Abstract
Dyslipidemia is an important risk factor for cardiovascular disease (CVD) and atherosclerosis. When dyslipidemia coincides with other metabolic disorders such as obesity, hypertension, and glucose intolerance, defined as the metabolic syndrome (MS), individuals present an elevated risk to develop type 2 diabetes (T2D) as well as CVD. Because the MS epidemic represents a growing public health problem worldwide, the development of therapies remains a major challenge. Alterations of bile acid pool regulation in T2D have revealed a link between bile acid and metabolic homeostasis. The bile acid receptors farnesoid X receptor (FXR) and TGR5 both regulate lipid, glucose, and energy metabolism, rendering them potential pharmacological targets for MS therapy. This review discusses the mechanisms of metabolic regulation by FXR and TGR5 and the utility relevance of natural and synthetic modulators of FXR and TGR5 activity, including bile acid sequestrants, in the treatment of the MS.
Keywords: atherosclerosis, FXR, glucose metabolism, lipid metabolism, TGR5
Dyslipidemia is a major risk factor for cardiovascular disease (CVD). The deposition of plasma lipids in the arterial intima induces a local inflammatory response and extensive vascular remodeling, resulting in the formation of atherosclerotic plaques (1). Plaque rupture or erosion causes myocardial infarction or stroke. The prevalence of dyslipidemia, characterized by elevated plasma triglycerides (TGs) and low HDL-cholesterol (HDL-C), in combination with obesity, elevated blood glucose levels, and/or hypertension termed the metabolic syndrome (MS), increases worldwide, mainly due to a sedentary lifestyle (increased caloric intake, decreased exercise). In addition to a higher incidence of CVD, individuals with the MS are at a higher risk to develop type 2 diabetes (T2D), which further increases the risk for CVD, the most common cause of death from T2D (http://www.who.int/mediacentre/factsheets/fs312/en/index.html). Obesity and T2D are associated with nonalcoholic fatty liver disease (NAFLD), a pathophysiological accumulation of lipids in the liver (steatosis), which, associated with inflammation, evolves into nonalcoholic steatohepatitis (NASH). NASH may cause cirrhosis and cancer, and in the case of complete liver failure, transplantation is the only option (2). Therefore, an appropriate management of the MS is essential to prevent these associated metabolic disorders. The most efficacious treatment consists of lifestyle changes, which is, however, often difficult to implement due to a lack of patient compliance. A combination of drugs may thus be needed, targeting each single disorder to manage the patient's global risk. Thus, the development of drugs with a combined effect on different risk factors may yield a more effective and improved treatment of the MS.
Before the arrival of statins, hypercholesterolemia was mainly treated with bile acid sequestrants (BAS), which bind bile acids (BA) in the intestine and remove them from the BA pool (3). BAS were subsequently found to reduce fasting plasma glucose and HbA1c levels in T2D patients (4). This link between BA and metabolic homeostasis has been further evidenced by the demonstration that BA themselves regulate metabolism mainly by signaling through two receptors, the nuclear farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5/M-BAR. The modulation of FXR and TGR5 activity either directly by BA or pharmacological compounds or indirectly by intestinal BA sequestration has helped to unravel the function of these BA receptors in metabolic control. Subsequently, both receptors have emerged as promising targets for the treatment of metabolic disorders associated with the MS.
This review focuses on recent advances in the understanding of BA metabolism and the function of FXR and TGR5 in lipid, glucose, and energy metabolism as well as in atherosclerosis. We further discuss the clinical potential of FXR and TGR5 modulators for MS therapy.
BILE ACIDS AS SIGNALING MOLECULES
Bile acid metabolism
BA are amphipatic molecules and major constituents of bile. Their synthesis from cholesterol in the hepatocyte represents an important way to eliminate cholesterol from the human body. Stored in the gallbladder, BA are secreted into the intestine after a meal, where they facilitate the absorption of lipid nutrients and lipid-soluble vitamins. Of the BA pool, 95% is reabsorbed in the ileum and returns to the liver, a pathway known as the enterohepatic cycle. The remaining 5% of the BA pool is lost with the feces and replaced by hepatic de novo synthesis.
The synthesis of BA involves two distinct pathways: the classic and the alternative pathway with cholesterol-7α-hydroxylase (CYP7A1) and sterol-27-hydroxylase (CYP27A1), respectively, as key enzymes (5). In a first step, 7α-hydroxysterol is formed from cholesterol by microsomal CYP7A1, which is then converted into the primary BA chenodeoxycholic acid (CDCA) and cholic acid (CA) by the action of several enzymes, including sterol 12α-hydroxylase (CYP8B1), 25-hydroxycholesterol-7α-hydroxylase (CYP7B1), and CYP27A1 (6). CYP8B1 is the rate-limiting enzyme in the synthesis of CA, determining the CA/CDCA ratio in humans and CA/muricholic acid (MCA) ratio in mice. Primary BA are finally conjugated to glycine (humans) or taurine (mice) by BA-CoA synthetase (BACS) and BA-CoA amino acid N-acyltransferase (BAT) (7, 8). Once excreted into the small intestine, primary BA are deconjugated and partially converted into secondary BA by dehydroxylation (9). These modifications are catalyzed by enzymes of the microbial flora, which in turn are regulated by BA (10). In humans, the most abundant secondary BA are lithocholic acid (LCA) and deoxycholic acid (DCA) (9, 11). Hydrophobicity of these BA is increased, facilitating their absorption by the colon epithelium. The BA pool of mice and humans is different. In mice, CDCA is quantitatively converted into MCA, rendering the pool more hydrophilic than in humans.
BA, which at high concentrations are toxic detergents, regulate their own metabolism. In addition to inhibiting the expression of their transepithelial transporters apical sodium/bile acid cotransporter (ASBT) and organic solute transporter (OST)α/β in the intestine (12), they are strong transcriptional inhibitors of CYP7A1, exerting a negative feedback regulation of their own synthesis (6). These regulatory functions of BA are mediated by signaling through their receptors, particularly FXR.
Bile acid receptors
The best-studied BA receptors are the nuclear receptor FXR and the membrane receptor TGR5 (13). Noteworthy, BA can also activate other nuclear receptors, such as the human steroid and xenobiotic receptor (SXR) and its rodent homolog pregnane X receptor (PXR), vitamin D receptor (VDR), and constitutive androstane receptor (CAR) (14, 15). These receptors are also implicated in lipid and glucose metabolism, but their activation seems to occur at nonphysiological BA concentrations. Thus, we focus on FXR- and TGR5-mediated functions of BA in this review (Fig. 1).
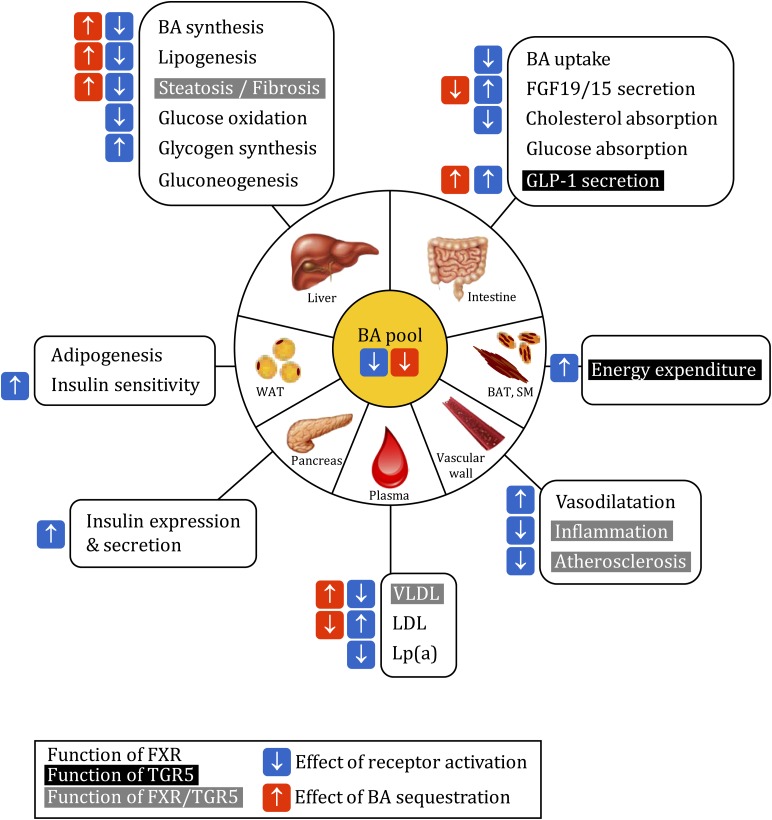
Fig. 1.
Functions of FXR and TGR5 in metabolic homeostasis and effect of bile acid receptor modulation. See text for details. The indication of agonist or BAS effects was omitted in case of contradictory data in the literature. Functions of FXR, black on white; functions of TGR5, white on black; functions of both receptors, white on gray. Blue flash, effect of receptor activation; red flash, effect of bile acid sequestration. SM, skeletal muscle; WAT, white adipose tissue.
FXR is expressed in several tissues, such as liver, intestine, adipose tissue, the vascular wall, pancreas, and kidney (16). When FXR is activated by a ligand, it binds to FXR response elements (FXRE) in the promoter of its target genes, either as a monomer or as a heterodimer with the retinoid X receptor (RXR), regulating their transcription. FXR can also indirectly regulate gene expression notably via the induction of other transcription factors like the small heterodimer partner (SHP). Thus, in the liver, FXR-activated SHP inhibits the liver receptor homolog (LRH)-1, liver X receptor (LXR), and hepatocyte nuclear factor (HNF)4α, all necessary for optimal CYP7A1 transcription (17–19). BA further induce an intestinal-hepatic signaling axis by activating FXR in the intestine, which enhances the transcription of fibroblast growth factor (FGF)19 in humans and FGF15 in mice. FGF19/15 is secreted into the circulation and activates the FGF receptor (FGFR)4 on hepatocytes, leading to the repression of CYP7A1 expression via the activation of the c-jun N-terminal kinase (JNK) (20, 21). The transport of BA across the ileal epithelium by ASBT and OSTα/β is rate limiting for the activation of hepatic as well as intestinal FXR signaling. FXR exerts a negative feedback loop by inhibiting the expression of both transporters (12). Furthermore, FXR is subject to posttranscriptional modifications, such as phosphorylation or acetylation, which regulate its activity (22, 23).
TGR5 is a member of the rhodopsin-like superfamily of G protein-coupled receptors, with most important expression levels in gallbladder, ileum, and colon (24). Primary and secondary BA activate TGR5 (13, 24, 25). TGR5 activation leads to receptor internalization and liberation of the Gαs subunit, which activates adenylate cyclase, subsequently inducing cAMP production and protein kinase A (PKA) activation. PKA phosphorylates the cAMP-response element-binding protein (CREB) and induces the transcription of its target genes (13, 26).
