Abstract
Background
Laminectomy/laminotomy and foraminotomy are well established surgical techniques for treatment of symptomatic lumbar spinal stenosis. However, these procedures have significant limitations, including limited access to lateral and foraminal compression and postoperative instability. The purpose of this cadaver study was to compare bone, ligament, and soft tissue morphology following lumbar decompression using a minimally invasive MicroBlade Shaver® instrument versus hemilaminotomy with foraminotomy (HL).
Methods
The iO-Flex® system utilizes a flexible over-the-wire MicroBlade Shaver instrument designed for facet-sparing, minimally invasive “inside-out” decompression of the lumbar spine. Unilateral decompression was performed at 36 levels in nine human cadaver specimens, six with age-appropriate degenerative changes and three with radiographically confirmed multilevel stenosis. The iO-Flex system was utilized on alternating sides from L2/3 to L5/S1, and HL was performed on the opposite side at each level by the same investigator. Spinal canal, facet joint, lateral recess, and foraminal morphology were assessed using computed tomography.
Results
Similar increases in soft tissue canal area and decreases in ligamentum flavum area were noted in nondiseased specimens, although HL required removal of 83% more laminar area (P < 0.01) and 95% more bone resection, including the pars interarticularis and facet joints (P < 0.001), compared with the iO-Flex system. Similar increases in lateral recess diameter were noted in nondiseased specimens using each procedure. In stenotic specimens, the increase in lateral recess diameter was significantly (P = 0.02) greater following use of the iO-Flex system (43%) versus HL (7%). The iO-Flex system resulted in greater facet joint preservation in nondiseased and stenotic specimens. In stenotic specimens, the iO-Flex system resulted in a significantly greater increase in foraminal width compared with HL (24% versus 4%, P = 0.01), with facet joint preservation.
Conclusion
The iO-Flex system resulted in significantly better decompression of the lateral recess and foraminal areas compared with HL, while preserving posterior spinal elements, including the facet joint.
Keywords: decompression, laminectomy, lumbar, minimally invasive, stenosis, MicroBlade Shaver, iO-Flex system
Introduction
Lumbar spinal stenosis (LSS) is a common cause of low back pain and lower extremity pain in older adults and is responsible for 1.2 million physician office visits each year in the US.1 Degenerative LSS is characterized by narrowing of the spinal canal as a result of bony and intraspinal soft tissue degeneration, including intervertebral disc herniation, facet joint and ligamentum flavum hypertrophy, and/or osteophyte formation.2 LSS may involve the central spinal canal, lateral recess, or foramina, with affected areas frequently coexisting in the elderly.3 The characteristic clinical manifestations of LSS include periodic exacerbations of low back and leg pain and neurogenic claudication,4 with resulting compromise in health-related quality of life, mobility, and independence.5 The burden of LSS is expected to rise concomitant with quadrupling of the number of persons 60 years and older by the year 2050.
The optimal treatment strategy for LSS has not been defined. Nonoperative treatment including limitation of activity, posture modification, and epidural steroid injections, are useful for LSS symptoms of mild-to-moderate severity.6 Decompressive surgery is indicated for severe neurogenic claudication, neural compromise, and/or intractable radicular symptoms. Surgical decompression of symptomatic LSS is the most common indication for low back surgery in patients over 65 years of age.7 Decompression with spinal fusion is utilized as the primary treatment in selected LSS patients, even in the absence of gross instability, in part because of the limitations of traditional decompression tools. The effectiveness of decompression surgery for the treatment of lateral recess or foraminal stenosis is hindered by the difficulty associated with gaining access to these areas using standard rigid instrumentation, such as Kerrison ronguers, without partial or total resection of the posterior elements. Resection of the facet joints and midline structures during decompression surgery increases the risk of spinal instability, which may eventually necessitate revision surgery with fusion.8,9 A minimally invasive approach that selectively targets the lateral recess and foramen with preservation of the posterior elements may be useful in reducing perioperative complications and minimizing the need for revision surgery after lumbar decompression.
The iO-Flex® system (Baxano Inc, San Jose, CA) utilizes a flexible, over-the-wire MicroBlade Shaver® instrument designed to allow for direct decompression of impinging tissue, particularly in the lateral recess and foramen, while avoiding disruption of the pars interarticularis, the facet joints, and the midline structures. The purpose of this feasibility study was to quantify changes in lumbar spinal anatomy using computed tomography (CT) following decompression with the iO-Flex system versus hemilaminotomy with foraminotomy (HL) in cadaveric lumbar specimens.
Materials and methods
Specimens
This study was conducted using 36 lumbar motion segments in nine human cadaveric lumbar (L1 to sacrum) specimens. Six specimens (mean cadaveric age 78 years) exhibited age-appropriate degenerative changes and three specimens (mean cadaveric age 87 years) had CT-verified degenerative LSS with moderate-to-severe multilevel foraminal stenosis.
