Abstract
Neuronal migration, a key event during brain development, remains largely unexplored in the mesencephalon, where dopaminergic (DA) and GABA neurons constitute two major neuronal populations. Here we study the migrational trajectories of DA and GABA neurons and show that they occupy ventral mesencephalic territory in a temporally and spatially specific manner. Our results from the Pitx3-deficient aphakia mouse suggest that pre-existing DA neurons modulate GABA neuronal migration to their final destination, providing novel insights and fresh perspectives concerning neuronal migration and connectivity in the mesencephalon in normal as well as diseased brains.
Keywords: Midbrain, Neuronal migration, Parkinson’s disease
INTRODUCTION
Neuronal migration is a fundamental process in the development of the central nervous system because neurons eventually dwell in regions distinct from their origin. From ventricular zones, neurons and/or neuronal progenitors navigate along diverse courses, radially and tangentially, to their final destination and integrate into specific brain circuits (Corbin et al., 2001; Hatten, 2002; Marín and Rubenstein, 2001; Parnavelas, 2000). A concerted and/or sequentially regulated migration of both excitatory and inhibitory neurons is essential for the emergence of their proper connectivity and brain functions. Unlike in the telencephalon, where neuronal migration has been well elucidated, in the mesencephalon this vital event has been understudied and key factors remain to be defined. Dopaminergic (DA) neurons located in the three anatomically defined areas of the ventral mesencephalon (VM) – the substantia nigra (SN), ventral tegmental area (VTA) and retrorubral field (RRF) – are involved in controlling diverse brain functions, including motor control and cognition, emotion and reward behaviors (Björklund and Dunnett, 2007; Damier et al., 1999; Ding et al., 2011; Lennington et al., 2011; Schultz, 2001; Seeman et al., 1993; Smidt and Burbach, 2007). The migration routes of DA neurons are not well understood and the related literature is contradictory (Hanaway et al., 1971; Kawano et al., 1995), while the route of GABA neurons to the VM is unknown.
Here, we study the migratory trajectories of DA and GABA neurons and find that proper migration of GABA neurons to their final location in the VM is dependent on the pre-existing DA neuron palisade. These results provide several new concepts regarding functional interactions between DA and GABA neurons necessary for their proper migration and final connectivity that may translate into novel understanding of potential etiology as well as therapeutic development for many neurological diseases.
MATERIALS AND METHODS
Animals
Timed pregnant CD1 mice were purchased from Charles River Laboratories. Colonies of GAD65-GFP and ak/ak mice were maintained in our institutional animal facility. Day of plug discovery was designated embryonic day (E) 0. Animal experiments were in full compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the McLean Institutional Animal Care and Use Committee.
BrdU labeling and immunohistochemistry
A single BrdU injection (50 μg/g body weight) was administered to pregnant dams carrying E10 or E11 embryos. Embryonic brains were removed after 2 hours, at E13, E15, E17 and postnatal (P) day 0 stages, immersed in zinc fixative (BD Pharmingen) for 24 hours and processed for paraffin wax histology. BrdU immunohistochemistry was performed on 10-μm sections with a mouse monoclonal anti-BrdU antibody (1:75, BD Pharmingen). Other antibodies used were anti-TH (1:200, Millipore), anti-Otx2 (1:200, Neuromics), anti-GAD65/67 (Gad2/1 – Mouse Genome Informatics) (1:400, Millipore), anti-Lmx1b (1:100; Drs Carmen Birchmeier and Thomas Muller, Max-Delbrück-Center for Molecular Medicine, Berlin, Germany), anti-Foxa2 (1:100, Santa Cruz), anti-Lmx1a (1:100, Millipore), anti-Ki67 (1:30, Sigma), anti-Pax6 (1:30, Sigma), anti-DAT (Slc6a3 – Mouse Genome Informatics) (1:200, Millipore), anti-Helt (1:30, Sigma) and anti-calbindin (1:100, Swant). BrdU+ cells in the red nucleus and BrdU+ GAD65/67+ co-labeled cells in the VM were counted using ImageJ software (NIH).
Explant cultures
Basal plate (BP) and VM explants were dissected from mesencephalic slices of E15 wild-type (WT) and ak/ak embryos. Explants were plated in Matrigel (BD Biosciences) at a distance of 600 μm, overlaid with Neurobasal medium (1×, Invitrogen/Life Technologies) and co-cultured for 36 hours. Explants were fixed in 4% paraformaldehyde, stained with Hoechst (Sigma) and imaged. For quantification, BP explants were subdivided into proximal (P) and distal (D) quadrants. The areas occupied by migrating cells in each quadrant were determined using ImageJ. The P/D ratio was calculated and used as a measure of chemoattraction in each case.
