Abstract
In animal cells, the primary repositories of esterified fatty acids and alcohols (neutral lipids) are lipid droplets that form on the lumenal and/or cytoplasmic side of the endoplasmic reticulum (ER) membrane. A monolayer of amphipathic lipids, intermeshed with key proteins, serves to solubilize neutral lipids as they are synthesized and desorbed. In specialized cells, mobilization of the lipid cargo for delivery to other tissues occurs by secretion of lipoproteins into the plasma compartment. Serum lipoprotein assembly requires an obligate structural protein anchor (apolipoprotein B) and a dedicated chaperone, microsomal triglyceride transfer protein. By contrast, lipid droplets that form on the cytoplasmic face of the ER lack an obligate protein scaffold or any required chaperone/lipid transfer protein. Mobilization of neutral lipids from the cytosol requires regulated hydrolysis followed by transfer of the products to different organelles or export from cells. Several proteins play a key role in controlling droplet number, stability, and catabolism; however, it is our premise that their formation initiates spontaneously, solely as a consequence of neutral lipid synthesis. This default pathway directs droplets into the cytoplasm where they accumulate in many lipid disorders.
Keywords: apolipoproteins, fatty acid, lipid droplets, diacylglycerol acyltransferase
Lipids are critical determinants of membrane integrity, important sources of energy, and in some cells, substrates for the synthesis of hormones. Endogenous lipid synthesis consumes significant chemical energy and is therefore tightly regulated and coordinated with frugal transport processes to assimilate them from the environment and/or store them safely. Lipids enter the cytoplasm as acids (free fatty acids) or alcohols (e.g., free cholesterol). High concentrations of free fatty acids and sterols are injurious to cells, whereas alcohols such as diacylglycerol are bioactive at low concentrations as signaling molecules. Consequently, efficient systems have evolved to limit their concentrations but retain their availability by coesterification of the acids and alcohols into neutral lipids.
Neutral lipids confer several selective advantages to the cell and organism in which they reside. They provide a conduit for detoxification of free fatty acids and a key reservoir of membrane components and energy. Esterification of sterols with FA to form steryl esters (SE) [e.g., cholesteryl ester (CE)] provides for future membrane rebuilding and remodeling. Triacylglycerol (TG), a fatty acyl ester derivative of glycerol, represents the major energy depot of all eukaryotic and some bacterial cells. The energy of complete oxidation of the alkyl chains of TG (38 KJ/g) is more than twice the same weight of carbohydrate or protein, and unlike polysaccharide, TG carries no extra weight as water of solvation. Similarly, esterified long- and short-chain alcohols, such as wax esters and diesters, form an important water repellant permeability barrier in the skin and fur of mammals and the cuticle of plants.
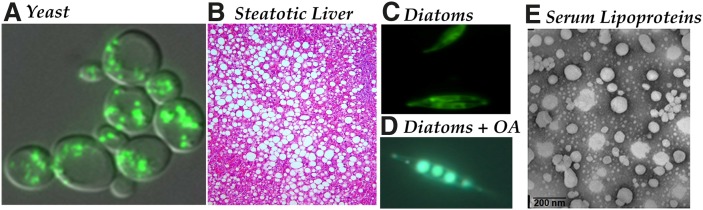
The majority of neutral lipid synthesis is completed at the endoplasmic reticulum (ER). However, neutral lipid synthesis poses two problems: neutral lipids have limited solubility in the ER membrane bilayer, and they are immiscible with the hydrophilic intracellular environment. These quandaries are solved by lipid sequestration into cytoplasmic lipid droplets (CLD), a process that nullifies any impact on the osmolarity of the cytosol. The formation of CLDs is a phenomenon common to all eukaryotes (for examples, see Fig. 1) that has been maintained for over 2 billion years of evolution, presumably to confer protection against toxic free fatty acids and sterols.
Fig.1.
Lipid droplets are ubiquitous. All eukaryotes produce and often accumulate neutral lipids as cytoplasmic or secreted particles. A: In budding yeast (Saccharomyces cerevisiae), lipid droplets (envisaged here by fluorescence staining with Nile red) accumulate as cells approach stationary phase. B: The accumulation of fatty deposits in the liver (oil red O staining; Liu and Sturley, unpublished data) is the pathological determinant of steatosis and nonalcoholic fatty liver disease (NAFLD). C, D: Lipid deposition in algae (e.g., diatoms stained with Nile red grown in media lacking and containing free fatty acids, respectively; Ruggles and Sturley, unpublished data) form the basis of our oil reserve. E: A similar structure, triglyceride-rich lipoproteins (negative staining, electron micrograph; Iqbal and Hussain, unpublished data), such as very low density lipoproteins, form in the ER lumen and accumulate in plasma of many patients at risk for coronary artery disease. OA, oleic acid.
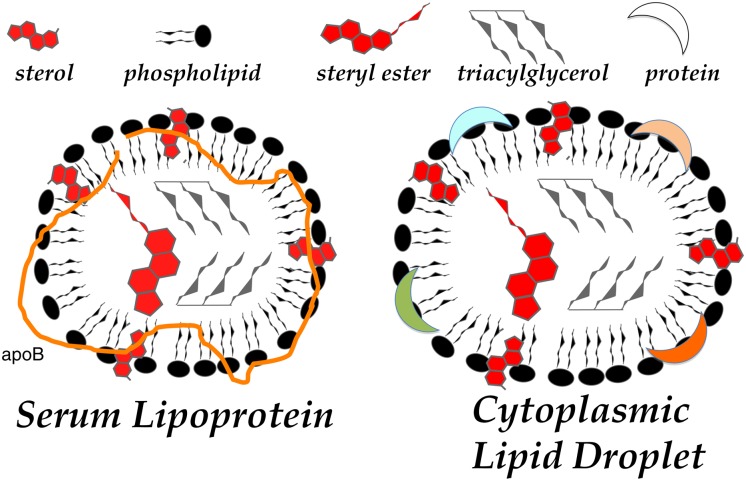
In metazoans with circulatory systems, neutral lipids are actively transported between organs, from the sites of dietary acquisition (i.e., the intestine) to distribution (e.g., the liver) for either storage (e.g., adipose) or catabolism (e.g., skeletal and cardiac muscle cells) via emulsion particles called “lipoproteins.” Intracellular assembly of neutral lipid-rich lipoproteins begins in the lumen of the ER of specialized cells. These lipoprotein particles exhibit a marked resemblance to the aforementioned CLDs (Fig. 1). Indeed, lipid droplets and lipoprotein particles both have a neutral lipid core surrounded by a phospholipid/sterol monolayer into which key proteins are interdigitated (Fig. 2). The role of these proteins and the means by which they are incorporated into the particle has been carefully scrutinized with significantly more success with respect to lipoprotein assembly than with CLDs. Lipoprotein formation requires an obligate scaffold of related proteins [lipophorins, vitellogenins, or in mammals, apolipoprotein B (apoB)] and a dedicated chaperone [microsomal triglyceride transfer protein (MTP)]. Interestingly, an obligate scaffolding protein or chaperone has yet to be identified for CLD biogenesis.
