Abstract
Nitric oxide synthases (NOSs) have been shown to modulate thermal hyperalgesia and mechanical hypersensitivity in inflammatory and neuropathic pain. However, little is known about the effect of NOSs on baseline function of sensory nerve fibers. Using genetic deficiency and pharmacologic inhibition of NOSs, we examined the impact of the three isoforms NOS1, NOS2, and NOS3 on baseline nocifensive behavior by measuring current vocalization threshold in response to electrical stimulation at 5, 250, 2000 Hz that preferentially stimulate C, Aδ, and Aβ fibers. In response to 5, 250 and 2000 Hz, NOS1-deficient animals had significantly higher current vocalization thresholds compared with wild-type. Genetic deficiency of NOS2 was associated with higher current vocalization thresholds in response to 5 Hz (C-fiber) stimulation. In contrast, NOS3-deficient animals had an overall weak trend toward lower current vocalization thresholds at 5 Hz and significantly lower current vocalization threshold compared with wild-type animals at 250 and 2000 Hz. Therefore, NOSs distinctively affect baseline mouse current vocalization threshold and appear to play a role on nocifensive response to electrical stimulation of sensory nerve fibers.
Keywords: Nociception, Pain, Vocalization, Nitric oxide, Mouse
Introduction
A growing body of literature indicates that the three isoforms of nitric oxide synthases (NOSs) as well as their end-product, nitric oxide (NO), have modulatory effects on nociception [1,2]. Researchers have shown that there is upregulation of NOS1 expression and activity in spinal cord and dorsal root ganglia in inflammatory and neuropathic pain [3–7]. In addition, NOS1 deficiency or inhibition attenuates thermal hyperalgesia and mechanical hypersensitivity associated with inflammatory pain after intraplantar injection of complete Freund’s adjuvant [6,8–10] or formalin [11] and that associated with neuropathic pain [7,12] in mice. Therefore, NOS1 appears to have a pivotal role in modulating thermal and mechanical hyperalgesia associated with inflammatory and neuropathic pain. The other constitutive isoform, NOS3, has also been implicated in mechanisms of nociception. In rats, NOS3 expression is upregulated in the ipsilateral lumbar sympathetic chain after constriction of the sciatic nerve [13] and NOS3-deficient mice have faster recovery from thermal hyperalgesia after intraplantar injection of complete Freund’s adjuvant [9]. In contrast, in studies using an NOS3 inhibitor, NOS3 did not modulate hyperalgesia associated with inflammatory pain [10]. Therefore, while the role of NOS1 in nociception has been supported by many studies, that of NOS3 is less clear.
The inducible NOS, NOS2, has also been implicated in nociception as genetic deficiency and pharmacologic inhibition of NOS2 are associated with decreases in mechanical allodynia and thermal hyperalgesia after sciatic nerve injury and inflammatory pain [3,7,9,10,14]. However, others have reported that targeted disruption of NOS2 did not affect thermal hyperalgesia whereas, the use of an NOS1 inhibitor in NOS2-deficient animals reduced the late phase thermal hyperalgesia after carrageenan injection, thus suggesting that NOS2 is sufficient but not essential for the late phase of carrageenan-induced hyperalgesia [15]. Therefore, despite some discrepant results and incompletely understood mechanisms, there is ample evidence to suggest that all three NOSs have a role in inflammatory and neuropathic pain.
While all NOSs appear to have a role in neuropathic and inflammatory pain, the impact of genetic deletion and pharmacologic inhibition of NOSs during basal conditions is less clear. In the present investigation, we hypothesized that genetic manipulation of NOSs would affect baseline nocifensive response to electrical stimulation. To test this hypothesis, we used a nociceptive assay that delivers electrical stimulation at 5, 250, and 2000 Hz to preferentially stimulate C, Aδ, and Aβ sensory nerve fibers to determine the effect of NOSs during basal condition.
Materials and methods
Animals
This investigational protocol was approved by the Animal Care and Use Committees from the NIH Clinical Center, NIH, Bethesda, MD, and from the Children’s National Medical Center, Washington, DC. Female and male B6129SF2/J (wild-type controls for NOS1-KO) and B6;129S4-NOS1tm1Plh (NOS1-knock out, NOS1-KO) and female C57Bl/6J (wild-type controls for NOS2-KO and NOS3-KO), B6.129P2-Nos2tm1Lau/J (NOS2-knock out, NOS2-KO), and B6.129P2-Nos3tm1Unc/J (NOS3-knock out, NOS3-KO) mice (Jackson Laboratory, Bar Harbor, ME) were used in this investigation.
Nociception assay and delivery of electrical stimulus
We used electrical stimulation delivered at three different frequencies: 5, 250, and 2000 Hz which are believed to preferentially stimulate C, Aδ, and Aβ fibers respectively as described elsewhere [16]. Briefly, the electrical stimulus is generated by a neurostimulator (Neurotron, Inc., Baltimore, MD), and is controlled through a standard RS-232 serial port by a custom software. Animals are gently restrained and electrodes are placed on the tail approximately one cm apart [16]. The amperage of the electrical stimulus that elicits vocalization (nocifensive behavior) is defined as current vocalization threshold (cVT). The unit of measurement of cVT is “unit” which corresponds to 100 times the intensity (amperage) that elicited vocalization. For given frequencies, stimuli are delivered at predetermined intervals and in incremental intensities as described [16].
Study design and experimental protocol
We used a Latin square design where each animal was exposed to all possible permutations of electrical frequencies over a two week period. During the two-week period, each study session was conducted every other day (session) when a given sequence of frequencies was evaluated (Table 1).
Table 1.