Procedures
The cranial portion of L1 and the caudal sacrum were stripped of muscle and adipose tissue and secured in glass-reinforced filler (Bondo; 3M Bondo, Atlanta, GA). Each specimen was then fixed in an acrylic frame in the neutral position and scanned intact using thin-slice (0.75 mm) axial CT (Somatom Sensation 4; Siemens, Munich, Germany). After completion of the CT scan, the specimen was removed and bilateral tissue exposure of the L2–S1 lamina was performed by one of five experienced surgeons (three neurosurgeons and two orthopedic spinal surgeons). All procedures on a given specimen were performed using a consistent technique by the same surgeon in order to minimize bias. The decompression procedure, ie, iO-Flex system or standard HL, was randomly selected and performed for the first level and the alternate procedure was performed next on the contralateral side for each level from L2/3 to L5/S1.
Surgeons aimed to remove the central ligamentum flavum and any excess bone and soft tissue in the lateral recess and foramen that would typically be expected to contribute to LSS symptoms, while sparing as much of the nonoffending structures as possible (eg, lamina, pars, and facet joints). A total of 36 levels were treated (six nondiseased and three stenotic specimens, with four treated levels each). After the procedures were performed, CT was repeated using the technique described above.
Surgical technique used for the iO-Flex system
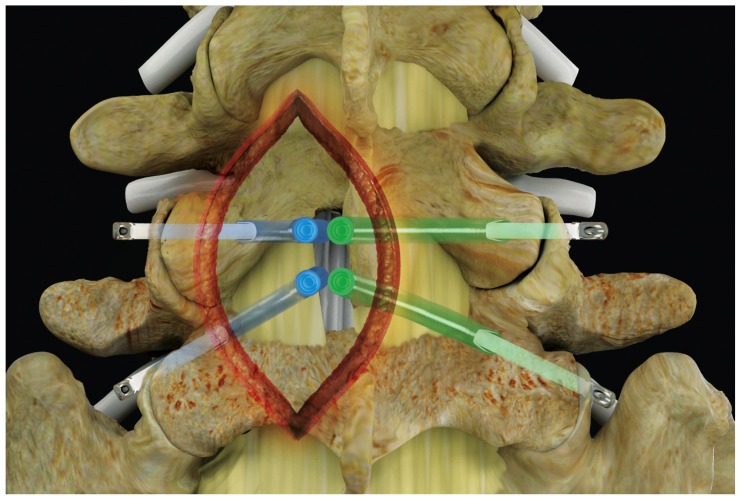
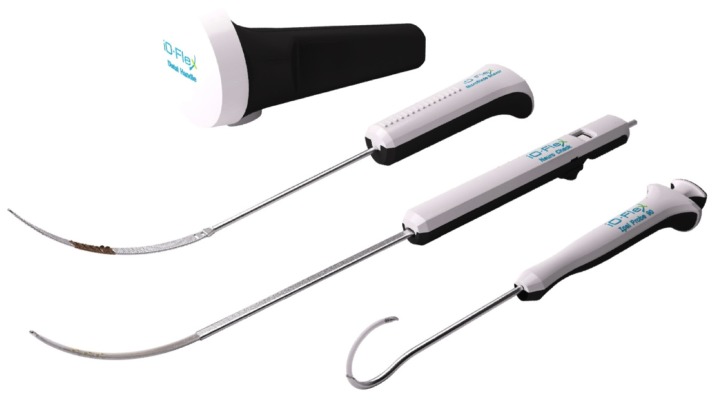
The iO-Flex system is a set of over-the-wire instruments that allows ventral-to-dorsal decompression of impinged neural elements in the lumbar spine while sparing uninvolved bone and soft tissue with controlled bimanual reciprocations. Specifically, the MicroBlade Shaver instrument allows for “inside-out” decompression by removing the ligamentum flavum and shaving bony overgrowth on the superior articular process and under the pars interarticularis, while requiring minimal resection of the facet joints and midline structures. Up to four nerve roots (traversing and exiting roots on the ipsilateral and contralateral side) may be decompressed via a single interlaminar access point using the MicroBlade Shaver (Figure 1). The procedural steps for the iO-Flex system consist of an iO-Flex probe to gain access, the Neuro Check® device for localization of neural structures, and the MicroBlade Shaver instrument for targeted tissue removal and decompression (Figure 2).
Figure 1.
Posterior spine indicating the four nerve roots traversing and exiting on the ipsilateral and contralateral side, decompressed through a single interlaminar access point.
Figure 2.
The iO-Flex® system consists of (from top to bottom) a distal handle, a MicroBlade Shaver® instrument (available in 5.5 mm, 7.5 mm [shown], 10 mm, and 12 mm widths), the Neuro Check® device, and a probe (ipsilateral [shown], contralateral [not shown]).
Note: The wire is not shown.
In this study, surgeons typically performed a small ipsilateral laminotomy to create an interlaminar window just large enough to remove the central ligament, directly visualize the dura, and to fit the MicroBlade Shaver instrument so that it could be used to remove bone and soft tissue in the lateral recess and foramen. Additional structural anatomy was not removed because, in a clinical setting, the Neuro Check device is used to confirm positions dorsal to the lateral neural structures without direct visualization of the traversing root and because the flexible nature of the shaver allows for decompression around the facet joints without their removal.