Fig.2.
Cytoplasmic lipid droplet and serum lipoprotein structures. A commonality to cytoplasmic and serum lipid carriers that reflects their shared origin is the membrane of the endoplasmic reticulum. Both particles bud from the ER membranes as neutral lipid is synthesized and approaches insolubility in the membrane. Insolubility is overcome by micellar association with phospholipids and, to a lesser extent, sterols, from the ER and proteins, such as apoB (shown), acyltransferases, lipases, or perilipins (generically indicated by crescents).
In 1892, in a series of lectures at Columbia University, NY, E. B. Wilson characterized cytoplasmic lipid droplets as “lifeless bodies (metaplasm) suspended in the cytoplasmic reticulum” (1). We now have a different perspective; this organelle is by no criteria inert or innocuous. The pathophysiological accumulation of neutral lipids, both within cells and in serum, is a clear harbinger of ill-health. In terms of health risk in Western societies, elevated cellular deposition of neutral lipid, such as CE in macrophages and smooth muscle cells or TG in adipocytes, typifies the twin “epidemics” of atherosclerosis and obesity, respectively. Similarly, elevated plasma levels of CE or TG in low-density lipoprotein particles represent independent risk factors for atherosclerosis. Moreover, as specialized fat storage tissues become saturated beyond capacity, other tissues start storing lipids, leading to diseases such as diabetes and insulin resistance due to lipotoxicity at pancreatic β-cells and hepatocytes/myocytes. The fate of excess lipids is thus a critical component of lipid homeostasis that provokes some of the most severe diseases confronting our society.
Here we present our perspective that the thermodynamic properties of neutral lipids suffice to promote droplet formation and that the evolutionary constraint of lipotoxicity then promoted stabilization and regulated catabolism of the CLD. This process was likely coopted by certain animal cells to create circulatory lipoproteins for organ-to-organ lipid distribution and hydrolysis. The major purpose of CLDs and lipoprotein particles is to retain or release their cargo in response to cellular need. Triacylglycerols and cholesteryl esters represent valuable commodities that are lipolyzed by highly regulated pathways involving phosphorylation/dephosphorylation of several key lipases and their cofactors. The breakdown products are then transported by poorly characterized mechanisms most likely involving protein-facilitated processes. By contrast, specialized tissues such as intestine and liver have evolved a sophisticated system to mobilize neutral lipids en masse by the intracellular assembly of lipoproteins.
NEUTRAL LIPID SYNTHESIS IS COMPLETED AT THE ENDOPLASMIC RETICULUM MEMBRANE
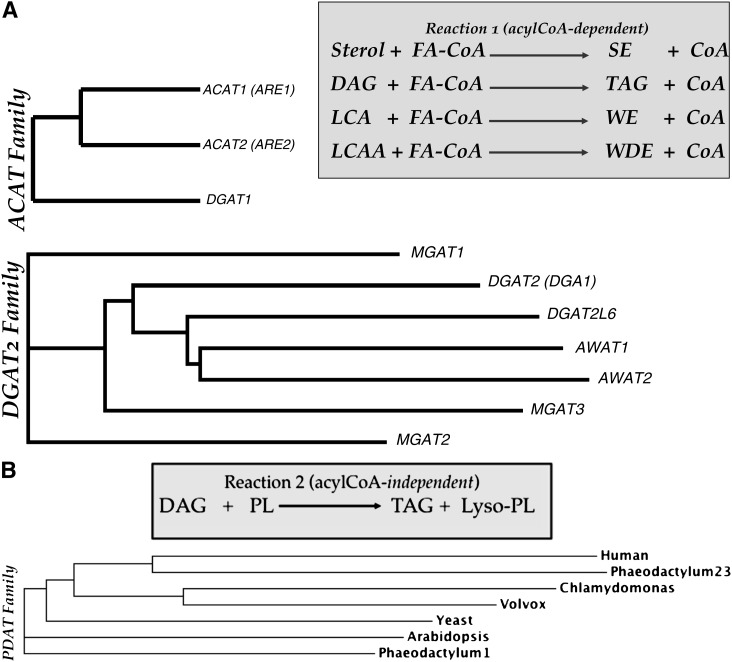
Numerous enzymatic reactions located in several organelles direct the incorporation of acetate into neutral lipids; however, the terminal and committed step of conjugation of alcohols (e.g., diacylglycerol and sterols) with free fatty acids is primarily performed at the ER membrane. Metabolic labeling studies describe the appearance of newly synthesized TG on both lumenal and cytosolic sides of the membrane (2). To date, three distinct acyltransferase gene families have been identified that accomplish the esterification reactions (Fig. 3) (3). The esterification of sterol is mediated by members of the acyl-CoA cholesterol acyltransferase (ACAT) family. ACAT1-mediated esterification is ubiquitous, whereas expression of ACAT2 is restricted to lipogenic tissues such as liver and intestine. Diacylglycerol (DAG) esterification using an acyl-CoA donor is primarily the activity of DGAT1 (also a member of the ACAT family based on sequence conservation) or DGAT2 (a member of the evolutionarily unrelated diacylglycerol acyltransferase 2 gene family). DGAT2 mRNA expression is highest in the liver and adipose tissue, whereas DGAT1 mRNA expression is relatively low in these tissues (4). Loss of DGAT2 in induced mutant mice results in a failure to thrive that is not compensated by expression of DGAT1, suggesting that the DGAT2 enzyme is responsible for the majority of TG synthesis (4). Further, combined deletion of DGAT1 and DGAT2 does not obviate TG synthesis in murine cells, indicating the presence of alternative DAG esterification pathways (5). In humans, the DGAT2 gene family is complex, comprising six additional members, all of which demonstrably synthesize TG in vitro (6, 7). Within each gene family, it is likely that the multiple gene duplication events have facilitated divergence of active sites and thus alternative substrate preferences. Indeed, members of the DGAT2 family of TG synthases also direct esterification of monoacylglycerol (8–10), long-chain alcohols (6), and long-chain dialcohols (Turkish et al., unpublished data). The latter two reactions are particularly important in the mammalian sebocyte and meibocyte, where wax monoesters and diesters comprise key components of the epidermal and corneal permeability barrier. Similarly, the cuticle of insects and plants is neutral lipid rich; accordingly, the genomes of many model systems (e.g., Drosophila, Arabidopsis, and Nicotiana) predict orthologs that encode these reactions (12).
Fig.3.
Gene families involved in terminal steps of neutral lipid biosynthesis. A: Acyl-CoA-dependent acyltransferases. Two unrelated gene families (ACAT and DGAT2) encode acyltransferases with similar activities that transfer the acyl moiety of acyl-CoA. B: Acyl-CoA-independent acyltransferases. In yeast and likely mammalian cells, triglyceride biosynthesis proceeds in the absence of members of the ACAT and DGAT2 gene families. Members of the PDAT gene family perform this reaction independently of acyl-CoA by using phospholipids, such as phosphatidylcholine, as acyl donors. FA-CoA, fatty acyl CoEnzyme A; SE, steryl ester; CoA, CoEnzyme A; DAG, diacylglycerol; TAG, triacylglycerol; LCA, long chain alcohol; WE, wax ester; LCAA, long chain dialcohol; WDE, wax diester, PL, phospholipid. Gene symbols represent establish nomenclature for human acyltransferases and yeast orthologs (in parentheses).