Experimental design indicating the sequence of stimuli delivered to individual mice over a two week study period.*
| Mouse | Week 1 Sessions |
Week 2 Sessions |
||||
|---|---|---|---|---|---|---|
| Monday | Wednesday | Friday | Monday | Wednesday | Friday | |
| 1 | 2000 Hz, 250 Hz, 5 Hz | 250 Hz, 5 Hz, 2000 Hz | 5 Hz, 2000 Hz, 250 Hz | 250 Hz, 2000 Hz, 5 Hz | 5 Hz, 250 Hz, 2000 Hz | 2000 Hz, 5 Hz, 250 Hz |
| 2 | 250 Hz, 5 Hz, 2000 Hz | 5 Hz, 2000 Hz, 250 Hz | 5 Hz, 250 Hz, 2000 Hz | 2000 Hz, 5 Hz, 250 Hz | 250 Hz, 2000 Hz, 5 Hz | 2000 Hz, 250 Hz, 5 Hz |
| 3 | 5 Hz, 2000 Hz, 250 Hz | 2000 Hz, 250 Hz, 5 Hz | 2000 Hz, 5 Hz, 250 Hz | 5 Hz, 250 Hz, 2000 Hz | 250 Hz, 5 Hz, 2000 Hz | 250 Hz, 2000 Hz, 5 Hz |
| 4 | 250 Hz, 2000 Hz, 5 Hz | 5 Hz, 250 Hz, 2000 Hz | 2000 Hz, 250 Hz, 5 Hz | 250 Hz, 5 Hz, 2000 Hz | 2000 Hz, 5 Hz, 250 Hz | 5 Hz, 2000 Hz, 250 Hz |
| 5 | 5 Hz, 250 Hz, 2000 Hz | 2000 Hz, 5 Hz, 250 Hz | 250 Hz, 2000 Hz, 5 Hz | 5 Hz, 2000 Hz, 250 Hz | 2000 Hz, 250 Hz, 5 Hz | 250 Hz, 5 Hz, 2000 Hz |
| 6 | 2000 Hz, 5 Hz, 250 Hz | 250 Hz, 2000 Hz, 5 Hz | 250 Hz, 5 Hz, 2000 Hz | 2000 Hz, 250 Hz, 5 Hz | 5 Hz, 2000 Hz, 250 Hz | 5 Hz, 250 Hz, 2000 Hz |
Animals were studied in groups of six (a total of six groups of six animals). The assignment of their random order of electrical stimulus was maintained for each group of animals. Measurements of current vocalization threshold were obtained over two-weeks.
In order to evaluate the effects of NO donors or NOS inhibitors (see below), electrical stimulation was delivered using the same sequence (2000, 250, and 5 Hz) in all measurements and cVTs were obtained before and after drug injections.
Intrathecal injections and experimental drugs
Intrathecal injections were performed with isoflurane anesthesia as described [17]. Briefly, using a 30 G needle and a microliter syringe, study drug or vehicle were injected intrathecally at the L5–L6 intervertebral space.
Diethylenetriamine NONOate, a NO donor with a half life of 20 h, (DETA/NO, Cayman Chemical; Ann Arbor, MI) dissolved in 0.1 M buffered saline or corresponding concentration of DETA (vehicle control for DETA_NO, Sigma/Aldrich, St. Louis, MO) was injected intrathecally at doses of 5, 50 or 500 µg/kg in a volume of 10 µl. Measurements were obtained before and 1.5 and 6 h after DETA/NO or DETA injections. These doses of DETA/NO were selected based on reports that 10 µg/kg doses of intrathecal DETA/NO reverses vasospasm in dogs [18] and on preliminary studies indicating that while higher doses were well tolerated, they were associated with decreases in tail blood pressure in mice.
7-Nitroindazole (7NI, NOS1-specific inhibitor) (Cayman Chemical; Ann Arbor, MI) dissolved in 20% dimethyl sulfoxide (DMSO) at 10 mM/mouse or vehicle (20%DMSO) in 10 µl volume was injected intrathecally and cVT was measured before and 1.5 and 4 h after injections. This dose of 7NI was selected as it has been shown to reverse carrageenan-induced thermal hyperalgesia in mice [8]. l-NG-Nitroarginine methyl ester (a non-specific NOS inhibitor of all NOSs, l-NAME) or its vehicle control were injected intrathecally (30 µg/mouse) or intraperitoneally (l-NAME 100 µg/mouse) and cVT was measured before and at 1 and 6 h after injection. These doses of l-NAME were selected based on their reported antinociceptive effects in models of neuropathic and inflammatory pain [12,19].
Hot plate test
In order to evaluate nocifensive response to thermal stimuli mice were placed on a 55 °C hotplate (Harvard Apparatus, Holliston, Massachusetts) and latency response for pain avoiding behaviors (jumping, stomping or repeated lifting or licking of hind paws) were measured. Once one of these behaviors was noted, mice were removed from the hotplate and time in seconds was recorded as heat response latency.
NOS enzyme activity
NOS enzyme activity was measured using the NOS Activity Assay Kit (Cayman Chemical, Ann Arbor, MI). Briefly, spinal cords were isolated and homogenized in 1X homogenization buffer and spun for 15 min at 10,000g. Supernatants were added to reaction buffer containing 10 mM NADPH [3H] Arginine (1 µCi/ml), 6 mM CaCl2 and water. Reactions were incubated at room temperature for 15 min and stop solution was added. Reactions were then spun through an equilibrated resin and flow through was quantitated on a liquid scintillation counter. The percent citrulline was calculated by the following formula: ((cpm reaction-cpm background)/total cpm) * 100.
Nitrate/nitrite measurements
In a cohort of animals at baseline conditions nitrate/nitrite levels were determined in cerebral cortex and spinal cord tissue extracts using the reduction of vanadium chloride on a Sievers Instruments Model 280 Nitric Oxide Analyzer (NOA; GE Instrument, GE Analytical, Boulder, CO). The tissue was homogenized in deionized water. After centrifugation at 13,000 rpm for 10 min, an aliquot of the supernatant was set aside to determine the protein concentration, the remainder was used for further analyses. Tissue extracts were filtered using Microcon Centrifugal Filter devices (Ultracel YM-10, Millipore, Billerica, MA) to remove protein. The nitrate nitrite NOx contents in tissues were extrapolated from the calibration curves using known amounts of sodium nitrate (NaNO3) in the concentration range 1–150 µM and expressed in µM/g of protein. Data were collected using the NO Analysis™ software (Sievers, Boulder, CO, USA), and analysis was performed using Origin 8.5 SR6 software (OriginLab Corp., Northampton, MA).