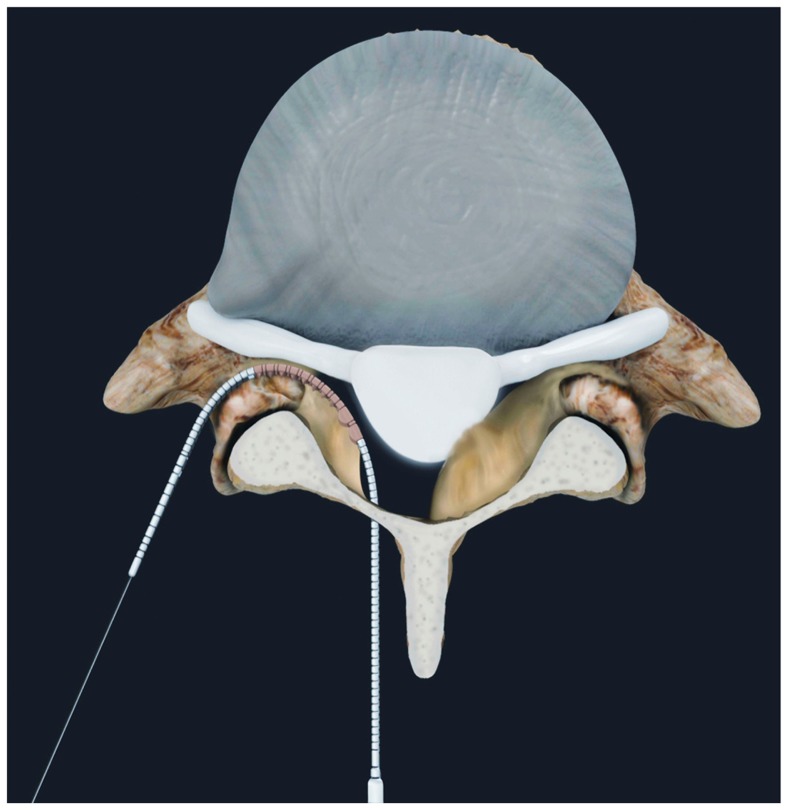
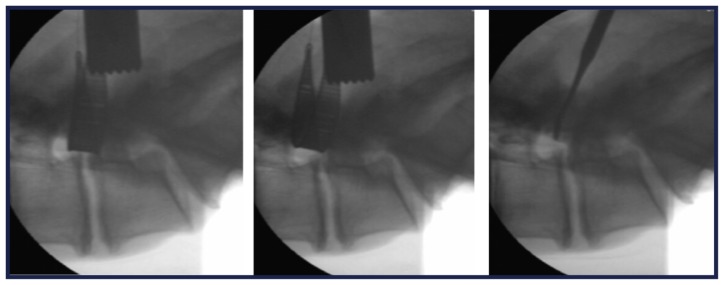
After the laminotomy, the probe, with its cannulated catheter in the retracted position, is passed out of the neural foramen, just rostral to the caudal pedicle. The position of the probe inside the foramen is confirmed using lateral fluoroscopy and the inner catheter is then deployed. A nitinol wire is passed through the probe and out through the dorsal skin where it is locked into a distal handle. The catheter is then retracted and the probe removed, leaving the wire in place. In a clinical setting, the neural localization step with the Neuro Check device would follow next, but was not performed in this cadaver study. The MicroBlade Shaver instrument is then attached to the wire via a proximal exchange tip and pulled into the lateral recess and foramen using a distal handle (Figure 3). The dorsal side of the instrument has small cutting teeth designed to excise bone and ligament, while the ventral side is smooth to protect the neural structures. Decompression is achieved using gentle upward tension and a bimanual reciprocating motion with the handle of the MicroBlade Shaver instrument and the distal handle. Tissue removal and completeness of decompression is assessed using foraminal probes and lateral fluoroscopy (Figure 4). When the surgeon deems that decompression is complete, all instruments are removed.
Figure 3.
After the probe has been properly inserted and the wire has been delivered, the iO-Flex® MicroBlade Shaver® is passed through the epidural space and out of the lateral foramen.
Note: Tissue is removed using a bimanual reciprocating motion.
Figure 4.
Preoperative and postoperative assessment of decompression using fluoroscopy and a Woodson probe (lateral images [left to right]: pretreatment with MicroBlade Shaver® instrument, post-treatment with MicroBlade Shaver instrument, and post-treatment assessment with Woodson probe).
Hemilaminotomy with foraminotomy technique
Surgeons used a traditional HL technique to decompress the neural elements adequately.10,11 Traditional surgical instruments, such as spinal curettes, high speed burrs, tissue, and Kerrison rongeurs were used. After the laminae were exposed, bone from the inferior edge of the superior lamina as well as the superior edge of the inferior lamina was removed. The ligamentum flavum was detached and removed, leaving the interlaminar space open. Kerrison rongeurs were used to decompress further laterally by removing the medial aspect of the facet. Visual inspection and standard foraminal probes were used throughout the procedure to assess the amount of space in the neural foramen and to determine when the decompression was complete. Surgeons typically removed significant portions of the lamina and the facet joints in order to visualize the shoulder of the traversing nerve root for safety and due to the constraints of rigid instruments, such as Kerrison rongeurs, in attempting to access impinging tissue in the lateral recess and foramen. However, each surgeon carefully balanced the tradeoff between the ability to reach this impinging tissue with the desire to spare the structural anatomy for stability.