In model systems ranging from the unicellular eukaryote Saccharomyces cerevisiae to multicellular systems, such as nematodes and insects, ACAT-related enzymes (in yeast, Are1p and Are2p) are responsible for the esterification of sterols (13–16). Diacylglycerol esterification is mediated by members of the DGAT2 gene family [in yeast, the DGA1 gene product (15)] and DGAT1 of the ACAT family (Fig. 3A). Additionally, yeast, algae, and green plants use an acyl-CoA-independent, phospholipid diacylglycerol acyltransferase (PDAT, encoded by the LRO1 gene in yeast) reaction that derives the acyl group from phospholipids for esterification of diacylglycerol (Fig. 3B) (17). PDAT gene family members (Fig. 3B) are typified by the mammalian lecithin cholesterol acyltransferase (LCAT) enzyme, the predominant product of which is cholesteryl ester. This reaction resides primarily in the plasma compartment and uses lipoprotein-associated sterols as substrates. The mammalian LCAT enzyme also directs the production of TG in vitro (18), although its contribution to TG levels in vivo is undetermined. By contrast, the major physiological role of fungal and plant PDATs (17, 19) is to synthesize triacylglycerol from DAG and phospholipids as an alternative acyl-donor. Thus, neutral lipid synthesis in various organisms occurs in the ER by various enzymes belonging to three distinct gene families. The precise role of these individual enzymes remains to be determined. Particularly, why have so many genes (at least 12 genes and three distinct reactions in humans) evolved for the same step in neutral lipid synthesis?
IS NEUTRAL LIPID DEPOSITION SPONTANEOUS OR PROTEIN-MEDIATED?
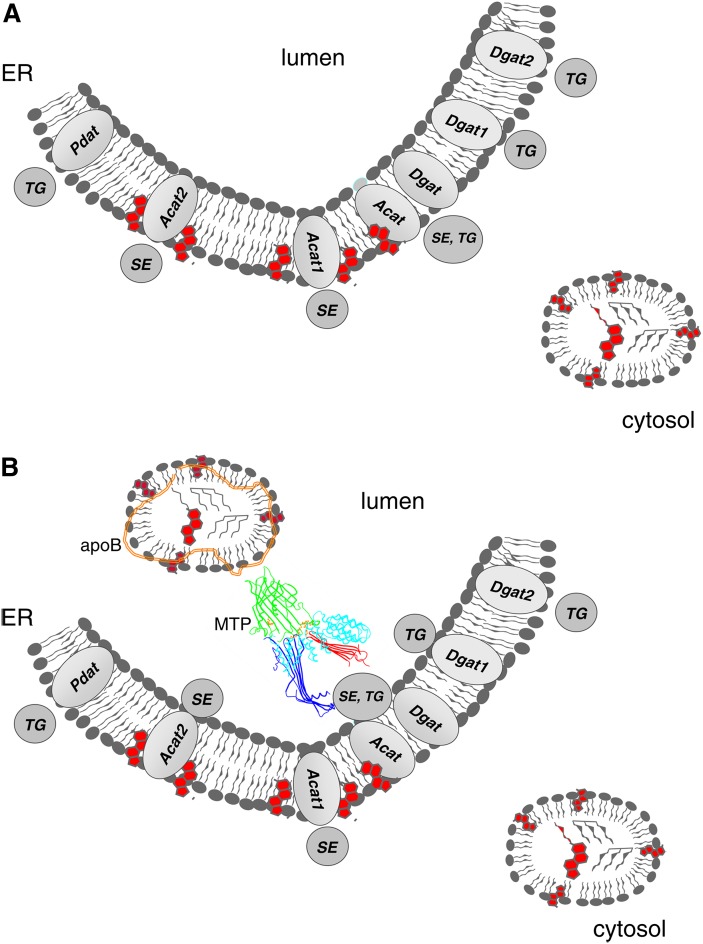
Subsequent to synthesis, neutral lipids are rapidly and efficiently deposited into cytoplasmic lipid droplets (CLD) in all cells (Fig. 4A) and into lipoprotein particles in specialized cells, such as hepatocytes, enterocytes, and cardiomyocytes (Fig. 4B). It remains to be determined whether CLD formation is a spontaneous process that is stabilized or whether proteins are needed to assemble them immediately after neutral lipids are synthesized. The biophysical and thermodynamic properties of neutral lipids have prompted the suggestion that an oil droplet spontaneously forms as a “lens” in the ER bilayer. Alternatively, the neutral lipid may diffuse freely throughout the ER membrane until it reaches a critical concentration that requires an amphipathic shell of phospholipids for solubility. This process in itself likely deforms the membrane of the ER, and as a consequence, the droplets “bud” from the organelle. This “bilayer to emulsion” transition was first proposed by Donald Small (20) to be the initiating step in the biogenesis of neutral lipid-rich serum lipoproteins. Transport of neutral lipids into the ER lumen is dependent on MTP activity and the presence of a scaffold such as apolipoprotein B (21). MTP acts as both a chaperone by physically interacting with nascent apoB, as well as a lipid transfer protein that loads lipids onto the nascent apoB polypeptide. The MTP reaction catalyzes transfer of several lipids but is functionally pertinent because of its ability to transfer neutral lipids. It has been suggested that MTP can also help in the formation of lumenal lipid droplets that lack apoB (21, 22). This function could be related to its ability to interact with lipid vesicles as well as its ability to transfer lipids (23). Thus, MTP activity creates a concentration gradient that is essential for transferring neutral lipids into the lumen of the ER that is then further stabilized by apoB for secretion. The directionality of this process is reversible, in that liver-specific genetic ablation or chemical inhibition of MTP impedes VLDL formation and enhances accumulation of CLDs in the cytoplasm, resulting in hepatic steatosis (24).
Fig.4.
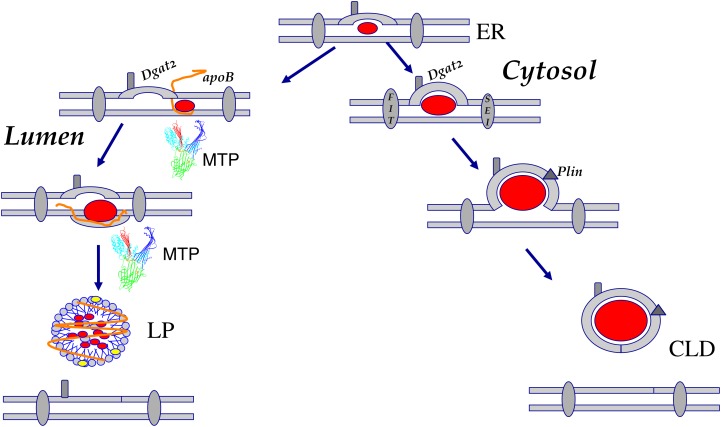
A: Deposition of neutral lipids into the cytosol. In all eukaryotic cells and some bacteria, neutral lipids are deposited into the cytoplasm as phopholipid monolayers surrounding an insoluble core of oil. In mammalian cells and most other eukaryotes, the only necessary factor appears to be neutral lipid biosynthetic reactions, such as the DGAT and PDAT reactions. B: Deposition of neutral lipids into the vesicular secretion pathway. In certain specialized tissues and cells (e.g., hepatocytes, enterocytes), neutral lipids are actively packaged into lipoprotein particles by chaperones and are ultimately secreted. Acyltransferases (Pdat, Acat1, Acat2, Dgat1, Dgat2), lumenal transfer/anchor proteins (MTP and apoB), and neutral lipids (SE and TG) are as described in the text. In some instances, the lipid core of the lipid particle is heterogeneous and of obscure origin in terms of acyltransferase isoform (which is depicted without a numerical assignment).
