rock2a apical intensity was measured from the average fluorescence intensity in the apical domain (apical one-third of the cell) compared with the rest of the cell (the basal two-thirds).
Transplantation experiments
Transplantation experiments were carried out as previously described (Nechiporuk and Raible, 2008). Host embryos expressed claudinB:EGFP, whereas donor cells contained Rhodamine-dextran (Invitrogen). Chimeric embryos were collected at 28 hpf and heat shocked as described above.
Statistical analysis
We used JMP (SAS) to perform one-way ANOVA, Wilcoxon and Tukey-Kramer post-hoc tests. Student’s t-tests were performed in Microsoft Excel. Data are presented as mean ± s.e.m.
RESULTS AND DISCUSSION
Rosette formation is dependent on the Fgfr-Ras-MAPK signaling cascade
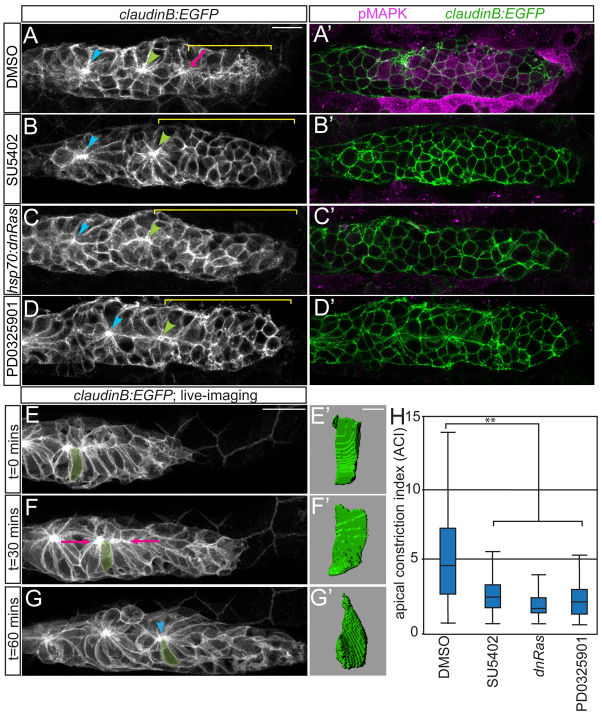
To address how Fgf signals are intracellularly interpreted within the pLLp, we tested whether the MAP kinase (MAPK) signaling pathway, a common mediator of Fgfr signaling, is active in the pLLp. Activation of the MAPK signaling cascade, as detected by phosphorylated MAPK (pMAPK) immunostaining, was observed in pLLp cells and surrounding tissues (Fig. 1A). Although the MAPK expression pattern was somewhat variable, the majority of cells with high levels of pMAPK were positioned in forming or existing rosettes (Fig. 1A). pMAPK was not detectable in the leading-most progenitor cells of the pLLp (McGraw et al., 2011). Blocking Fgfr signaling with the chemical inhibitor SU5402 (Mohammadi et al., 1997) resulted in pMAPK loss, indicating that Fgfr activity is required for MAPK cascade activation (Fig. 1B). Consistent with previous observations (Lecaudey et al., 2008; Nechiporuk and Raible, 2008), inhibiting Fgfr activity also interfered with rosette formation in the leading zone (Fig. 1A,B, yellow bracket). Blocking Ras-MAPK signaling using hsp70:dn-Ras heat-shock-inducible dominant-negative transgenics or the MAPK kinase (MAPKK) inhibitor PD0325901 resulted in loss of the pMAPK signal and a failure of leading rosette formation, phenotypes similar to those observed following Fgfr inhibition (Fig. 1C,D). Additionally, prolonged treatment with PD0325901 caused a progressive expansion of the rosette-free region (data not shown). We thus conclude that Fgfr-Ras-MAPK signaling is necessary for MAPK activation, which is in turn required for rosette formation.
Fig. 1.