Real time quantitative PCR
In cohorts of animals that had not undergone any experiments, RNA was isolated from whole brain, cerebral cortex, and spinal cord using RNeasy Mini kit (Qiagen, Valencia, CA). RNA quality was evaluated by Agilent 2100 Bioanalyzer using RNA 6000 Nano kits (Agilent Technologies, Santa Clara, CA) and quantity was measured using NanoDrop (NanoDrop Technologies, Wilmington, DE). Whole brain, cerebral cortex, and spinal cord NOS2, NOS3, and C–C chemokine receptor type 1 (CCR1) and type 5 (CCR5) and chemokine (C–C motif) ligand 3 (CCL3) and ligand 5 (CCL5) gene expression were quantified by real-time polymerase chain reaction (qPCR) using One-Step qRTPCR Kit in the Abi Prism® 7900 (both from Applied Biosystems, Foster City, CA).
Statistical analysis
A variety of analyses of variances (ANOVA) were performed on the raw data. One-way to multi-factor factorial ANOVAs were used to analyze fixed effect models. Repeated measures ANOVAs were used to model longitudinal data. For all ANOVAs, residuals were examined for normality and homogeneity and residuals were partitioned if found to be heterogeneous. Non-parametric tests (Wilcoxon rank sum) were used to compare distributions if the data were not normally distributed and/or the sample sizes were very small. LS-Means (model based means) and their standard errors are reported unless indicated otherwise. In view of the multiple tests performed, we consider 0.01 < p ≤ 0.05 as a weak trend, 0.005 < p ≤ 0.01 as a strong trend, and p ≤ 0.005 as statistically significant.
Fold changes in gene expression were calculated after normalization to endogenous GAPDH and were expressed relative to that detected in brain, cerebral cortex, and spinal cord of wild-type control animals (normalized to 1) using the comparative Ct method and the relative expression software tool, REST© version 2009 [20].
Results
Table 2 lists the number of animals enrolled in each study group. Throughout experimental sessions, there were no signs of lasting distress or injury to the animals and audible vocalizations were only observed with electrical stimuli.
Table 2.
Number of animals enrolled in the nociception studies.*
| Sex/treatment | NOS1 | NOS2 | NOS3 | |||
|---|---|---|---|---|---|---|
| Knock out | Wild-type (B6129SF2/J) | Knock out | Wild-type (C57Bl/6 J) | Knock out | Wild-type (C57Bl/6 J) | |
| Male | 12 | 12 | ||||
| Female | 18 | 18 | 12 | 12 | 12 | 12 |
| DETA | 12 | 11 | ||||
| DETA/NO | 36 (12/each concentration) | 36 (12/each concentration) | ||||
| 7-NI | 11 | 10 | ||||
| 7-NI vehicle | 4 | 4 | ||||
| l-NAME (i.p.) | 8 | 8 | ||||
| l-NAME (i.p.) vehicle | 4 | 4 | ||||
| l-NAME (i.t.) | 10 | 11 | ||||
| l-NAME (i.t.) vehicle | 4 | 5 | ||||
NOS represents nitric oxide synthase; DETA, diethylenetriamine; DETA/NO, diethylenetriamine NONOate; 7-NI, 7-nitroindazole; and l-NAME, Nω-nitro-l-arginine methyl ester; i.p. intraperitoneal; i.t. intrathecal.
Effect of genetic deficiency of NOS1 on cVT