FUNCTIONS OF BILE ACID RECEPTORS
FXR and lipid metabolism
The FXR-mediated regulation of BA synthesis, which represents a major pathway of cholesterol catabolism, suggests an implication of FXR in the control of lipid metabolism. Indeed, mice deficient for FXR are dyslipidemic, with elevated plasma TG and HDL-C as well as non-HDL-C levels (27–29).
The increase of non-HDL-C in FXR-deficient mice occurs both in the VLDL and LDL lipoprotein fraction, illustrated by an elevation of plasma apoB concentrations (27, 28). Hepatic LDL receptor (LDLR) expression is not different, but the production of TG-rich, apoB-containing lipoproteins as well as intestinal cholesterol absorption are increased, probably causing the elevated non-HDL-C levels upon FXR deficiency (27). In the same line, FXR activation in mice with a synthetic FXR agonist reduces intestinal cholesterol absorption by 50% (30). The authors propose a reduction in BA pool size and hydrophobicity index (due to an increased tauro-β-MCA/TCA ratio) upon FXR activation (31) as underlying mechanism, as a reduced BA pool hydrophobicity has been associated with a decrease in cholesterol absorption (32).
The activation of FXR by CDCA in a human hepatocyte cell line results in an increase of LDLR expression and activity (33, 34). In addition, FXR activation potentiates LDLR activity by inhibiting proprotein convertase subtilisin/kexin type (PCSK)9, a LDLR inhibitor (35). Both effects suggest that FXR activation could lower plasma LDL-cholesterol (LDL-C) in vivo. However, CDCA treatment in humans has no effect or even increases LDL-C (36, 37), possibly due to the FXR-dependent inhibition of CYP7A1 resulting in a decreased demand in cholesterol for BA synthesis and a decreased LDL uptake. In the same line, BAS, which deactivate FXR by limiting the availability of endogenous ligands, lower LDL-C (3, 38). Due to the increase in BA synthesis, endoplasmatic reticulum cholesterol is depleted, thus activating the sterol regulatory element binding protein (SREBP)2, which in turn upregulates LDLR expression (38, 39).
Recently, it has been shown that CA represses human LPA gene expression in transgenic mice in a FXR-dependent manner. The corresponding protein, apo(a), covalently binds to apoB forming lipoprotein(a) [Lp(a)], a plasma lipoprotein strongly associated with a high risk for the development of atherothrombotic diseases (40). Further, in patients with biliary obstruction and thus elevated plasma BA levels, plasma Lp(a) concentrations are very low but return to normal levels after successful removal of the obstruction (40). Thus, regarding the management of Lp(a) levels and the associated atherosclerosis risk, FXR activation, which may exert positive effects on proatherogenic apoB-containing lipoproteins, appears to be indicated.
The elevation of HDL-C observed in FXR-deficient mice is associated with the presence of larger HDL particles and a reduced uptake of HDL-C esters by the liver due to a decreased expression of the HDL receptor scavenger receptor class B member (SR-B) 1 (27, 30). Accordingly, the administration of an FXR ligand decreases HDL-C levels, which is associated with an increase of hepatic SR-B1 expression and of hepatic cholesterol clearance (30). HDL-C modulation by FXR is apoAI-independent, as apoAI expression is not altered in liver or ileum of FXR-deficient mice (27) or upon FXR activation (30). FXR further induces the expression of the phospholipid transfer protein (PLTP) (41), which contributes to HDL remodeling (42). These data suggest that FXR activation in mice promotes reverse cholesterol transport, however, reflected by a decrease in HDL-C.
In contrast, the human APOA1 gene is repressed by BA treatment in primary human hepatocytes in vitro and in mice overexpressing the human transgene (43). Possible mechanisms include FXR binding to a negative FXRE in the APOA1 promoter (43), the induction of SHP, which inhibits LRH-1-mediated APOA1 induction (44), or FXR-independent mechanisms (45). Thus, the effects of FXR activation on HDL-C in humans remain uncertain.
As mentioned above, FXR-deficient mice present increased plasma TG levels, implying a role for FXR in TG metabolism. In the absence of FXR, the hepatic synthesis rate of TG-rich lipoproteins is elevated even though mRNA levels of apoB are unaltered, and mRNA levels of microsomal TG transfer protein (MTP), a protein involved in VLDL assembly and secretion, are decreased (27). FXR activation improves plasma TG clearance in wild-type, but not in FXR-deficient mice, by increasing apoCII and decreasing apoCIII expression, a lipoprotein lipase activator and inhibitor, respectively (46, 47). In addition, FXR activation induces VLDL receptor expression, which is known to contribute to plasma TG clearance (48). Furthermore, FXR inhibits hepatic lipogenesis by repressing SREBP1c in a SHP-dependent manner (49).
In genetically obese mice, FXR deficiency strongly increases plasma TG levels (50), as observed in lean animals (27, 28). Although the hepatic production of VLDL is unaltered in this model, the decreased expression of apoCII points toward reduced clearance as the underlying cause (50). Conversely, the administration of natural or synthetic FXR agonists decreases plasma TG in mouse and hamster models of obesity (49, 51). In both studies, VLDL production decreases upon FXR activation, which has been linked to a reduction of hepatic lipogenesis and TG content (49, 51).
In normo- and hypertriglyceridemic patients, reduction of the BA pool by BAS treatment results in elevated plasma TG levels due to an increased VLDL production (52). Likewise, patients with CYP7A1 deficiency display elevated plasma TG (53). In vitro, FXR activation in a human hepatocyte cell line decreases MTP expression and the secretion of VLDL (54). Consequently, the administration of CDCA to patients with hypertriglyceridemia reduces plasma TG (55, 56).
In line with the inhibitory function of FXR on hepatic lipogenesis (49), FXR deficiency leads to a mild steatosis in lean mice (28) and to an exacerbation of hepatic TG content in genetic (ob/ob) obesity (50), whereas FXR activation with different agonists improves hepatic steatosis (49, 57). These findings have raised the intriguing possibility that FXR may also be implicated in the progression of steatosis to NASH. Indeed, FXR deficiency in LDLR-deficient mice fed a high-fat diet leads to the development of NASH with infiltration of inflammatory cells and deposition of extracellular collagen (58). The administration of a synthetic FXR agonist reverses inflammatory infiltration and fibrosis in a FXR-dependent manner in mice fed a methionine- and choline-deficient diet (59).
Overall, in both mice and humans, FXR activation improves hypertriglyceridemia and decreases steatosis. Thus, FXR activation might be useful to improve hepatic steatosis and even NASH.
FXR and atherosclerosis
Loss of FXR function in mouse models of atherosclerosis has produced contradictory results, depending on the animal model and gender. On the one hand, the absence of FXR in apoE-deficient mice fed an atherogenic diet leads to more severe atherosclerosis with a decreased survival rate (60). On the other hand, FXR deficiency in LDLR-deficient mice is atheroprotective (61, 62). The effect of FXR activation on decreasing atherosclerotic plaque burden seems in part due to an improvement of the lipid profile (63–65).
In addition to controlling metabolic risk factors of atherosclerosis, FXR is expressed in different cell types of the blood vessel, including vascular smooth muscle cells (VSMC) of coronary arteries and the aorta (66). FXR activation enhances apoptosis (66) and inhibits inflammation and migration (67) of rat VSMC, effects that may attenuate vascular remodeling and atherosclerosis development. Further, FXR directly promotes the transcription of angiotensin type 2 receptor (AT2R) (68), which prevents neointimal formation in balloon-injured rat carotid arteries (69) and participates in the hypotensive effects of angiotensin type 1 receptor (AT1R) blockers (70) and the cardioprotective effects of peroxisome proliferator activated receptor (PPAR)γ ligands (71).
FXR is also expressed in endothelial cells (72). FXR activation regulates the vascular tone by increasing endothelial nitric oxide synthase (eNOS) expression and production of the vasodilator nitrogen oxide (NO), while decreasing expression of the vasoconstrictor endothelin (ET)-1 (72–74). Furthermore, FXR stimulates the catabolism of circulating asymmetric dimethylarginine (ADMA), an endogenous NO synthase inhibitor (75, 76). Thus, FXR exerts vasodilatator effects. However, impairment of endothelium-dependent relaxation due to decreased sensitivity of VSMC to NO has been reported after chronic FXR stimulation in cultured rabbit arteries (77).
Overall, FXR activation appears to protect against atherosclerotic plaque formation.
FXR and glucose metabolism
The expression of both FXR and CYP7A1 is increased by glucose and decreased by insulin (78), indicating a link between BA and glucose metabolism. Indeed, FXR modulates glucose homeostasis. The gluconeogenic genes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase), have been shown to be regulated by FXR, however, with discordant results in the literature (79, 80). FXR activation increases PEPCK expression and glucose output in primary rat hepatocytes (80). In contrast, CA treatment decreases the expression of multiple gluconeogenic genes, including PPARγ coactivator (PGC)-1α, PEPCK, and G6Pase, and decreases fasting blood glucose levels in wild-type mice, but not in FXR- or SHP-deficient mice, indicating a FXR-SHP axis dependency (81). In addition, CDCA decreases PEPCK and G6Pase expression by decreasing HNF4α activity in a FXR-independent manner (82, 83). Further, FXR-deficient mice reveal an accelerated induction of glycolytic and lipogenic genes upon high-carbohydrate refeeding, whereas FXR activation decreases the induction of glucose-responsive genes (79). FXR-deficient mice have less hepatic glycogen (78), and conversely, FXR activation leads to an increase of glycogen synthesis (57). Moreover, FXR deficiency is associated with a delayed intestinal glucose absorption due to an enhanced glucose phosphorylation cycle in proximal enterocytes (84), another action site of FXR in the regulation of glucose metabolism.
Three groups have shown independently that FXR-deficient mice display peripheral insulin resistance (29, 57, 81). FXR plays a role in maintaining adipocyte differentiation and function (29, 85, 86), which might explain why the absence of FXR affects peripheral insulin sensitivity (29). Glucose-stimulated insulin secretion by pancreatic islets is impaired in FXR-deficient mice (87). Furthermore, FXR increases the expression of insulin in a glucose-dependent manner via the induction of the transcription factor KLF11 (88). In addition, FXR increases insulin secretion by enhancing GLUT2 translocation to the plasma membrane by a nongenomic effect via Akt phosphorylation (88).
The effects of synthetic FXR agonists on glucose homeostasis are less clear. Two studies have reported that activation of FXR in db/db or ob/ob mice decreases gluconeogenic gene expression and improves hyperglycemia and peripheral insulin resistance (29, 57). In contrast, a recent third paper shows that FXR activation by the synthetic agonist GW4064 in mice with diet-induced obesity aggravates glucose intolerance by reducing the BA pool size. Consequently, the administration of BA restores the BA pool and reverses the GW4064-induced metabolic effects (89). In line with this concept, FXR deficiency has been shown to protect from genetic and diet-induced obesity (50, 90) and to improve glucose homeostasis by increasing peripheral glucose clearance and adipose tissue insulin sensitivity (50). The deletion of hepatic FXR has no protective effect (50), highlighting the importance of extra-hepatic FXR.