In contrast to lipoprotein formation at the lumenal face of the ER, the deposition of neutral lipids in the cytoplasm is more obscure but more prevalent. Neutral lipid accumulation drives a “bilayer to emulsion” transition (20) that forces the formation of the CLD (25, 26). Other models propose that neutral lipid may leave the ER through a pore as it coalesces. Alternatively, a preexisting shell of phospholipid associates with the ER and is then filled with neutral lipid as it is synthesized (25). None of these models are mutually exclusive; certainly no “smoking gun” to any of them is currently available.
ROLE OF NEUTRAL LIPID SYNTHESIZING ENZYMES IN LIPID DROPLET FORMATION
Surprisingly, very little is known about the role of the neutral lipid biosynthetic reactions in formation of the oil droplet in eukaryotes. The reaction is definitely required; strains harboring deletions of all four acyltransferase genes (are1Δ, are2Δ, dga1Δ, and lro1Δ) are viable but completely lack neutral lipids or CLDs (27). Loss of the CLD compartment in such mutant strains results in marked sensitivity to excess fatty acids, a common phenomenon known as lipotoxicity (27). Similarly, loss of murine DGAT1 and DGAT2 markedly diminishes cellular CLD content (5). Importantly, this demonstrates the necessity of neutral lipid synthesis for CLD formation. Unlike, for example, peroxisome biogenesis (28), there is no detectable “CLD phospholipid ghost” awaiting neutral lipid deposition. Whether neutral lipid synthesis is sufficient for CLD formation remains to be determined; it clearly is necessary.
The majority (>90%) of the neutral lipid cargo in CLDs and mammalian lipoproteins is TG. Diacylglycerol esterification at the ER is predominantly acyl-CoA-dependent, directed by DGAT1 (13) and DGAT2 (6). In 1997, Zammit and coworkers (29) described two classes of DGAT activities, latent and overt, perhaps reflecting the disposition of the active sites of these enzymes. Overt activity was proposed to represent enzymes with their active sites facing the cytoplasm, whereas latent activity faced toward the lumen of the ER. This activity was recently implicated as arising from two populations of DGAT1 with alternate topologies with respect to active site orientation (30). This might suggest that different sources of TG are used for CLD and lipoprotein formation, however, it is also clear that TG from CLDs is incorporated into lipoproteins after liberation of fatty acids from the CLDs and resynthesis of TG at the ER (31). Therefore, whether TG is synthesized by overt or latent DGAT activity, it is ultimately incorporated with nascent lipoproteins. Similarly, the identification of the mammalian ACAT2 enzyme (14, 32, 33) and subsequent elucidation of its expression profile and active site topology has prompted models in which the individual ACAT isoforms are hypothesized to form steryl esters on either side of the ER. A similar model for TG deposition into the lumen of the ER of yeast was suggested when the active site of Lro1p, the sole yeast PDAT, was oriented to the lumenal side of the ER membrane (34). However, the physiological relevance of this observation is unclear given the absence of secreted neutral lipid in yeast.
An integral role of specific acyltransferases in CLD and lipoprotein assembly has been further implicated by unexpected observations regarding viral particle biogenesis. The subcellular hydrophobic environment provided by neutral lipids has been hijacked for hepatitis C virus propagation and secretion. Hepatitis C virus assembly and its secretion requires lipoprotein biogenesis and intact DGAT1 activity (35). In addition, the hepatitis C virus is physically associated with the CLD compartment. To explain the effects of hepatitis C virus on both lumenal and cytosolic lipid droplet metabolism, we propose that hepatitis C virus associates with lipids in the ER membrane where it becomes part of apoB lipoproteins as they assemble. When apoB lipoproteins are desorbed from the ER membrane, the virus becomes part of these lipoproteins and follows their secretory pathway. However, when apoB lipoprotein assembly is inhibited or when viral replication exceeds apoB lipoprotein assembly, then the virus becomes associated with CLDs as part of the default mechanism that helps in the formation of cytosolic lipid droplets. This process prompts core steatosis, i.e., fatty liver that is associated with rampant viral infection (36). An issue with this scenario is the observation that hepatitis C virus infection is associated with reduced MTP activity (37). This observation has been explained by hypothesizing that in the early stages of viral infection there might be an increase in MTP activity (21, 38). During later stages of infection, the virus might inhibit MTP to become part of CLDs in order to defy immune surveillance.
The synthesis of any neutral lipid at the ER and subsequent incorporation into lumenal lipoproteins and/or cytoplasmic lipid droplets can be explained by invoking a model similar to that proposed for the synthesis of cholesteryl esters (39). In this model, generation of cholesteryl ester and, by analogy, synthesis of TG and other neutral lipids occur at the plane of the membrane, and the products are sequestered within the bilayer. However, neutral lipids have limited solubility in the membrane (20), and when concentrations surpass solubility limits, lipid droplets likely bud toward either side of the bilayer, perhaps at random. The presence of MTP within the ER forces an equilibrium toward the secretion pathway by formation of nascent apoB lipoproteins or lipid droplets that can ultimately fuse with primordial apoB lipoproteins for secretion. On the cytosolic side, the number and size of CLDs are likely modulated by proteins present in the cytosol or acquired from the ER membrane.