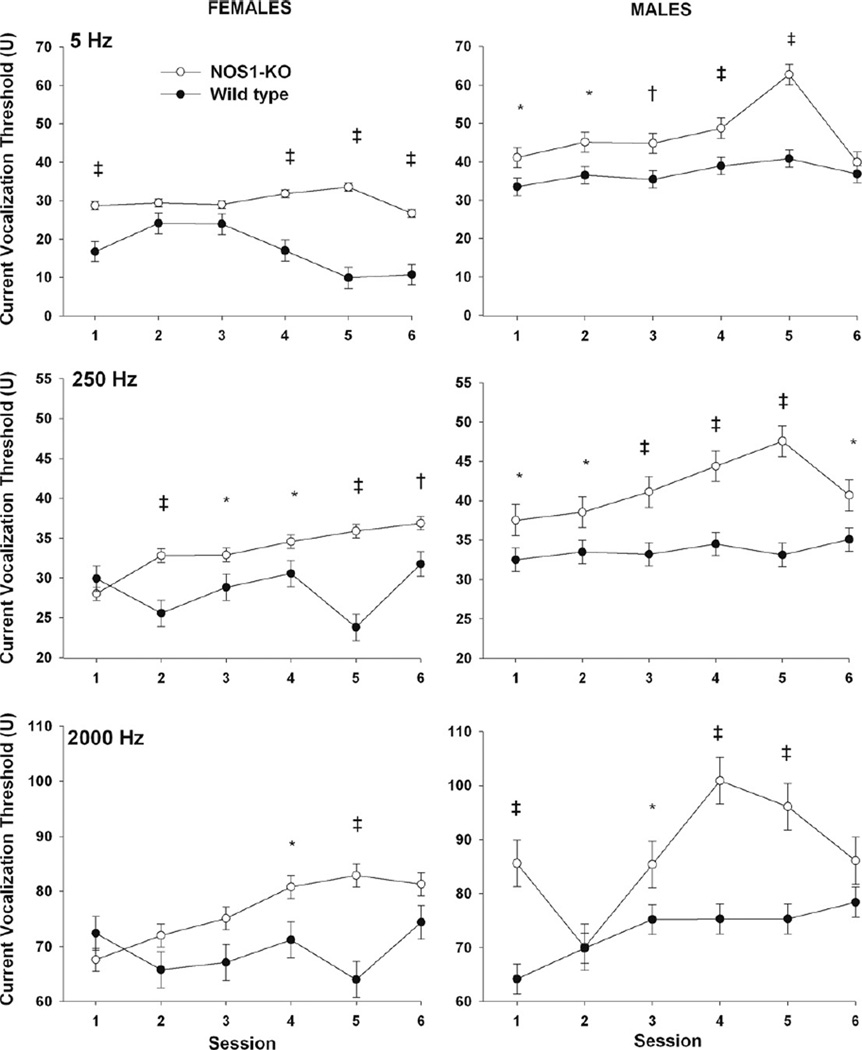
In wild-type and NOS1-KO mice, males had significantly higher cVTs in response to 5, 250, and 2000 Hz stimulation compared with females (Fig. 1, all p ≤ 0.0005, pooled over study sessions and strain). In addition, in response to 5, 250 and 2000 Hz stimulation NOS1-KO animals had overall significantly higher cVTs compared with wild-type (Fig. 1, all p < 0.0001, pooled over study sessions and gender). While the overall effect of strain indicates that NOS1-KO animals had higher cVTs for all frequencies, there were significant gender, session, and strain interactions for 5 Hz (p = 0.0003), 250 (p = 0.030), and 2000 Hz (p < 0.0001) stimulation. Specifically, in response to 5 Hz, female NOS1-KO had significantly higher cVTs compared with female wild-type mice on first, fourth, fifth, and sixth study sessions (all p < 0.0001) and male NOS1-KO mice had significantly higher cVTs compared with male wild-type on first (p = 0.032), second (p = 0.015), third (p = 0.008), fourth (0.005), and fifth (p < 0.0001) study sessions. For 250 Hz female NOS1-KO had significantly higher cVTs compared with female wild-type mice on second (p = 0.0003), third (p = 0.043), fourth (p = 0.044), fifth (p < 0.0001), and sixth (p = 0.008) study sessions and male NOS1-KO mice had significantly higher cVTs compared with male wild-type on the first (p = 0.043), second (p = 0.041), third (p = 0.001), fourth (p < 0.0001), fifth (p < 0.0001), and sixth (p = 0.024) study sessions. For 2000 Hz, female NOS1-KO had significantly higher cVTs compared with female wild-type mice on the fourth (p = 0.023) and fifth (p < 0.0001) study sessions and male NOS1-KO mice had significantly higher cVTs compared with male wild-type on the first (p < 0.0001), third (p = 0.047), fourth (p < 0.0001), and fifth (p < 0.0001) sessions.
Fig. 1.
Effect of NOS1 deficiency on current vocalization threshold in female (L panels) and male (R panels) mice in response to electrical stimulations at 5, 250 and 2000 Hz that preferentially stimulate C, Aδ, and Aβ sensory fibers respectively. Least square means ± SEM threshold are shown.*indicates 0.01 < p < 0.05, † 0.005 < p ≤ 0.01, and ‡ p ≤ 0.005.
With regards to thermal sensitivity, NOS1-deficient animals had similar thermal response latency compared to wild-type (13.2 ± 1.18 vs. 14.9 ± 1.37 seconds (mean ± SEM), NOS1-deficient vs. controls respectively, p = 0.36).
Effect of genetic deficiency of NOS2 on cVT
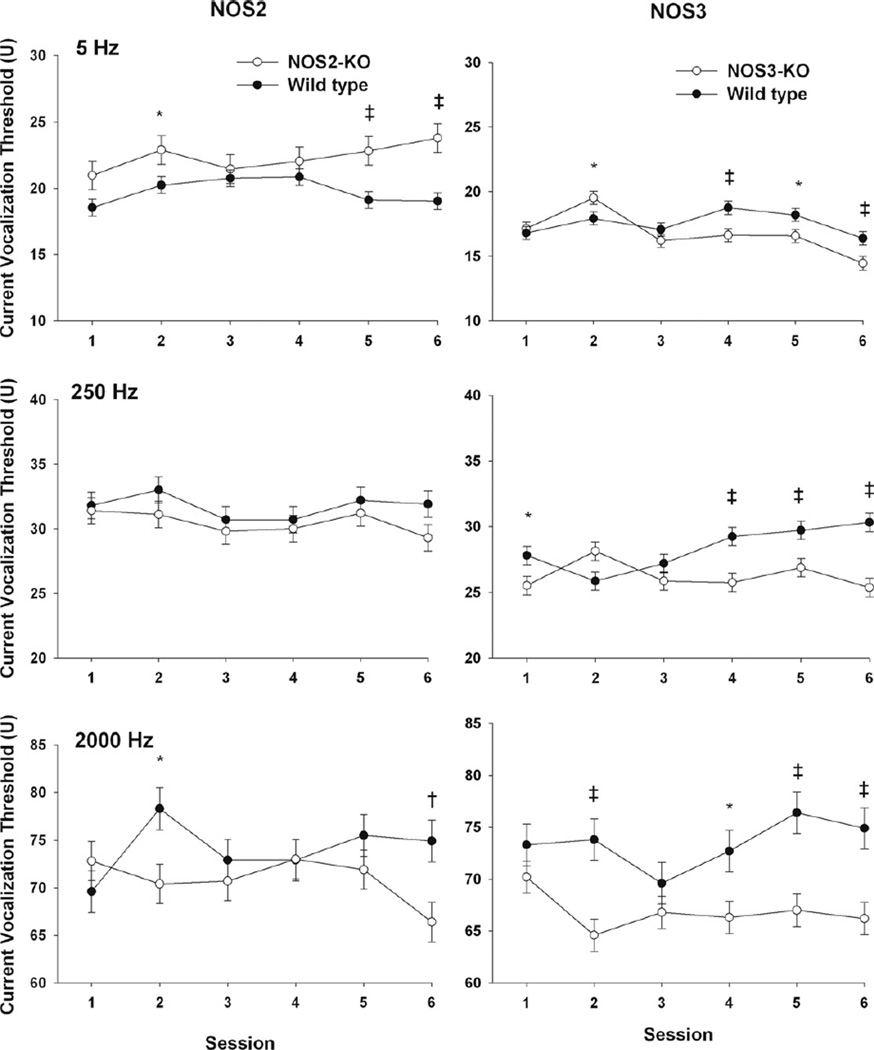
The effect of NOS2 on cVT varied by frequency. Specifically, with 5 Hz stimulation, female NOS2-KO animals had significantly higher cVTs compared with wild-type (p = 0.002 pooled over study sessions). In contrast, female NOS2-KO mice had similar cVTs in response to 250 Hz compared to wild-type (Fig. 2, p = 0.16). At 2000 Hz the strain effect (knock out vs. wild-type) varied with session as indicated by the strain and session interaction effect (Fig. 2, p = 0.008).