Outcomes
For nondiseased specimens, CT scans were evaluated by an independent core laboratory (Medical Metrics, Houston, TX) using validated quantitative image analysis software.12 All measurements were taken from a digital composite of the pretreatment and post-treatment scans, an automated technique that reflects only the change attributable to treatment by eliminating the error arising from manual selection of anatomical landmarks on separate examinations.
Reconstructed left and right parasagittal images were used to measure anteroposterior foramen width and area (Figure 5). Reconstructed mid-disc axial images were used to measure the spinal canal area, soft tissue canal area, ligamentum flavum area, laminar width (medial to lateral distance), lateral recess diameter (anterior-posterior diameter of lateral recess), and facet width (medial to lateral articulation distance), as shown in Figure 6. Joint (articulation) cross-sectional area was measured from a plane bisecting the facet joint at each level. The magnitude of laminar removal was measured as a change in width from an axial slice or as a change in area from a coronal slice.
Figure 5.
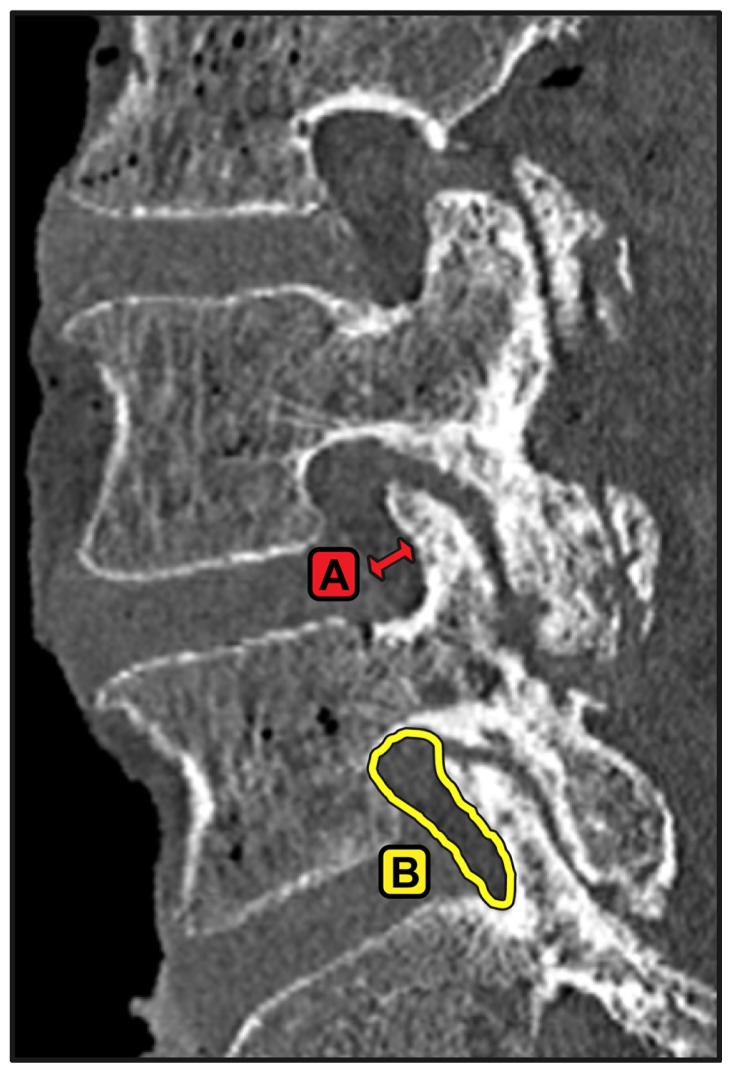
Example of measurements made from reconstructed parasagittal computed tomography slices bisecting the cranial and caudal pedicles at the level of interest. (A) Foraminal width, measured at the narrowest part of the foramen and (B) foraminal area.
Figure 6.
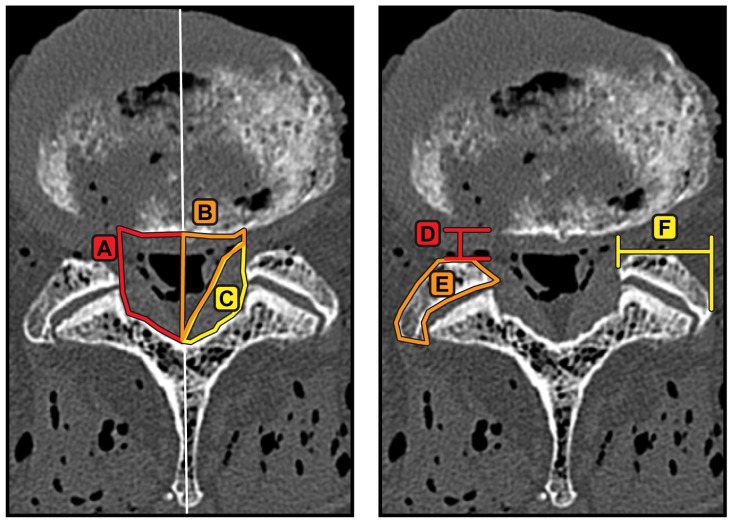
Example of measurements made from reconstructed axial slices through the center of the intervertebral disc space aligned with the inferior endplate at the level of interest. (A) Bony canal area, (B) soft tissue canal area, (C) ligamentum flavum area, (D) lateral recess diameter, (E) facet area, and (F) facet width.
