Summary
Intact cholesterol homeostasis helps to maintain hematopoietic stem and multipotential progenitor cell (HSPC) quiescence. Mice with defects in cholesterol efflux pathways due to deficiencies of the ATP binding cassette transporters ABCA1 and ABCG1 displayed a dramatic increase in HSPC mobilization and extramedullary hematopoiesis. Increased extramedullary hematopoiesis was associated with elevated serum levels of G-CSF due to generation of IL-23 by splenic macrophages and dendritic cells. This favored hematopoietic lineage decisions towards granulocytes rather than macrophages in the bone marrow leading to impaired support for osteoblasts and decreased Cxcl12/SDF-1 production by mesenchymal progenitors. Greater HSPC mobilization and extramedullary hematopoiesis were reversed by raising HDL levels in Abca1−/−Abcg1−/− and Apoe−/− mice or in a mouse model of myeloproliferative neoplasm mediated by Flt3-ITD mutation. Our data identify a novel role of cholesterol efflux pathways in the control of HSPC mobilization. This may translate into novel therapeutic strategies for atherosclerosis and hematologic malignancies.
Introduction
Hematopoietic stem and multipotential progenitor cells (HSPCs) reside in specialized environments called stem cell niches in the medulla of the bone. While the endosteal ‘osteoblastic niche’ (composed of a subset of specialized osteoblasts in the inner surface of the bone cavity) is believed to maintain HSPCs in a quiescent state in poorly perfused regions, the ‘vascular niche’ (adjacent to the bone marrow (BM) vasculature) may serve as a transit pathway that senses environmental signals and shuttles HSPCs out of the BM (Kiel et al., 2008)(Lymperi et al., 2010)(Ehninger et al., 2011).
In hematologic malignancies such as leukemias and myeloproliferative neoplasms, the spleen and the liver resume their fetal hematopoietic functions causing organomegaly in a process called extramedullary hematopoiesis (Kraus et al., 1998)(O’Malley et al., 2005). Symptomatic splenomegaly is common and causes significant morbidity in these patients. An enlarged spleen can cause pain, early satiety, pancytopenia, portal hypertension and hypercatabolic changes. While not fully understood, extramedullary hematopoiesis is believed to result from conditions that disrupt the BM microenvironment, facilitating the egress of progenitor and precursor cells. Mobilization of hematopoietic stem and multipotential progenitor cells (HSPCs), mainly to the spleen, may provide a more permissive microenvironment for proliferation and myeloid differentiation (Morrison et al., 1997). Deregulation of this system contributes to the progression of myeloproliferative diseases (Perry et al., 2007)(Raaijmarkers et al., 2010).
ABCA1 and ABCG1 play an important role in cholesterol homeostasis by promoting cellular cholesterol efflux to lipid poor apoA-I and HDL particles, respectively (Wang et al., 2007)(Yvan-Charvet et al., 2007). Intrinsic deficiency of these transporters in HSPCs led to expansion and proliferation of HSPCs in BM. (Wang et al., 2007)(Yvan-Charvet et al., 2007)(Yvan-Charvet et al., 2010). However, this mechanism did not explain splenomegaly and myeloid cell infiltration of different organs observed in Abca1−/−Abcg1−/− mice. An investigation of these processes led to the discovery of dramatic HSPC mobilization in Abca1−/−Abcg1−/− mice reflecting increased G-CSF production. Prior studies have identified a feedback loop controlling G-CSF and neutrophil production (Stark et al., 2005). When macrophages phagocytose apoptotic neutrophils, there is suppression of production of the cytokine IL-23 leading to decreased G-CSF and neutrophil production. Our studies show that the production of IL-23 was increased in macrophages and dendritic cells deficient in ABCA1 and ABCG1, and that the resulting increase in G-CSF led to changes in the bone marrow milieu favouring release of HSPCs into the circulation.
Results
Enhanced HSPC mobilization and extramedullary hematopoiesis in Abca1−/− Abcg1−/− mice
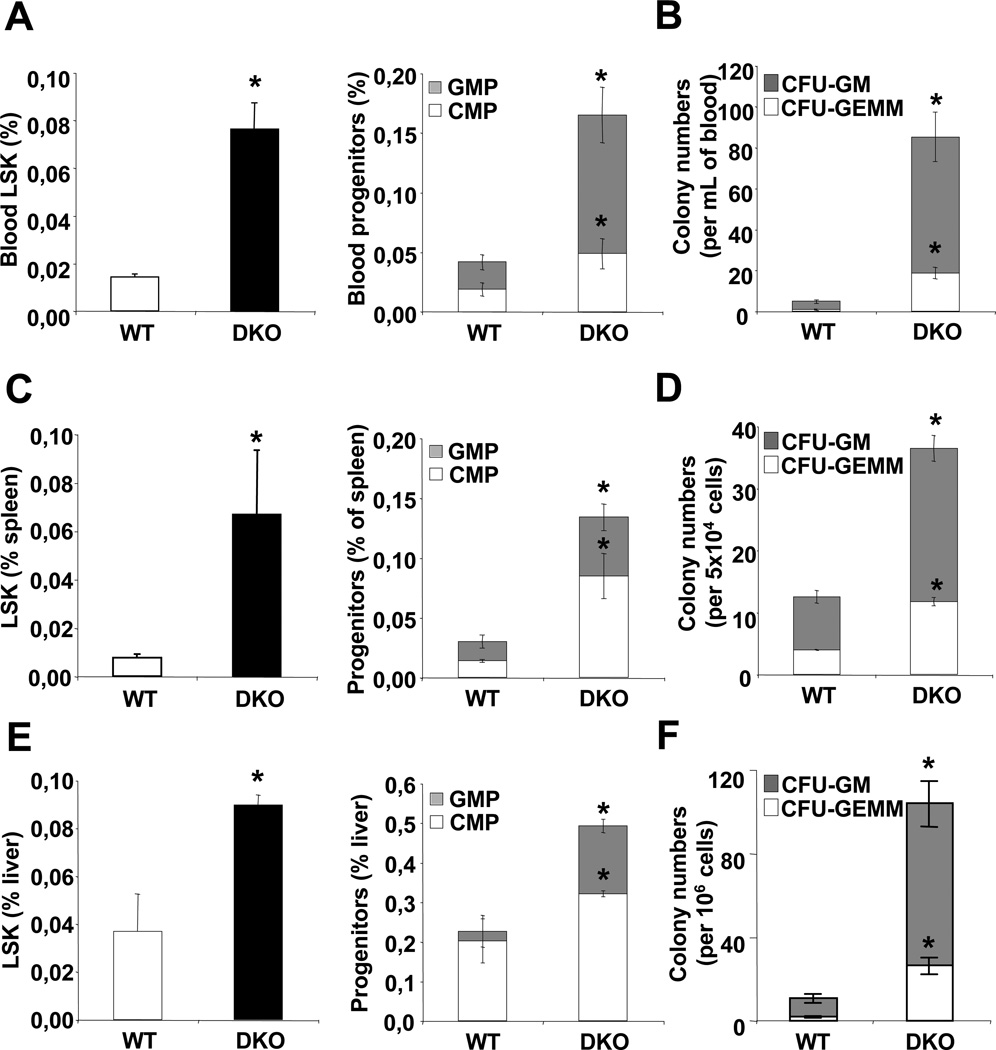
Flow cytometry analysis of HSPC, common myeloid progenitors (CMP) and granulocyte macrophage progenitors (GMP) and colony forming unit assays of multipotential progenitors (CFU-GEMM) and GMP (CFU-GM), revealed a 3-fold increase in the number of these cells in the blood of chow-fed Abca1−/−Abcg1−/− mice (Fig. 1A–B and S1A) indicating enhanced HSPC, CMP and GMP mobilization. Circulating LSK Flk2− cells and LSK CD34− cells were also proportionally increased in these mice (Fig. S1B). This was associated with a parallel 3-fold increase in the number of HSPCs, CMP and GMP progenitors and CFU-GEMM/GM in the spleen (Fig. 1C–D and S1D) and liver (Fig. 1E–F and S1E) and increased CFUs in lung and heart cell extracts (Fig. S1C). These changes indicate HSPC mobilization and extramedullary hematopoiesis in multiple organs in Abca1−/−Abcg1−/− mice.
Figure 1. Extramedullary hematopoiesis in Abca1−/−Abcg1−/− mice.
Quantification of hematopoietic stem and multipotential progenitor cells (HSPCs) by flow cytometry (LSK, Lin−Sca1+c-Kit+) or common myeloid progenitors (CMP) and granulocye/macrophage progenitors (GMP) from (A) the blood, (C) the spleen or (E) the liver of chow fed WT and Abca1−/−Abcg1−/− mice. Colony forming unit assays of multipotential progenitors and granulocyte macrophage progenitors (CFU-GEMM and CFU-GM, respectively) from (B) the blood, (D) the spleen, or (F) the liver of chow fed WT and Abca1−/−Abcg1−/− mice. Results are ± SEM of 6 animals per group. *P<0.05 vs. WT.