Fgfr-Ras-MAPK signaling in the pLLp is required for rosette formation. (A-D) Confocal projections showing rosette formation in the DMSO control (A) and with Fgfr (B), Ras (C) and MAPKK (D) inhibition in claudinB:EGFP zebrafish embryos at 30 hpf. Arrowheads indicate centers of the trailing rosettes. Pink arrow indicates nascent rosette. Brackets indicate rosette-free region. (A′-D′) pMAPK immunolabeling in single planes from projections in A-D. Fgfr and MAPKK inhibition with 100 μM SU5402 and 7 μM PD0325901, respectively, was from 28-30 hpf. Ras inhibition via hsp70:dn-Ras induction was at 28 hpf; embryos were fixed 2 hours following heat shock. (E-G) Stills from time-lapse movie of pLLp in a wild-type claudinB:EGFP embryo (supplementary material Movie 1). (F) Cells align apical ends along the midline (pink arrows). (G) Center of the nascent rosette (arrowhead). (E′-G′) Three-dimensional reconstructions of the highlighted cell in E-G. (H) ACIs for embryos treated with DMSO, SU5402, PD0325901 or following induction of hsp70:dn-Ras. n=180 cells from six embryos per condition. **P<0.0001, Wilcoxon test. Error bars indicate s.e.m. Scale bars: 20 μm in A-G; 4 μm in E′-G′.
Similar to Fgfr inhibition, inhibiting MAPKK repressed Fgf target gene expression (fgfr1 and pea3), suggesting that MAPK mediates Fgf-dependent patterning in the pLLp (supplementary material Fig. S1) (Nechiporuk and Raible, 2008). Combinatorial treatment with suboptimal doses of Fgfr and MAPKK inhibitors between 30 and 48 hpf arrested pLLp migration, implying that Fgf and MAPK function in the same pathway (supplementary material Fig. S2). Together, these data show that MAPK is the intracellular transducer for Fgfr signaling in the pLLp.
The Fgfr-Ras-MAPK signaling cascade is required for apical constriction in leading pLLp cells
Apical constriction has been suggested to underlie pLLp rosette formation (Hava et al., 2009; Chitnis et al., 2012). Cell shape analysis during live imaging of rosette formation revealed that, over a 1-hour period, columnar cells become apically constricted as nascent rosettes form (Fig. 1E-G), confirming that apical constriction promotes rosette formation.
Because blocking Fgfr-Ras-MAPK signaling caused a failure of leading rosette formation, we examined whether Fgfr-Ras-MAPK inhibition disrupts apical constriction. We limited our analyses to the leading 30 pLLp cells, as our live imaging indicated that this population gives rise to new rosettes. We measured apical constriction indices (ACIs; ratios of lateral height to apical width) of pLLp cells after 2 hours of Fgfr, Ras or MAPKK inhibition, and compared them with DMSO-treated controls. Control cells had widely ranging ACIs (Fig. 1H), with cells becoming more constricted as they moved away from the leading edge (supplementary material Fig. 3A,E). By contrast, the ACIs of treated embryos were smaller, as the cells did not constrict (Fig. 1H; supplementary material Fig. S3B-E). From these data, we conclude that Fgfr-Ras-MAPK signaling is required for apical constriction.
Fgfr-Ras-MAPK signaling is not required for subcellular distribution of apical polarity and cytoskeletal components
Analyses in other models have shown that apical polarity establishment and actomyosin network assembly are important for apical constriction (Sawyer et al., 2010). Because Fgfr-Ras-MAPK signaling underlies apical constriction in the pLLp, we examined whether MAPK activation modulates the localization of polarity [Par3 (Pard3) and γ-tubulin] or cytoskeletal (Cadherin 2, F-actin and Myosin II) components. We found that Par3 and γ-tubulin were apically localized in the leading cells, caudal to the first rosette, demonstrating that cells are polarized prior to apical constriction and rosette formation (supplementary material Fig. S4A-H and Table S1). Inhibiting Fgfr or MAPKK did not disrupt the apical localization of these polarity components (supplementary material Fig. S4A-H). Cadherin 2, F-actin and Myosin II were also localized to apical domains, but slightly more rostral than to the polarity markers (supplementary material Fig. S4I-T). The localization of these components was unchanged following inhibition of Fgfr or MAPKK (supplementary material Fig. S4I-T). These data indicate that Fgfr-Ras-MAPK signaling is not required for the localization or assembly of cytoskeletal polarity machinery in the pLLp.