THE LIPOPROTEIN PARTICLE PROTEOME
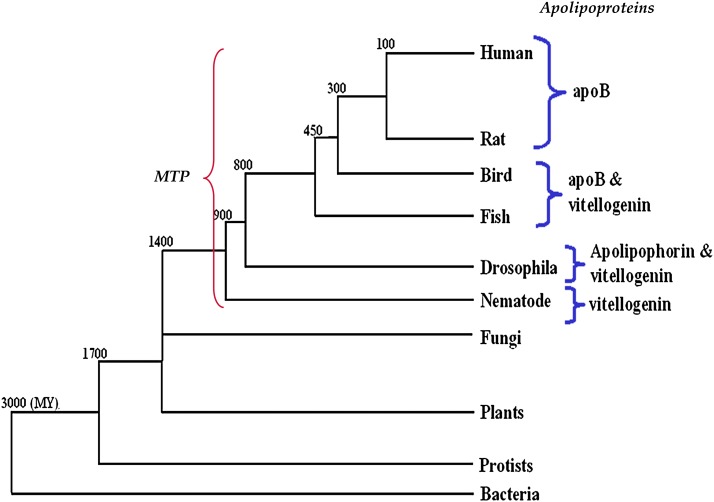
Lipoprotein particles in plasma have a well-characterized protein composition; numerous proteins exchange between and within lipoprotein classes mediating cell uptake, lipid exchange, and lipid hydrolysis (40). Many of these exchangeable factors can be detected in the ER lumen where they often decorate the lipoprotein particles; however, there is only one nonexchangeable mammalian protein, apoB, which tracks with TG/CE-rich chylomicrons, very low and low-density lipoproteins (VLDL and LDL). Several proteins in other animals have similarly coevolved with this pathway, such as lipohorin III and vitellogenin. Together, these proteins represent a metazoan family of large lipid transfer proteins (LLTP) (41) that are unequivocally required for lipoprotein formation in the ER (Fig. 5). It has been hypothesized that MTP might be the founder member of this family. LLTPs are typified by a conserved large lipid transfer (LLT) domain, which is commonly present at the NH2-terminal ∼900 residues and includes paired amphipathic β-sheets as a lipid binding pocket (41).
Fig.5.
Evolution of large lipid transfer proteins. Members of large lipid transfer proteins, such as MTP, apoB, and vitellogenin, share sequence homology. Sequence relationships depict a coevolution that correlates to the appearance of circulatory systems in insects and nematodes. Note that MTP is present in all organisms and is perhaps the first protein to evolve. Throughout evolution, different lipid carrier proteins have arisen, including vitellogenin, apolipophorin, and apoB. Approximate time of divergence is shown at break points. Modified from Ref. 71.
The translocation of apoB, the obligate scaffold of chylomicrons, and VLDL and LDL particles into the ER represents a key decision point in terms of secretion of TG. In the absence of ongoing TG synthesis, apoB is rapidly degraded as a consequence of an interaction with components of the translocon, ER chaperones and the cytosolic proteasome. In observations that suggest apoB accesses the cytosol during its lifetime, a crescent structure that is immunopositive for apoB surrounds the CLD, most notably in lipid- or MTP-limiting conditions or where the proteasome has been inhibited (42). The apoB crescent likely represents an intermediate or pause in apoB degradation, the CLD providing a hydrophobic solubilizing lipid core such that apoB is not retrieved by the retrotranslocon and thus continues on its path of destruction.
THE CLD PROTEOME
The profile of proteins associated with the CLD is complex and surprisingly well conserved across genera. The structure clearly is complex and highly ordered as predicted by liquid crystal polarizing microscopy which depicts a highly birefringent shell to the lipid droplet structure (Fig. 6). The lipid droplet proteome (“adiposome”) (43) has been defined across organisms as diverse as Chinese hamster ovary cells, human 3T3-L1 adipocytes, algae and yeast. This has revealed a surprisingly well-conserved array of CLD-associated proteins, including enzymes of lipid metabolism, regulators and mediators of protein transport (SNARES, RABs), signaling molecules, structural proteins, and proteins commonly associated with caveolae membrane rafts (43–45).
Fig.6.
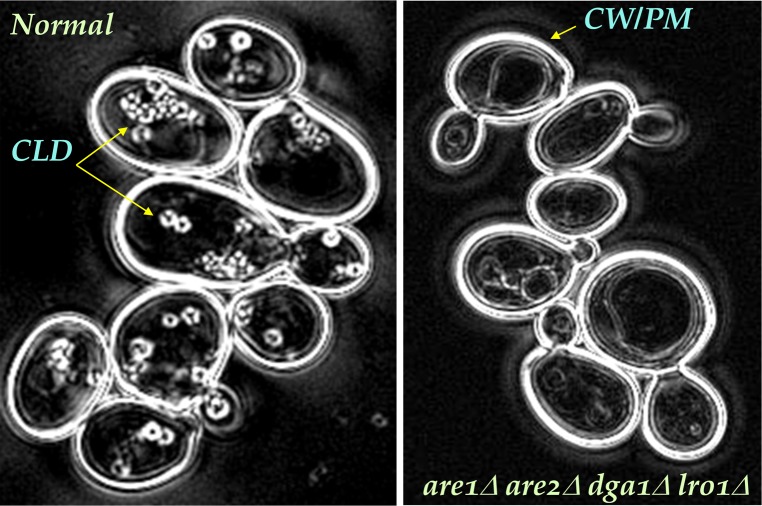
Polarizing light microscopy of yeast strains. Budding yeast imaged with the liquid crystal polarizing microscope (LC-PolScope, Caliper Life Sciences). Cells are surrounded by a highly birefringent cell wall, shown in white in these retardance images. Inside cells, the highly birefringent shell of the lipid droplets (blue arrows) are also visible. In acyltransferase (neutral lipid-deficient, mutant cells shown on the right), lipid droplets are absent. Unlike traditional polarized light microscopes, the so-called retardance image generated by the LC-PolScope represents birefringent structures in shades of gray that are proportional to the amount of birefringence, irrespective of the orientation of the structure. The technical for the amount of birefringence measured by the LC-PolScope is retardance and is expressed as a distance. In these images, white corresponds to 3 nm retardance, medium gray is 1.5 nm retardance. The more that highly ordered lipids and embedded membrane proteins surround the lipid droplets, the more retardance is measured. Images provided by Rudolf Oldenbourg, Marine Biology Laboratory, Woods Hole, MA.
The role of the majority of the CLD proteome in CLD formation, stability, or metabolism remains to be determined. In marked contrast to lumenal lipoprotein particles, an obligate role for a scaffold has yet to be defined for any CLD protein. The majority of CLDs proteins derive from the ER and are lacking in true transmembrane domains (TMD), although most are hydrophobic. This is consistent with the origin of the CLD; “oiling out” of the ER lipid bilayers likely coopts a subset of ER proteins. Currently, there is no algorithm that predicts which proteins locate to the droplet; no common targeting motif has been identified, although the “transmembrane” regions of some CLD proteins have been characterized as necessary and sufficient for CLD association (46). Indeed, the neutral lipid hydrophobic core of lipid droplets has been suggested to represent a holding station for misfolded proteins, preventing their aggregation and thus safeguarding the cell. Several amyloid proteins likely follow this route, for example, αSynuclein (47). It is plausible that the CLD represents a key intermediary in ER-associated degradation (ERAD) of proteins (48). Some components of the CLD proteome may be artifactual in that they merely reflect an improved solubility of certain proteins in this oil-like environment, as opposed to any functional role.