BM-dependent regulation of HSPC mobilization in Abca1−/−Abcg1−/− mice
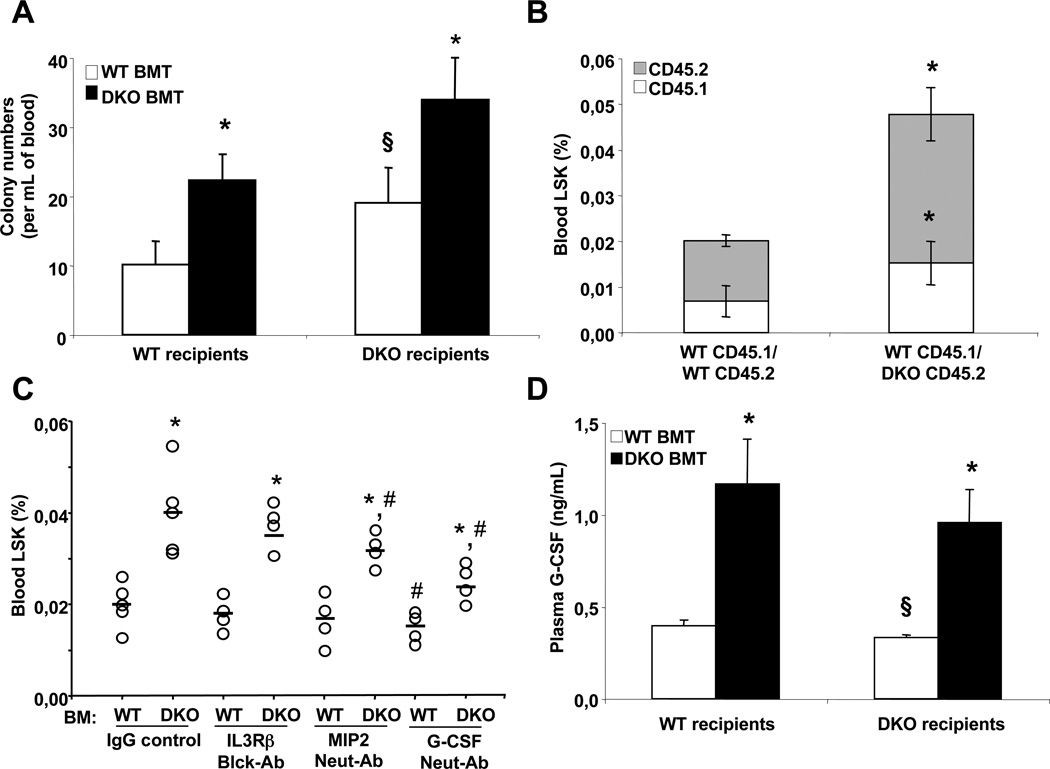
Transplantation of Abca1−/−Abcg1−/− BM into lethally irradiated WT recipients showed a 2-fold increase in the number of CFU in the blood of these mice while reconstitution of Abca1−/−Abcg1−/− mice with WT BM reduced the number of CFU by 40% (Fig. 2A). Analysis of the number of HSPCs in the spleen by flow cytometry showed parallel changes (Fig. S2A). Consistent with our earlier findings, we observed that the increased number of BM LSK cells reflected a BM-dependent role of ABCA1 and ABCG1 and correlated with the cell surface expression of the IL3Rβ on HSPCs (Fig. S2B and S2C) (Yvan-Charvet et al., 2010). Surprisingly, in a competitive BM transplantation experiment with equally mixed BM cells from CD45.1 WT mice and either CD45.2 WT BM or CD45.2 Abca1−/−Abcg1−/− BM, we found that the mobilization of CD45.1 WT HSPCs was increased 2-fold in presence of CD45.2 Abca1−/−Abcg1−/− BM cells, similar to the effect on CD45.2 Abca1−/−Abcg1−/− HSPCs, suggesting that the enhanced HSPC mobilization in these mice involved a cell extrinsic factor (Fig. 2B). This was consistent with the lack of alteration in the cell surface expression of key cytokine receptors involved in the migratory response of HSPCs in Abca1−/−Abcg1−/− mice including the integrinα4β1 receptor (VLA4), the glycoprotein CD44, the stem cell growth factor receptor c-kit and angiopoietin receptor Tie2 (Fig. S2D). Cell surface expression of CD47 in BM and peripheral HSPCs, used by these cells to hide from normal scavenging by myeloid cells (Jaiswal et al., 2009), was also unaltered in Abca1−/−Abcg1−/− mice (Fig. S2E). In addition, analysis of the circadian oscillation of the number of CFU showed that the appearance of HSPCs in the blood during the early phase of the diurnal cycle was increased 2.7-fold in WT recipients transplanted with Abca1−/−Abcg1−/− BM compared to 1.7-fold in controls (Fig. S2F). This pattern suggested that the appearance of HSPCs rather than their clearance from the blood was increased in these mice. Thus, in contrast to the cell autonomous effect of these transporters on HSPC proliferation (Yvan-Charvet et al., 2010), HSPC mobilization in Abca1−/−Abcg1−/− mice was mediated by cell extrinsic factors.
Figure 2. G-CSF-dependence of HSPC mobilization in Abca1−/−Abcg1−/− mice.
Colony-forming unit numbers of multipotential progenitors (CFU-GEMM) from the blood of WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (A). Competitive BM transplantation of WT CD45.1+ BM equally mixed with either CD45.2+ WT BM or CD45.2+ DKO BM and transplanted into WT recipient mice. Circulating HSPCs were analyzed by flow cytometry (B). WT recipients transplanted with WT and DKO BM 14 weeks post-reconstitution were i.p injected with IgG control, 100µg IL3Rβ blocking antibody, MIP2 (CXCL2) neutralizing antibody or G-CSF neutralizing antibody for 16h and analyzed for circulating HSPC (C). Plasma G-CSF levels in WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (D). Results are ± SEM of 5 to 6 animals per group. *P<0.05 vs. WT recipients transplanted with WT BM. #P<0.05 vs. IgG control. §P<0.05 vs. Abca1−/−Abcg1−/− recipients transplanted with Abca1−/−Abcg1−/− BM.
G-CSF-dependence of HSPC mobilization in Abca1−/−Abcg1−/− BM transplanted mice
We next tested the roles of IL-3, CXCL2 (or macrophage inflammatory protein, MIP2), and G-CSF, factors that were previously shown to be increased in Abca1−/−Abcg1−/− mice (Yvan-Charvet et al., 2007)(Yvan-Charvet et al., 2008), and also known to have key roles in the regulation of HSPC mobilization (Guillaume et al., 1993)(Fibbe et al., 1999)(Metcalf et al., 2008)(Greenbaum et al., 2011). While injection of IL3Rβ or CXCL2 blocking antibodies did not prevent the enhanced HSPC mobilization of WT recipients transplanted with Abca1−/−Abcg1−/− BM, the G-CSF neutralizing antibody caused a major reduction in the mobilization of HSPCs in these mice (Fig. 2C), and in the number of HSPCs in the spleen (data not shown). Alteration in the expression of the G-CSF receptor was unlikely to explain these effects as there was no significant change in its cell surface expression or mRNA level in different BM populations of Abca1−/−Abcg1−/− mice (Fig. S3A–S3C). Consistent with a lack of expression of G-CSF receptor on HSPCs (Liu et al., 2000), addition of G-CSF to in vitro BM cultures did not promote expansion of either WT or Abca1−/−Abcg1−/− HSPCs (Fig. S3D) and the G-CSF neutralizing antibody did not modulate the number of BM HSPC in these mice (Fig. S3E). The increased plasma G-CSF in Abca1−/−Abcg1−/− mice was a BM-mediated effect (Fig. 2D). Thus, the absence of ABCA1 and ABCG1 in myeloid BM-derived cells led to an increase in plasma G-CSF that in turn caused increased mobilization of HSPCs.