Rock is required for apical constriction and activation of MRLC
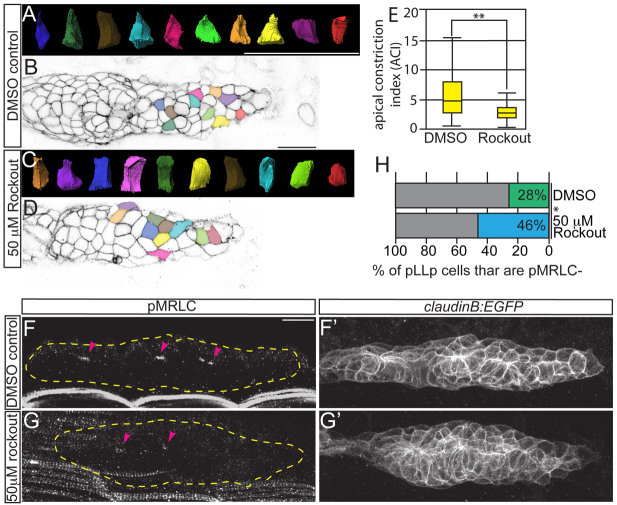
In order for Myosin II to generate force, it must be activated through MRLC phosphorylation (Vicente-Manzanares et al., 2009). Because Rho-associated kinase (Rock) is known to phosphorylate MRLC in other contexts (Ishiuchi and Takeichi, 2011; Plageman et al., 2011), we examined whether Rock is required for myosin activation during rosette formation in the pLLp. We treated embryos with the Rock inhibitor Rockout (Weiser et al., 2007) for 2 hours beginning at 28 hpf, and assayed constriction and MRLC phosphorylation. We observed apical constriction failures in leading-edge cells following Rockout treatment (Fig. 2A-E). Rock inhibition also resulted in failure of MRLC phosphorylation in the leading edge (Fig. 2F-H). From these data, we conclude that Rock activity is required to activate MRLC in the pLLp and to drive apical constriction during rosette formation.
Fig. 2.
Rho kinase activity is required for rosette formation, apical constriction and MRLC activation. claudinB:EGFP zebrafish embryos were treated from 28 to 30 hpf with the Rho kinase inhibitor Rockout. (A-D) ACIs were then measured for the leading-most 30 cells (C,D) and compared with DMSO-treated controls (A,B). Colors of surfaces in A,C correspond to cell positions in B,D. (E) ACI measurements from embryos treated with Rockout or DMSO. n=180 cells from six embryos per condition. **P<0.0001, Wilcoxon test. Error bars indicate s.e.m. (F-G′) Immunolabeling showing loss of pMRLC from the leading region following Rockout treatment as compared with control. (H) Quantification of the leading region, where pMRLC is not detected. *P<0.0001, Student’s t-test. Percentages were derived by counting the number of cells caudal to the distalmost pMRLC signal (i.e. the number of leading cells that lacked the signal) and normalizing to the total number of cells in the pLLp. Scale bars: 20 μm.
Fgfr-Ras-MAPK signaling is required for subcellular distribution of Rock2a
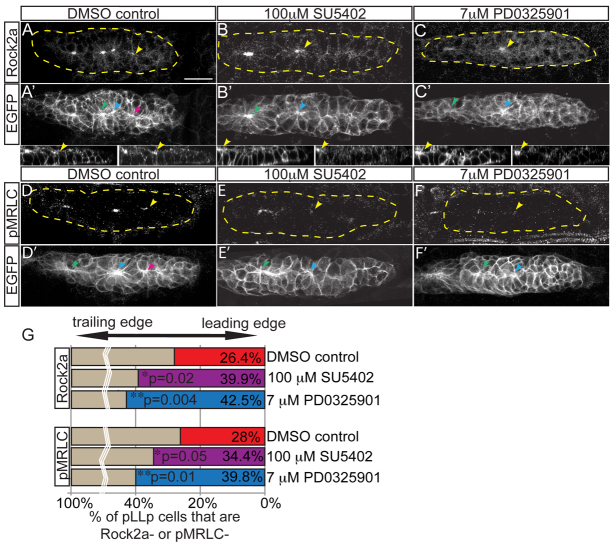
Next, we investigated whether Fgfr-Ras-MAPK regulates the subcellular localization of Rock during pLLp apical constriction. We focused on Rock2a as it is the predominant Rho kinase in neuronal tissues (Amano et al., 2010) and is expressed in the pLLp (supplementary material Fig. S5). A second isoform, Rock2b, does not appear to be expressed in the pLLp (Wang et al., 2011). In control embryos, Rock2a was apically localized in leading cells. However, following Fgfr or MAPKK inhibition, Rock2a failed to segregate apically and appeared dispersed throughout the cell (Fig. 3A-C). rock2a expression was unaffected by MAPKK inhibition, suggesting that MAPK does not regulate rock2a transcription (supplementary material Fig. S5).