Notes: The spinal canal was defined to have a width equal to one-third of the left to right width of the intervertebral disc to avoid large variations in measurements. Left and right canal measurements were taken from the midline.
Stenotic specimens were evaluated by an independent neuroradiologist using identical CT image analysis software. Following initial analysis of nondiseased lumbar specimens, only the most clinically relevant outcomes were preselected for analysis in the stenotic specimens.
Data analysis
Data are presented as the mean ± standard deviation. The Wilcoxon signed-rank test was used to assess anatomical changes before and after each procedure, as well as to compare anatomical changes between treatments on paired sides of each treated level.
Results
Central access and facet preservation
In nondiseased cadaver specimens, open HL required excision of significantly more bone than did the iO-Flex system. HL resulted in removal of 83% greater laminar area (P < 0.01), including resection of the structural pars interarticularis and removal of 95% more bone in the central canal (P < 0.001) versus the iO-Flex system. Similarly, in stenotic specimens, HL resulted in significantly more resection of structural posterior elements, with a 54% decrease in laminar width versus 28% when using the iO-Flex system (P = 0.03, Tables 1 and 2). Decreases in facet width were approximately threefold greater in nondiseased (P < 0.01) and stenotic (P = 0.03) specimens following HL versus the iO-Flex system. In nondiseased specimens, facet area was similarly decreased after each treatment (11% versus 14%, P = 0.94). However, decreases in joint cross-sectional area were significantly less following use of the iO-Flex system (3%) versus HL (7%, P < 0.01, Tables 1 and 2).
Table 1.
Anatomical lumbar changes following use of the iO-Flex® system and of hemilaminotomy with foraminotomy in nondiseased cadaveric lumbar specimens
| Variable, mean ± SD | iO-Flex system (n = 24) | Hemilaminotomy with foraminotomy (n = 24) | P§ | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Pre | Post | Δ | Pre | Post | Δ | ||
| Spinal canal, cm2 | |||||||
| Laminar area removed | − | 0.47 ± 0.45 | − | − | 0.86 ± 0.37 | − | <0.01 |
| Bone canal area | 2.01 ± 0.56 | 2.23 ± 0.58 | 0.22* | 2.05 ± 0.60 | 2.47 ± 0.65 | 0.43* | <0.001 |
| Soft tissue canal area | 1.17 ± 0.38 | 1.72 ± 0.49 | 0.55* | 1.17 ± 0.40 | 1.83 ± 0.53 | 0.66* | 0.16 |
| Ligamentum flavum area | 0.47 ± 0.15 | 0.10 ± 0.17 | −0.37* | 0.46 ± 0.15 | 0.08 ± 0.18 | −0.38* | 0.94 |
| Facet morphology | |||||||
| Joint cross-sectional area, cm2 | 4.15 ± 1.08 | 4.04 ± 1.08 | −0.11† | 4.05 ± 1.21 | 3.75 ± 1.25 | −0.30* | <0.01 |
| Facet area, cm2 | 1.56 ± 0.34 | 1.34 ± 0.35 | −0.22‡ | 1.60 ± 0.43 | 1.41 ± 0.38 | −0.18* | 0.94 |
| Facet width, cm | 1.70 ± 0.28 | 1.65 ± 0.27 | −0.05† | 1.69 ± 0.31 | 1.52 ± 0.30 | −0.17* | <0.01 |
| Lateral recess | |||||||
| Lateral recess diameter, cm | 0.52 ± 0.17 | 0.68 ± 0.23 | 0.17* | 0.51 ± 0.18 | 0.61 ± 0.20 | 0.10† | 0.14 |
| Foramen | |||||||
| Foraminal area, cm2 | 1.51 ± 0.38 | 1.58 ± 0.36 | 0.07* | 1.49 ± 0.37 | 1.53 ± 0.39 | 0.04‡ | 0.09 |
| Foraminal width, cm | 0.83 ± 0.19 | 0.92 ± 0.17 | 0.09* | 0.81 ± 0.21 | 0.84 ± 0.21 | 0.04‡ | 0.06 |
Notes: Within-group significance value:
P < 0.001;
P < 0.01;
P < 0.05.
Between-group significance value.
Table 2.
Anatomical lumbar changes following use of the iO-Flex® system and of hemilaminotomy with foraminotomy in stenotic cadaveric specimens
| Variable, mean ± SD | iO-Flex system (n = 12) | Hemilaminotomy with foraminotomy (n = 12) | P§ | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Pre | Post | Δ | Pre | Post | Δ | ||
| Spinal canal | |||||||
| Laminar width, cm | 1.63 ± 0.27 | 1.17 ± 0.46 | −0.46† | 1.53 ± 0.25 | 0.71 ± 0.36 | −0.82† | 0.03 |
| Facet morphology | |||||||
| Facet width, cm | 1.62 ± 0.39 | 1.33 ± 0.48 | −0.28† | 1.61 ± 0.45 | 0.91 ± 0.50 | −0.70* | 0.03 |
| Lateral recess | |||||||
| Lateral recess diameter, cm | 0.42 ± 0.11 | 0.60 ± 0.18 | 0.18† | 0.42 ± 0.14 | 0.45 ± 0.15 | 0.03 | 0.02 |
| Foramen | |||||||
| Foraminal width, cm | 0.89 ± 0.23 | 1.10 ± 0.24 | 0.21* | 0.91 ± 0.25 | 0.95 ± 0.28 | 0.04 | 0.01 |
Notes: Within-group significance value:
P < 0.001;
P < 0.01.