Even though partly controversial, these data provide evidence for a regulatory role of FXR in glucose metabolism and insulin sensitivity. FXR might exert differential effects in lean versus obese mice. Tissue-specific models might be necessary to dissect the complex effects of FXR on insulin resistance. From a pharmacological viewpoint, it is unclear whether inhibition or activation of FXR would be more efficient to correct altered glucose metabolism.
TGR5 and energy metabolism
Mice deficient for TGR5, the membrane receptor for BA, exhibit a decreased BA pool size even though fecal BA excretion is unaltered (91). In addition, they are protected from gallstone formation (92). TGR5 is expressed in human cholangiocytes (gallbladder epithelial cells) (93) and is involved in regulating bile composition by inducing chloride secretion by cholangiocytes (94). Treatment with the TGR5 agonist INT-777 stimulates gallbladder filling (95).
Interestingly, TGR5 also regulates metabolic homeostasis. Whereas TGR5-deficient mice have normal weight and fat mass (92), when fed a high-fat diet, females display increased weight gain resulting from increased fat accumulation (91). Conversely, TGR5 activation by supplementing CA to the diet prevents the high-fat-induced changes in adipose tissue mass and morphology (96). The role of TGR5 in weight loss has been associated with increased energy expenditure rather than decreased caloric intake (96). TGR5 activation induces deiodinase-2 expression in murine brown adipose tissue (BAT) and human skeletal muscle cells by increasing cAMP levels. Deiodinase-2-mediated conversion of inactive thyroxine (T4) to active 3,5,3′-tri-iodothyronine (T3) enhances the expression of uncoupling proteins (UCP), mitochondrial oxidative phosphorylation and energy expenditure in BAT and skeletal muscle (96). Comparable results have been obtained with semisynthetic (INT-777) (96) and natural (oleanolic acid) (97) TGR5, but not with FXR agonists (98). Thus, TGR5 agonists might promote energy expenditure.
TGR5 and lipid metabolism
TGR5 also affects hepatic lipid content. Female TGR5-deficient mice present an increase in hepatic fat content, especially when fed a high-fat diet (92). In line, TGR5 activation with INT-777 improves liver function by decreasing steatosis and preventing fibrosis (98). Additionally, plasma TG and nonesterified fatty acid levels are decreased upon TGR5 activation (98). Thus, TGR5 activation might be useful for the treatment of NAFLD.
TGR5 and atherosclerosis
A recent study has reported anti-atherosclerotic effects of TGR5 activation (99). The administration of INT-777 to LDLR-deficient mice attenuates atherosclerotic lesion size only in the presence of TGR5, due to a decrease in plaque macrophage content and intraplaque inflammation. This effect is attributed to TGR5-induced cAMP signaling in macrophages, causing the inhibition of nuclear factor κ light-chain enhancer of activated B cells (NF-κB) activity and the subsequent production of pro-inflammatory cytokines (99).
TGR5 and glucose homeostasis
In mice fed a high-fat diet, TGR5 activation lowers serum glucose and insulin levels and improves glucose tolerance (97, 98). TGR5-transgenic (TGR5-tg) mice also display improved diet-induced glucose intolerance without changes in body weight (98). TGR5 activation induces glucagon-like peptide (GLP)-1 production by enteroendocrine cells (100), hence enhancing postprandial insulin secretion (101). TGR5 induction of GLP-1 in STC-1 (enteroendocrine) cells is mediated by an increase in cAMP levels and cytochrome C oxydase (Cox) activity, leading to enhanced cellular oxygen consumption and rise in the ATP/ADP ratio. This signal induces the closing of KATP channels and the opening of calcium-gated voltage channels (CAv), triggering GLP-1 secretion (98). Furthermore, enhanced plasma GLP-1 in TGR5-tg mice fed a high-fat diet is associated with a normal pancreatic islet distribution and higher insulin content compared with hypertrophic islets with low insulin content in control mice fed a high-fat diet (96). In contrast, TGR5-deficient mice have impaired glucose tolerance, and INT-777 treatment has no effect on GLP-1 secretion in these mice (98). In humans, a genetic variation within the TGR5 gene is, however, not associated with the development of prediabetic phenotypes (102).
MODULATORS OF BILE ACID RECEPTOR ACTIVITY AND THEIR CLINICAL APPLICATION
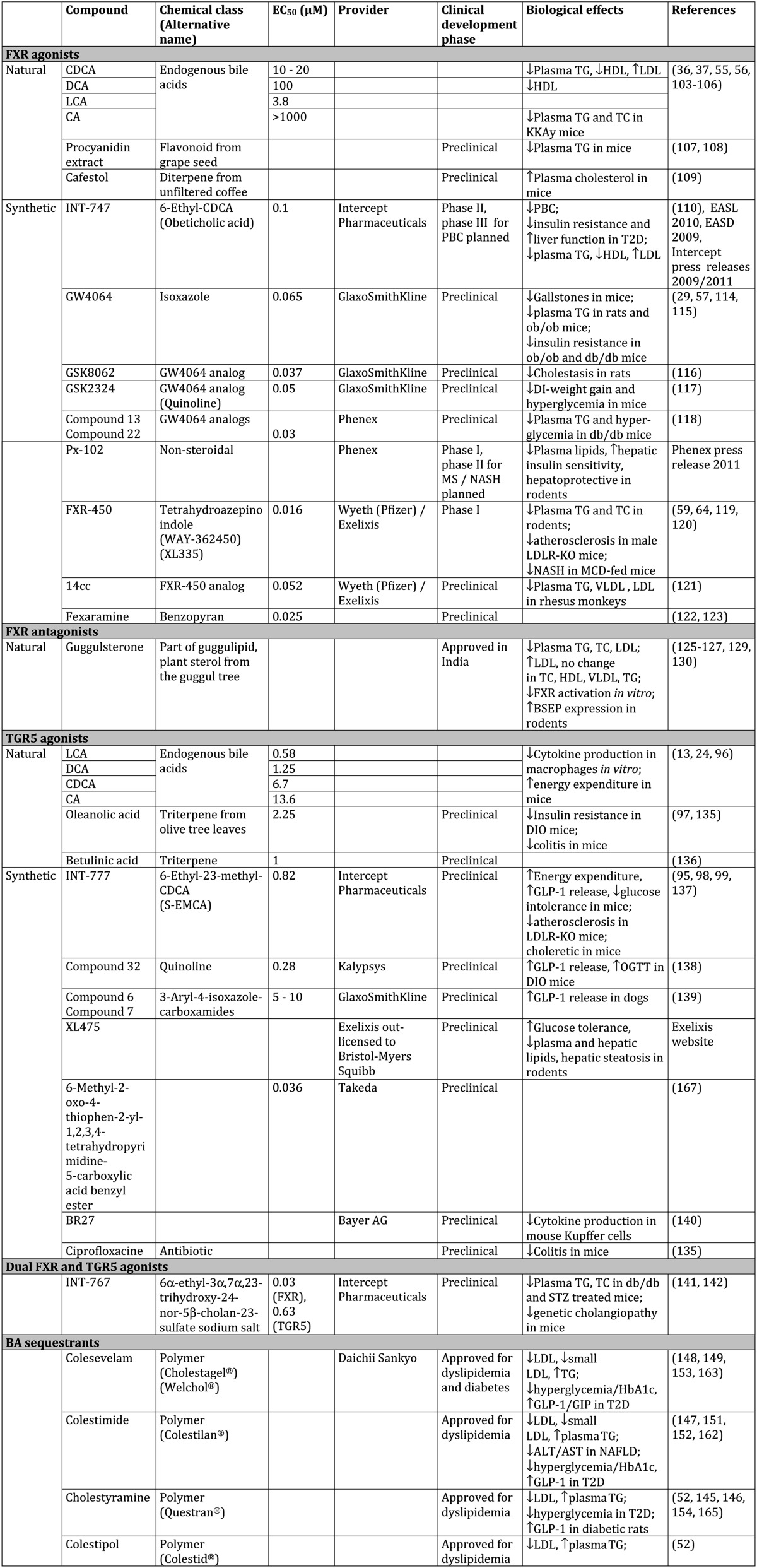
As outlined above, FXR and TGR5 contribute to the regulation of a panel of pathways linking BA and metabolic homeostasis. As a result, both receptors are potential targets for the treatment of cholestasis, hyperlipidemia, NAFLD/NASH, CVD, and T2D. Consequently, an extensive effort has been put into the identification and development of modulators of FXR and TGR5 activity. In the following sections, we discuss the available compounds and the status of their (pre)clinical evaluation (Table 1 and Fig. 1).
TABLE 1.
Modulators of BA receptor activity and BA metabolism
DI, diet-induced; DIO, diet-induced obesity; GIP, gastric inhibitory protein; MCD, methionine-choline-deficient; OGTT, oral glucose tolerance test; STZ, streptozotocin; TC, total cholesterol.
FXR agonists
Endogenous primary (CDCA, CA) and secondary (LCA, DCA) BA activate FXR with different efficacy (CDCA > LCA = DCA > CA), with the conjugated forms being less potent (103, 104). Mainly CDCA and CA have been used to study FXR function in vitro and in vivo. However, their conversion into secondary BA species in vivo and the fact that their specificity is not restricted to FXR often complicates the interpretation of the results. Thus, all four BA also activate TGR5 and, with exception of CDCA, PXR (15, 26).
Before BA were known to activate FXR, CDCA was tested for the treatment of gallstones to enrich the bile and dissolve the stone. Four studies in small patient cohorts (n = 8–15) find a reduction in plasma TG (55, 56, 105, 106) but no change in total plasma cholesterol (55, 56, 106), and one reports decreased HDL-C levels (105). The only study conducted in a large number of patients (n = 916) observes an increase of LDL-C upon CDCA administration (36). Thus, even though CDCA treatment lowers elevated plasma TG levels, the impact on cholesterol metabolism is not conclusive and might be unfavorable. In addition, elevated liver enzymes and diarrhea might occur as side effects (36, 56), discouraging the use of CDCA in the clinic. Nevertheless, a study has recently been launched in patients with MS and dyslipidemia (scheduled n = 48) to test the effect of CDCA on plasma TG levels, defined as primary endpoint, hepatic fat content to be determined by nuclear magnetic resonance and hepatic glucose metabolism to be measured by hyperinsulinemic euglycemic clamp analysis (http://clinicaltrials.gov; NCT00465751).