Fig. 2.
Effect of NOS2 (L panels) and NOS3 (R panels) on current vocalization threshold in female mice in response to electrical stimulations at 5, 250 and 2000 Hz that preferentially stimulate C, Aδ, and Aβ sensory fibers respectively. Least square means ± SEM threshold are shown.*indicates 0.01 < p < 0.05, † 0.005 < p ≤ 0.01, and ‡ p ≤ 0.005.
Effect of genetic deficiency of NOS3 on cVT
The effect of NOS3 on cVT varied by frequency and study session (Fig. 2). Specifically, with 5 Hz stimulation, NOS3-deficient animals had a variable trend in the first three sessions and in the last three sessions tended toward lower cVTs compared with wild-type that reached significance on the fourth (p = 0.004) and sixth sessions (p = 0.008). With 250 and 2000 Hz stimulation, NOS3 animals had overall significantly lower cVT compared with wild-type (both p < 0.0001).
Effect of DETA/NO on cVT
Overall at 1.5 and 6 h after intrathecal injection of increasing doses of DETA/NO, wild-type and NOS1-KO animals had similar estimated slopes for cVT changes from baseline in response to all frequencies studied (all p > 0.1, data not shown). During pilot studies, we observed that, while animals showed no overt behavior changes and tolerated DETA/NO well, intrathecal injections of DETA/NO at higher doses were associated with decreases in blood pressure to below detectable levels using an inflatable tail cuff.
Effect of pharmacological inhibition of NOSs with 7-NI and l-NAME on cVT
Five hours after intrathecal injection, the overall effect of 7-NI on arginine conversion to citrulline in spinal cord was significantly different comparing wild-type and NOS1-deficient animals (Table 3, p < 0.001). Specifically, 7-NI compared with vehicle significantly decreased arginine conversion to citrulline in wild-type (p < 0.001) whereas it yielded no significant changes in NOS1-deficient mice (p = 0.007). Overall, over 4 h after intrathecal 7-NI, there were no significant differences in cVT in response to electrical stimulation at 5 and 250 Hz frequencies comparing NOS-KO and wild-type animals. However, at 2000 Hz, the wild-type group had a larger difference in cVT compared with NOS1-KO animals (−6.77 ± 2.20 vs. −0.1 ± 2.15, respectively, p = 0.030).
Table 3.
Arginine conversion to citrulline in spinal cords after intrathecal injection of 7-nitroindazole or vehicle.*
| Genotype | Treatment | Least square means ± SEM (%) | 95% Confidence interval (%) |
|---|---|---|---|
| NOS1-KO | 7-Nitroindazole | 1.17 ± 0.698 | 0.22, 5.95 |
| NOS1-KO | Vehicle | 1.4 ± 1.08 | 0.2, 11.1 |
| Wild-type | 7-Nitroindazole | 17.4 ± 1.01 | 14.8, 20.4 |
| Wild-type | Vehicle | 29.2 ± 1.72 | 24.7, 34.2 |
NOS1-KO indicates NOS1-knock out animals.
Overall, over 6 h after injection of l-NAME intraperitoneally, NOS1-KO animals had greater increases in cVT from baseline compared with wild-type mice in response to 250 Hz stimulations (p = 0.007 pooled over dose and hours). Specifically, compared with wild-type, NOS1-KO mice had greater increases in cVT at 1 h (0.7 ± 2.54 vs. 11.5 ± 2.54, wild-type vs. NOS1-KO respectively, p = 0.005) and 6 h (2.3 ± 2.54 vs. 10.7 ± 2.54, wild-type vs. NOS1-KO respectively, p = 0.026). After intraperitoneal injection of l-NAME, with 5 Hz stimulations, there was a significant interaction between mouse strain and hour, p = 0.048 (pooled over dose). Specifically, NOS1-KO animals had greater increases in cVT after 6 h compared to 1 h (7.7 ± 1.66 vs. 4.7 ± 2.17, respectively, p = 0.061), whereas wild-type animals had lesser increases in cVT after 6 h compared to 1 h (5.2 ± 1.66 vs. 6.7 ± 2.17, respectively, p = 0.33). After intrathecal l-NAME injections, with 250 Hz stimulations, there was a significant interaction between strain and hour, p = 0.015 (pooled over dose). Specifically, NOS1-KO animals had greater increases in cVT after 6 h compared to 1 h (7.3 ± 2.17 vs. 3.0 ± 1.65, respectively, p = 0.007), whereas wild-type had similar cVT changes after 6 h compared to 1 h (3.8 ± 2.17 vs. 4.9 ± 1.65, respectively, p = 0.47).
Nitrite/nitrate levels during basal conditions and after neurostimulation
During basal conditions (without neurostimulation), whole brain nitrate/nitrite levels are significantly lower in NOS1-KO mice compared with wild-type animals (0.107 ± 0.135 vs. 0.297 ± 0.329 µM NOx/g of protein, mean ± SEM, NOS1-KO vs. wild-type respectively, p = 0.003). In contrast, cerebral cortex nitrate/ nitrite levels were similar in NOS1-KO and wild-type controls (p = 0.7, see below). In a different cohort of animals, we measured nitrate/nitrite levels after cVT measurements with 5, 250, and 2000 Hz. After neurostimulation compared to baseline, levels of nitrite/nitrate in cerebral cortex of both wild-type (4.45 ± 0.78 vs. 4.92 ± 0.81 µM NOx/g of protein, mean ± SEM, after cVT measurements vs. basal conditions respectively, p = 0.7) and NOS1-KO animals (5.06 ± 0.98 vs. 5.48 ± 0.79 µM NOx/g of protein, mean ± SEM, after cVT measurements vs. basal conditions respectively, p = 0.5) were similar.