Increased IL-23/IL-17 drives G-CSF-dependent HSPC mobilization in Abca1−/−Abcg1−/− BM transplanted mice
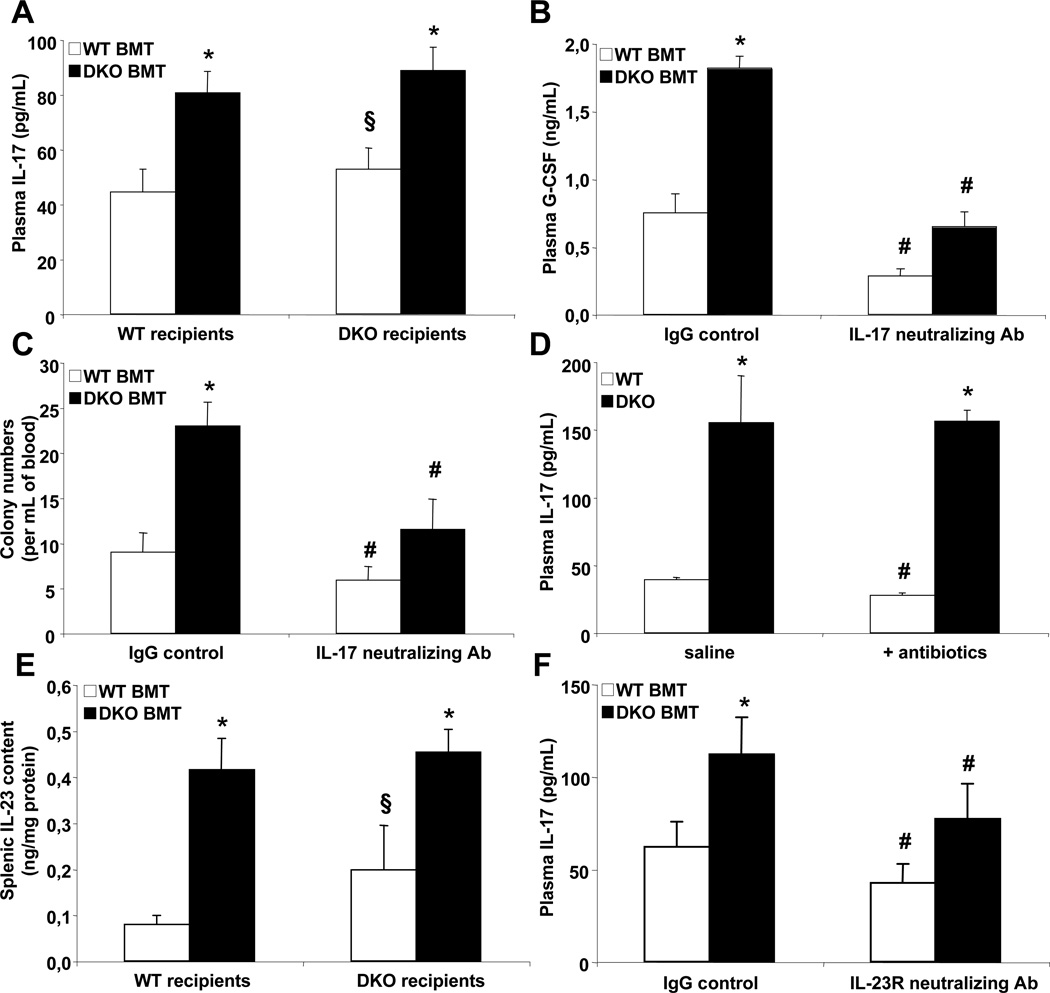
Consistent with the central role of interleukin-17 (IL-17) as a potent inducer of G-CSF (Fossiez et al., 1996), we found increased plasma IL-17 levels in Abca1−/−Abcg1−/− BM transplanted mice (Fig. 3A). We also showed that the administration of an IL-17 blocking antibody was able to normalize both plasma G-CSF levels (Fig. 3B) and CFU numbers (Fig. 3C) in WT recipients transplanted with Abca1−/−Abcg1−/− BM. The production of IL-17 can be mediated by commensal bacteria from the intestinal flora (Ivanov et al., 2008) or by the secretion of interleukin-23 (IL-23) from splenic phagocytic cells (Stark et al., 2005). Despite hypertrophy of intestinal Peyer’s patches in Abca1−/−Abcg1−/− mice (Yvan-Charvet et al., 2010), treatment of these mice with broad spectrum antibiotics including vancomycin (Yvan-Charvet et al., 2010) to suppress segmented filament bacteria involved in the induction of intestinal Th17 (Ivanov et al., 2008), did not reduce their plasma IL-17 or G-CSF levels (Fig. 3D and S3F). In contrast, we found a 4-fold increase in IL-23 concentration in the spleen of these mice, an effect that was dependent on the absence of ABCA1 and ABCG1 in BM-derived myeloid cells (Fig. 3E). Treatment with an IL-23R neutralizing antibody reduced both plasma IL-17 and G-CSF levels in WT recipients transplanted with Abca1−/−Abcg1−/− BM (Fig. 3F and S3G) and also the number of CFU in the blood (Fig. S3H).
Figure 3. IL-23/IL-17-dependent G-CSF regulation in Abca1−/−Abcg1−/− mice.
Plasma IL-17 levels in WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (A). Plasma G-CSF levels in WT recipients transplanted with WT and Abca1−/−Abcg1−/− BM 14 weeks post-reconstitution and i.p injected with IgG control or 250µg IL-17 neutralizing antibody for 16h (B). Colony-forming unit numbers from the blood of these mice (C). Plasma IL-17 levels in WT and Abca1−/−Abcg1−/− mice treated with broad-spectrum antibiotics for 2 weeks to deplete commensal bacteria (D). IL-23 protein content in the spleen of WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (E). Plasma IL-17 levels in WT recipients transplanted with WT and Abca1−/−Abcg1−/− BM 14 weeks post-reconstitution and i.p injected with IgG control or 200µg IL-23R neutralizing antibody for 16h (F). Results are ± SEM of 5 to 6 animals per group. *P<0.05 vs. WT recipients transplanted with WT BM. #P<0.05 vs. IgG control. §P<0.05 vs. Abca1−/−Abcg1−/− recipients transplanted with Abca1−/−Abcg1−/− BM.
ABCA1 and ABCG1 deficiency in macrophages and dendritic cells promote HSPC mobilization
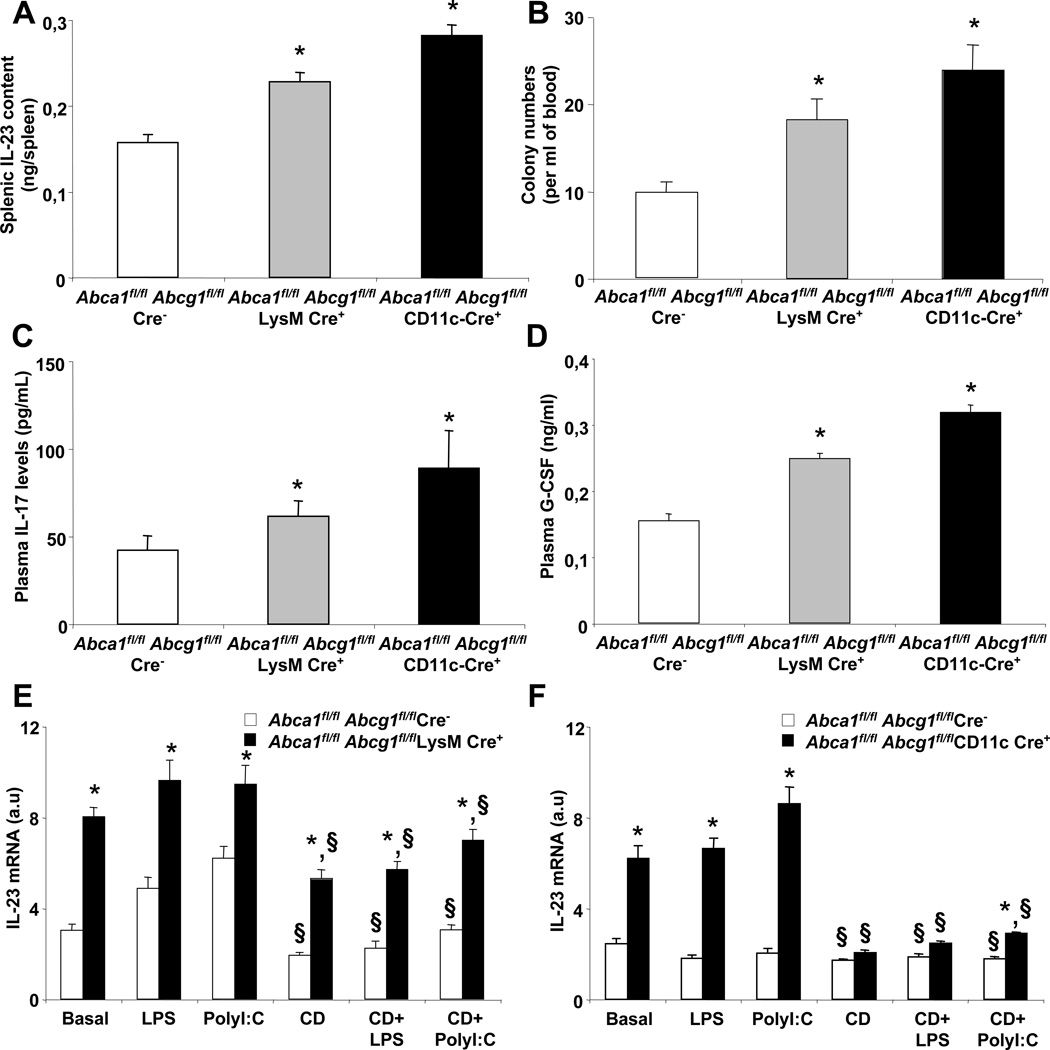
Since IL-23 is secreted by phagocytic macrophages and dendritic cells (Stark et al., 2005), we generated macrophage-specific ABCA1/ABCGG1 knockouts (LysM-cre Abca1fl/flAbcg1fl/fl) and dendritic cell-specific ABCA1/ABCG1 knockouts (CD11c-cre Abca1fl/flAbcg1fl/fl) to examine the contribution of these cells to HSPC mobilization. LysM-cre Abca1fl/flAbcg1fl/fl and CD11c-cre Abca1fl/flAbcg1fl/fl mice showed >95% reductions in ABCA1/ABCG1 expression in macrophages and dendritic cells, respectively, and no difference in ABCA1/ABCG1 expression in HSPCs (data not shown). We observed that LysM-cre Abca1fl/flAbcg1fl/fl and CD11c-cre Abca1fl/flAbcg1fl/fl mice exhibited a 1.5-fold and 1.8-fold increase in IL-23 content in their spleen compared to their respective controls (Fig. 4A), with a parallel increase in the number of CFU in the blood (Fig. 4B). This was also associated with an increase in plasma IL-17 (Fig. 4C) and G-CSF levels (Fig. 4D). In vitro experiments showed that IL-23 mRNA levels were increased in LysM-cre Abca1fl/flAbcg1fl/fl macrophages and CD11c-cre Abca1fl/flAbcg1fl/fl dendritic cells under steady state or LPS and PolyI:C stimulated conditions (Fig. 4E). In addition, plasma membrane cholesterol depletion by cyclodextrin significantly reduced the mRNA expression levels of IL-23 in LysM-cre Abca1fl/flAbcg1fl/fl macrophages under steady state or LPS and PolyI:C stimulated conditions (Fig. 4E) and completely reversed the increase in CD11c-cre Abca1fl/flAbcg1fl/fl dendritic cells (Fig. 4F). Thus, in addition to its known role in promoting granulopoiesis (Stark et al., 2005), IL23/IL17/G-CSF signaling is now shown to be associated with enhanced HSPC mobilization. Cholesterol efflux pathways in macrophages and dendritic cells suppressed this signaling pathway.