A procyanidin extract from grape seeds reduces postprandial plasma TG levels in wild-type but not in FXR- or SHP-deficient mice, decreases the expression of lipogenic genes, such as SREBP1c, and enhances the transcriptional activity of FXR, although only in the presence of CDCA (107, 108). However, it is unknown which component of the extract exerts these effects. Further, cafestol, a diterpene present in unfiltered coffee, activates FXR as well as PXR. Cafestol treatment of APOE3-Leiden mice increases total plasma cholesterol and consistently represses CYP7A1 and CYP8B1 expression in wild-type mice but not in FXR-deficient mice (109).
The most clinically advanced FXR agonist is the semisynthetic compound INT-747 (6-ethyl-CDCA or obeticholic acid), which was designed based on the structure of CDCA and is 10 times more potent (110). Results from in vitro and in vivo studies indicate that INT-747 exerts a hepatoprotective effect in acute LCA-induced necrosis (110), fibrosis (111), and cholestasis (112), and it further protects from insulin resistance and hepatic steatosis in genetically obese rats (113).
A phase II study has confirmed the hepatoprotective effect of INT-747 in primary biliary cirrhosis (PBC), an autoimmune disease in which progressive destruction of the small bile ducts causes cholestasis, fibrosis, and cirrhosis. INT-747 treatment at doses of 10 mg and 50 mg daily for 12 weeks decreases plasma levels of alkaline phosphatase, a diagnostic parameter of PBC, alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT), as well as the inflammatory markers C reactive protein (CRP) and immunoglobulin M (Intercept Pharmaceuticals press release 31/03/2011, communication at the European Association for the Study of the Liver 2010). Curiously, the lower dose appears more effective. Half of the patients show pruritus (itching) as side effect. Subsequently, a phase III PBC program is planned (Intercept Pharmaceuticals press release 31/03/2011). A second phase II study has evaluated the effect of INT-747 (25 mg and 50 mg daily for 6 weeks) in T2D patients with NAFLD. INT-747 treatment results in a small (1–2%) but significant weight loss and an improved insulin sensitivity (10–18% increase of the glucose disposal rate in hyperinsulinemic euglycemic clamps). Further, plasma ALT and GGT, as well as plasma TG levels, decrease. However, HDL-C levels decrease whereas LDL-C levels increase (Intercept Pharmaceuticals press release 01/10/2009; communication at the European Association for the Study of Diabetes 2009), confirming that FXR activation may be beneficial to lower plasma TG but not cholesterol, similar to CDCA (36, 105). A third phase II study (“FLINT”, http://clinicaltrials.gov; NCT01265498) run by the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is currently ongoing, testing by means of liver biopsy whether INT-747 administration of 25 mg daily for 72 weeks can improve the NAFLD activity score in NASH patients.
Natural (steroidal) FXR ligands enter the enterohepatic cycle and may be metabolized to secondary BA in an uncontrollable manner, thus raising the interest in nonsteroidal compounds devoid of such metabolism. The first synthetic, nonsteroidal FXR agonist GW4064, was identified by screening a stilbene carboxylic acid library (114). GW4064 has been extensively used to study FXR functions in vitro and in vivo [e.g., it is reported to protect against cholesterol gallstones in mice (115), lower plasma TG and cholesterol, and to improve insulin resistance in genetic mouse models of obesity (29, 49, 57)]. However, GW4064 presents some major pharmacodynamic limitations: a certain toxicity due to the stilbene moiety, a poor bioavailability, and the difficulty to target extrahepatic tissues due to fast metabolism by the liver. Thus, quite an effort has been made to develop GW4064 analogs with comparable potency and improved biological compatibility (116, 117); however, none has yet passed preclinical testing. In addition, a number of GW4064 analogs are handled by BA transporters and conjugated like BA, leading to very low plasma levels and hepatic accumulation (118).
The synthetic, nonsteroidal compound Px-102 has entered phase I to test tolerance and pharmacokinetic properties in men. A subsequent phase II study is planned in patients with MS and NAFLD to test whether Px-102 improves hypertriglyceridemia, insulin resistance, and NASH. The lipid-lowering effect of Px-102 in animals seems to be due to a reduction in intestinal uptake and an increase in excretion of neutral lipids and cholesterol (Phenex Pharmaceuticals AG press release 15/09/2011).
A chemical class of synthetic, nonsteroidal FXR agonists, different from GW4064 analogs, has been developed with FXR-450 (WAY-362450, XL335) as the leading compound (63). FXR-450 lowers plasma TG and cholesterol in different rodent models of dyslipidemia (119) and reduces atherosclerotic lesions in LDLR-deficient mice fed a high-fat diet (63). The FXR-450 analog 14cc further decreases plasma TG, VLDL, and LDL-C in normolipemic rhesus monkeys, associated with reduced CYP7A1 and increases LDLR mRNA expression (120). FXR-450 has entered phase I studies in 2007 to analyze safety, tolerability, and pharmacokinetics in healthy Japanese men (http://clinicaltrials.gov; NCT00509756) and in healthy men and women in the US (http://clinicaltrials.gov; NCT00499629), but the results have not been reported.
A third class of synthetic, nonsteroidal FXR agonists is represented by fexaramine (121). Surprisingly, different gene profiles are induced by fexaramine, CDCA, or GW4064 in mouse hepatocytes (122). The conformation of the ligand-receptor complex of FXR with fexaramine differs from that with CDCA or MFA-1, a synthetic steroidal FXR agonist (122, 123), which might be linked to the differential induction of target genes by the respective ligands (SBARM concept).
Altogether, accumulating proof from animal and human studies shows that FXR activation reduces hypertriglyceridemia and improves insulin resistance and NAFLD, but it has unfavorable effects on cholesterol concentrations. Further studies are necessary to assess the full potential of FXR agonists in clinical settings.
FXR antagonists
The only FXR antagonist so far described is guggulsterone, found in the oleoresin of the guggul tree (= guggulipid). Guggulsterone inhibits FXR activation by CDCA or GW4064 in vitro by impairing coactivator recruitment and corepressor release from the receptor (124). However, alone it is without effect on FXR activity (124, 125). In wild-type but not FXR-deficient mice fed a cholesterol-enriched diet, guggulsterone lowers hepatic cholesterol content (125), whereas it lowers plasma TG and increases HDL-C in rats (126). Curiously, guggulsterone enhances the FXR agonist-mediated induction of the BA transporter bile salt export pump (BSEP) and is thus rather a selective FXR modulator (SBARM) than a full antagonist (126). Guggulsterone further activates PXR (125), steroid and xenobiotic receptor (SXR) (124), and the progesterone receptor and binds to a variety of other receptors (127), hence lacking selectivity for FXR.
Guggulipid is traditionally used in Aryuvedic medicine, and it has been approved since 1987 in India to treat hyperlipidemia. Several clinical studies in Indian populations have been published, but only one has been conducted in a double-blind, randomized, placebo-controlled manner using a guggulipid with standardized guggulsterone content. Hypercholesterolemic adults (n = 61) were treated with 50 mg guggulipid twice daily for 24 weeks in addition to a fruit- and vegetable-enriched diet. The treatment decreases plasma TG as well as total and LDL-C levels, whereas HDL-C does not change (128). In a second double-blind, randomized, placebo-controlled study performed in a Western population of hyperlipidemic adults consuming a normal Western diet (n = 103), a guggul extract containing 2.5% guggulsterone was administered three times daily (1 g and 2 g per dose) for 8 weeks. No changes are observed in plasma TG, VLDL, total cholesterol, or HDL-C, but LDL-C levels increase 4–5% compared with placebo controls (129). The reasons for these contradicting results are unclear.
Selective bile acid receptor modulators
As mentioned above, FXR agonists of different chemical classes may activate different target gene patterns (122). Structurally different ligands can bind differently to the FXR ligand binding pocket, whose intrinsic plasticity is defined by two cavities that can fit ligand substructures (130, 131). Depending on the (stereo)chemical properties of the ligand, the exact site of binding, the closeness of the ligand-receptor conformation, and the strength of the binding can differ (122, 123, 131). The specific ligand-receptor conformation can determine the ability of coregulators to act (i.e., activators to bind or repressors to dissociate), which defines the gene selectivity of a given ligand. Those effects might also explain opposing biological “outputs” of different ligands on the same target. They further offer the possibility to develop selective bile acid receptor modulators (SBARM), which can exert a desired beneficial effect, such as the inhibition of lipogenesis to lower plasma TG, while avoiding side effects (e.g., lowering HDL-C or increasing LDL-C).
AGN34 is a selective FXR modulator, which in the intestinal cell line Caco-2 inhibits the intestinal BA binding protein (IBABP) without affecting SHP expression, both direct FXR target genes (132). In HepG2 cells, AGN34 again has no effect on SHP and decreases CYP7A1 expression, leading to the conclusion that AGN34 acts as an antagonist on IBABP expression but as an agonist on CYP7A1 expression, hence, as a gene-selective modulator (132). However, the mechanism by which AGN34 lowers CYP7A1 expression in a SHP-independent manner in isolated hepatocytes remains unknown.
TGR5 agonists
Compared with FXR, no TGR5 agonist has entered a clinical trial yet. However, TGR5 modulators are expected to be useful for the treatment of T2D, and an emerging function of TGR5 in monocytes and macrophages (133) might open up therapeutic options to target the inflammatory component of CVD and T2D.
TGR5 is activated by the same endogenous BA as FXR, although in a different order of potency (LCA > DCA > CDCA > CA) (24). CA activation of TGR5 has revealed a role for BA in the TGR5-dependent regulation of energy expenditure (96).
A naturally occurring triterpene from olive tree leaves, oleanolic acid also activates TGR5. Addition of oleanolic acid to a high-fat diet tends to reduce weight gain and improves glucose tolerance in mice (97). Oleanolic acid also attenuates colitis in a murine model, suggesting a role for TGR5 in intestinal barrier function (134). Another triterpene, betulinic acid, also exhibits TGR5-activating properties. However, derivatives of the compound with an improved potency have no effect on obesity or glucose tolerance in vivo (135).
As for FXR, a semisynthetic, steroidal derivative of CDCA has been developed that is a TGR5 agonist. INT-777 is well absorbed in vivo and undergoes enterohepatic cycling, largely without conjugation (136). The compound exerts a choleretic effect in rats (136) with TGR5 dependency having recently been demonstrated in mice (95). INT-777 increases GLP-1 release from intestinal L-cells ex vivo (136) and in vivo, and it improves glucose tolerance (98). Similar to BA, INT-777 increases energy expenditure in mice fed a high-fat diet (98). INT-777 also inhibits atherosclerosis by exerting anti-inflammatory effects in macrophages (99). Thus, the compound today presents the most promising TGR5 agonist developed into a therapeutic for metabolic disorders.