Heterochronic microtransplants
BP tissue obtained from E15 GAD65-GFP mesencephalic slices was inserted into ak/ak mesencephalon using fine tungsten needles under a high-magnification stereomicroscope. For some of these ak/ak slices (with transplanted BP), the VM was discarded and substituted by VM from WT slices. Slices were transferred to polycarbonate membrane filters (Invitrogen) in sterile six-well plates containing Neurobasal medium and cultured for 48 hours. Slices were fixed and TH immunohistochemistry performed. The number of GFP+ cells that migrated to the VM was counted in all slices and average values obtained.
Statistics
Statistical significance of differences between groups was analyzed by two-tailed Student’s t-test (Prism, GraphPad software). Results were expressed as mean ± s.d. and statistical significance reported at P<0.05.
RESULTS AND DISCUSSION
Neuronal migration silhouette in the mesencephalon
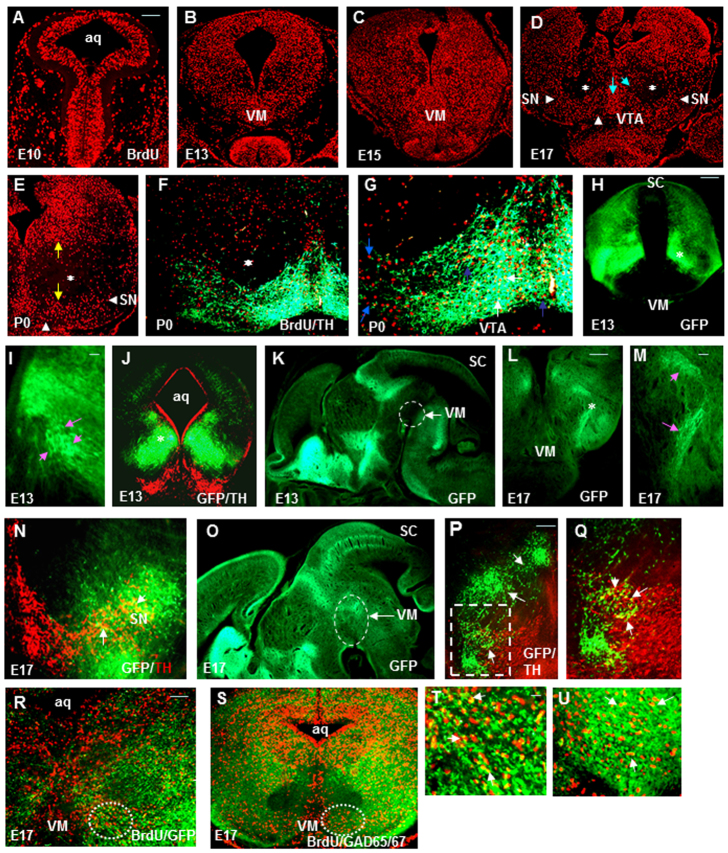
BrdU birthdating studies have been widely used to study neuronal migration in the developing neocortex (López-Bendito et al., 2008; Mathis et al., 2010; Ori-McKenney and Vallee, 2011; Soriano and Del Rio, 1991; Supèr et al., 2000; Wines-Samuelson et al., 2005). Because BrdU is integrated into the DNA of S-phase progenitor cells, it serves as a stable marker for cells born around the time of injection. First, we performed a thorough and systematic BrdU birthdating study to understand mesencephalic neuronal migration in CD1 mice. We labeled neuronal progenitors born at E10 with a single BrdU pulse and followed their migration in the mesencephalon until P0 (Fig. 1A-G). BrdU-labeled cells first spread out uniformly in the mesencephalon from E10-15 (Fig. 1A-C). However, a major change in neuronal migration was observed at E17 (Fig. 1D), by which time neurons had migrated both ventrally and perpendicular to the aqueduct to form the distinct anatomical architecture of the boat-shaped SN and VTA (Fig. 1D). The red nucleus area was significantly depleted of E10-labeled neuronal progenitors by this perpendicular migration. By P0, most of the neurons had segregated to both dorsal and ventral mesencephalon (Fig. 1E). The boat-shaped architecture was confirmed by tyrosine hydroxylase (TH) staining (Fig. 1F,G). E11-labeled neuronal progenitors followed the same route as E10-labeled neuronal progenitors and formed the distinct anatomical architecture of SN and VTA in VM (supplementary material Fig. S1A). Our results corroborate those of previous studies indicating that neurons of the SN and VTA in the mouse are generated on or before E12 (Bayer et al., 1995). E13-labeled neuronal progenitors migrated predominantly to the dorsal mesencephalon and E15-labeled progenitors gave rise to a limited number of lateral neurons (supplementary material Fig. S1B,C).
Fig. 1.