Figure 4. Deficiency of ABCA1 and ABCG1 in macrophages and dendritic cells promotes the G-CSF-dependent HSPC mobilization.
Splenic IL-23 protein content (A), Colony-forming unit numbers (B), plasma G-CSF levels (C), and plasma IL-17 levels (D) in 28 weeks old Abca1fl/flAbcg1fl/fl (controls), LysM-Cre Abca1fl/flAbcg1fl/fl, and CD11c-Cre Abca1fl/flAbcg1fl/fl mice. Results are ± SEM of 5 to 6 animals per group. *P<0.05 vs. controls. Modulation of IL-23 mRNA expression levels in Abca1fl/flAbcg1fl/fl and LysM-Cre Abca1flfl-Abcg1fl/fl BM-derived macrophages (E) and CD11c-Cre Abca1fl/flAbcg1fl/fl BM-derived dendritic cells (F) after manipulation of plasma membrane cholesterol. Cells were incubated with 5mmol/L cyclodextrin (CD) for 30minutes before treatment with 50ng/mL lipopolysaccharide (LPS, TLR4 ligand) or 2,5µg/mL PolyI:C (TLR3 ligand) for 3 hours. IL-23 transcript levels were normalized to m36B4 mRNA amount. RNA levels were expressed as arbitrary units (a.u). Values are mean ± SEM of an experiment performed in quadruplicate. *P<0.05 vs. respective controls. §P<0.05 vs. conditions without cyclodextrin treatment.
Increased proteolytic cleavage of CXCR4 in Abca1−/−Abcg1−/− HSPCs is not sufficient to promote their mobilization
These experiments have been described in the online data supplement.
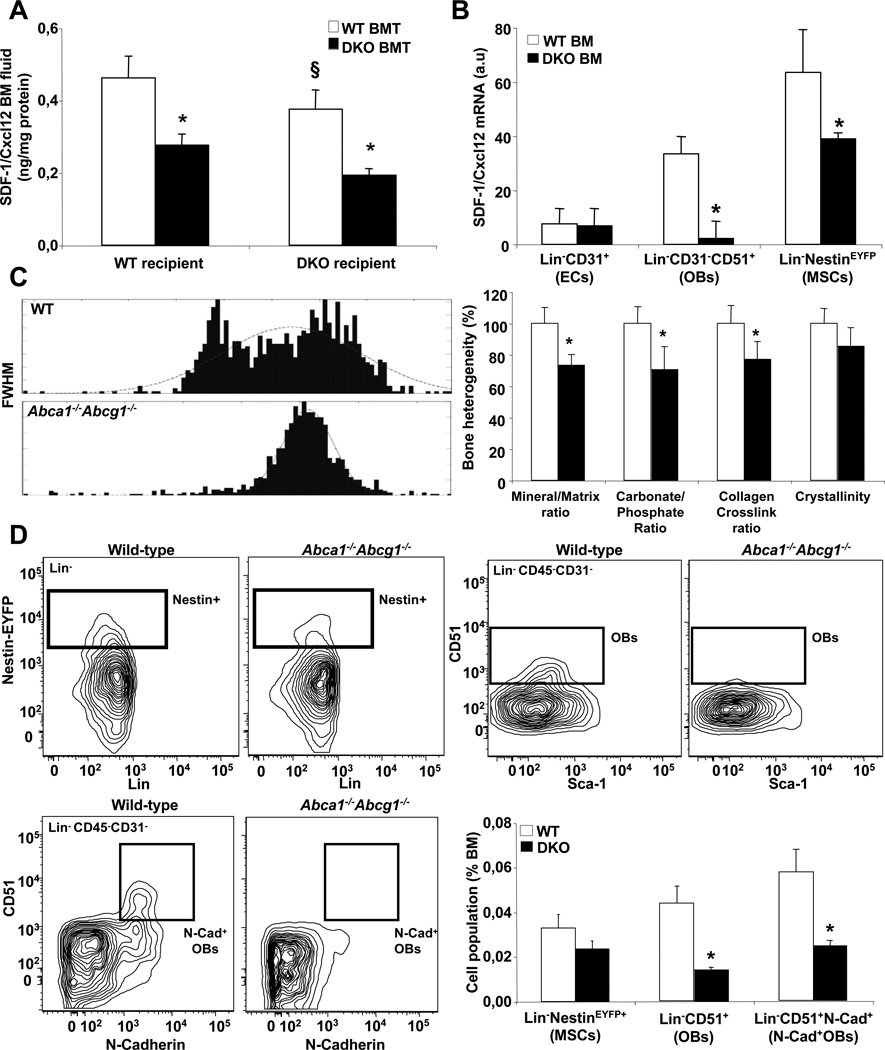
Decreased Cxcl12 expression in BM of Abca1−/−Abcg1−/− mice due to decreased expression in osteoblasts and Nestin+ progenitors
These observations suggested that an alternative pathway was involved in the enhanced HSPC mobilization of Abca1−/−Abcg1−/− mice. Recent evidence suggests that G-CSF may decrease Cxcl12/SDF-1 expression (Semerad et al., 2005). Cxcl12/SDF-1 was reduced by 25% in BM fluid of WT recipients transplanted with Abca1−/−Abcg1−/− BM, with or without CXCR4 lentiviral overexpression (Fig. S4J). There was a 50% decrease in Cxcl12/SDF-1 mRNA expression and protein content in the BM (Fig. S5A). As the number of dipeptidyl peptidase-4 (CD26/DPPIV) positive cells measured by flow cytometry was reduced by 60% in Abca1−/−Abcg1−/− BM (Fig. S5B), this suggested that the altered production rather than the cleavage of Cxcl12/SDF-1 was impaired in these mice. Consistent with this observation, the number of SDF-1 expressing reticular (CAR) cells (Sugiyama et al., 2006) but not non SDF-1 expressing cells was reduced in Abca1−/−Abcg1−/− BM (Fig. S5C). Together these findings suggested that the enhanced HSPC mobilization of Abca1−/−Abcg1−/− BM transplanted mice could not be attributed to increased neutrophil elastase 2-dependent CXCR4 cleavage but rather reflected reduced SDF-1/Cxcl12 mRNA expression. In addition, we found that the 50% decrease in SDF-1/Cxcl12 BM fluid content was mainly attributable to the lack of ABCA1 and ABCG1 in BM-derived myeloid cells (Fig. 5A). Endothelial cells, osteoblasts (OBs) and Nestin+ mesenchymal stem cells (MSCs) were reported to be the major Cxcl12/SDF-1 expressing cell types within the BM (Dar et al., 2005)(Semerad et al., 2005)(Chistopher et al., 2009)(Mendez-Ferrer et al., 2010). To gain more insight into the regulation of Cxcl12/SDF-1 in Abca1−/−Abcg1−/− mice, we bred these mice with mice expressing yellow fluorescent protein (YFP) under the control of regulatory elements of the nestin promoter to allow the identification of Nestin+ MSCs. We compared the mRNA expression levels of Cxcl12/SDF-1 in sorted CD45-Ter119−Nestin+ MSCs, Lin−CD45−CD31−Sca1−CD51+ OB lineage cells and Lin−CD45−CD31+ endothelial cells (Fig. S5D). High Osteocalcin and VE-cadherin expression confirmed that bone Lin−CD45−CD31−Sca1−CD51+ cells and Lin−CD45−CD31+ cells were enriched in OBs and endothelial cells, respectively (Fig. S5E). As previously reported, BM endothelial cells expressed low amount of Cxcl12/SDF-1 compared to OBs (Semerad et al., 2005), and no change was observed between WT and Abca1−/−Abcg1−/− mice (Fig. 5B). While ABCA1 and ABCG1 mRNA expression were barely detectable in Nestin+ MSCs and OBs (data not shown), there was a 50% decrease in Cxcl12/SDF-1 expression in Nestin+ MSCs and almost complete absence of Cxcl12/SDF-1 expression in OBs isolated from Abca1−/−Abcg1−/− BM compared to controls (Fig. 5B). Vascular cell adhesion molecule-1 (VCAM-1), another HSPC maintenance gene, was also reduced in these cells (data not shown). Thus, lack of ABCA1 and ABCG1 indirectly modulates the expression of the key HSPC maintenance gene Cxcl12/SDF-1 in OBs and their Nestin+ progenitors.