Fig. 3.
Fgf and MAPK signaling are required for localization of Rock2a and MRLC activation. (A-F′) Fgfr or MAPKK inhibition with SU5402 or PD0325901, respectively, from 28-30 hpf in claudinB:EGFP zebrafish embryos. Lower panels show sagittal view of leading pLLp region (left, EGFP; right, Rock2a). Post-treatment, Rock2a (A-C) and pMRLC (D-F) were assayed by immunolabeling. Yellow arrowheads indicate the caudal-most apical accumulation of Rock2a. Note that Rock2a is not localized to apical ends of cells in the leading region following treatments. pMRLC staining shows failure of leading-region MRLC activation following treatments. (G) Quantification of the leading region (performed as in Fig. 2H). Rock2a (n=6 embryos; P<0.03, ANOVA) and pMRLC (n=6 embryos; P<0.003, ANOVA) are not apically localized. Note there are fewer leading cells with apically localized Rock2a in Fgf-inhibited (39.9±1.1%) and MAPKK-inhibited (42.5±1.4%) embryos compared with the control (26.4±1.5%). For pMRLC staining: DMSO, 28.0±0.7%; SU5402, 34.4±0.6%; and PD0325901, 39.8±1.0%. Scale bar: 20 μm.
As Rock is required for phosphorylation of MRLC, we examined whether Fgfr-Ras-MAPK activity is also necessary for MRLC phosphorylation. Indeed, pMRLC was lost from leading pLLp cells in embryos treated with Fgfr or MAPKK inhibitors (Fig. 3D-F). This implies that proper Rock2a localization is required for the activation of MRLC. Interestingly, the loss of apical Rock2a and of MRLC activation were unchanged in trailing rosettes following Fgfr-Ras-MAPK inhibition, indicating that this pathway is required for the initiation of Rock2a apical positioning but not its maintenance. Finally, combining suboptimal inhibition of Ras-MAPK signaling with suboptimal inhibition of Rockout resulted in a failure of MRLC activation in the leading pLLp cells (supplementary material Fig. S6 and Table S2), suggesting that Ras-MAPK and Rock2a act in the same pathway.
Ras-MAPK signaling is required cell-autonomously for Rock2a localization and apical constriction
We next investigated whether individual pLLp cells lacking Ras-MAPK signaling are capable of apically constricting in the context of wild-type neighbors. We generated mosaic embryos that contained a small number of hsp70:dn-Ras or wild-type (control) donor cells. Mosaic embryos were heat shocked at 28 hpf to inhibit Ras activity, and pMAPK and apical constriction were assayed at 30 hpf. As expected, induction of dn-Ras in donor cells resulted in loss of pMAPK (Fig. 4A,B). Reconstructed cell shapes from before and after heat shock showed that wild-type cells constricted over the 2-hour period (Fig. 4C). By contrast, the average ACIs of Ras-deficient cells did not change, indicating constriction failures (Fig. 4D-E). Loss of Ras-MAPK activity also corresponded to cell-autonomous loss of apical Rock2a in dn-Ras cells (Fig. 4H-I). However, this loss had no obvious effect on the overall distribution of Rock2a (Fig. 4F-G′). Our data show that individual cells constrict in response to the apical accumulation of Rock2a initiated by intracellular Ras-MAPK, and not as a result of neighbor interactions.
Fig. 4.