Neuronal migrational profiles in the mesencephalon. (A-G) E10 BrdU-labeled progenitors in the mesencephalon at 2 hours (A), E13 (B), E15 (C), E17 (D) and P0 (E). Blue arrows in D point to ventral and perpendicular migration that depletes the red nucleus of BrdU-labeled cells (white asterisks, D-F) to form the anatomical architecture (arrowheads, D,E) of VTA and SN. Yellow arrows in E show dorsally and ventrally segregated cells. (F) The anatomical architecture of SN and VTA confirmed by co-labeling of BrdU (red) and TH (green). (G) A 20× magnification of F showing that some BrdU+ cells are TH+ (white arrows), whereas others are TH– (blue arrows). (H-K) GABA neurons are absent from the VM in coronal (H-J) and sagittal (K) slices (30 μm) from E13 GAD65-GFP embryos. White asterisk indicates GABA neurons in the basal plate (BP) (H,J) and pink arrows indicate possible ventral migration in a magnified BP (I). (J) No overlap of TH neurons (red) with GABA neurons was seen. (K) Dashed circle indicates the absence of GABA neurons from VM. (L-O) GABA neurons appear in VM in coronal (L-N) and sagittal (O) slices from E17 GAD65-GFP embryos. White asterisk indicates the GABA neuron stream from BP to VM (L), as magnified in M (pink arrows). GABA neurons and TH neurons are in intimate contact (white arrows, N). (O) Dashed circle indicates GABA neurons in the VM. (P,Q) GFP profiles of E17 mesencephalon collected from a second GAD65-GFP transgenic line (GAD65_Ncol/gfp/1e_6e) also showed a similar picture. (P) White arrows indicate GABA neurons descending to VM and integrating with TH+ DA neurons (red). The boxed region in P is magnified in Q and TH-GABA neuron contact is indicated (arrows). (R-U) Birthdating experiment in GAD65-GFP (R,T) and CD1 (S,U) mice. Many of the BrdU+ cells that migrated into VM by E17 were GFP+ (R) or stained positively for GAD65/67 (S). VM regions of R and S are magnified in T and U and co-expressing cell nuclei appear yellow (arrows). n=5. aq, aqueduct; SN, substantia nigra; VTA, ventral tegmentum area; VM, ventral mesencephalon; SC, superior colliculus. Scale bars: 100 μm in A-H,J-L,N-S; 50 μm in I,M,T,U.
Although many BrdU+ neurons in SN and VTA at P0 were dopaminergic/TH+ (Fig. 1F,G), there were also many TH– neurons. Given that DA and GABA neurons constitute two major neuronal populations in the ventral midbrain, these neurons might be GABA neurons. Although GAD mRNA expression starting at E10.5 has been reported in BP, alar plate and dorsal mesencephalon (Guimera et al., 2006; Katarova et al., 2000), and the number, frequency and topography of GABA neurons in SN and VTA regions of the adult brain have been characterized (Korotkova et al., 2004; Nair-Roberts et al., 2008; Olson and Nestler, 2007), it is not known when and how GABA neurons become admixed with ventral mesencephalic DA neurons during development. Are GABA neurons of the VM born elsewhere, then come to reside there to form the final connectivity? To address these fundamental questions we used the GAD65-GFP mouse model, in which GABA neurons can be clearly visualized (López-Bendito et al., 2004). Strikingly, we found that whereas at E13 the VM was completely devoid of GABA neurons (Fig. 1H-K), by E17 GABA neurons were substantially intermingled with DA neurons (Fig. 1L-Q). At E13, many BP GFP+ neurons were oriented ventrally (Fig. 1H,I) and by E17 a cohort of GFP+ neurons were positioned in stream-like routes to the VM (Fig. 1L,M,P,Q). Further, high-magnification images showed how GABA neurons of the VM are in close physical contact with DA neurons (Fig. 1N,P,Q). Birthdating experiments revealed that many E11-labeled neuronal progenitors migrated to contribute to GABA neurons of the VM by E17 (Fig. 1R-U).
Abnormal neuronal migration in the ak/ak mesencephalon
This novel finding that DA and GABA neurons occupy separate territories in the E13 mesencephalon (Fig. 1J) prompted us to hypothesize that the early-formed DA neuron palisade might have a role in GABA neuron migration into the VM at later developmental stages. In line with this possibility, the direction and entry of the GABA neuron stream were oriented towards VM territory (Fig. 1H,I), leading to intimate contact with DA neurons (Fig. 1L-Q). To address our hypothesis and to further investigate mesencephalic neuron migration, we postulated that the Pitx3- deficient aphakia (ak) mouse might provide an ideal animal model with a defective DA neuron architecture as Pitx3 is one of the crucial regulators of mesencephalic DA neuron development (Ding et al., 2011; Kim et al., 2007; Nunes et al., 2003; Smidt et al., 2004; van den Munckhof et al., 2003) and there is selective and early loss of A9 DA neurons in the SN of ak/ak mice (Hwang et al., 2005; Smidt et al., 2004; van den Munckhof et al., 2003).