Figure 5. Reduced osteoblastic SDF-1/Cxcl12 production in Abca1−/−Abcg1−/− mice.
Quantification of the SDF-1/Cxcl12 protein content in the BM fluid of WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (A). SDF-1/Cxcl12 mRNA levels normalized to ribosomal m36B4 in sorted Lin−CD45−CD31+ endothelial cells (ECs), Lin−CD45−CD31−Sca1−CD51+ OB lineage cells (OBs) and Lin-Nestin+ progenitors (MSCs) from Nestin-Cre+Gt(Rosa)26Sor and Abca1−/−Abcg1−/− Nestin-Cre+Gt(Rosa)26Sor mice (B). Representative histograms and percentage changes in the bone heterogeneity of the indicated bone mineral and matrix properties quantified by Fourier Transform Infrared (FTIR) spectroscopy as the full width at half maximum (FWHM) of the pixel histogram distribution (PHD) (C). Representative dot plots and quantification of Lin-Nestin+ MSCs, Lin−CD45−CD31−Sca1−CD51+ OB lineage cells and Lin−CD45−CD31−CD51+N-Cadherin+ OBs by flow cytometry (D). Results are ± SEM of 5 to 6 animals per group. *P<0.05 vs. WT. §P<0.05 vs. Abca1−/−Abcg1−/− recipients transplanted with Abca1−/−Abcg1−/− BM.
Reduced Cxcl12/SDF-1 expression in Abca1−/−Abcg1−/− BM is associated with decreased N-Cadherin+ osteoblasts
In addition to reduced Cxcl12/SDF-1 expression in OBs and their Nestin+ progenitors, it has been shown that reduced osteoblast numbers, especially N-Cadherin+ bone-lining, spindle-shaped OBs could contribute to the lack of HSPC retention within the BM (Zhang et al., 2003)(Visnjic et al., 2004)(Lymperi et al., 2008). Bone histomorphometry analysis in Abca1−/−Abcg1−/− mice revealed no significant change in the percentage of bone cancellous surface occupied by various heterogeneous populations of OBs including endosteal, trabecular and periosteal OBs as well as osteoclasts (Fig. S5F). These data were consistent with similar bone mineral density measured in vivo by DEXA (Fig. S5G). Fourier transform infrared imaging (FTIRI) analysis of tibial cortical bone also revealed similar mineral/matrix, carbonate/phosphate and collagen crosslink ratio as well as crystallinity between WT and Abca1−/−Abcg1−/− mice (Fig. S5H). Thus, the enhanced HSPC mobilization in Abca1−/−Abcg1−/− mice was not the consequence of altered mineral or matrix bone properties. However, a decrease in the heterogeneous distribution of bone tissue material properties was noted for almost all of the measured FTIRI parameters in the bone of Abca1−/−Abcg1−/− mice (Fig. 5C). This reflected reduced bone remodeling with fewer regions of newly formed bone tissue (Gourion-Arsiquaud et al., 2010) and suggested some specificity in osteoblastic lineage fate in these mice. While the reduced number of Nestin+ MSCs in Abca1−/−Abcg1−/− BM did not reach significance (Fig. 5D), there was a pronounced 3-fold decrease in the number of Lin−CD45−CD31−Sca1−CD51+ OB lineage cells (Fig. 5D). We also observed similar reductions in Lin−CD45−CD31−CD51+N-Cadherin+ OB population in the BM of these mice (Fig. 5D). Of note, all Lin−CD45−CD31−CD51+Sca1− OBs were N-Cadherin+ (data not shown). Thus, the reduced production of SDF-1/Cxcl12 in Abca1−/−Abcg1−/− BM reflected not only reduced expression of this HSPC maintenance gene but also decreased numbers of N-Cadherin+ OB-derived Nestin+ MSCs.
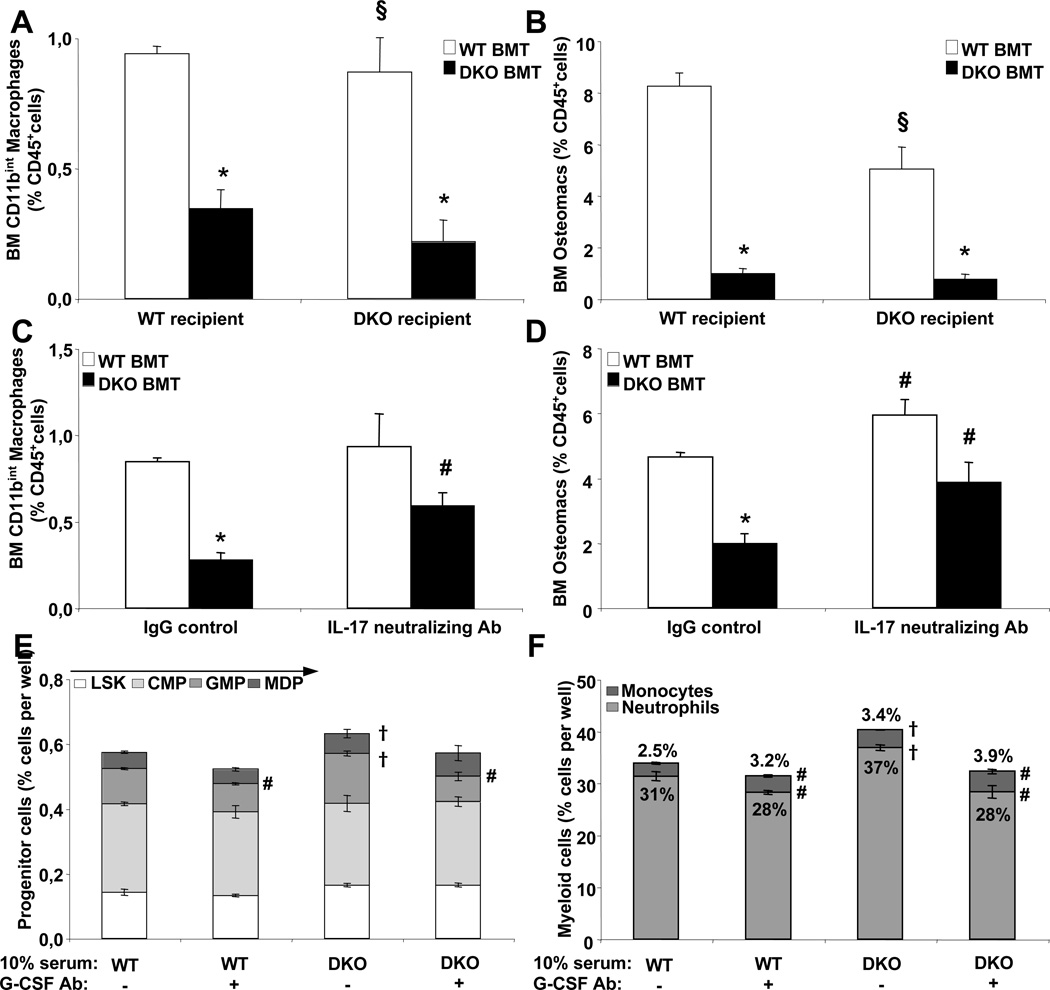
Depletion of BM macrophages contributes to reduced Cxcl12/SDF-1 expression in Abca1−/−Abcg1−/− BM
Recent studies have highlighted a role of BM macrophages in supporting osteoblast differentiation and SDF-1/Cxcl12 production (Winkler et al. 2010)(Christopher et al., 2011)(Chow et al., 2011). We next examined BM macrophages by flow cytometry in WT and Abca1−/−Abcg1−/− mice transplanted with either WT or Abca1−/−Abcg1−/− BM. Two different populations of macrophages have been described in the BM namely CD11bhi osteomacs (Winkler et al. 2010) and CD11bintCD169+ macrophages (Chow et al., 2011) that are localized in different hypoxic area of the BM (Ehninger et al., 2011). The gating strategy is provided in Fig. S6A. Both population of macrophages were dramatically reduced, by 2 and 4-fold, respectively in WT recipients transplanted with Abca1−/−Abcg1−/− BM and this was dependent on a role of ABCA1 and ABCG1 within the BM-derived myeloid population as transplantation of WT BM into Abca1−/−Abcg1−/− recipients almost completely reversed this loss (Fig. 6A–6B). Interestingly, the role of G-CSF in mediating HSPC mobilization was recently shown to involve the G-CSF receptor within cells of the monocytic lineage (Christopher et al., 2011). Thus, we next took advantage of the finding that the IL-17 neutralizing antibody reduced the plasma G-CSF levels in these mice. Treatment of WT recipients transplanted with Abca1−/−Abcg1−/− BM with this antibody largely rescued the loss of both BM macrophage populations (Fig. 6C–6D) and the reduced SDF-1/Cxcl12 BM fluid content (Fig. S6B). Similar findings were observed with the use of IL-23R neutralizing antibody (Fig. S6C–D) and reduced BM macrophage populations were also observed in LysM-cre Abca1fl/flAbcg1fl/fl and CD11c-cre Abca1fl/flAbcg1fl/fl mice (Fig. S6E). This revealed that the enhanced IL23/IL-17/G-CSF axis contributed substantially to the depletion