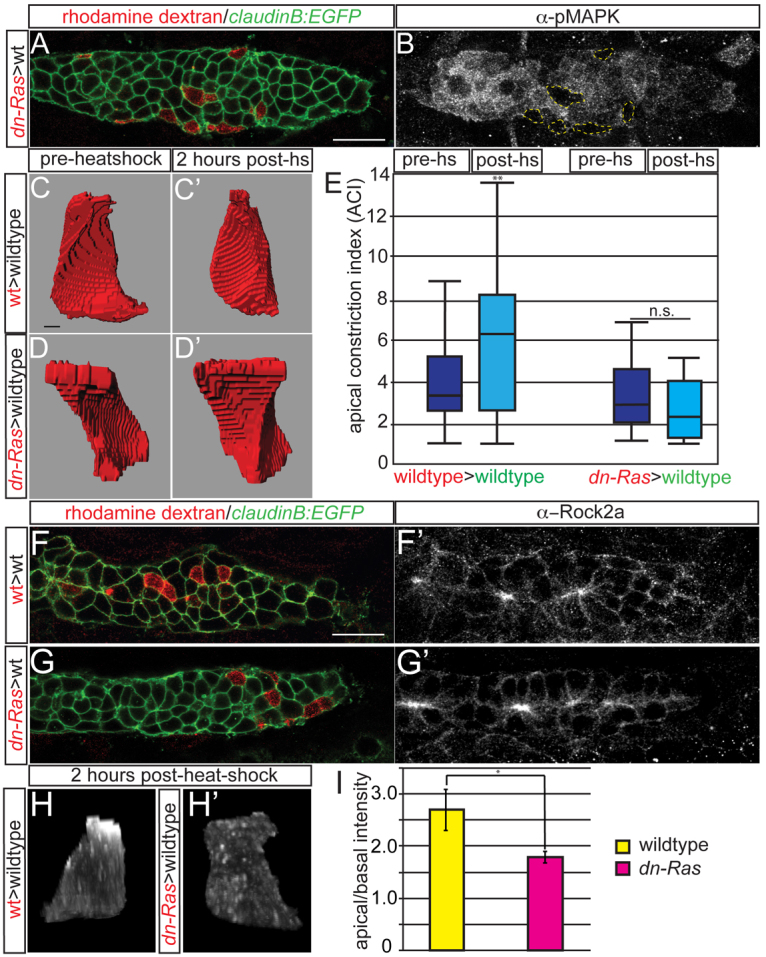
Ras-MAPK signaling mediates cell-autonomous apical constriction and Rock2a localization. (A,B) claudinB:EGFP-positive mosaic zebrafish embryos containing hsp70:dn-Ras donor cells (red) at 30 hpf. Note the lack of pMAPK labeling in dn-Ras cells. (C-D′) Before heat shock, both dn-Ras and wild-type donor cells are columnar; 2 hours after heat shock, the wild-type cell is constricted, whereas the dn-Ras cell remains columnar. (E) Quantification of ACIs of transplanted wild-type and hsp70:dn-Ras cells before and after heat shock. n=50 cells from ten embryos. **P<0.009, Wilcoxon test. n.s., not significant. (F-G′) Transplantation of hsp70:dn-Ras or wild-type cells causes no obvious differences in global Rock2a distribution. (H,H′) Rock2a distribution in a single transplanted cell shows failure of Rock2a apical localization when Ras is inhibited. (I) Ratio of apical to basal fluorescence intensity in transplanted wild-type cells and dn-Ras cells. n=15 cells from four embryos per condition. *P<0.03, ANOVA. Error bars indicate s.e.m. Scale bars: 20 μm in A,F; 2 μm in C.
An intriguing question raised by this work is that of how Fgfr-Ras-MAPK acts to regulate the apical localization of Rock2a. Although Rock does not contain a consensus sequence for MAPK phosphorylation, MAPK might regulate Rock via phosphorylation and suppression of p190A RhoGAP activity and subsequent promotion of RhoA/Rock activity (Pullikuth and Catling, 2010).
Conclusions
Our observations reveal a novel role for Fgf signaling during morphogenesis that might represent a general strategy for controlling apical constriction. Fgf signaling has previously been implicated in apical constriction via activation of basally localized Myosin II during inner ear morphogenesis (Sai and Ladher, 2008). However, our data provide the first example that Fgfr activation, through the Ras-MAPK pathway, can regulate the localization of Rock without affecting other polarity or cytoskeletal components. When combined with the role of Fgf in pLLp hair cell specification (Nechiporuk and Raible, 2008), this model elucidates the elegant manner in which Fgf couples morphogenesis and patterning of the pLLp.
Supplementary Material
Acknowledgments
We thank Philip Stork, Hillary McGraw and Maya Culbertson for comments on the manuscript and Catherine Drerup for experimental assistance.