NOS2, NOS3 and chemokine gene expression in NOS1-KO mice during basal conditions
Given the role that NOS2, NOS3, and chemokines and their receptors (CCL3, CCR1, CCL5, CCR5) play in the development of thermal and mechanical hyperalgesia during nerve injury [21–23], we examined the effect of NOS1 deficiency in gene expression of NOS2 and NOS3 as well as chemokines receptors and their ligands in whole brain of wild-type and NOS1-KO animals at baseline. During basal conditions, in whole brain, NOS1-KO have significant upregulation of CCR1 expression compared with wild-type animals [expression ratio NOS1-KO vs. wild-type 3.86 (1.72–7.86), mean (95% CI), p = 0.003]. In contrast, in whole brain NOS1-KO and wild-type animals had similar expression of NOS2, CCL5, CCR5 and CCL3 (p = NS, data not shown).
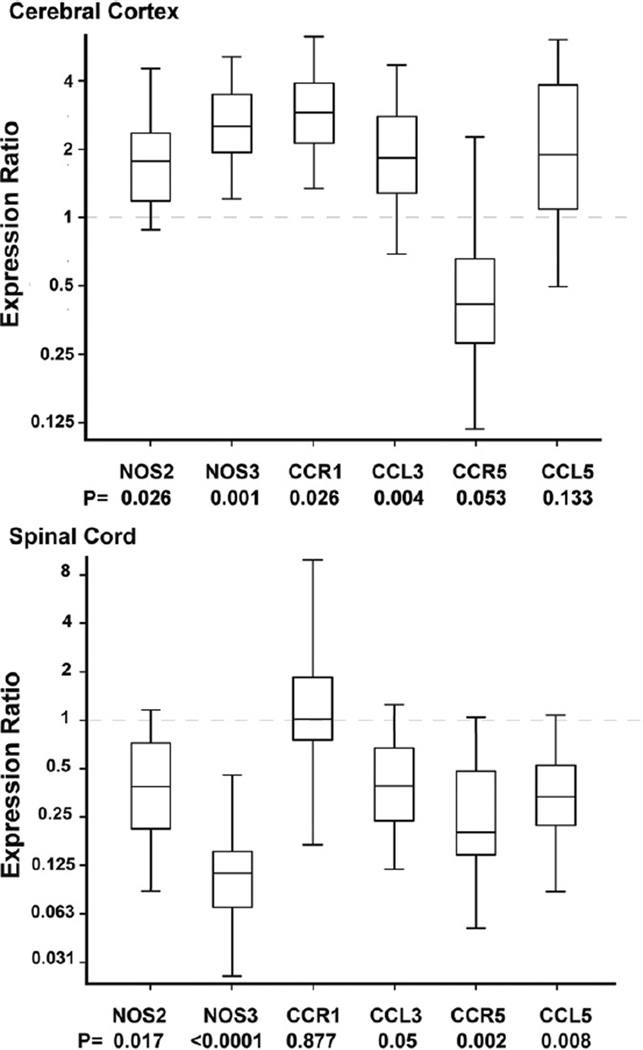
Also during basal conditions in a different cohort of B6129SF2/J (wild-type, N = 5) and NOS1-KO (N = 5) animals, RNA was isolated from the cerebral cortex and from the lower half of spinal cords. In cerebral cortex, NOS1-KO animals have significant upregulation of NOS2 (p = 0.026), NOS3 (p = 0.001), CCR1 (p = 0.026), and CCL3 (p = 0.004) but similar expression of CCR5 (p = 0.053) and CCL5 (p = 0.133) compared with wild-type animals (Fig. 3). In contrast, in spinal cord, gene expression profile was different than that of cerebral cortex. Specifically, NOS1-KO animals have significant downregulation of NOS2 (p = 0.017), NOS3 (p < 0.0001), CCL3 (p = 0.05), CCR5 (p = 0.002) and CCL5 (p = 0.008) expression but similar expression of CCR1 (p = 0.877) compared with wild-type animals.
Fig. 3.
Effect of NOS1 deficiency on NOS2, NOS3, and chemokines gene expression in cerebral cortex and spinal cord. The box plots represent the 25th to 75th interquartile expression ratio (the middle 50% of observations) and the line in the box, the median gene expression ratio comparing NOS1 deficient vs. wild-type animals used as calibrators (expression of 1). Whiskers represent minimum and maximum observations. Numbers below each listed gene represent the p value of expression ratio comparing NOS1-KO vs. wild-type animals. In cerebral cortex, NOS1 deficient animals have significant upregulation of NOS2, NOS3, C–C chemokine receptor type 1 (CCR1), C–C chemokine ligand 3 (CCL3) but similar expressions of C–C chemokine receptor 5 (CCR5) and C–C chemokine ligand 5 (CCL5) compared with wild-type controls. In contrast in spinal cord, NOS1 deficient animals have significant downregulation of NOS2, NOS3, CCL3, CCR5, and CCL5 but similar expression of CCR1 compared with wild-type controls. N = 5 animals per group.
Discussion
In response to stimulation with electrical frequencies of 5, 250, and 2000 Hz that preferentially stimulate C, Aδ, and Aβ sensory fibers, animals with NOS1-deficiency have higher cVT during baseline suggesting that NOS1-deficient animals have an increased tolerance to noxious stimulus during basal conditions. In contrast, genetic deficiency of NOS2 increases cVT response to 5 Hz but not to 250 or 2000 Hz. Surprisingly, in animals lacking the NOS3 gene, cVTs were actually lower in all frequencies, suggesting that NOS3-deficiency lowers tolerance to electrical stimulus at baseline. Taken together, these findings suggest that the three isoforms of NOSs distinctively alter baseline cVTs in mice and that all NOSs appear to play a role in nocifensive response to electrical stimulation of sensory nerve fibers.