A number of synthetic nonsteroidal compounds have been described, but they have very few in vivo analyses. Compound 32, a quinoline, shows modest bioavailability, but increases GLP-1 release and improves oral glucose tolerance in a diet-induced obesity mouse model (137). A group of isoxazolecarboxamides are potent TGR5 activators, increasing GLP-1 release in dogs while reducing portal vein glucose concentrations (138). XL475, a third compound, was designed to selectively target TGR5 in the intestine without exerting systemic effects. According to the manufacturer's website, XL475 increases GLP-1 secretion in multiple species, improves glucose tolerance, plasma, and hepatic lipid levels, and reduces hepatic steatosis in preclinical models of T2D (www.exelixis.com/pipeline/xl475). Information from patent literature further identifies a pyrimidine benzyl ester that potently activates TGR5 (139). The synthetic agonist BR27 has been reported to inhibit LPS-induced cytokine release from Kupffer cells (140), suggesting anti-inflammatory activities. Recently, ciprofloxazine has been found to activate TGR5 and reduce colitis in a mouse model (134).
Thus, TGR5 agonists might improve glucose intolerance by increasing energy expenditure and/or GLP-1 secretion, in addition to being potentially anti-inflammatory.
The semisynthetic steroidal compound INT-767 has dual agonist properties for TGR5 and FXR, being 10 times more potent for FXR than for TGR5. INT-767 decreases plasma TG and cholesterol in db/db and streptozotocin-treated mice (141). Further, it has hepatoprotective effects in a mouse model of genetic cholangiopathy, illustrated by an improvement of plasma liver enzymes, hepatic inflammation, and biliary fibrosis (142). Curiously, such effect is not observed with the single FXR or TGR5 agonists INT-747 and INT-777 (142). Although the combined potential benefit of FXR and TGR5 activation on lipid and glucose metabolism is intriguing, the synergistic advantage of INT-767 over single FXR or TGR5 agonists, as demonstrated for cholangiopathy, still needs to be proved for metabolic disorders.
Bile acid sequestrants
BA homeostasis and, by deduction, the activity of BA receptors can be modulated by BAS, nonabsorbable resins that complex BA in the intestinal lumen and direct them toward excretion. Consequently, the BA pool is depleted by about 40% (143), which is compensated for by an increase in BA synthesis. BAS are cross-linked polymers, which interact with the hydrophobic core of the BA, and they possess cationic side-chains that bind the BA's carboxyl group by electrophysical interaction. Binding characteristics vary for different BAS. Cholestyramine and colestipol, BAS of the first generation, preferentially bind dihydroxy (CDCA and DCA) instead of trihydroxy BA (CA), rendering the pool composition more hydrophilic (143) and probably diminishing the efficacy of BA binding to the resin due to the decreasing proportion of dihydroxy BA. Second-generation BAS, such as colesevelam, are considered to bind di- and trihydroxy BA equally well. However, BA pool composition analysis upon colesevelam treatment revealed an increased hydrophilicity due to an elevated CA synthesis and a decreased CDCA and DCA pool size, suggesting that the specificity of colesevelam might not be so different from other BAS (144). A reduced pool size of CDCA and DCA, potent endogenous FXR ligands, might be synonymous with a decreased FXR activation, making it conceivable that the effects of BAS on metabolic homeostasis are in part mediated by FXR.
Since decades, BAS are established as treatment for hypercholesterolemia. An early study showed that resin treatment of dyslipidemic patients decreases LDL-C up to 30% without affecting HDL-C (145), and a multicenter long-term trial in a large number of patients showed that the cholesterol-lowering effect of BAS is associated with a 19% decrease in the risk for CVD death and/or nonfatal myocardial infarction (146). However, BAS treatment is counterindicated when TG levels are elevated, as VLDL production may further increase upon treatment (52). Since then, a large body of evidence has confirmed the overall LDL-C-lowering and TG-increasing properties of different BAS with discordant results on HDL-C concentrations (reviewed in Ref. 38). BAS specifically reduce LDL particle number, with a preference for small LDL (147–149), which are considered especially atherogenic. Further, BAS therapy is frequently used in combination with other lipid-lowering drugs (e.g., statins, fibrates, ezetimibe, niacin) to obtain a better global risk control or in cases of drug intolerance (see Ref. 38).
Part of the LDL-C-lowering and TG-increasing effects of BAS might be mediated by FXR. Relieve of FXR-mediated inhibition of lipogenesis increases hepatic steatosis at least in rodents (150). Administration of colestimide for 24 weeks lowers ALT and aspartate aminotransferase (AST), but not GGT in NASH patients. Unfortunately, the effect on NASH itself has not been evaluated by histological analysis of a follow-up biopsy (151). Thus, the effect of BAS on hepatic steatosis and NASH remains to be determined.
BAS also lower blood glucose in T2D patients, reducing both fasting plasma glucose and HbA1c levels (152–154). Inadequately controlled T2D patients on insulin, sulfonylurea, or metformin therapy also benefit from BAS administration in terms of glucose control (155–157). Surprisingly, the glucose-lowering effect of BAS appears not to be mediated by an improvement of insulin sensitivity as measured by hyperinsulinemic euglycemic clamps (158), glucose, or meal tolerance tests (159–161). In contrast, one week administration of colestimide in T2D patients (n = 16) lowers plasma glucose and increases postprandial GLP-1 (162). A second study evaluating glucose kinetics in T2D patients after BAS treatment finds improved glucose clearance and increased GLP-1 and gastric inhibitory peptide (GIP) concentrations (163). In diabetic rat models, BAS decrease plasma glucose and improve glucose tolerance associated with an increase in plasma GLP-1 (164, 165). Beysen et al. further observe no effect of BAS treatment on hepatic glucose production (163), similar to that reported in a diabetic mouse model (166). Thus, the contribution of FXR to the effects of BAS is unclear. Instead, it has been proposed that BAS-sequestered BA can activate TGR5 expressed in L-cells of the intestinal wall (100) or that, due to impaired micelle formation, unabsorbed long-chain fatty acids stimulate GPR40 (158), both effects promoting GLP-1 secretion.
CONCLUSION
Today, the link between BA and metabolic homeostasis is well established. Data demonstrating the contribution of BA to the regulation of different pathways in lipid, glucose, and energy metabolism by FXR- and TGR5-mediated signaling keeps accumulating. Recent clinical studies have confirmed that the activation of FXR is beneficial for the treatment of hypertriglyceridemia but detrimental for plasma cholesterol levels, whereas deactivation of FXR by BAS lowers elevated plasma cholesterol at the expense of increasing TG. FXR activation further appears to improve insulin resistance and liver status in T2D patients. The mechanism of the glucose-lowering action of BAS remains under investigation, but it seems to be mediated by TGR5-mediated stimulation of the incretin system rather than by FXR signaling pathways.
Clearly, the modulation of FXR as well as TGR5 activity has a growing potential for the treatment of the MS. The development of selective modulators of action (SBARM) or combinatory FXR/TGR5 agents might increase the therapeutic possibilities.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- ASBT
- apical sodium/bile acid cotransporter
- AST
- aspartate aminotransferase
- BA
- bile acid
- BAS
- bile acid sequestrant
- BAT
- brown adipose tissue
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- CVD
- cardiovascular disease
- DCA
- deoxycholic acid
- ET-1
- endothelin-1
- FGF19
- fibroblast growth factor 19
- FXR
- farnesoid X receptor
- FXRE
- FXR response element
- G6Pase
- glucose 6-phosphatase
- GGT
- γ-glutamyl transferase
- GLP-1
- glucagon-like peptide-1
- HbA1c
- hemoglobin A1c
- HDL-C
- HDL-cholesterol
- HNF4α
- hepatocyte nuclear factor 4α
- IBABP
- intestinal bile acid binding protein
- LCA
- lithocholic acid
- LDL-C
- LDL-cholesterol
- LDLR
- LDL receptor
- Lp(a)
- lipoprotein (a)
- LRH-1
- liver receptor homolog-1
- MCA
- muricholic acid
- MS
- metabolic syndrome
- MTP
- microsomal triglyceride transfer protein
- NAFLD
- non-alcoholic fatty liver disease
- NASH
- non-alcoholic steatohepatitis
- NO
- nitrogen oxide
- OST
- organic solute transporter
- PBC
- primary biliary cirrhosis
- PEPCK
- phosphoenolpyruvate carboxykinase
- PKA
- protein kinase A
- PXR
- pregnane X receptor
- SBARM
- selective bile acid receptor modulator
- SHP
- small heterodimer partner
- SREBP
- sterol regulatory element binding protein
- T2D
- type 2 diabetes
- TG
- triglyceride
This work was supported by grants from the Agence Nationale de la Recherche (ANR), INSERM, Région Nord-Pas-De-Calais, Contrats de Projets Etat Région (CPER), and LABEX EGID. G.P. is financed by the INSERM and the Région Nord-Pas-De-Calais, and J.P. by the CPER “Cell-specific regulation of atherosclerosis and vascular function by the nuclear receptor FXR.” B.S. is a member of the Institut Universitaire de France.