Footnotes
Funding
This work was supported by funds from the National Institutes of Health [HD055303] and March of Dimes [5-FY09-116] to A.V.N. and a National Science Foundation Graduate Research Fellowship to M.J.H. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.082271/-/DC1
References
- Abramoff M. D. (2004). Image processing with ImageJ. Biophotonics International 11, 36–42 [Google Scholar]
- Amano M., Nakayama M., Kaibuchi K. (2010). Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann P., Ungos J., Raible D. W. (2002). Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 251, 45–58 [DOI] [PubMed] [Google Scholar]
- Chitnis A. B., Dalle Nogare D., Matsuda M. (2012). Building the posterior lateral line system in zebrafish. Dev. Neurobiol. 72, 234–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudière C. (2007). The lateral line microcosmos. Genes Dev. 21, 2118–2130 [DOI] [PubMed] [Google Scholar]
- Grandel H., Draper B. W., Schulte-Merker S. (2000). dackel acts in the ectoderm of the zebrafish pectoral fin bud to maintain AER signaling. Development 127, 4169–4178 [DOI] [PubMed] [Google Scholar]
- Haas P., Gilmour D. (2006). Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell 10, 673–680 [DOI] [PubMed] [Google Scholar]
- Hava D., Forster U., Matsuda M., Cui S., Link B. A., Eichhorst J., Wiesner B., Chitnis A., Abdelilah-Seyfried S. (2009). Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J. Cell Sci. 122, 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi T., Takeichi M. (2011). Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat. Cell Biol. 13, 860–866 [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Cakan-Akdogan G., Norton W. H. J., Gilmour D. (2008). Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135, 2695–2705 [DOI] [PubMed] [Google Scholar]
- Lee Y., Hami D., De Val S., Kagermeier-Schenk B., Wills A. A., Black B. L., Weidinger G., Poss K. D. (2009). Maintenance of blastemal proliferation by functionally diverse epidermis in regenerating zebrafish fins. Dev. Biol. 331, 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw H. F., Drerup C. M., Culbertson M. D., Linbo T., Raible D. W., Nechiporuk A. V. (2011). Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development 138, 3921–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Raible D. W. (2008). FGF-dependent mechanosensory organ patterning in zebrafish. Science 320, 1774–1777 [DOI] [PubMed] [Google Scholar]
- Ortiz-Hidalgo C. (1992). Pol André Bouin, MD (1870-1962). Bouin’s fixative and other contributions to medicine. Arch. Pathol. Lab. Med. 116, 882–884 [PubMed] [Google Scholar]
- Plageman T. F., Jr, Chauhan B. K., Yang C., Jaudon F., Shang X., Zheng Y., Lou M., Debant A., Hildebrand J. D., Lang R. A. (2011). A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination. Development 138, 5177–5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth A. K., Catling A. D. (2010). Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP. Mol. Cell. Biol. 30, 3233–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F., Brand M. (2001). Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech. Dev. 107, 105–117 [DOI] [PubMed] [Google Scholar]
- Sai X., Ladher R. K. (2008). FGF signaling regulates cytoskeletal remodeling during epithelial morphogenesis. Curr. Biol. 18, 976–981 [DOI] [PubMed] [Google Scholar]
- Sawyer J. M., Harrell J. R., Shemer G., Sullivan-Brown J., Roh-Johnson M., Goldstein B. (2010). Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpp S., Groth C., Lohs C., Lardelli M., Brand M. (2004). Zebrafish fgfr1 is a member of the fgf8 synexpression group and is required for fgf8 signalling at the midbrain-hindbrain boundary. Dev. Genes Evol. 214, 285–295 [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. (2003). Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. (2006). The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979–987 [DOI] [PubMed] [Google Scholar]
- Tsang M., Dawid I. B. (2004). Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci. STKE 2004, pe17 [DOI] [PubMed] [Google Scholar]
- Ungos J. M., Karlstrom R. O., Raible D. W. (2003). Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development 130, 5351–5362 [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Trotha J. W., Campos-Ortega J. A., Reugels A. M. (2006). Apical localization of ASIP/PAR-3:EGFP in zebrafish neuroepithelial cells involves the oligomerization domain CR1, the PDZ domains, and the C-terminal portion of the protein. Dev. Dyn. 235, 967–977 [DOI] [PubMed] [Google Scholar]
- Wang G., Cadwallader A. B., Jang D. S., Tsang M., Yost H. J., Amack J. D. (2011). The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer’s vesicle in zebrafish. Development 138, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser D. C., Pyati U. J., Kimelman D. (2007). Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation. Genes Dev. 21, 1559–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd edn. Eugene, OR: University of Oregon Press; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.