In wild-type animals, acute pharmacologic inhibition of NOS1 activity with 7-NI did not alter the baseline response to electrical stimulus in any frequency studied. These results suggest that acute inhibition of NOS1 in wild-type animals does not recapitulate the effects of deletion of NOS1 during embryologic development in cVT. In addition, baseline increases in cVT were unchanged after intrathecal injections of DETA/NO. These results might suggest that in genetically altered mice, lack of NOS1 throughout embryologic development likely leads to compensatory or structural changes that can not be recapitulated by acute alterations in NO availability or NOS1 enzymatic activity. Interestingly, after acute pharmacologic inhibition of all NOSs with l-NAME, cVTs further increased in NOS1-deficient compared with wild-type mice. It is possible that NOS1-deficient animals have compensatory changes in other NOSs as previously shown by others [24,25] and that nonspecific inhibition of other NOSs would further increases cVT. Taken together, these findings suggest that NOS1 alters baseline cVT in response to electrical stimulation by mechanisms that appear to be independent of NO availability or acute changes in NOS activity.
It is unclear how NOS1 alters cVTs. One possibility is that NOS1-deficient animals have motor impairment and delayed vocalization. However, this possibility seems unlikely as researchers have shown that NOS1-deficient animals have increased locomotor activity [24,25] and normal motor nerve conduction velocity [26]. In contrast, researchers have reported sensory nerve function changes in NOS1-deficient mice [26] and after pharmacologic inhibition of NOS1 in rats [27]. In mice, NOS1-deficiency is associated with slight decreases in sensory nerve conduction velocity and significant (32%) decreases in intraepidermal nerve fiber density compared to wild-type animals [26]. In anesthetized rats, pharmacologic inhibition of NOS1 with 7-NI significantly decreased intracranial somatosensory evoked potentials associated with forepaw electrical stimulation [27]. Therefore, our findings of increased vocalization thresholds to electrical stimulation of C, Aδ, and Aβ sensory fibers and that of others showing peripheral nerve dysfunction in NOS1-deficient animals suggest that NOS1 is important for the integrity of sensory nerve fibers response.
The relevance of our findings that NOS1 deficiency alters the gene expression of chemokines and their receptors in different patterns in whole brain, cerebral cortex, and spinal cord is unclear. However, it is conceivable that altered chemokine gene expression could be related to the increases in tolerance to noxious stimulus seen in NOS1-KO animals compared with wild-type. We and others have shown that NOS1 impacts cytokine, CCL3, and NOS2 gene expression during sepsis [28–31]. Researchers have also shown that in astrocytes, NOS1 regulates activity of the transcription factor NF-κB and NOS2 expression suggesting that NOS1 modulates brain cytokine expression by regulating NF-κB-regulated genes [31]. Therefore, it is clear that NOS1 impacts on the expression of inflammatory mediators including chemokines. This is interesting as chemokines have been shown to play a role in brain development, modulation of excitability, and neuroimmune response [32,33]. Further, researchers have shown that chemokines and their receptors are expressed in sensory neurons [21], can sensitize C-fibers [34] and have an important role in the development of tactile allodynia and thermal hyperalgesia associated with nerve injury [22,23]. Here we found that during basal conditions NOS1-KO animals are more tolerant to electrical stimulus and have altered expression of chemokines and their receptors in the brain and spinal cord compared to wild-type control. While we did not establish that the changes in nocifensive behavior in NOS1-KO animals are related to distinct patterns of NOS2, NOS3, and chemokine gene expression throughout the central nervous system, this is certainly a hypothesis worthy of further investigations.
The findings that NOS1-deficient mice have intact response to the hot plate test during baseline conditions are in concert with those reported by others using radiant heat methods [8,9]. These findings of intact thermosensation at baseline and increased baseline cVTs in NOS1-deficient animals might not necessarily be contradictory. With the nociceptive assay used here, the electrical current bypasses cutaneous nociceptors and directly stimulates sensory nerve fibers. Further, as our endpoint is vocalization which is thought to represent a complex supra spinal response, we postulate that this assay enables the examination of complex nocifensive behavior compared with evaluation of thermosensation with the hot plate test. Therefore our findings suggest that this nociceptive assay could be valuable for evaluation of changes in nociception in transgenic mice.
In NOS2-deficient mice, we observed increases in cVT to 5 Hz (C fiber) but not in response to 250 (Aδ.fiber) or 2000 Hz (Aβ fiber). Using other noxious stimuli, researchers have shown that NOS2-deficient mice have intact thermal response and mechanical withdrawal thresholds compared with wild-type [7,9,15]. With regards to NOS3, we found that in NOS3-deficient animals, cVTs were lower in all frequencies, suggesting an increased sensitivity to electrical stimulus at baseline. Other have shown that the NOS3 gene does not alter baseline thermal response and mechanosensation [7,9]. While these might appear to be conflicting results, one must recognize that we are using different modalities of nociceptive stimulus and that comparing these results may lead to inaccurate conclusions. Instead, one could postulate that different nociceptive stimuli (heat vs. electrical stimuli) evaluate different aspects of the role of NOSs in mechanisms of nociception. Nevertheless, these results suggest that, by mechanisms incompletely understood, the role of NOSs on baseline nocifensive response is complex and each isoforms has distinct effects on response of sensory nerve fibers.
Interestingly, as seen in wild-type mice, we observed that NOS1-deficient males have higher cVT than females. Such findings of different nocifensive response comparing male and female mice are in concert with various reports of sex-related differences in nociception in mice [35–39]. To our knowledge, studies examining the role of NOS1 on nociception have not evaluated differences between male and female NOS1-deficient animals. Nevertheless, while NOS1 deficiency alters baseline nocifensive response to electrical stimulation, it does not alter sex-related differences in nocifensive response to electrical stimulation.
Conclusions
NOSs distinctively alter cVT in response to electrical stimulation of sensory nerve fibers. In NOS1-deficient animals, the alterations in cVT appear to occur by mechanisms independent of acute changes in NO availability and NOS1 activity. While one must be circumspect about extrapolating rodent data to other species, it is conceivable that because of its favorable effect on cVT, NOS1 could potentially serve as a potential therapeutic target to increase nociceptive threshold.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health Clinical Center, National Institutes of Health and by The Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s National Medical Center, Washington, DC.