Within the background associated with all proteomic studies, several abundant CLD proteins have been shown to participate in CLD function. These authentic CLD proteins include members of the cell death-inducing DFF45-like effector (CIDE) family (44, 49) [e.g., fat-specific protein 27 (FSP27) or CIDEC], which has been implicated as a fusogen (50), as well as several components of sterol biosynthesis [in yeast, at least four ERG genes (51)], acyl-CoA metabolism [e.g., acyl-CoA synthetase 3 (52)], and TG biosynthesis [e.g., members of the DGAT2 gene family in yeast (53) and murine cell lines (46, 54)]. In the adipocyte, the interactions of several highly abundant “structural” proteins, perilipins (55), are critical to the integrity and catabolism of CLDs (56), to the extent that deletion of the Perilipin 1 gene produces animals with reduced adiposity and resistance to dietary-induced obesity (57). A family of related proteins [collectively termed PAT proteins and recently enumerated as perilipin (PLIN) 1 through 5 (58)] have been characterized extensively with regard to their structure and function. An obligate role for the PLIN proteins in CLD formation is unfounded; however, they are the major players in the stability and consequently integrity of the CLD compartment of mammalian adipocytes.
All eukaryotic cells make CLDs and must regulate the lipid content of the droplet by lipolysis; otherwise, the utility of this compartment becomes meaningless. Enzymes involved in neutral lipid hydrolysis are commonly physically associated with the CLD (53, 54). A continuum of CLDs in of size and content reflects an activated or quiescent state that is primed or resistant, respectively, to hydrolysis. In mammals, “priming” by a phosphorylation cascade activates PLIN proteins and hormone-sensitive lipase. This mechanism is neither conserved nor ubiquitous; nevertheless, neutral lipid hydrolysis at the CLD remains a key determinant of the net neutral lipid content of all cells. For example, a primary event in CLD-associated TG hydrolysis is mediated by PNPLA2 (ATGL) or its orthologs, which are lipid droplet proteins in mammals (59), plants, algae, and yeast. It remains to be determined whether these proteins are also obliged to serve as CLD scaffolds, in addition to their roles in providing or hydrolyzing neutral lipids.
GENETIC CONTROL OF LIPID DROPLET STATUS
Although spontaneous deposition of neutral lipids into the cytoplasm can occur, it is likely that this is a highly regulated process. This premise is based on the observation that the only defect that nullifies this compartment is the complete loss of neutral lipid biosynthesis, although numerous factors appear to modulate the number, size, and distribution of CLDs.
Most CLD-associated proteins function in their catabolism, suggesting that the majority of these particles are undergoing catabolism. Numerous approaches to define the genetic control of lipid droplet composition have been undertaken. A set of fat storage-inducing transmembrane (FIT) proteins was initially identified as highly induced by fibrates in a PPARα-dependent manner (60). Subsequently these proteins, particularly FIT2, were shown to positively modulate CLD number and bind TG (61). The FIT proteins are not associated with the CLD but are residents of the ER.
Some advances have been made in model organisms, such as fruit flies or nematodes, to understand the processes that lead to adiposity. For example, loss of adp, a mutation in an evolutionary conserved WD40/tetratricopeptide-repeat-domain protein, (62) and proteins related to the Tubby transcription factors (63) confer adiposity in Drosophila. Similarly, a genome-wide ablation RNAi scan of genes involved in fat accumulation in Caenorhabditis elegans identified numerous loci that either promoted or reduced neutral lipid accumulation (64). In other instances, morphometric screens in yeast (65–67) or insect cells (68, 69) using fluorescent reporters of lipid droplet composition have been applied with great success but, surprisingly, little concordance. A common finding to many of these screens was the identification of the seipins, BSCL2 in mammals and FLD1 in yeast (reviewed in Ref. 70). Loss-of-function mutations in seipin account for a severe form of congenital lipodystrophy (Berardinelli-Seip congenital lipodystrophy) and striking changes in lipid droplet size, number, and distribution. Seipin localizes at the ER membrane, often close to possible nucleation sites of CLD formation, suggesting a key regulatory role in biogenesis of this compartment. It remains to be determined whether proteins such as seipins or FITs perform MTP-like roles by acting as chaperones/LLTPs in the assembly of CLDs. Unlike the MTP reaction, however, the activity of these proteins (or thus far any protein) is not required for CLD formation.
CONCLUSIONS
CLDs represent a newly appreciated organelle found in all eukaryotes, which to date has defied analysis of its origins. Most importantly, the role of the CLD as a safe harbor for toxic lipids suggests that modulating this compartment may affect several human syndromes, ranging from obesity and diabetes to neurodegeneration. Besides avoiding toxicity, neutral lipids represent the key energy resource of cells and tissues. Assembly and secretion of lipoproteins likely has provided significant evolutionary advantage whereby a bolus of energy could be transported en masse.
Here we contend that simple thermodynamic principles predict that synthesis of neutral lipids, in the absence of intervention by proteins other than the biosynthetic acyltransferases, is sufficient to form an oil droplet (Fig. 7). The most accepted initial trajectory for this insoluble lipid resource involves the formation of a “lens” of neutral lipid that then acquires a phospholipid coat from the ER bilayer. Evolution has acted, on either side of the ER membrane, to stabilize, regulate, and exploit this process to optimize the availability of this key resource. Several commonalities underlie the formation of lumenal lipoproteins and CLDs, most critical of which is a direct role of the terminal acyltransferases, ACATs, DGATs, and PDATs. In addition to their biosynthetic activities, several models suggest these molecules define the direction in which the particle buds. Active sites or simple biochemical activities have been purported to orientate to opposing sides of the membrane. However, it is clear that no single enzyme, whatever its topology, is exclusively required; deletion of any one of the acyltransferase genes still leaves cytoplasmic and secreted neutral lipids intact, if diminished. By contrast, the formation of lipoproteins in a secretion-competent form requires lipid transfer proteins and a protein scaffold. What is the role of the proteins involved? Do proteins like apoB “trap” neutral lipid, preventing it from going cytosolic? ApoB can spontaneously interact with neutral lipids and form “nucleation sites.” However, MTP actively brings more neutral lipids to these sites to initiate primordial lipoprotein formation. More importantly, MTP likely dislodges/desorbs nascent apoB from the ER membrane to form nascent primordial lipoproteins that are secretion-competent (Fig. 7). Membrane-associated apoB, despite being able to associate with neutral lipids, is not secretion-competent in the absence of MTP.
Fig.7.
Models for cytoplasmic droplet and serum lipoprotein formation in specialized cells. Lipid droplets form on either side of the ER membrane. Import into the cytoplasm reflects the thermodynamic nature of neutral lipids that oil-out the ER membrane. It is unclear whether the droplets are tethered to the ER or migrate away (as shown here). Accessory proteins, such as the fat storage-inducing transmembrane proteins (FIT) and BSCL2/Seipin (SEI), then stabilize and regulate the nascent particles. Dgat2, as a consequence of putative CLD localization, may initiate or supplement core neutral lipid loading while Perilipins (Plin) regulate hydrolysis by specific lipases. In the liver, neutral lipid deposition also occurs in the lumen of the ER and requires a defined set of proteins, specifically an apoB scaffold that acquires lipids, such as triglyceride, as both molecules are produced. The lipid transfer protein MTP, a complex of a transfer polypeptide and the molecular chaperone protein disulphide isomerase, is essential for efficient mobilization of these lipids.