Between-group significance value.
Spinal canal decompression
In nondiseased cadaver specimens, similar increases in soft tissue canal area (47% and 56%, P = 0.16) and decreases in ligamentum flavum area (79% and 83%, P = 0.94) were observed for the iO-Flex system and HL, respectively.
Lateral recess decompression
Lateral recess diameter increased by 33% (P < 0.001) in the nondiseased specimens following use of the iO-Flex system versus a 20% increase (P < 0.01) with HL (Table 1). Lateral recess diameter changes were particularly pronounced in stenotic specimens following decompression using the iO-Flex system, with an increase of 43%, which was significantly greater (P = 0.02) compared with HL (7%, Table 2).
Foraminal decompression
Foraminal width (P = 0.06) and area (P = 0.09) were marginally greater following use of the iO-Flex system in nondiseased specimens compared with HL (Table 1). However, in the stenotic specimens, HL resulted in a nonsignificant 4% increase in foraminal width. In contrast, decompression using the iO-Flex system resulted in a significant 24% (P < 0.001) improvement in foraminal width (P = 0.01 between groups, Table 2).
Discussion
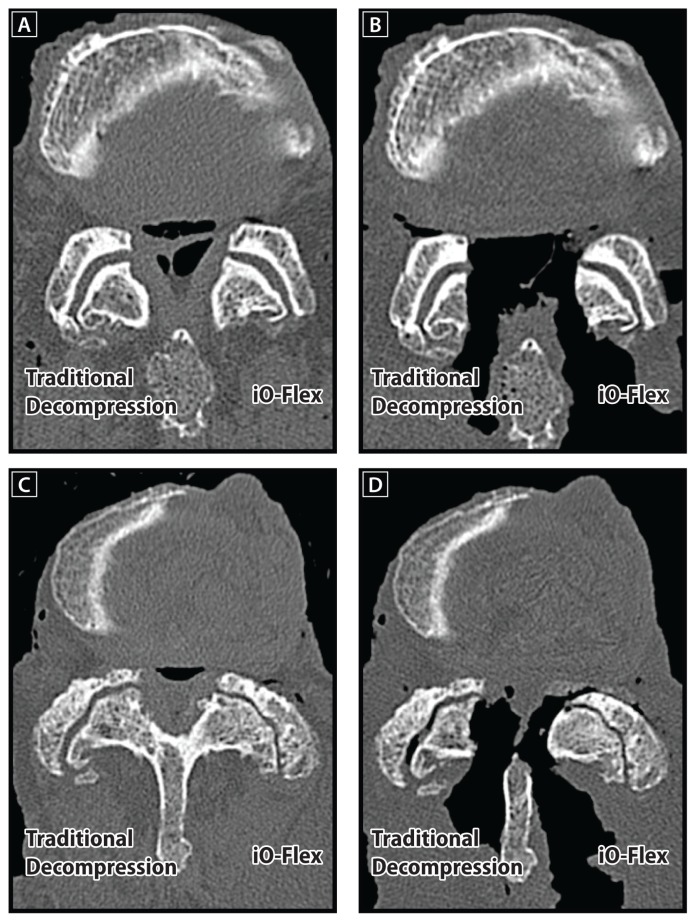
The iO-Flex system is a minimally invasive, facet-sparing approach that allows for direct decompression of impinging tissue via a ventral-to-dorsal action of the MicroBlade Shaver. In contrast, traditional decompression procedures utilize an invasive medial-to-lateral approach that removes a significant portion of the posterior elements at the treated level. Another advantage of the iO-Flex system is that, unlike open laminectomy that utilizes fixed-angle tools with a limited ability to address lateral recess and foraminal stenosis, the iO-Flex System uses low-profile flexible instrumentation that targets impinging tissue in the spinal canal, lateral recess, and foramen (Figure 7). This cadaver study demonstrated that the iO-Flex system allows for decompression of the spinal canal with limited resection of structural posterior elements and with selective resection of compressing structures in the lateral recess and foraminal regions.
Figure 7.
Reconstructed axial computed tomography scans illustrating the ability of the iO-Flex® system to decompress the lateral recess and foramen effectively while maintaining facet joint integrity. Images are provided for nondiseased preoperative (A) and postoperative (B) specimens as well as stenotic preoperative (C) and postoperative (D) specimens.
Although the clinical relevance of these study findings is unknown, the iO-Flex system has been utilized in two clinical studies with data published prior to this current study.13 A postmarket pilot study of nine patients with one-year follow-up demonstrated a median 73% reduction in pain, a 50% improvement in back function, 72% and 31% improvements in Zurich Claudication Questionnaire physical function and symptom severity, respectively, and a 36% improvement in health-related quality of life. A retrospective study of 67 patients treated with the iO-Flex system for LSS reported no reoperations or cases of neurologic impairment through approximately one year post-treatment. Thus far, the cumulative data on the iO-Flex system support safety and clinical utility, although additional study is required.