References
- 1.Miclescu A, Gordh T. Nitric oxide and pain: ‘something old, something new’. Acta Anaesthesiol. Scand. 2009;53:1107–1120. doi: 10.1111/j.1399-6576.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 2.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011 doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 4.Cizkova D, Lukacova N, Marsala M, Marsala J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res. Bull. 2002;58:161–171. doi: 10.1016/s0361-9230(02)00761-x. [DOI] [PubMed] [Google Scholar]
- 5.Lam HH, Hanley DF, Trapp BD, Saito S, Raja S, Dawson TM, Yamaguchi H. Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci. Lett. 1996;210:201–204. doi: 10.1016/0304-3940(96)12702-6. [DOI] [PubMed] [Google Scholar]
- 6.Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain. 2005;119:113–123. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Hervera A, Negrete R, Leanez S, Martin-Campos JM, Pol O. The spinal cord expression of neuronal and inducible nitric oxide synthases and their contribution in the maintenance of neuropathic pain in mice. PLoS One. 2010;5:e14321. doi: 10.1371/journal.pone.0014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao F, Tao YX, Zhao C, Dore S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience. 2004;128:421–430. doi: 10.1016/j.neuroscience.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur. J. Pain. 2007;11:810–818. doi: 10.1016/j.ejpain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Boettger MK, Reif A, Schmitt A, Uceyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol. Pain. 2010;6:13. doi: 10.1186/1744-8069-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolesnikov YA, Chereshnev I, Criesta M, Pan YX, Pasternak GW. Opposing actions of neuronal nitric oxide synthase isoforms in formalin-induced pain in mice. Brain Res. 2009;1289:14–21. doi: 10.1016/j.brainres.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol. Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy D, Tal M, Hoke A, Zochodne DW. Transient action of the endothelial constitutive nitric oxide synthase (ecNOS) mediates the development of thermal hypersensitivity following peripheral nerve injury. Eur. J. Neurosci. 2000;12:2323–2332. doi: 10.1046/j.1460-9568.2000.00129.x. [DOI] [PubMed] [Google Scholar]
- 14.De Alba J, Clayton NM, Collins SD, Colthup P, Chessell I, Knowles RG. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain. 2006;120:170–181. doi: 10.1016/j.pain.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, Raja SN, Johns RA. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience. 2003;120:847–854. doi: 10.1016/s0306-4522(03)00362-2. [DOI] [PubMed] [Google Scholar]
- 16.Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, Koziol D, Wesley R, Quezado ZM. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology. 2006;105:360–369. doi: 10.1097/00000542-200608000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hylden J. George Wilcox, Intrathecal morphine in mice. a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 18.Wolf EW, Banerjee A, Soble-Smith J, Dohan FC, Jr, White RP, Robertson JT. Reversal of cerebral vasospasm using an intrathecally administered nitric oxide donor. J. Neurosurg. 1998;89:279–288. doi: 10.3171/jns.1998.89.2.0279. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Singh JP, Lodge D. Experiments with nitric oxide synthase inhibitors in spinal nerve ligated rats provide no evidence of a role for nitric oxide in neuropathic mechanical allodynia. Neurosci. Lett. 2005;385:179–183. doi: 10.1016/j.neulet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiguchi N, Kobayashi Y, Maeda T, Saika F, Kishioka S. CC-chemokine MIP-1alpha in the spinal cord contributes to nerve injury-induced neuropathic pain. Neurosci. Lett. 2010;484:17–21. doi: 10.1016/j.neulet.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 23.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain. 2010;149:305–315. doi: 10.1016/j.pain.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol. Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoubovsky SP, Pogorelov VM, Taniguchi Y, Kim SH, Yoon P, Nwulia E, Sawa A, Pletnikov MV, Kamiya A. Working memory deficits in neuronal nitric oxide synthase knockout mice: potential impairments in prefrontal cortex mediated cognitive function. Biochem. Biophys. Res. Commun. 2011;408:707–712. doi: 10.1016/j.bbrc.2011.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG. Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int. J. Mol. Med. 2009;23:571–580. doi: 10.3892/ijmm_00000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanovic B, Schwindt W, Hoehn M, Silva AC. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J. Cereb. Blood Flow Metab. 2007;27:741–754. doi: 10.1038/sj.jcbfm.9600377. [DOI] [PubMed] [Google Scholar]
- 28.Cui X, Besch V, Khaibullina A, Hergen A, Quezado M, Eichacker P, Quezado ZM. Neuronal nitric oxide synthase deficiency decreases survival in bacterial peritonitis and sepsis. Intensive Care Med. 2007;33:1993–2003. doi: 10.1007/s00134-007-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu XW, Wang H, De Plaen IG, Rozenfeld RA, Hsueh W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001;15:439–446. doi: 10.1096/fj.99-0343com. [DOI] [PubMed] [Google Scholar]
- 30.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am. J. Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 31.Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, Dawson VL. Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc. Natl. Acad. Sci. USA. 1997;94:2676–2680. doi: 10.1073/pnas.94.6.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb. Exp. Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mines M, Ding Y, Fan GH. The many roles of chemokine receptors in neurodegenerative disorders: emerging new therapeutical strategies. Curr. Med. Chem. 2007;14:2456–2470. doi: 10.2174/092986707782023686. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl. Acad. Sci. USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 37.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain, analgesia: a consensus report. Pain. 2007;132(Suppl. 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chanda ML, Mogil JS. Sex differences in the effects of amiloride on formalin test nociception in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R335–R342. doi: 10.1152/ajpregu.00902.2005. [DOI] [PubMed] [Google Scholar]
- 39.Spornick N, Guptill V, Koziol D, Wesley R, Finkel J, Quezado ZM. Mouse current vocalization threshold measured with a neurospecific nociception assay: the effect of sex, morphine, and isoflurane. J. Neurosci. Meth. 2011;201:390–398. doi: 10.1016/j.jneumeth.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]