These observations suggest to us that neutral lipid deposition in the cytoplasm is the default process. If so, do lipid transfer or other proteins play a role in CLD biogenesis? Unlike the exclusive and required role of MTP in serum lipoprotein formation, no obligate lipid transfer protein for CLD formation has been identified. The closest candidates, the FIT and seipin protein families, clearly affect CLD formation but are not essential. In their absence, “ectopic” lipid droplets form spontaneously and randomly and thus have variable size, number, and possibly, stability. Therefore, it is a reasonable speculation that CLD-associated proteins act primarily as organizers or facilitators of CLD trafficking. We further contend that secreted lipoprotein assembly is a latecomer in evolution and requires proteins with high lipid binding affinity to overcome the default pathway. We propose that understanding and manipulating the manner in which lipid particle equilibrium on either side of the ER membrane is maintained will impact many aspects of human pathophysiology and disease.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CLD
- cytoplasmic lipid droplet
- DAG
- diacylglycerol
- DGAT
- diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- LP
- lipoprotein particle
- LTP
- lipid transfer protein
- MTP
- microsomal triglyceride transfer protein
- PDAT
- phospholipid-diacylglycerol acyltransferase
- PLIN
- perilipin
- SE
- steryl ester
- TG
- triacylglycerol
This work was supported by the American Heart Association, American Diabetes Association, Ara Parseghian Medical Research Foundation, and National Institutes of Health Grants DK054320 (to S.L.S.) and DK-46900 and HL-5924 (to M.M.H.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The concepts described here were prompted by interactions with David Silver and Molecular Nutrition MS students at Columbia University's Institute of Human Nutrition, Spring 2011.
REFERENCES
- 1.Wilson E. B. 1896. The Cell in Development and Inheritance Macmillan, New York. [Google Scholar]
- 2.Chao F. F., Stiers D. L., Ontko J. A. 1986. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J. Lipid Res. 27: 1174–1181. [PubMed] [Google Scholar]
- 3.Turkish A., Sturley S. L. 2007. Regulation of triglyceride metabolism. I. Eukaryotic neutral lipid synthesis: “Many ways to skin ACAT or a DGAT”. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G953–G957. [DOI] [PubMed] [Google Scholar]
- 4.Stone S. J., Myers H. M., Watkins S. M., Brown B. E., Feingold K. R., Elias P. M., Farese R. V., Jr 2003. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 279:11767–11176. [Google Scholar]
- 5.Harris C. A., Haas J. T., Streeper R. S., Stone S. J., Kumari M., Yang K., Han X., Brownell N., Gross R. W., Zechner R., et al. 2011. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J. Lipid Res. 52: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turkish A. R., Henneberry A. L., Cromley D., Padamsee M., Oelkers P., Bazzi H., Christiano A. M., Billheimer J. T., Sturley S. L. 2005. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J. Biol. Chem. 280: 14755–14764. [DOI] [PubMed] [Google Scholar]
- 7.Turkish A. R., Sturley S. L. 2009. The genetics of neutral lipid biosynthesis: an evolutionary perspective. Am. J. Physiol. Endocrinol. Metab. 297: E19–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen C. L., Stone S. J., Cases S., Zhou P., Farese R. V., Jr 2002. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA. 99: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen C. L., Farese R. V., Jr 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 10.Cheng D., Nelson T. C., Chen J., Walker S. G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J. T., Ramaker M., Feder J. N. 2003. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278: 13611–13614. [DOI] [PubMed] [Google Scholar]
- 11.Reference deleted in proof. [Google Scholar]
- 12.Bouvier-Navé P., Benveniste P., Oelkers P., Sturley S. L., Schaller H. 2000. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur. J. Biochem. 267: 85–96. [DOI] [PubMed] [Google Scholar]
- 13.Yang H., Bard M., Bruner D. A., Gleeson A., Deckelbaum R. J., Aljinovic G., Pohl T. M., Rothstein R., Sturley S. L. 1996. Sterol esterification in yeast: a two-gene process. Science. 272: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 14.Oelkers P., Behari A., Cromley D., Billheimer J. T., Sturley S. L. 1998. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 273: 26765–26771. [DOI] [PubMed] [Google Scholar]
- 15.Oelkers P., Cromley D., Padamsee M., Billheimer J. T., Sturley S. L. 2002. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 277: 8877–8881. [DOI] [PubMed] [Google Scholar]
- 16.Sandager L., Dahlqvist A., Banas A., Stahl U., Lenman M., Gustavsson M., Stymne S. 2000. An acyl-CoA:cholesterol acyltransferase (ACAT)-related gene is involved in the accumulation of triacylglycerols in Saccharomyces cerevisiae. Biochem. Soc. Trans. 28: 700–702. [PubMed] [Google Scholar]
- 17.Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J. T., Sturley S. L. 2000. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275: 15609–15612. [DOI] [PubMed] [Google Scholar]
- 18.Subbaiah P. V., Subramanian V. S., Liu M. 1998. Trans unsaturated fatty acids inhibit lecithin: cholesterol acyltransferase and alter its positional specificity. J. Lipid Res. 39: 1438–1447. [PubMed] [Google Scholar]
- 19.Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. 2000. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA. 97: 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small D. M. 1981. Membrane and plasma lipoproteins bilayer-to-emulsion and emulsion to bilayer transitions. In Membranes, Molecules, Toxins and Cells. K. Bloch, L. Bolis, and D. C. Tosteson, editors. PSG Publ. Co., Boston. 11–34. [Google Scholar]
- 21.Hussain M. M., Rava P., Walsh M., Rana M., Iqbal J. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond). 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 23.Bakillah A., Hussain M. M. 2001. Binding of microsomal triglyceride transfer protein to lipids results in increased affinity for apolipoprotein B: evidence for stable microsomal MTP-lipid complexes. J. Biol. Chem. 276: 31466–31473. [DOI] [PubMed] [Google Scholar]
- 24.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walther T. C., Farese R. V. 2009. The life of lipid droplets. Biochim. Biophys. Acta. 1791: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy D. J., Vance J. 1999. Mechanisms of lipid-body formation. Trends Biochem. Sci. 24: 109–115. [DOI] [PubMed] [Google Scholar]
- 27.Garbarino J., Padamsee M., Wilcox L., Oelkers P. M., D'Ambrosio D., Ruggles K. V., Ramsey N., Jabado O., Turkish A., Sturley S. L. 2009. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death. J. Biol. Chem. 284: 30994–31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamasaki M., Hashiguchi N., Fujiwara C., Imanaka T., Tsukamoto T., Osumi T. 1999. Formation of peroxisomes from peroxisomal ghosts in a peroxisome-deficient mammalian cell mutant upon complementation by protein microinjection. J. Biol. Chem. 274: 35293–35296. [DOI] [PubMed] [Google Scholar]
- 29.Owen M. R., Corstorphine C. C., Zammit V. A. 1997. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 323: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wurie H. R., Buckett L., Zammit V. A. 2011. Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells. J. Biol. Chem. 286: 36238–36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbons G. F., Wiggins D. 1995. The enzymology of hepatic very-low-density lipoprotein assembly. Biochem. Soc. Trans. 23: 495–500. [DOI] [PubMed] [Google Scholar]