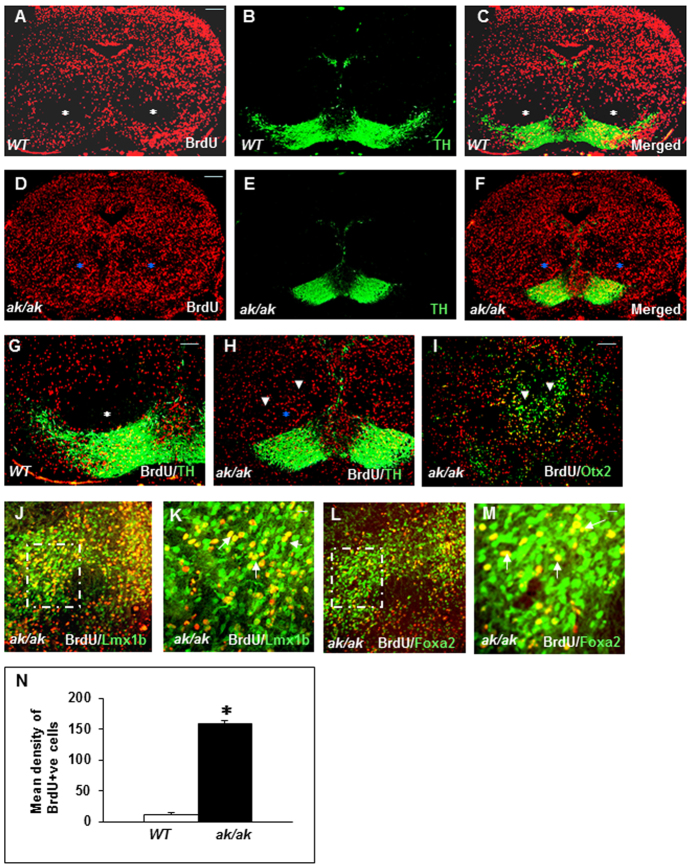
Since expression of both Pitx3 and Th begins at E11, we consistently studied the migration of E11-labeled neuronal progenitors in both WT and ak/ak mice (Fig. 2). Mesencephalic sections were analyzed at E17 with BrdU and TH markers (Fig. 2A-H). Strikingly, in the ak/ak mutant, BrdU+ cells were scattered aberrantly in BP regions and failed in their perpendicular migration to the SN. The distinct anatomical architecture of the boat-shaped SN and VTA outlined by BrdU+ cell migration did not form in the ak/ak mutant (Fig. 2D), in contrast to WT (Fig. 2A; supplementary material Fig. S1A). BrdU and TH double labeling revealed many E11-labeled cells displaying a dopaminergic phenotype after arriving at their final destination in both VTA and SN regions in WT mesencephalon (Fig. 2A-C,G), whereas in the ak/ak mutant the cells were unable to reach the SN and display their full dopaminergic phenotype (Fig. 2D-F,H). Instead, these E11-labeled cells appeared to be stuck or trailing in the middle of their migratory trajectory and distributed abnormally in the red nucleus area in the ak/ak mutant (Fig. 2F,H).
Fig. 2.
Abnormal neuronal migration and distribution of neurons in ak/ak mesencephalon. (A-M) The migrational profile of E11-labeled neuronal progenitors was analyzed in WT (A-C,G) and ak/ak mutant (D-F,H-M) mice at E17. White asterisks indicate areas free of BrdU-labeled cells in WT mesencephalon (A-C,G) and blue asterisks indicate abnormal cell clusters in the red nucleus of the ak/ak mutant (D-F,H). (G,H) Higher magnifications of C and F displaying failed perpendicular migration (arrowheads) in the ak/ak mutant. (I-M) Stalled cells in the ak/ak mutant are double positive for BrdU/Otx2 (arrowheads, I), BrdU/Lmx1b (J,K) and BrdU/Foxa2 (L,M). The boxed regions in J and L are magnified in K and M, respectively, and white arrows indicate double-positive cells. (N) Quantification of E11 BrdU-labeled cells distributed in the red nucleus (both hemispheres) of WT and ak/ak mutant (mean density of BrdU+ cells ± s.d.). A significant increase in BrdU+ cells was observed in the ak/ak mutant. *P<0.0001; n=5. Scale bars: 100 μm in A-J,L; 50 μm in K,M.