Although laminectomy is the traditional treatment of choice for patients with recalcitrant LSS, long-term outcomes are mixed.14,15 Laminectomy is often associated with postoperative pain, disability, and dysfunction, due to extensive resection of muscle, ligament, and bone. Resection of excessive bone from the posterior elements may contribute to subsequent lumbar instability and increased intradiscal pressure by establishing an alternate path of axial loading, transferring forces to the adjacent annulus and anterior longitudinal ligament.16 Consequently, disc degeneration may be accelerated following this procedure. Johnsson et al reported that 44% of patients presented with subsequent degenerative spondylolisthesis after decompressive lumbar laminectomy.17 Furthermore, Sanderson and Getty reported an average loss of 1.3 mm of disc height after partial undercutting facetectomy.18 Progression of stenotic symptoms following laminectomy is quite common19 and is due, in part, to inadequate decompression,20,21 local tissue trauma, and subsequent adhesion formation.22,23
Inadequate decompression of lateral recess stenosis has been shown to be responsible for 25%–56% of failed back surgery syndrome cases.24–26 Ultimately, 11%–23% of laminectomy patients undergo reoperation within 10 years.27,28 Results from this cadaver study confirmed that traditional decompression has a limited effect in improving lateral recess and foraminal area, especially in stenotic specimens. Decompression surgery using the iO-Flex system may reduce muscle trauma by allowing bilateral decompression through a single access point and, in theory, may result in a lower degree of destabilization, as seen in traditional decompression surgery.
Microdecompression procedures have recently been advocated due to a perceived lower risk of iatrogenic insult. However, these procedures require great technical skill and surgical experience, and evidence for these treatments is limited. In addition, while these procedures can be done via a less invasive exposure, fixed-angle tools still limit the ability to perform facet-sparing bilateral decompression of the lateral recess and foramen. Interspinous spacers have recently been advocated as an indirect method for relieving symptomatic LSS. Lumbar extension narrows the spinal canal and lateral recess by approximately 15% compared with a neutral posture,29 and therefore exacerbates LSS symptoms. Interspinous spacers limit back extension at the affected level by distracting the spinous processes of the degenerated segment, thereby unloading the posterior annulus fibrosus and facet joints. Despite promising mid-term outcomes, loss of radiographic correction after only 1.5 years is a well established phenomenon with these devices,30 and high clinical failure rates have been reported.31
Furthermore, interspinous spacers are only appropriate for patients with mild-to-moderate intermittent neurogenic claudication that is relieved by lumbar flexion and they do not directly address the underlying anatomical cause of pain, such as bony neuroforaminal encroachment and buckled ligamentum flavum. Because LSS is a progressive condition, interspinous spacers likely represent only a temporary solution to this disease. Overall, decompression using the iO-Flex system may have potential advantages over micro-decompression and interspinous spacers, including use of a simple minimally invasive approach and procedure, and the ability to treat patients with severe LSS symptoms.
This cadaver study had limitations. First, surgeons were not blinded to the procedures being performed in this study and, therefore, the possibility of intervention bias exists. However, all surgeons were well experienced and used a similar surgical technique, thus minimizing potential bias. Second, despite the noted advantages of the iO-Flex system in this cadaver study, the results do not necessarily translate into superior clinical outcomes compared with HL, especially given the variable relationship of LSS severity and clinical symptoms.32,33 Additional prospective human clinical studies are required to elucidate further the safety and effectiveness of this procedure. Two prospective studies measuring multiple clinical outcomes are currently underway. The first study is evaluating the clinical performance of the iO-Flex system in patients with one or two levels of LSS (NCT01067014), and the second study (STRiDE) is evaluating the iO-Flex system in patients with LSS and stable grade 1 spondylolisthesis (NCT01338766).
Conclusion
The minimally invasive iO-Flex system yields significantly better decompression of the lateral recess and foraminal area in cadaveric specimens with LSS, without extensive removal of the stabilizing posterior spinal elements, including the facet joint, compared with traditional HL.
Acknowledgments
We thank Joyce Lin Ballard and Robyn Capobianco for assistance with study execution and data acquisition, Randy Asher for graphical assistance, and Medical Metrics Inc, Houston, TX, and Mark Myers for quantitative computed tomography analysis.
Footnotes
Disclosure
This study was supported by Baxano Inc, San Jose, CA.