- 32.Cases S., Novak S., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Welch C. B., Lusis A. J., Spencer T. A., Krause B. R., et al. 1998. ACAT-2, A second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273: 26755–26764. [DOI] [PubMed] [Google Scholar]
- 33.Anderson R. A., Joyce C., Davis M., Reagan J. W., Clark M., Shelness G. S., Rudel L. L. 1998. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273: 26747–26754. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary V., Jacquier N., Schneiter R. 2011. The topology of the triacylglycerol synthesizing enzyme Lro1 indicates that neutral lipids can be produced within the luminal compartment of the endoplasmatic reticulum: implications for the biogenesis of lipid droplets. Commun. Integr. Biol. 4: 781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herker E., Harris C., Hernandez C., Carpentier A., Kaehlcke K., Rosenberg A. R., Farese R. V., Jr, Ott M. 2010. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med. 16: 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris C., Herker E., Farese R. V., Jr., Ott M. 2011. Hepatitis C virus core protein decreases lipid droplet turnover: a mechanism for core-induced steatosis. J. Biol. Chem. 286: 42615–42625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirandola S., Realdon S., Iqbal J., Gerotto M., Dal Pero F., Bortoletto G., Marcolongo M., Vario A., Datz C., Hussain M. M., et al. 2006. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology. 130: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 38.Mirandola S., Bowman D., Hussain M. M., Alberti A. 2010. Hepatic steatosis in hepatitis C is a storage disease due to HCV interaction with microsomal triglyceride transfer protein (MTP). Nutr. Metab. (Lond). 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang T. Y., Chang C. C., Lu X., Lin S. 2001. Catalysis of ACAT may be completed within the plane of the membrane: a working hypothesis. J. Lipid Res. 42: 1933–1938. [PubMed] [Google Scholar]
- 40.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smolenaars M. M., Madsen O., Rodenburg K. W., Van der Horst D. J. 2007. Molecular diversity and evolution of the large lipid transfer protein superfamily. J. Lipid Res. 48: 489–502. [DOI] [PubMed] [Google Scholar]
- 42.Ohsaki Y., Cheng J., Suzuki M., Fujita A., Fujimoto T. 2008. Lipid droplets are arrested in the ER membrane by tight binding of lipidated apolipoprotein B-100. J. Cell Sci. 121: 2415–2422. [DOI] [PubMed] [Google Scholar]
- 43.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279: 3787–3792. [DOI] [PubMed] [Google Scholar]
- 44.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically-stimulated 3T3–L1 adipocytes. J. Biol. Chem. 279:46835–46842. [DOI] [PubMed] [Google Scholar]
- 45.Leber R., Zinser E., Zellnig G., Paltauf F., Daum G. 1994. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast. 10: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 46.McFie P. J., Banman S. L., Kary S., Stone S. J. 2011. Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J. Biol. Chem. 286: 28235–28246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. 2002. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 277: 6344–6352. [DOI] [PubMed] [Google Scholar]
- 48.Klemm E. J., Spooner E., Ploegh H. L. 2011. Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control. J. Biol. Chem. 286: 37602–37614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F., Gu Y., Dong W., Li H., Zhang L., Li N., Li W., Song Y., Jiang L., Ye J., et al. 2010. Cell death-inducing DFF45-like effector, a lipid droplet-associated protein, might be involved in the differentiation of human adipocytes. FEBS J. 277: 4173–4183. [DOI] [PubMed] [Google Scholar]
- 50.Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R. G., Li P. 2011. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorger D., Athenstaedt K., Hrastnik C., Daum G. 2004. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J. Biol. Chem. 279: 31190–31196. [DOI] [PubMed] [Google Scholar]
- 52.Poppelreuther M., Rudolph B., Du C., Großmann R., Becker M., Thiele C., Ehehalt R., Füllekrug J. 2012. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 53: 888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorger D., Daum G. 2002. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 184: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone S. J., Levin M. C., Zhou P., Han J., Walther T. C., Farese R. V., Jr 2009. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284: 5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Londos C., Brasaemle D. L., Gruia-Gray J., Servetnick D. A., Schultz C. J., Levin D. M., Kimmel A. R. 1995. Perilipin: unique proteins associated with intracellular neutral lipid droplets in adipocytes and steroidogenic cells. Biochem. Soc. Trans. 23: 611–615. [DOI] [PubMed] [Google Scholar]
- 56.Londos C., Brasaemle D. L., Schultz C. J., Adler-Wailes D. C., Levin D. M., Kimmel A. R., Rondinone C. M. 1999. On the control of lipolysis in adipocytes. Ann. N. Y. Acad. Sci. 892: 155–168. [DOI] [PubMed] [Google Scholar]
- 57.Tansey J. T., Sztalryd C., Gruia-Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R., et al. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 98: 6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimmel A. R., Brasaemle D. L., McAndrews-Hill M., Sztalryd C., Londos C. 2010. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 60.Kadereit B., Kumar P., Wang W. J., Miranda D., Snapp E. L., Severina N., Torregroza I., Evans T., Silver D. L. 2008. Evolutionarily conserved gene family important for fat storage. Proc. Natl. Acad. Sci. USA. 105: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gross D. A., Zhan C., Silver D. L. 2011. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc. Natl. Acad. Sci. USA. 108: 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Häder T., Muller S., Aguilera M., Eulenberg K. G., Steuernagel A., Ciossek T., Kuhnlein R. P., Lemaire L., Fritsch R., Dohrmann C., et al. 2003. Control of triglyceride storage by a WD40/TPR-domain protein. EMBO Rep. 4: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronshaugen M., McGinnis N., Inglis D., Chou D., Zhao J., McGinnis W. 2002. Structure and expression patterns of Drosophila TULP and TUSP, members of the tubby-like gene family. Mech. Dev. 117: 209–215. [DOI] [PubMed] [Google Scholar]
- 64.Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S., Ahringer J., Ruvkun G. 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 421: 268–272. [DOI] [PubMed] [Google Scholar]
- 65.Szymanski K. M., Binns D., Bartz R., Grishin N. V., Li W. P., Agarwal A. K., Garg A., Anderson R. G., Goodman J. M. 2007. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA. 104: 20890–20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P., Brown A. J., Wenk M. R., Parton R. G., Yang H. 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fei W., Alfaro G., Muthusamy B. P., Klaassen Z., Graham T. R., Yang H., Beh C. T. 2008. Genome-wide analysis of sterol-lipid storage and trafficking in Saccharomyces cerevisiae. Eukaryot. Cell. 7: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beller M., Sztalryd C., Southall N., Bell M., Jäckle H., Auld D. S., Oliver B. 2008. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 6: e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y., Walther T. C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J. S., Vale R. D., Walter P., Farese R. V. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 453: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cartwright B. R., Goodman J. M. 2012. Seipin - from human disease to molecular mechanism. J. Lipid Res. 53:1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rava P., Hussain M. M. 2007. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry. 46: 12263–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]