REFERENCES
- 1.Libby P., Ridker P. M., Hansson G. K. 2011. Progress and challenges in translating the biology of atherosclerosis. Nature. 473: 317–325. [DOI] [PubMed] [Google Scholar]
- 2.Larter C. Z., Chitturi S., Heydet D., Farrell G. C. 2010. A fresh look at NASH pathogenesis. Part 1: the metabolic movers. J. Gastroenterol. Hepatol. 25: 672–690. [DOI] [PubMed] [Google Scholar]
- 3.Staels B., Kuipers F. 2007. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 67: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 4.Bays H. E., Goldberg R. B., Truitt K. E., Jones M. R. 2008. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch. Intern. Med. 168: 1975–1983. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191. [DOI] [PubMed] [Google Scholar]
- 6.Russell D. W., Setchell K. D. 1992. Bile acid biosynthesis. Biochemistry. 31: 4737–4749. [DOI] [PubMed] [Google Scholar]
- 7.Kwakye J. B., Barnes S., Diasio R. B. 1993. Identification of bile acid coenzyme A synthetase in rat kidney. J. Lipid Res. 34: 95–99. [PubMed] [Google Scholar]
- 8.Falany C. N., Johnson M. R., Barnes S., Diasio R. B. 1994. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 269: 19375–19379. [PubMed] [Google Scholar]
- 9.Ridlon J. M., Kang D., Hylemon P. B. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47: 241–259. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki T., Moschetta A., Lee Y., Peng L., Zhao G., Downes M., Yu R. T., Shelton J. M., Richardson J. A., Repa J. J., et al. 2006. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA. 103: 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setchell K. D., Lawson A. M., Tanida N., Sjövall J. 1983. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J. Lipid Res. 24: 1085–1100. [PubMed] [Google Scholar]
- 12.Kim I., Ahn S., Inagaki T., Choi M., Ito S., Guo G. L., Kliewer S. A., Gonzalez F. J. 2007. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48: 2664–2672. [DOI] [PubMed] [Google Scholar]
- 13.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., et al. 2003. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278: 9435–9440. [DOI] [PubMed] [Google Scholar]
- 14.Chiang J. Y. L. 2004. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J. Hepatol. 40: 539–551. [DOI] [PubMed] [Google Scholar]
- 15.Xie W., Radominska-Pandya A., Shi Y., Simon C. M., Nelson M. C., Ong E. S., Waxman D. J., Evans R. M. 2001. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA. 98: 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber R. M., Murphy K., Miao B., Link J. R., Cunningham M. R., Rupar M. J., Gunyuzlu P. L., Haws T. F., Kassam A., Powell F., et al. 2002. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 290: 35–43. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., et al. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 6: 517–526. [DOI] [PubMed] [Google Scholar]
- 18.Brendel C., Schoonjans K., Botrugno O. A., Treuter E., Auwerx J. 2002. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol. Endocrinol. 16: 2065–2076. [DOI] [PubMed] [Google Scholar]
- 19.De Fabiani E., Mitro N., Anzulovich A. C., Pinelli A., Galli G., Crestani M. 2001. The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J. Biol. Chem. 276: 30708–30716. [DOI] [PubMed] [Google Scholar]
- 20.Holt J. A., Luo G., Billin A. N., Bisi J., McNeill Y. Y., Kozarsky K. F., Donahee M., Wang D. Y., Mansfield T. A., Kliewer S. A., et al. 2003. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17: 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2: 217–225. [DOI] [PubMed] [Google Scholar]
- 22.Gineste R., Sirvent A., Paumelle R., Helleboid S., Aquilina A., Darteil R., Hum D. W., Fruchart J., Staels B. 2008. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol. Endocrinol. 22: 2433–2447. [DOI] [PubMed] [Google Scholar]
- 23.Kemper J. K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S., Chiang C., Veenstra T. D. 2009. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 10: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. 2002. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298: 714–719. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. 2008. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7: 678–693. [DOI] [PubMed] [Google Scholar]
- 26.Sato H., Macchiarulo A., Thomas C., Gioiello A., Une M., Hofmann A. F., Saladin R., Schoonjans K., Pellicciari R., Auwerx J. 2008. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 51: 1831–1841. [DOI] [PubMed] [Google Scholar]
- 27.Lambert G., Amar M. J. A., Guo G., Brewer H. B. J., Gonzalez F. J., Sinal C. J. 2003. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278: 2563–2570. [DOI] [PubMed] [Google Scholar]
- 28.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102: 731–744. [DOI] [PubMed] [Google Scholar]
- 29.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J., Gonzalez F. J., et al. 2006. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 281: 11039–11049. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Yin L., Anderson J., Ma H., Gonzalez F. J., Willson T. M., Edwards P. A. 2010. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J. Biol. Chem. 285: 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung D., Inagaki T., Gerard R. D., Dawson P. A., Kliewer S. A., Mangelsdorf D. J., Moschetta A. 2007. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J. Lipid Res. 48: 2693–2700. [DOI] [PubMed] [Google Scholar]
- 32.Wang D. Q., Tazuma S., Cohen D. E., Carey M. C. 2003. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G494–G502. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi T., Chen J., Cooper A. D. 1994. Regulation of cholesterol 7 alpha-hydroxylase gene expression in Hep-G2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J. Biol. Chem. 269: 10071–10078. [PubMed] [Google Scholar]
- 34.Nakahara M., Fujii H., Maloney P. R., Shimizu M., Sato R. 2002. Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J. Biol. Chem. 277: 37229–37234. [DOI] [PubMed] [Google Scholar]
- 35.Langhi C., Le May C., Kourimate S., Caron S., Staels B., Krempf M., Costet P., Cariou B. 2008. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 582: 949–955. [DOI] [PubMed] [Google Scholar]
- 36.Schoenfield L. J., Lachin J. M. 1981. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann. Intern. Med. 95: 257–282. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Jones P. J. H., Woollett L. A., Buckley D. D., Yao L., Granholm N. A., Tolley E. A., Heubi J. E. 2006. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans. Transl. Res. 148: 37–45. [DOI] [PubMed] [Google Scholar]
- 38.Insull W., Jr. 2006. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South. Med. J. 99: 257–273. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson L. M., Abrahamsson A., Sahlin S., Gustafsson U., Angelin B., Parini P., Einarsson C. 2007. Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem. Biophys. Res. Commun. 357: 707–711. [DOI] [PubMed] [Google Scholar]
- 40.Chennamsetty I., Claudel T., Kostner K. M., Baghdasaryan A., Kratky D., Levak-Frank S., Frank S., Gonzalez F. J., Trauner M., Kostner G. M. 2011. Farnesoid X receptor represses hepatic human APOA gene expression. J. Clin. Invest. 121: 3724–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urizar N. L., Dowhan D. H., Moore D. D. 2000. The farnesoid X-activated receptor mediates bile acid activation of phospholipid transfer protein gene expression. J. Biol. Chem. 275: 39313–39317. [DOI] [PubMed] [Google Scholar]
- 42.Rye K. A., Clay M. A., Barter P. J. 1999. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 145: 227–238. [DOI] [PubMed] [Google Scholar]
- 43.Claudel T., Sturm E., Duez H., Torra I. P., Sirvent A., Kosykh V., Fruchart J., Dallongeville J., Hum D. W., Kuipers F., et al. 2002. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delerive P., Galardi C. M., Bisi J. E., Nicodeme E., Goodwin B. 2004. Identification of liver receptor homolog-1 as a novel regulator of apolipoprotein AI gene transcription. Mol. Endocrinol. 18: 2378–2387. [DOI] [PubMed] [Google Scholar]
- 45.Gardès C., Blum D., Bleicher K., Chaput E., Ebeling M., Hartman P., Handschin C., Richter H., Benson G. M. 2011. Studies in mice, hamsters, and rats demonstrate that repression of hepatic apoA-I expression by taurocholic acid in mice is not mediated by the farnesoid-X-receptor. J. Lipid Res. 52: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claudel T., Inoue Y., Barbier O., Duran-Sandoval D., Kosykh V., Fruchart J., Fruchart J., Gonzalez F. J., Staels B. 2003. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 125: 544–555. [DOI] [PubMed] [Google Scholar]
- 47.Kast H. R., Nguyen C. M., Sinal C. J., Jones S. A., Laffitte B. A., Reue K., Gonzalez F. J., Willson T. M., Edwards P. A. 2001. Farnesoid X-activated receptor induces apolipoprotein C–II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 15: 1720–1728. [DOI] [PubMed] [Google Scholar]
- 48.Sirvent A., Claudel T., Martin G., Brozek J., Kosykh V., Darteil R., Hum D. W., Fruchart J., Staels B. 2004. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 566: 173–177. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113: 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prawitt J., Abdelkarim M., Stroeve J. H. M., Popescu I., Duez H., Velagapudi V. R., Dumont J., Bouchaert E., van Dijk T. H., Lucas A., et al. 2011. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 60: 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilz S., Samuel V., Morino K., Savage D., Choi C. S., Shulman G. I. 2006. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am. J. Physiol. Endocrinol. Metab. 290: E716–E722. [DOI] [PubMed] [Google Scholar]
- 52.Beil U., Crouse J. R., Einarsson K., Grundy S. M. 1982. Effects of interruption of the enterohepatic circulation of bile acids on the transport of very low density-lipoprotein triglycerides. Metabolism. 31: 438–444. [DOI] [PubMed] [Google Scholar]
- 53.Pullinger C. R., Eng C., Salen G., Shefer S., Batta A. K., Erickson S. K., Verhagen A., Rivera C. R., Mulvihill S. J., Malloy M. J., et al. 2002. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 110: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirokane H., Nakahara M., Tachibana S., Shimizu M., Sato R. 2004. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J. Biol. Chem. 279: 45685–45692. [DOI] [PubMed] [Google Scholar]
- 55.Bateson M. C., Maclean D., Evans J. R., Bouchier I. A. 1978. Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br. J. Clin. Pharmacol. 5: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller N. E., Nestel P. J. 1974. Triglyceride-lowering effect of chenodeoxycholic acid in patients with endogenous hypertriglyceridaemia. Lancet. 2: 929–931. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. 2006. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 103: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong B., Luyendyk J. P., Tawfik O., Guo G. L. 2009. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 328: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S., Wang J., Liu Q., Harnish D. C. 2009. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51: 380–388. [DOI] [PubMed] [Google Scholar]
- 60.Hanniman E. A., Lambert G., McCarthy T. C., Sinal C. J. 2005. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 46: 2595–2604. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y., Wang X., Vales C., Lee F. Y., Lee H., Lusis A. J., Edwards P. A. 2006. FXR deficiency causes reduced atherosclerosis in Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 26: 2316–2321. [DOI] [PubMed] [Google Scholar]