To further investigate the abnormally migrating cells, we tested whether the stalled E11-labeled cells in the ak/ak red nucleus area contain DA progenitor cells. Many cells were positive for Otx2 (Fig. 2I), a marker for DA progenitors (Chung et al., 2009; Vernay et al., 2005), but never expressed the TH marker of DA neurons (Fig. 2E,F,H), indicating impaired differentiation. The stalled cells were also positive for the markers Lmx1b (Fig. 2J,K) and Foxa2 (Fig. 2L,M), confirming their DA progenitor identity. They were positive for the proliferating progenitor markers Lmx1a, Ki67 and Pax6 and negative for DAT, a marker of immature postmitotic DA progenitors (supplementary material Fig. S2A-E). In addition, many BrdU+ cells were observed along perpendicular migration routes in a short-pulse experiment at E17 (supplementary material Fig. S2F), confirming the presence of abnormally proliferating progenitors in the ak/ak mesencephalon at late embryonic stages. The mean density of BrdU+ cells in the red nucleus of the ak/ak mutant was significantly higher than in WT (Fig. 2N).
Taken together, our data provide strong evidence of the perpendicular migration of DA neurons to the VM and that this is significantly disturbed in the ak/ak mutant. Thus, in the absence of Pitx3, DA neuronal migration is impaired, contributing to severe loss of A9 DA neurons in the SN. Interestingly, we also found that migration of E13-labeled neuronal progenitors was similarly affected in the ak/ak mutant (supplementary material Fig. S3).
Pre-existing DA neurons modulate GABA neuron migration to ventral mesencephalon
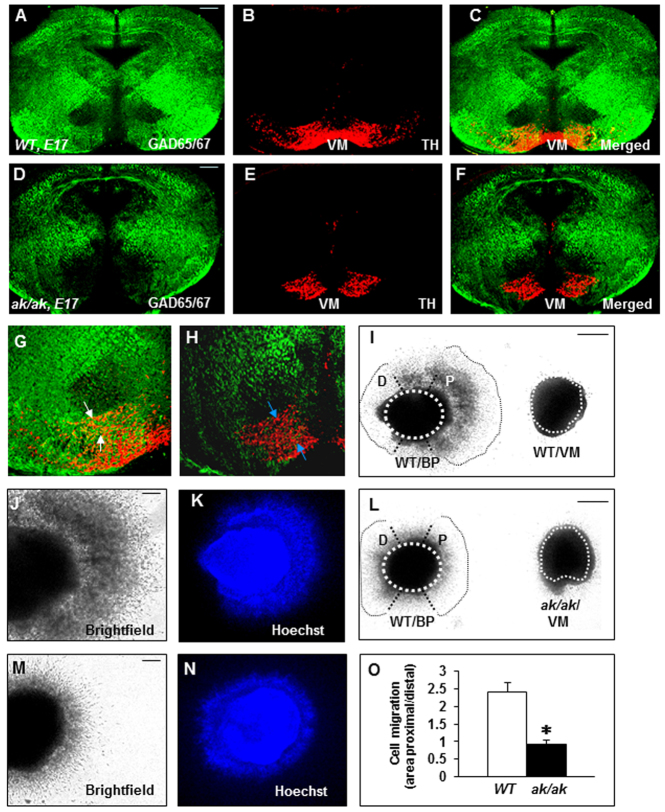
GABA neuron development was also significantly affected in the ak/ak mesencephalon. By E17, in WT mouse embryos GABA neurons had settled together in close physical contact with TH neurons (Fig. 3A-C,G). This profile was substantially altered in the ak/ak mutant, leading to significantly limited contact of these neurons in the VM (Fig. 3D-F,H). GABA neurogenesis was unaffected in the ak/ak mutant (supplementary material Fig. S4). The stalled cells on the route of perpendicular migration or in the VM were not apoptotic (supplementary material Fig. S5), and so we examined whether the decreased GABA neuron profile in the ak/ak mutant by late embryonic stages was due to impaired migration. To search for cellular sources of guidance cues in the VM for migratory BP neurons, explants of VM were confronted with explants of BP from WT mice. BP neurons were markedly attracted towards VM (Fig. 3I-K,O). BP explants from WT mice, by contrast, showed no attraction to VM from the ak/ak mutant (Fig. 3L-O).
Fig. 3.