of both BM macrophage populations and subsequent loss of BM SDF-1/Cxcl12 production. Finally, consistent with earlier work (Zhu et al., 2002)(Rieger et al., 2009), in vitro BM cultures showed that G-CSF treatment of Abca1−/−Abcg1−/− BM cultures resulted in increased neutrophil but decreased monocyte production, while IL-3 treatment increased both neutrophils and monocytes, suggesting that G-CSF instructed hematopoietic progenitor determination to favor neutrophilic over monocytic lineages (Fig. S6F–G). Consistent with these findings, we observed that in vitro treatment of WT BM cells with 10% serum from Abca1−/−Abcg1−/− mice favored mainly GMP and to some extent MDP expansion compared to WT serum (Fig. 6E and S6H–I) leading to a major increase in the number of neutrophils (Fig. 6F). The use of the G-CSF neutralizing antibody in these cultures revealed that the inhibition of GMP and neutrophil expansion (Fig. 6E and 6F) was associated with a significant increase in the number of monocytes (Fig. 6F) indicating that G-CSF acted at the level of GMP to tightly control the leukocyte fate. Thus, we propose that enhanced IL-23/IL17 dependent G-CSF production by Abca1−/−Abcg1−/− splenic phagocytic cells induced HSPC mobilization by altering the fate of GMPs such that there was decreased formation of monocyte/macrophage-lineage cells and decreased macrophage-dependent osteoblastic support and SDF-1/Cxcl12 production.
Figure 6. Depletion of BM macrophages in Abca1−/−Abcg1−/− mice.
Quantification of BM macrophage subsets by flow cytometry in WT and Abca1−/−Abcg1−/− recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 8 weeks post-reconstitution (A and B) and in WT recipients transplanted with WT and Abca1−/−Abcg1−/− BM 14 weeks post-reconstitution and i.p injected with IgG control or 250µg IL-17 neutralizing antibody for 16h (C and D). Results are ± SEM of 5 to 6 animals per group. *P<0.05 vs. WT. §P<0.05 vs. Abca1−/−Abcg1−/− recipients transplanted with Abca1−/−Abcg1−/− BM. Quantification of hematopoietic progenitors (E), monocytes and neutrophils (F) in WT BM cultures grown for 48h in liquid culture in presence of 10% of the indicated serum and 50ng/mL G-CSF neutralizing antibody (+) or control non-specific IgG (−). Results are ± SEM of 3 independent experiments. #P<0.05 vs. IgG control. †P<0.05 vs. 10% WT serum.
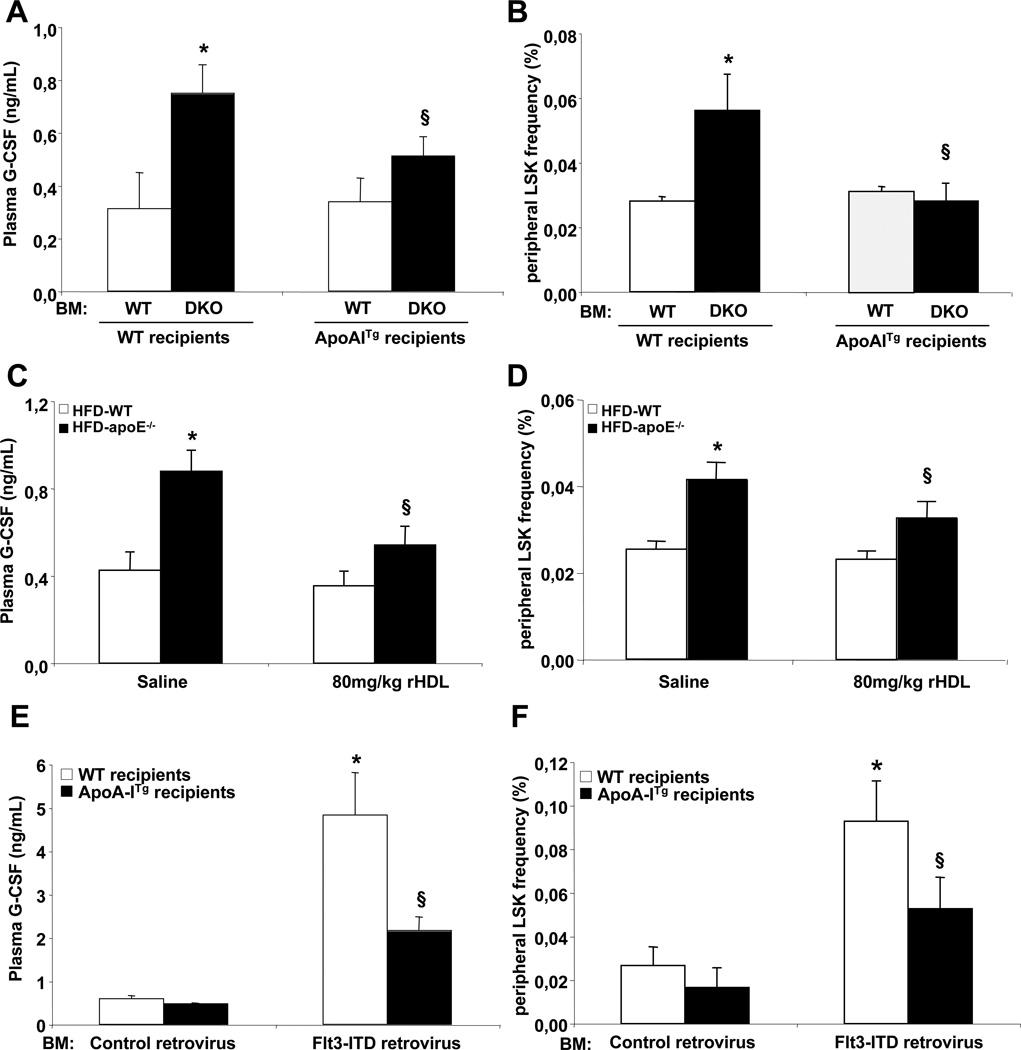
Raising HDL-cholesterol suppresses HSPC mobilization in mouse models of hypercholesterolemia and myeloproliferative disorders
Expression of the human apoA-I transgene led to reduced plasma G-CSF (Fig. 7A) and reduced HSPC mobilization in Abca1−/−Abcg1−/− BMT mice (Fig. 7B), providing a mechanism to explain the reversal of extramedullary hematopoiesis, splenomegaly and myeloid cell proliferation by the apoA-I transgene. This was associated with reduced splenic mRNA expression of IL-23 (data not shown). We next tested whether the suppression of HSPC mobilization by raising HDL levels in Abca1−/−Abcg1−/− BM transplanted mice was peculiar to this model or could be observed as a more general phenomenon in other mouse models of hypercholesterolemia or MPN. We recently reported that hypercholesterolemic Apoe−/− mice exhibited a similar phenotype to Abca1−/−Abcg1−/− mice and that infusion of rHDL suppressed their leukocytosis (Murphy et al., 2011). Apoe−/− mice fed a high fat diet for 4 weeks exhibited a 2-fold increased plasma G-CSF levels (Fig. 7C) that correlated with their number of circulating HSPCs (Fig. 7D). Infusion of 80mg/kg rHDL reduced by approximately 40% not only the plasma G-CSF levels (Fig. 7C) but also the number of circulating HSPCs in these mice 96hours post-infusion (Fig. 7D). Finally, we transplanted BM transduced with retroviruses encoding Flt3-ITD, an oncogenic mutation previously identified in acute myeloid leukaemia (AML) that confers a fully penetrant myeloproliferative disorder in mice (Kelly et al., 2002) into either WT or apoA-I transgenic mice. Reduced plasma HDL-cholesterol levels have been previously reported in myeloproliferative disorders (Gilbert et al., 1981)(Dessi et al., 1995). Consistent with these reports, mice bearing the Flt3-ITD mutation exhibited a 1.5-fold decrease in their plasma HDL-cholesterol levels (Fig. S7A) and this was associated with reduced mRNA expression levels of ABCA1 but not ABCG1 in their spleen (Fig. S7B). Remarkably, there was a 4-fold increase in plasma G-CSF levels and circulating HSPC numbers in mice bearing the Flt3-ITD mutation, and both parameters were markedly reduced by the human apoA-I transgene (Fig. 7E and 7F). This was associated with reduced mRNA expression of IL-23 in their spleen (Fig. S7C), reduced plasma IL-17 (Fig. S7D) and suppression of splenomegaly (Fig. S7E) and splenic HSPC infiltration (Fig. S7F). Finally, the depletion of HSPCs in the BM of mice bearing the Flt3-ITD mutation was prevented by overexpression of the apoA-I transgene (Fig. S7G) reflecting increased mRNA expression levels of SDF-1/Cxcl12 (Fig. S7H). Thus, similar mechanisms of HSPC mobilization and reversal by increased HDL levels appear to be involved in both the Abca1−/−Abcg1−/− and the Flt3-ITD models of MPNs.