References
- 1.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine (Phila Pa 1976) 1995;20(1):11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Porter RW. Spinal stenosis and neurogenic claudication. Spine (Phila Pa 1976) 1996;21(17):2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 3.Onel D, Sari H, Donmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine (Phila Pa 1976) 1993;18(2):291–298. [PubMed] [Google Scholar]
- 4.Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine lumbar spine study. Spine (Phila Pa 1976) 2000;25(5):556–562. doi: 10.1097/00007632-200003010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DK. Office management of chronic pain in the elderly. Am J Med. 2007;120(4):306–315. doi: 10.1016/j.amjmed.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA. Back surgery – who needs it? N Engl J Med. 2007;356(22):2239–2243. doi: 10.1056/NEJMp078052. [DOI] [PubMed] [Google Scholar]
- 7.Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 1994;19(11):1207–1212. doi: 10.1097/00007632-199405310-00002. [DOI] [PubMed] [Google Scholar]
- 8.Tuite GF, Doran SE, Stern JD, et al. Outcome after laminectomy for lumbar spinal stenosis. Part II: radiographic changes and clinical correlations. J Neurosurg. 1994;81(5):707–715. doi: 10.3171/jns.1994.81.5.0707. [DOI] [PubMed] [Google Scholar]
- 9.Tuite GF, Stern JD, Doran SE, et al. Outcome after laminectomy for lumbar spinal stenosis. Part I: clinical correlations. J Neurosurg. 1994;81(5):699–706. doi: 10.3171/jns.1994.81.5.0699. [DOI] [PubMed] [Google Scholar]
- 10.Tsai RY, Yang RS, Bray RS., Jr Microscopic laminotomies for degenerative lumbar spinal stenosis. J Spinal Disord. 1998;11(5):389–394. [PubMed] [Google Scholar]
- 11.Delamarter RB, McCulloch JA. Microdiscectomy and microsurgical spinal laminotomies. In: Frymoyer JW, editor. The Adult Spine. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 12.Pearson AM, Spratt KF, Genuario J, et al. Precision of lumbar inter-vertebral measurements: does a computer-assisted technique improve reliability? Spine (Phila Pa 1976) 2011;36(7):572–580. doi: 10.1097/BRS.0b013e3181e11c13. [DOI] [PubMed] [Google Scholar]
- 13.Lauryssen C. Technical advances in minimally invasive surgery: direct decompression for lumbar spinal stenosis. Spine (Phila Pa 1976) 2010;35(Suppl 26):S287–S293. doi: 10.1097/BRS.0b013e3182023268. [DOI] [PubMed] [Google Scholar]
- 14.Postacchini F. Surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 1999;24(10):1043–1047. doi: 10.1097/00007632-199905150-00020. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haher TR, O’Brien M, Dryer JW, Nucci R, Zipnick R, Leone DJ. The role of the lumbar facet joints in spinal stability. Identification of alternative paths of loading. Spine (Phila Pa 1976) 1994;19(23):2667–2670. [PubMed] [Google Scholar]
- 17.Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine (Phila Pa 1976) 1986;11(2):107–110. doi: 10.1097/00007632-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson PL, Getty CJ. Long-term results of partial undercutting facetectomy for lumbar lateral recess stenosis. Spine (Phila Pa 1976) 1996;21(11):1352–1356. doi: 10.1097/00007632-199606010-00015. [DOI] [PubMed] [Google Scholar]
- 19.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson B, Annertz M, Sjoberg C, Stromqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: five-year follow-up by an independent observer. Spine (Phila Pa 1976) 1997;22(24):2938–2944. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Lipson SJ, Larson MG, McInnes JM, Fossel AH, Liang MH. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am. 1991;73(6):809–816. [PubMed] [Google Scholar]
- 22.Airaksinen O, Herno A, Kaukanen E, Saari T, Sihvonen T, Suomalainen O. Density of lumbar muscles 4 years after decompressive spinal surgery. Eur Spine J. 1996;5(3):193–197. doi: 10.1007/BF00395513. [DOI] [PubMed] [Google Scholar]
- 23.Weiner BK, Walker M, Brower RS, McCulloch JA. Microdecompression for lumbar spinal canal stenosis. Spine (Phila Pa 1976) 1999;24(21):2268–2272. doi: 10.1097/00007632-199911010-00016. [DOI] [PubMed] [Google Scholar]
- 24.Burton CV, Kirkaldy-Willis WH, Yong-Hing K, Heithoff KB. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981;157:191–199. [PubMed] [Google Scholar]
- 25.Waguespack A, Schofferman J, Slosar P, Reynolds J. Etiology of longterm failures of lumbar spine surgery. Pain Med. 2002;3(1):18–22. doi: 10.1046/j.1526-4637.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 26.Slipman CW, Shin CH, Patel RK, et al. Etiologies of failed back surgery syndrome. Pain Med. 2002;3(3):200–214. doi: 10.1046/j.1526-4637.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- 27.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine (Phila Pa 1976) 2005;30(8):936–943. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 28.Jansson KA, Nemeth G, Granath F, Blomqvist P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years – a national analysis of 9,664 operations. Eur Spine J. 2005;14(7):659–663. doi: 10.1007/s00586-004-0851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976) 1996;21(21):2412–2420. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Sobottke R, Schluter-Brust K, Kaulhausen T, et al. Interspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J. 2009;18(10):1494–1503. doi: 10.1007/s00586-009-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhoof OJ, Bron JL, Wapstra FH, van Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17(2):188–192. doi: 10.1007/s00586-007-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geisser ME, Haig AJ, Tong HC, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain. 2007;23(9):780–785. doi: 10.1097/AJP.0b013e31815349bf. [DOI] [PubMed] [Google Scholar]
- 33.Sirvanci M, Bhatia M, Ganiyusufoglu KA, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008;17(5):679–685. doi: 10.1007/s00586-008-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]