Impaired GABA neuron development in ak/ak mesencephalon. (A-H) GAD65/67 and TH labeling at E17 in WT (A-C,G) and ak/ak (D-F,H) mouse mesencephalon. Higher magnification of VM from C and F show intimate association of GABA neurons and DA neurons in WT (co-label in yellow, arrows in G) and decreased GAD65/67 label and limited physical contact with TH neurons in the ak/ak mutant (blue arrows, H). (I-O) WT BP explants (outlined) show robust cell migration towards WT VM (I-K,O), whereas WT BP explants fail to migrate towards ak/ak VM (L-O) in proximal quadrants. WT BP explants from I and L are magnified in J and M, respectively. (K,N) Hoechst staining of WT BP explants from I and L, respectively. (O) Quantification of chemoattraction expressed as a ratio of the quadrant (dotted lines) facing the VM explants (proximal, P) relative to the area occupied by cells in the opposite quadrant (distal, D). *P<0.01; n=25; error bars indicate s.d. Scale bars: 100 μm in A-H,J,K,M,N; 200 μm in I,L.
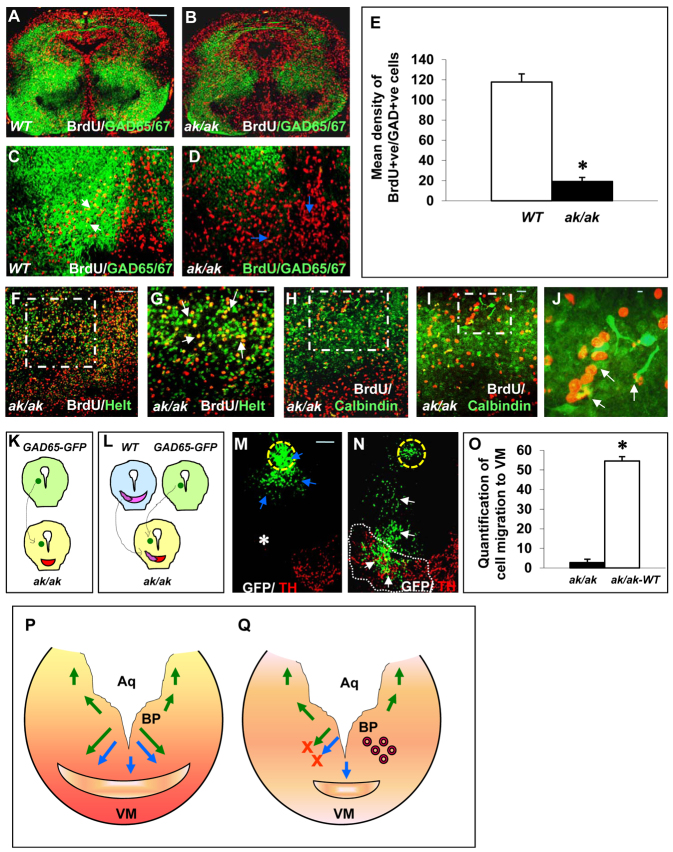
Birthdating studies indicated that E11-labeled neuronal progenitors contributed to GABA neurons of VM in WT embryos and many BrdU+ GAD65/67+ co-labeled cells were observed (Fig. 4A,C). In the ak/ak mutant, E11-labeled neuronal progenitors did not contribute significantly to GABA neurons of the VM, as illustrated by the significant decrease in BrdU GAD65/67 co-labeling (Fig. 4B,D) and mean density of BrdU+ GAD65/67+ cells in the VM area (Fig. 4E). The stalled cells along the perpendicular migration routes expressed the GABA neuron progenitor markers Helt (Fig. 4F,G) and calbindin (Fig. 4H-J). Heterochronic microtransplants were also performed to understand mesencephalic GABA neuron migration. When a BP explant from a GAD65-GFP mouse was transplanted into ak/ak mesencephalon, GFP+ cells appeared to be stalled around the transplantation site and were unable to migrate and integrate into VM (Fig. 4K,M,O). By contrast, when ak/ak VM was substituted with a VM from WT mouse, GFP+ cells exited the transplantation site and migrated robustly to integrate with DA neurons (Fig. 4L,N,O).
Fig. 4.