Figure 7. Raising HDL-cholesterol prevents HSPC mobilization.
Plasma G-CSF levels and quantification of circulating HSPC cells by flow cytometry in WT and ApoA-I transgenic recipient mice transplanted with either WT or Abca1−/−Abcg1−/− BM 12 weeks post-reconstitution (A and B), in 4-weeks high fat diet-fed WT and apoE−/− mice infused or not with 80mg/kg rHDL for 96h (C and D) and in WT and ApoA-I transgenic recipient mice transplanted with BM transduced with retroviruses encoding the human oncogenic Flt3-ITD mutation 6weeks post-reconstitution (E and F). Results are ± SEM of 4 to 6 animals per group. *P<0.05 vs. WT. §P<0.05, effect of HDL-raising treatment (hapoA-ITg or rHDL).
Discussion
We have identified a novel role of cholesterol efflux pathways in limiting the mobilization of HSPCs and prevention of clinically detrimental extramedullary hematopoiesis in mouse models of myeloproliferative disorders. In mice with knockout of transporters ABCA1 and ABCG1 in all BM cells, or exclusively in macrophages or dendritic cells, increased IL-23-dependent production of growth factor G-CSF alters the BM niche leading to HSPCs mobilization and infiltration of distant organs. Concordant with a recent study (Rieger et al., 2009), increased G-CSF acts via GMPs to direct myeloid production toward neutrophils rather than monocytes, eventuating in BM macrophage depletion. Consequently, OB populations as well as the production of SDF-1/Cxcl12 are reduced. Importantly, our findings indicate a beneficial effect of increased HDL levels in preventing or reversing HSPC mobilization and extramedullary hematopoiesis, not only in Abca1−/−Abcg1−/− mice and Apoe−/− mice but also in a mouse model of hematological malignancy. These findings suggest a novel therapeutic application of rHDL infusion in the prevention of extramedullary hematopoiesis in both atherosclerosis and hematologic malignancies.
Ley and colleagues have defined a negative feedback loop controlling granulopoiesis in which uptake of apoptotic neutrophils by macrophages and dendritic cells leads to suppression of IL-23 production, decreased release of IL-17 by lymphocytes and decreased production of G-CSF by bone marrow stromal cells (Stark et al., 2005). Our results provide the first evidence that activation of the IL23/IL17/G-CSF signaling axis in mice with defective cholesterol efflux pathways leads to increased HSPC mobilization. Moreover, by using cell-specific knockouts of ABCA1 and ABCG1, we show for the first time the importance of the cholesterol efflux pathways in macrophages and dendritic cells in the control of IL-23 production. This links the control of HSPC mobilization to cholesterol homeostasis in the innate immune system.
In agreement with previous studies (Rieger et al., 2009), increased plasma G-CSF levels in Abca1−/−Abcg1−/− mice direct hematopoietic lineages in vitro toward neutrophil rather than monocyte/macrophage production. This led to an expansion of neutrophils and loss of macrophage populations in the BM. Neutrophil expansion in Abca1−/−Abcg1−/− mice transformed the BM into a highly proteolytic environment (Levesque et al., 2002) leading to cleavage of CXCR4 by neutrophil elastase 2 (Petit et al., 2002)(Levesque et al., 2003). However, this alone could not explain enhanced HSPC mobilization as deletion of Elane or lentiviral overexpression of CXCR4 did not rescue this phenotype. Rather our studies indicated an essential role of reduced BM SDF-1/Cxcl12 production in HSPC mobilization. This is consistent with the recently demonstrated role of decreased SDF-1/Cxcl12 in mediating the effect of G-CSF on HSPC mobilization (Levesque et al., 2004)(Semerad et al., 2005)(Christopher et al., 2008).
While macrophage depletion has been shown to promote HSPC mobilization by disruption of the osteoblastic niche through impaired osteoblast (OB) activity and SDF-1/Cxcl12 production (Winkler et al., 2010)(Gordy et al., 2011)(Chow et al., 2011)(Christopher et al., 2011), the link with endogenous plasma G-CSF appears to be novel. Greater endogenous plasma G-CSF induced the loss of BM macrophage populations in Abca1−/−Abcg1−/− mice leading to decreased SDF-1/Cxcl12 production. This involved decreases in N-Cadherin+ OB subsets and Nestin+ progenitors. Previous studies have shown that these cell populations have a key role in maintaining HSPCs in their niche (Lymperi et al., 2008)(Visnjic et al., 2004)(Mendez-Ferrer et al., 2010)(Raaijmarkers et al., 2010). Thus, our study shows that cholesterol efflux pathways in splenic macrophages and dendritic cells are linked to control of the osteoblastic niche and HSPC mobilization via the IL23/IL17/G-CSF signaling axis.
Overexpression of the human apoA-I transgene to raise HDL levels prevented the myeloproliferative disorders of Abca1−/−Abcg1−/− BM transplanted mice (Yvan-Charvet et al., 2010). We now provide evidence that this effect was partly mediated by reduced plasma G-CSF levels and HSPC mobilization. We recently showed that Apoe−/− mice have expanded and proliferating HSPCs (Murphy et al., 2011). ApoE is expressed on the surface of HSPCs where it appears to interact with ABCA1 and ABCG1 to promote cholesterol efflux (Laffitte et al., 2001)(Murphy et al., 2011). Apoe−/− mice also develop hepatosplenomegaly on WTDs likely reflecting extramedullary hematopoiesis (Murphy et al., 2011). A recent study has shown that the spleen represents an important reservoir of monocytes in Apoe−/− mice, contributing directly to atherosclerotic lesions (Robbins et al., 2012). Together with our study, this suggests that extramedullary hematopoiesis resulting from increased G-CSF in Apoe−/− mice plays a role in atherogenesis. Moreover, infusion of cholesterol-poor phospholipid/apoA-I complexes (i.e, rHDL)(Murphy et al., 2011), suppressed plasma G-CSF levels and HSPC mobilization, uncovering a potential novel anti-atherogenic effect of this treatment.
The relevance of the human apoA-I transgene was also tested in a mouse model of myeloproliferative disorder induced by an activating mutation in the Flt3 gene (Flt3-ITD), the most common mutation associated with acute myeloid leukemia (AML) in humans. Consistent with their myeloproliferative disorder, mice that received BM transduced with retroviruses encoding Flt3-ITD exhibited splenomegaly (Kelly et al., 2002) and this was associated with increased splenic expression of IL-23, increased plasma G-CSF and HSC mobilization, likely reflecting an underlying expansion of DCs by Flt3 signaling (Liu et al., 2010). The MPN was associated with reduced plasma cholesterol and raising HDL levels though overexpression of the human apoA-I transgene partially prevented these defects. Our findings indicate that HDL-mediated cholesterol efflux pathways in macrophages and dendritic cells act to suppress HSPC mobilization and extramedullary hematopoiesis, and that HDL raising treatments could have beneficial effects on these processes in atherosclerosis and haematological malignancies.