- 62.Guo G. L., Santamarina-Fojo S., Akiyama T. E., Amar M. J. A., Paigen B. J., Brewer B. J., Gonzalez F. J. 2006. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 1761: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flatt B., Martin R., Wang T., Mahaney P., Murphy B., Gu X., Foster P., Li J., Pircher P., Petrowski M., et al. 2009. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J. Med. Chem. 52: 904–907. [DOI] [PubMed] [Google Scholar]
- 64.Hartman H. B., Gardell S. J., Petucci C. J., Wang S., Krueger J. A., Evans M. J. 2009. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR-/- and apoE-/- mice. J. Lipid Res. 50: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mencarelli A., Renga B., Distrutti E., Fiorucci S. 2009. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 296: H272–H281. [DOI] [PubMed] [Google Scholar]
- 66.Bishop-Bailey D., Walsh D. T., Warner T. D. 2004. Expression and activation of the farnesoid X receptor in the vasculature. Proc. Natl. Acad. Sci. USA. 101: 3668–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y. T. Y., Swales K. E., Thomas G. J., Warner T. D., Bishop-Bailey D. 2007. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 27: 2606–2611. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q., He F., Kuruba R., Gao X., Wilson A., Li J., Billiar T. R., Pitt B. R., Xie W., Li S. 2008. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc. Res. 77: 560–569. [DOI] [PubMed] [Google Scholar]
- 69.Molavi B., Chen J., Mehta J. L. 2006. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am. J. Physiol. Heart Circ. Physiol. 291: H687–H693. [DOI] [PubMed] [Google Scholar]
- 70.Nakajima M., Hutchinson H. G., Fujinaga M., Hayashida W., Morishita R., Zhang L., Horiuchi M., Pratt R. E., Dzau V. J. 1995. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc. Natl. Acad. Sci. USA. 92: 10663–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savoia C., Touyz R. M., Volpe M., Schiffrin E. L. 2007. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 49: 341–346. [DOI] [PubMed] [Google Scholar]
- 72.He F., Li J., Mu Y., Kuruba R., Ma Z., Wilson A., Alber S., Jiang Y., Stevens T., Watkins S., et al. 2006. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ. Res. 98: 192–199. [DOI] [PubMed] [Google Scholar]
- 73.Rask-Madsen C., King G. L. 2007. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 3: 46–56. [DOI] [PubMed] [Google Scholar]
- 74.Li J., Wilson A., Kuruba R., Zhang Q., Gao X., He F., Zhang L., Pitt B. R., Xie W., Li S. 2008. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc. Res. 77: 169–177. [DOI] [PubMed] [Google Scholar]
- 75.Hu T., Chouinard M., Cox A. L., Sipes P., Marcelo M., Ficorilli J., Li S., Gao H., Ryan T. P., Michael M. D., et al. 2006. Farnesoid X receptor agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J. Biol. Chem. 281: 39831–39838. [DOI] [PubMed] [Google Scholar]
- 76.Li J., Wilson A., Gao X., Kuruba R., Liu Y., Poloyac S., Pitt B., Xie W., Li S. 2009. Coordinated regulation of dimethylarginine dimethylaminohydrolase-1 and cationic amino acid transporter-1 by farnesoid X receptor in mouse liver and kidney and its implication in the control of blood levels of asymmetric dimethylarginine. J. Pharmacol. Exp. Ther. 331: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kida T., Murata T., Hori M., Ozaki H. 2009. Chronic stimulation of farnesoid X receptor impairs nitric oxide sensitivity of vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 296: H195–H201. [DOI] [PubMed] [Google Scholar]
- 78.Duran-Sandoval D., Mautino G., Martin G., Percevault F., Barbier O., Fruchart J., Kuipers F., Staels B. 2004. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 53: 890–898. [DOI] [PubMed] [Google Scholar]
- 79.Duran-Sandoval D., Cariou B., Percevault F., Hennuyer N., Grefhorst A., van Dijk T. H., Gonzalez F. J., Fruchart J., Kuipers F., Staels B. 2005. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J. Biol. Chem. 280: 29971–29979. [DOI] [PubMed] [Google Scholar]
- 80.Stayrook K. R., Bramlett K. S., Savkur R. S., Ficorilli J., Cook T., Christe M. E., Michael L. F., Burris T. P. 2005. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 146: 984–991. [DOI] [PubMed] [Google Scholar]
- 81.Ma K., Saha P. K., Chan L., Moore D. D. 2006. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 116: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Fabiani E., Mitro N., Gilardi F., Caruso D., Galli G., Crestani M. 2003. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J. Biol. Chem. 278: 39124–39132. [DOI] [PubMed] [Google Scholar]
- 83.Yamagata K., Daitoku H., Shimamoto Y., Matsuzaki H., Hirota K., Ishida J., Fukamizu A. 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 279: 23158–23165. [DOI] [PubMed] [Google Scholar]
- 84.van Dijk T. H., Grefhorst A., Oosterveer M. H., Bloks V. W., Staels B., Reijngoud D., Kuipers F. 2009. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice. J. Biol. Chem. 284: 10315–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdelkarim M., Caron S., Duhem C., Prawitt J., Dumont J., Lucas A., Bouchaert E., Briand O., Brozek J., Kuipers F., et al. 2010. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways. J. Biol. Chem. 285: 36759–36767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzo G., Disante M., Mencarelli A., Renga B., Gioiello A., Pellicciari R., Fiorucci S. 2006. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol. Pharmacol. 70: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 87.Popescu I. R., Helleboid-Chapman A., Lucas A., Vandewalle B., Dumont J., Bouchaert E., Derudas B., Kerr-Conte J., Caron S., Pattou F., et al. 2010. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 584: 2845–2851. [DOI] [PubMed] [Google Scholar]
- 88.Renga B., Mencarelli A., Vavassori P., Brancaleone V., Fiorucci S. 2010. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim. Biophys. Acta. 1802: 363–372. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M., Horai Y., Houten S. M., Morimoto K., Sugizaki T., Arita E., Mataki C., Sato H., Tanigawara Y., Schoonjans K., et al. 2011. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 286: 26913–26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Ge X., Heemstra L. A., Chen W., Xu J., Smith J. L., Ma H., Kasim N., Edwards P. A., Novak C. M. 2012. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol. Endocrinol. 26: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T., Miyamoto Y., Kanatani A., Tamai Y. 2006. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol. 191: 197–205. [DOI] [PubMed] [Google Scholar]
- 92.Vassileva G., Hu W., Hoos L., Tetzloff G., Yang S., Liu L., Kang L., Davis H. R., Hedrick J. A., Lan H., et al. 2010. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J. Endocrinol. 205: 225–232. [DOI] [PubMed] [Google Scholar]
- 93.Keitel V., Ullmer C., Häussinger D. 2010. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol. Chem. 391: 785–789. [DOI] [PubMed] [Google Scholar]
- 94.Keitel V., Cupisti K., Ullmer C., Knoefel W. T., Kubitz R., Häussinger D. 2009. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 50: 861–870. [DOI] [PubMed] [Google Scholar]
- 95.Li T., Holmstrom S. R., Kir S., Umetani M., Schmidt D. R., Kliewer S. A., Mangelsdorf D. J. 2011. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol. 25: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 97.Sato H., Genet C., Strehle A., Thomas C., Lobstein A., Wagner A., Mioskowski C., Auwerx J., Saladin R. 2007. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 362: 793–798. [DOI] [PubMed] [Google Scholar]
- 98.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pols T. W. H., Nomura M., Harach T., Lo Sasso G., Oosterveer M. H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., et al. 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katsuma S., Hirasawa A., Tsujimoto G. 2005. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329: 386–390. [DOI] [PubMed] [Google Scholar]
- 101.Baggio L. L., Drucker D. J. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology. 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 102.Müssig K., Staiger H., Machicao F., Machann J., Schick F., Schäfer S. A., Claussen C. D., Holst J. J., Gallwitz B., Stefan N., et al. 2009. Preliminary report: genetic variation within the GPBAR1 gene is not associated with metabolic traits in white subjects at an increased risk for type 2 diabetes mellitus. Metabolism. 58: 1809–1811. [DOI] [PubMed] [Google Scholar]
- 103.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 104.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 105.Leiss O., von Bergmann K. 1982. Different effects of chenodeoxycholic acid and ursodeoxycholic acid on serum lipoprotein concentrations in patients with radiolucent gallstones. Scand. J. Gastroenterol. 17: 587–592. [DOI] [PubMed] [Google Scholar]
- 106.Bell G. D., Whitney B., Dowling R. H. 1972. Gallstone dissolution in man using chenodeoxycholic acid. Lancet. 2: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 107.Del Bas J. M., Ricketts M., Vaqué M., Sala E., Quesada H., Ardevol A., Salvadó M. J., Blay M., Arola L., Moore D. D., et al. 2009. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol. Nutr. Food Res. 53: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Del Bas J. M., Ricketts M. L., Baiges I., Quesada H., Ardevol A., Salvadó M. J., Pujadas G., Blay M., Arola L., Bladé C., et al. 2008. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol. Nutr. Food Res. 52: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 109.Ricketts M. L., Boekschoten M. V., Kreeft A. J., Hooiveld G. J. E. J., Moen C. J. A., Müller M., Frants R. R., Kasanmoentalib S., Post S. M., Princen H. M., et al. 2007. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 21: 1603–1616. [DOI] [PubMed] [Google Scholar]
- 110.Pellicciari R., Fiorucci S., Camaioni E., Clerici C., Costantino G., Maloney P. R., Morelli A., Parks D. J., Willson T. M. 2002. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 45: 3569–3572. [DOI] [PubMed] [Google Scholar]
- 111.Fiorucci S., Antonelli E., Rizzo G., Renga B., Mencarelli A., Riccardi L., Orlandi S., Pellicciari R., Morelli A. 2004. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 127: 1497–1512. [DOI] [PubMed] [Google Scholar]
- 112.Fiorucci S., Clerici C., Antonelli E., Orlandi S., Goodwin B., Sadeghpour B. M., Sabatino G., Russo G., Castellani D., Willson T. M., et al. 2005. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J. Pharmacol. Exp. Ther. 313: 604–612. [DOI] [PubMed] [Google Scholar]
- 113.Cipriani S., Mencarelli A., Palladino G., Fiorucci S. 2010. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 51: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., et al. 2000. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43: 2971–2974. [DOI] [PubMed] [Google Scholar]
- 115.Moschetta A., Bookout A. L., Mangelsdorf D. J. 2004. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat. Med. 10: 1352–1358. [DOI] [PubMed] [Google Scholar]
- 116.Akwabi-Ameyaw A., Bass J. Y., Caldwell R. D., Caravella J. A., Chen L., Creech K. L., Deaton D. N., Jones S. A., Kaldor I., Liu Y., et al. 2008. Conformationally constrained farnesoid X receptor (FXR) agonists: naphthoic acid-based analogs of GW 4064. Bioorg. Med. Chem. Lett. 18: 4339–4343. [DOI] [PubMed] [Google Scholar]
- 117.Bass J. Y., Caravella J. A., Chen L., Creech K. L., Deaton D. N., Madauss K. P., Marr H. B., McFadyen R. B., Miller A. B., Mills W. Y., et al. 2011. Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg. Med. Chem. Lett. 21: 1206–1213. [DOI] [PubMed] [Google Scholar]