DA neurons modulate GABA neuron migration to ventral mesencephalon. (A-E) E11-labeled neuronal progenitors were examined at E17 for BrdU and GAD65/67 markers in WT (A,C) and ak/ak mutant (B,D). VM from A and B is magnified in C and D, respectively. White arrows show BrdU GAD65/67 co-labeling in WT mouse and blue arrows indicate the lack thereof in the ak/ak mutant. (E) BrdU+ GAD65/67+ cells in the VM of WT and ak/ak mutant were quantified (mean density of BrdU+ GAD65/67+ cells ± s.d.) and a significant reduction was observed in the mutant. *P<0.0001; n=5; error bars indicate s.d. (F-J) The stalled cells in ak/ak mesencephalon were Helt+ (F,G) and calbindin+ (H-J). The boxed regions in F, H and I are magnified in G, I and J, respectively. Arrows indicate BrdU+ Helt+ (G) and BrdU+ calbindin+ (J) co-labeled cells. (K,L) Scheme of transplantation of GAD65-GFP BP (green circle) and WT VM (pink shape) into ak/ak mesencephalon. Red crescent marks the defective DA neuron architecture of the ak/ak mesencephalon. (M,N) Blue arrows (M) indicate GFP+ cells close to site of transplantation (yellow dotted circle), white asterisk (M) indicates the lack of migration to ak/ak VM, and white arrows (N) indicate significant migration and integration into transplanted WT VM (white border). (O) Quantification of migrated GFP+ cells to ak/ak VM (K,M) and ak/ak VM substituted with WT VM (ak/ak-WT, L,N). *P<0.0001; n=25; error bars indicate s.d. (P,Q) Model of mesencephalic DA (blue arrows) and GABA (green arrows) neuron migration in WT (P), which involves ventral migration of VTA precursors (vertical arrow) and perpendicular migration of SN precursors and some VTA precursors (perpendicular arrows). In the ak/ak mutant (Q), perpendicular migration of DA neurons to VM is significantly affected (lower red cross) and cells cluster abnormally in the red nucleus. Then, GABA neurons also cannot migrate to VM (upper red cross). Aq, aqueduct; BP, basal plate; VM, ventral mesencephalon. Scale bars: 100 μm in A-D,F,H,M,N; 50 μm in G,I; 25 μm in J.
Together, these results strongly support our idea that the intact DA system of the VM guides the GABA neuron system to descend to VM and establish its connectivity with DA neurons. Furthermore, the reduction in GABA neurons observed in the ak/ak mesencephalon was reflected in adult (4 month old) mice as well (supplementary material Fig. S6).
Our data provide novel insights into neuronal migration in the embryonic mouse mesencephalon and its relevance for final ventral mesencephalic neuronal population and connectivity. First, our data support a model for DA and GABA neuron migration in the mesencephalon that depicts vertical migration of VTA precursors and perpendicular migration of both SN and VTA precursors (Fig. 4P). Second, our analysis of ak/ak mice indicates how A9 DA progenitor cells show blocked perpendicular migration and accumulate in the red nucleus area (Fig. 4Q). Perpendicular migration is therefore essential to set up the proper anatomical architecture of ventral mesencephalic structures. Third, we found that DA and GABA neurons occupy VM in a temporally sequential manner. Thus, at E13, the primary structure of DA neurons in the VM is completely devoid of GABA neurons, whereas by E17 GABA neurons come to reside along with DA neurons. Remarkably, proper migration of GABA neurons to their final location is dependent on the complete DA neuron architecture in the VM, strongly indicating an important interaction between GABA and DA neurons for final location and connectivity.
Given that major brain disorders such as schizophrenia, attention deficit hyperactivity disorder (ADHD) and Parkinson’s disease are considered to be caused by abnormal early brain development, this study will serve as a gateway to a whole new field of exploration to identify substrates and mechanisms of neuronal migration in the mesencephalon that might provide novel insights into the underlying pathophysiology of these brain disorders.
Supplementary Material
Acknowledgments
We thank Drs Carmen Birchmeier and Thomas Müller for the generous gift of Lmx1b antibody.
Footnotes
Funding
Supported by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award to A.V. and National Institutes of Health grants [NS064386, NS073635 to A.V., MH48866, MH087903 and NS070577 to K.-S.K.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.078394/-/DC1
References
- Bayer S. A., Wills K. V., Triarhou L. C., Ghetti B. (1995). Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp. Brain Res. 105, 191–199 [DOI] [PubMed] [Google Scholar]
- Björklund A., Dunnett S. B. (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202 [DOI] [PubMed] [Google Scholar]
- Chung S., Leung A., Han B. S., Chang M. Y., Moon J. I., Kim C. H., Hong S., Pruszak J., Isacson O., Kim K. S. (2009). Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell 5, 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. G., Nery S., Fishell G. (2001). Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat. Neurosci. 4 Suppl., 1177–1182 [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E. C., Agid Y., Graybiel A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122, 1437–1448 [DOI] [PubMed] [Google Scholar]