Experimental procedures
Mice and treatments
Human apoA-1 transgenic (B6.Tg(ApoA1)1Rub/J), Apoe−/− (B6.129P2-Apoetm1Unc), Elane−/− (B6.129X1-Elanetm1Sds/J), Nestin-Cre (B6.Cg-Tg(nescre) 1kln/J) and RosaEYFP (B6.129×1Gt(Rosa)26Sortm1EYFP/J) mice were obtained from The Jackson laboratory. Wild-type and Abca1−/−Abcg1−/− littermates in a mixed C57BL/6 × DBA background (Yvan-Charvet et al., 2007), were used for this study, unless otherwise noted. LysM-Cre Abca1fl/flAbcg1fl/fl mice and CD11c-Cre Abca1fl/flAbcg1fl/fl mice were obtained by crossing Abca1fl/fl mice (kindly provided by Dr. Parks, Wake Forest University) and Abcg1fl/fl mice (generated at Columbia University) with LysM-Cre mice (B6.129P2-Lyz2tm1(cre)Ifo/J) and CD11c-Cre mice (C57BL/6J-Tg(Itgax-cre,-EGFP)) from The Jackson laboratory, respectively. Cre efficiency was confirmed by RT-PCR against the targeted ABCA1 and ABCG1 genes in macrophages and dendritic cells, respectively (data not shown). Mice were fed either a regular chow diet or a Western diet (TD 88137, Harlan Teklad) as indicated in the figure legends. Bone marrow (BM) transplantation into the genetically uniform F1 generation obtained by crossing C57BL/6 wild-type or hapoA-1Tg mice with wild-type DBA mice (The Jackson laboratory) was performed as previously described (Yvan-Charvet et al., 2007). For neutralizing antibody experiments, WT and Abca1−/−Abcg1−/− BM transplanted mice were i.p injected 16h before analysis with the following antibodies: anti-IL-3Rβ AF549, anti-Cxcl2 MAB452, anti-IL-23R MAB1686, anti-IL-17 MAB421 and anti-G-CSF MAB414 (all from from R&Dsystems). Treatments with rHDL (provided by CSL Australia) or vehicle (saline) was administered via the tail vein. All mice were housed at Columbia University Medical Center according to animal welfare guidelines. Mice were maintained on a 12h light/12hdarkness lighting schedule. Animals had ad libitum access to both food and water.
Colony-Forming Assay
All experiments were performed between 12:00 and 1:00pm to limit variability between experiments. For peripheral blood CFU assays, 300µl of blood were collected into EDTA tubes before red blood cell lysis, filtration and centrifugation for 5min at 1,000rpm. Splenocytes were extracted by pressing spleens through a stainless steel grid. Single-cell suspension was submitted to red blood cell lysis, filtration and centrifugation for 5min at 1,000rpm. For liver, lung and heart CFU assays, tissues were cut in small piece and digested with 1mg/mL collagenase D (Roche) for 30min at 37°C before red blood cell lysis, filtration and centrifugation for 5min at 1,000rpm. Peripheral cells, splenocytes (5×104), liver, lung and heart cells (1×106) were plated in methylcellulose-based media containing a cocktail of recombinant cytokines including SCF, IL-3 and IL-6 (Methocult, Stemcell) supplemented with 2% FCS to generate multipotential progenitor cells (CFU-GEMM) and in presence or absence of 10% IL-3 supplement media (BD Bioscience) and GM-CSF (2ng/mL) (Mix conditions) (R&D Systems) to generate granulocyte-macrophage progenitors (CFU-GM). The number of CFUs per dish was scored after 10 days of differentiation.
Flow cytometry analysis and cell sorting
BM cells, peripheral blood leukocytes and splenocytes were collected as described above. Cells were stained with the appropriate antibodies for 30 min on ice. Intracellular stainings were performed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s protocol. Endothelial cells, osteoblasts and Nestin+ cells were isolated at previously described (Semerad et al., 2005). Briefly, tibias and femurs were flushed thoroughly of BM cells, chopped with a scapel, and washed three times to further remove residual BM cells. The bone fragments were then digested with collagenase type IA (Sigma-Aldrich) for 30min at at 37°C before red blood cell lysis, filtration and centrifugation for 5min at 1,000rpm. Multiparameter analyses of stained cell suspensions were performed on a four-laser BD LSRII cell analyzer or sorted on a BD FACSAria Cell Sorter both running with DiVa software (BD Biosciences). Viable cells were gated by light scatter or exclusion of CD45− cells. Data were analyzed using FlowJo software (Tree Star Inc.).
Statistical analysis
Statistical significance was assessed by performing two-tailed parametric student’s t test or by one-way analysis of variance (ANOVA, 4-group comparisons) with a Bonferroni multiple comparison post-test (GraphPad software, San Diego, CA).
Supplementary Material
Highlights.
Cholesterol efflux in splenic DCs and macrophages regulate HSPC mobilization.
Cholesterol efflux causes reduced G-CSF production and alters the osteoblastic niche.
Cholesterol efflux prevents HSPC mobilization and extramedullary hematopoiesis.
Acknowledgments
We thank Dr. Kristie Gordon for assistance with flow cytometry, CSL Australia for providing the rHDL used in this study and Dr. David W. Dempster for help on bone histomorphometry analysis. This work was supported by grants to L.Y.C from the American Heart Association (SDG2160053), to M.W. from the Netherlands Organization of Scientific Research (NWO VENI 916.11.072) and to A.R.T from the NIH (HL54591).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information. Supplemental information for this article includes Supplemental Experimental Procedures and Figures S1–S16.
References
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J. Bone Miner Res. 2008;23:1765–1774. doi: 10.1359/JBMR.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXC12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, Resnick I, Rot A, Lapidot T. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat. Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- Dessi S, Batetta B, Spano O, Sanna F, Tonello M, Giacchino M, Tessitore L, Costelli P, Baccino FM, Madon E. Clinical remission is associated with restoration of normal high-density lipoprotein cholesterol levels in children with malignancies. Clin Sci (lond) 1995;89:505–510. doi: 10.1042/cs0890505. [DOI] [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibbe WE, Pruijt JF, Velders GA, Opdenakker G, van Kooyk Y, Figdor CG, Willemze R. Biology of IL-8-induced stem cell mobilization. Ann. N. Y. Acad. Sci. 1999;872:71–82. doi: 10.1111/j.1749-6632.1999.tb08454.x. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. Faseb. J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HS, Ginsberg H, Fagerstrom R, Brown WV. Characterization of hypocholesterolemia in myeloproliferative disease. Relation to disease manifestations and activity. Am J Med. 1981;71:595–602. doi: 10.1016/0002-9343(81)90212-6. [DOI] [PubMed] [Google Scholar]
- Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2010;117:618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46:666–672. doi: 10.1016/j.bone.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- Guillaume T, D’Hondt V, Symann M. IL-3 and peripheral blood stem cell harvesting. Stem Cells. 1993;11:173–181. doi: 10.1002/stem.5530110303. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finaly BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-Cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kraus MD, Bartlett NL, Fleming MD, Dorfman DM. Splenic pathology in myelodysplasia: a report of 13 cases with clinical correlation. Am. J. Surg. Pathol. 1998;22:1255–1266. doi: 10.1097/00000478-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, Scharpe S, Van Damme J, De Meester I. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J. Biol. Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp. Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by G-CSF or cyclophosphamide. J. Clin. Invest. 2003;110:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Poursine-Laurent J, Link D. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95:3025–3031. [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol. Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- Lymperi S, Ferraro F, Scadden DT. The HSC nice concept has turned 31. Has our knowledge matured? Ann. N. Y. Acad. Sci. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–836. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DP, Kim YS, Perkins SL, Baldridge L, Juliar BE, Orazi A. Morphological and immunohistochemical evaluation of splenic hematopoietic proliferations in neoplastic and benign disorders. Mod. Pathol. 2005;18:1550–1561. doi: 10.1038/modpathol.3800480. [DOI] [PubMed] [Google Scholar]
- Perry JM, Li L. Disrupting the stem cell niche: good seeds in bad soil. Cell. 2007;129:1045–1047. doi: 10.1016/j.cell.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdelos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immnunol. 2002;3:687–787. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:853–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Semerad CL, Christopher MK, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by Cxcl12-Cxcr4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DL. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Jonhson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen, Alexander KA, Ragatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhu J, Emerson SG. Hematopoietic cytokines transcription factors and lineage commitment. Oncogene. 2002;21:3295–3313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.