- 118.Abel U., Schlüter T., Schulz A., Hambruch E., Steeneck C., Hornberger M., Hoffmann T., Perović-Ottstadt S., Kinzel O., Burnet M., et al. 2010. Synthesis and pharmacological validation of a novel series of non-steroidal FXR agonists. Bioorg. Med. Chem. Lett. 20: 4911–4917. [DOI] [PubMed] [Google Scholar]
- 119.Evans M. J., Mahaney P. E., Borges-Marcucci L., Lai K., Wang S., Krueger J. A., Gardell S. J., Huard C., Martinez R., Vlasuk G. P., et al. 2009. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G543–G552. [DOI] [PubMed] [Google Scholar]
- 120.Lundquist J. T., Harnish D. C., Kim C. Y., Mehlmann J. F., Unwalla R. J., Phipps K. M., Crawley M. L., Commons T., Green D. M., Xu W., et al. 2010. Improvement of physiochemical properties of the tetrahydroazepinoindole series of farnesoid X receptor (FXR) agonists: beneficial modulation of lipids in primates. J. Med. Chem. 53: 1774–1787. [DOI] [PubMed] [Google Scholar]
- 121.Nicolaou K. C., Evans R. M., Roecker A. J., Hughes R., Downes M., Pfefferkorn J. A. 2003. Discovery and optimization of non-steroidal FXR agonists from natural product-like libraries. Org. Biomol. Chem. 1: 908–920. [PubMed] [Google Scholar]
- 122.Downes M., Verdecia M. A., Roecker A. J., Hughes R., Hogenesch J. B., Kast-Woelbern H. R., Bowman M. E., Ferrer J., Anisfeld A. M., Edwards P. A., et al. 2003. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol. Cell. 11: 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soisson S. M., Parthasarathy G., Adams A. D., Sahoo S., Sitlani A., Sparrow C., Cui J., Becker J. W. 2008. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc. Natl. Acad. Sci. USA. 105: 5337–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu J., Xia C., Meier J., Li S., Hu X., Lala D. S. 2002. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol. Endocrinol. 16: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 125.Urizar N. L., Liverman A. B., Dodds D. T., Silva F. V., Ordentlich P., Yan Y., Gonzalez F. J., Heyman R. A., Mangelsdorf D. J., Moore D. D. 2002. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 296: 1703–1706. [DOI] [PubMed] [Google Scholar]
- 126.Cui J., Huang L., Zhao A., Lew J., Yu J., Sahoo S., Meinke P. T., Royo I., Pelaez F., Wright S. D. 2003. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 278: 10214–10220. [DOI] [PubMed] [Google Scholar]
- 127.Burris T. P., Montrose C., Houck K. A., Osborne H. E., Bocchinfuso W. P., Yaden B. C., Cheng C. C., Zink R. W., Barr R. J., Hepler C. D., et al. 2005. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol. Pharmacol. 67: 948–954. [DOI] [PubMed] [Google Scholar]
- 128.Singh R. B., Niaz M. A., Ghosh S. 1994. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc. Drugs Ther. 8: 659–664. [DOI] [PubMed] [Google Scholar]
- 129.Szapary P. O., Wolfe M. L., Bloedon L. T., Cucchiara A. J., DerMarderosian A. H., Cirigliano M. D., Rader D. J. 2003. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA. 290: 765–772. [DOI] [PubMed] [Google Scholar]
- 130.Mi L. Z., Devarakonda S., Harp J. M., Han Q., Pellicciari R., Willson T. M., Khorasanizadeh S., Rastinejad F. 2003. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol. Cell. 11: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 131.Pellicciari R., Gioiello A., Costantino G., Sadeghpour B. M., Rizzo G., Meyer U., Parks D. J., Entrena-Guadix A., Fiorucci S. 2006. Back door modulation of the farnesoid X receptor: design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J. Med. Chem. 49: 4208–4215. [DOI] [PubMed] [Google Scholar]
- 132.Dussault I., Beard R., Lin M., Hollister K., Chen J., Xiao J., Chandraratna R., Forman B. M. 2003. Identification of gene-selective modulators of the bile acid receptor FXR. J. Biol. Chem. 278: 7027–7033. [DOI] [PubMed] [Google Scholar]
- 133.Pols T. W. H., Noriega L. G., Nomura M., Auwerx J., Schoonjans K. 2011. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 54: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cipriani S., Mencarelli A., Chini M. G., Distrutti E., Renga B., Bifulco G., Baldelli F., Donini A., Fiorucci S. 2011. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE. 6: e25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Genet C., Strehle A., Schmidt C., Boudjelal G., Lobstein A., Schoonjans K., Souchet M., Auwerx J., Saladin R., Wagner A. 2010. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J. Med. Chem. 53: 178–190. [DOI] [PubMed] [Google Scholar]
- 136.Pellicciari R., Gioiello A., Macchiarulo A., Thomas C., Rosatelli E., Natalini B., Sardella R., Pruzanski M., Roda A., Pastorini E., et al. 2009. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52: 7958–7961. [DOI] [PubMed] [Google Scholar]
- 137.Herbert M. R., Siegel D. L., Staszewski L., Cayanan C., Banerjee U., Dhamija S., Anderson J., Fan A., Wang L., Rix P., et al. 2010. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg. Med. Chem. Lett. 20: 5718–5721. [DOI] [PubMed] [Google Scholar]
- 138.Evans K. A., Budzik B. W., Ross S. A., Wisnoski D. D., Jin J., Rivero R. A., Vimal M., Szewczyk G. R., Jayawickreme C., Moncol D. L., et al. 2009. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J. Med. Chem. 52: 7962–7965. [DOI] [PubMed] [Google Scholar]
- 139.Fumio I. 2004. Receptor antagonists. WO2004067008. [Google Scholar]
- 140.Keitel V., Donner M., Winandy S., Kubitz R., Häussinger D. 2008. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 372: 78–84. [DOI] [PubMed] [Google Scholar]
- 141.Rizzo G., Passeri D., De Franco F., Ciaccioli G., Donadio L., Rizzo G., Orlandi S., Sadeghpour B., Wang X. X., Jiang T., et al. 2010. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol. Pharmacol. 78: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baghdasaryan A., Claudel T., Gumhold J., Silbert D., Adorini L., Roda A., Vecchiotti S., Gonzalez F. J., Schoonjans K., Strazzabosco M., et al. 2011. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2-/- (Abcb4-/-) mouse cholangiopathy model by promoting biliary HCO output. Hepatology. 54: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Einarsson K., Ericsson S., Ewerth S., Reihnér E., Rudling M., Ståhlberg D., Angelin B. 1991. Bile acid sequestrants: mechanisms of action on bile acid and cholesterol metabolism. Eur. J. Clin. Pharmacol. 40(Suppl 1): S53–S58. [PubMed] [Google Scholar]
- 144.Brufau G., Stellaard F., Prado K., Bloks V. W., Jonkers E., Boverhof R., Kuipers F., Murphy E. J. 2010. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 52: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 145.Shepherd J., Packard C. J., Morgan H. G., Third J. L., Stewart J. M., Lawrie T. D. 1979. The effects of cholestyramine on high density lipoprotein metabolism. Atherosclerosis. 33: 433–444. [DOI] [PubMed] [Google Scholar]
- 146.Lipid Research Clinics Program. 1984. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 251: 351–364. [DOI] [PubMed] [Google Scholar]
- 147.Kishimoto N., Fujii S., Chiba H., Sakuma I., Tsutsui H. 2010. Colestimide, an anion exchange resin agent, can decrease the number of LDL particles without affecting their size in patients with hyperlipidemia. J. Cardiol. 55: 65–68. [DOI] [PubMed] [Google Scholar]
- 148.Rosenson R. S., Abby S. L., Jones M. R. 2009. Colesevelam HCl effects on atherogenic lipoprotein subclasses in subjects with type 2 diabetes. Atherosclerosis. 204: 342–344. [DOI] [PubMed] [Google Scholar]
- 149.Rosenson R. S. 2006. Colesevelam HCl reduces LDL particle number and increases LDL size in hypercholesterolemia. Atherosclerosis. 185: 327–330. [DOI] [PubMed] [Google Scholar]
- 150.Herrema H., Meissner M., van Dijk T. H., Brufau G., Boverhof R., Oosterveer M. H., Reijngoud D., Müller M., Stellaard F., Groen A. K., et al. 2010. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology. 51: 806–816. [DOI] [PubMed] [Google Scholar]
- 151.Taniai M., Hashimoto E., Tobari M., Yatsuji S., Haruta I., Tokushige K., Shiratori K. 2009. Treatment of nonalcoholic steatohepatitis with colestimide. Hepatol. Res. 39: 685–693. [DOI] [PubMed] [Google Scholar]
- 152.Yamakawa T., Takano T., Utsunomiya H., Kadonosono K., Okamura A. 2007. Effect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemia. Endocr. J. 54: 53–58. [DOI] [PubMed] [Google Scholar]
- 153.Zieve F. J., Kalin M. F., Schwartz S. L., Jones M. R., Bailey W. L. 2007. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin. Ther. 29: 74–83. [DOI] [PubMed] [Google Scholar]
- 154.Garg A., Grundy S. M. 1994. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann. Intern. Med. 121: 416–422. [DOI] [PubMed] [Google Scholar]
- 155.Bays H. E. 2011. Colesevelam hydrochloride added to background metformin therapy in patients with type 2 diabetes mellitus: a pooled analysis from 3 clinical studies. Endocr. Pract. 17: 933–938. [DOI] [PubMed] [Google Scholar]
- 156.Fonseca V. A., Rosenstock J., Wang A. C., Truitt K. E., Jones M. R. 2008. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 31: 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goldberg R. B., Fonseca V. A., Truitt K. E., Jones M. R. 2008. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch. Intern. Med. 168: 1531–1540. [DOI] [PubMed] [Google Scholar]
- 158.Henry R. R., Aroda V. R., Mudaliar S., Garvey W. T., Chou H. S., Jones M. R. 2012. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 14: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Handelsman Y., Goldberg R. B., Garvey W. T., Fonseca V. A., Rosenstock J., Jones M. R., Lai Y., Jin X., Misir S., Nagendran S., et al. 2010. Colesevelam hydrochloride to treat hypercholesterolemia and improve glycemia in prediabetes: a randomized, prospective study. Endocr. Pract. 16: 617–628. [DOI] [PubMed] [Google Scholar]
- 160.Vega G. L., Dunn F. L., Grundy S. M. 2011. Effect of colesevelam hydrochloride on glycemia and insulin sensitivity in men with the metabolic syndrome. Am. J. Cardiol. 108: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 161.Schwartz S. L., Lai Y., Xu J., Abby S. L., Misir S., Jones M. R., Nagendran S. 2010. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab. Syndr. Relat. Disord. 8: 179–188. [DOI] [PubMed] [Google Scholar]
- 162.Suzuki T., Oba K., Igari Y., Matsumura N., Watanabe K., Futami-Suda S., Yasuoka H., Ouchi M., Suzuki K., Kigawa Y., et al. 2007. Colestimide lowers plasma glucose levels and increases plasma glucagon-like PEPTIDE-1 (7–36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J. Nihon Med. Sch. 74: 338–343. [DOI] [PubMed] [Google Scholar]
- 163.Beysen C., Murphy E. J., Deines K., Chan M., Tsang E., Glass A., Turner S. M., Protasio J., Riiff T., Hellerstein M. K. 2012. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 55: 432–442. [DOI] [PubMed] [Google Scholar]
- 164.Shang Q., Saumoy M., Holst J. J., Salen G., Xu G. 2010. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 298: G419–G424. [DOI] [PubMed] [Google Scholar]
- 165.Chen L., McNulty J., Anderson D., Liu Y., Nystrom C., Bullard S., Collins J., Handlon A. L., Klein R., Grimes A., et al. 2010. Cholestyramine reverses hyperglycemia and enhances GLP-1 release in Zucker diabetic fatty rats. J. Pharmacol. Exp. Ther. 334: 164–170. [DOI] [PubMed] [Google Scholar]
- 166.Meissner M., Herrema H., van Dijk T. H., Gerding A., Havinga R., Boer T., Müller M., Reijngoud D., Groen A. K., Kuipers F. 2011. Bile acid sequestration reduces plasma glucose levels in db/db mice by increasing its metabolic clearance rate. PLoS ONE. 6: e24564. [DOI] [PMC free article] [PubMed] [Google Scholar]