- Ding Y., Won L., Britt J. P., Lim S. A., McGehee D. S., Kang U. J. (2011). Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. USA 108, 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera J., Weisenhorn D. V., Wurst W. (2006). Megane/Heslike is required for normal GABAergic differentiation in the mouse superior colliculus. Development 133, 3847–3857 [DOI] [PubMed] [Google Scholar]
- Hanaway J., McConnell J. A., Netsky M. G. (1971). Histogenesis of the substantia nigra, ventral tegmental area of Tsai and interpeduncular nucleus: an autoradiographic study of the mesencephalon in the rat. J. Comp. Neurol. 142, 59–73 [DOI] [PubMed] [Google Scholar]
- Hatten M. E. (2002). New directions in neuronal migration. Science 297, 1660–1663 [DOI] [PubMed] [Google Scholar]
- Hwang D. Y., Fleming S. M., Ardayfio P., Moran-Gates T., Kim H., Tarazi F. I., Chesselet M. F., Kim K. S. (2005). 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson’s disease. J. Neurosci. 25, 2132–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katarova Z., Sekerková G., Prodan S., Mugnaini E., Szabó G. (2000). Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. J. Comp. Neurol. 424, 607–627 [DOI] [PubMed] [Google Scholar]
- Kawano H., Ohyama K., Kawamura K., Nagatsu I. (1995). Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res. Dev. Brain Res. 86, 101–113 [DOI] [PubMed] [Google Scholar]
- Kim J., Inoue K., Ishii J., Vanti W. B., Voronov S. V., Murchison E., Hannon G., Abeliovich A. (2007). A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T. M., Ponomarenko A. A., Brown R. E., Haas H. L. (2004). Functional diversity of ventral midbrain dopamine and GABAergic neurons. Mol. Neurobiol. 29, 243–259 [DOI] [PubMed] [Google Scholar]
- Lennington J. B., Pope S., Goodheart A. E., Drozdowicz L., Daniels S. B., Salamone J. D., Conover J. C. (2011). Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J. Neurosci. 31, 13078–13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G., Sturgess K., Erdélyi F., Szabó G., Molnár Z., Paulsen O. (2004). Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb. Cortex 14, 1122–1133 [DOI] [PubMed] [Google Scholar]
- López-Bendito G., Sánchez-Alcañiz J. A., Pla R., Borrell V., Picó E., Valdeolmillos M., Marín O. (2008). Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 28, 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O., Rubenstein J. L. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790 [DOI] [PubMed] [Google Scholar]
- Mathis C., Schröter A., Thallmair M., Schwab M. E. (2010). Nogo-a regulates neural precursor migration in the embryonic mouse cortex. Cereb. Cortex 20, 2380–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts R. G., Chatelain-Badie S. D., Benson E., White-Cooper H., Bolam J. P., Ungless M. A. (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152, 1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I., Tovmasian L. T., Silva R. M., Burke R. E., Goff S. P. (2003). Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc. Natl. Acad. Sci. USA 100, 4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V. G., Nestler E. J. (2007). Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse 61, 87–95 [DOI] [PubMed] [Google Scholar]
- Ori-McKenney K. M., Vallee R. B. (2011). Neuronal migration defects in the Loa dynein mutant mouse. Neural Dev. 6, 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas J. G. (2000). The origin and migration of cortical neurones: new vistas. Trends Neurosci. 23, 126–131 [DOI] [PubMed] [Google Scholar]
- Prakash N., Puelles E., Freude K., Trumbach D., Omodei D., Di Salvio M., Sussel L., Ericson J., Sander M., Simeone A., Wurst W. (2009). Nkx6-1 controls the identity and fate of red nucleus and oculomotor neurons in the mouse midbrain. Development 136, 2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2001). Reward signaling by dopamine neurons. Neuroscientist 7, 293–302 [DOI] [PubMed] [Google Scholar]
- Seeman P., Guan H. C., Van Tol H. H. (1993). Dopamine D4 receptors elevated in schizophrenia. Nature 365, 441–445 [DOI] [PubMed] [Google Scholar]
- Smidt M. P., Burbach J. P. (2007). How to make a mesodiencephalic dopaminergic neuron. Nat. Rev. Neurosci. 8, 21–32 [DOI] [PubMed] [Google Scholar]
- Smidt M. P., Smits S. M., Bouwmeester H., Hamers F. P., van der Linden A. J., Hellemons A. J., Graw J., Burbach J. P. (2004). Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131, 1145–1155 [DOI] [PubMed] [Google Scholar]
- Soriano E., Del Rio J. A. (1991). Simultaneous immunocytochemical visualization of bromodeoxyuridine and neural tissue antigens. J. Histochem. Cytochem. 39, 255–263 [DOI] [PubMed] [Google Scholar]
- Supèr H., Del Río J. A., Martínez A., Pérez-Sust P., Soriano E. (2000). Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb. Cortex 10, 602–613 [DOI] [PubMed] [Google Scholar]
- van den Munckhof P., Luk K. C., Ste-Marie L., Montgomery J., Blanchet P. J., Sadikot A. F., Drouin J. (2003). Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130, 2535–2542 [DOI] [PubMed] [Google Scholar]
- Vernay B., Koch M., Vaccarino F., Briscoe J., Simeone A., Kageyama R., Ang S. L. (2005). Otx2 regulates subtype specification and neurogenesis in the midbrain. J. Neurosci. 25, 4856–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines-Samuelson M., Handler M., Shen J. (2005). Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev. Biol. 277, 332–346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.