Abstract
During atherogenesis, the extracellular pH of atherosclerotic lesions decreases. Here, we examined the effect of low, but physiologically plausible pH on aggregation of modified LDL, one of the key processes in atherogenesis. LDL was treated with SMase, and aggregation of the SMase-treated LDL was followed at pH 5.5–7.5. The lower the pH, the more extensive was the aggregation of identically prelipolyzed LDL particles. At pH 5.5–6.0, the aggregates were much larger (size >1 µm) than those formed at neutral pH (100–200 nm). SMase treatment was found to lead to a dramatic decrease in α-helix and concomitant increase in β-sheet structures of apoB-100. Particle aggregation was caused by interactions between newly exposed segments of apoB-100. LDL-derived lipid microemulsions lacking apoB-100 failed to form large aggregates. SMase-induced LDL aggregation could be blocked by lowering the incubation temperature to 15°C, which also inhibited the changes in the conformation of apoB-100, by proteolytic degradation of apoB-100 after SMase-treatment, and by HDL particles. Taken together, sphingomyelin hydrolysis induces exposure of protease-sensitive sites of apoB-100, whose interactions govern subsequent particle aggregation. The supersized LDL aggregates may contribute to the retention of LDL lipids in acidic areas of atherosclerosis-susceptible sites in the arterial intima.
Keywords: sphingomyelinase, aggregation, atherosclerosis, apolipoprotein, low density lipoprotein
During atherogenesis, LDL particles enter the subendothelial space of the arterial wall, where they are entrapped by binding to components, particularly to proteoglycans, of the extracellular matrix (1, 2). In the arterial intima, the retained LDL particles become modified and can aggregate (3). Aggregation of LDL particles increases their binding strength to the proteoglycans and in this way may enhance the extracellular retention of LDL in the arterial intima (3, 4). SMase, which catalyzes the hydrolysis of sphingomyelin into phosphorylcholine and ceramide, is suggested to be one of the key enzymes responsible for LDL aggregation during atherogenesis (3). Indeed, in aggregated LDL isolated from human atherosclerotic lesions, the ceramide content is 10–50-fold higher than in corresponding plasma LDL, and treatment of LDL with human recombinant secretory SMase (hrSMase) in vitro induces the formation of lesion-like LDL aggregates (5). More recently, a causal role for secretory SMase in the development of atherosclerotic lesions was shown in vivo: a deficiency of acid SMase was associated with a reduction of lipoprotein retention and atherosclerotic lesions in mice (6).
Macrophages and endothelial cells secrete SMase in vitro (7). Immunoreactive secretory SMase is found in human atherosclerotic lesions, where it is mostly associated with the extracellular matrix (8). The secretory SMase has an acidic pH optimum and is able to hydrolyze LDL at neutral pH only if the LDL particles have been premodified, e.g., by phospholipase A2, oxidative agents, or by proteolytic enzymes (9, 10). All these modifications lead to changes in the surface structure of the LDL particles. Analogously, we may infer that premodification of the surface structure of LDL particles is necessary for the ability of the secretory SMase to attack LDL particles at neutral pH. Thus, when present in the superficial layer of atherosclerotic lesions, where the extracellular pH is neutral, secretory SMase is likely to act on LDL only after the particles have been premodified by the other agents capable of modifying LDL at this pH.
The arterial intima is an avascular tissue, and oxygen can reach the intima only by diffusion from the lumen or from the medial layer of the arterial wall. The diffusion distance of oxygen in tissues is only about 200 µm (11), and hypoxic areas develop in the lesions already at this depth (12, 13). Even the normal arterial intima of atherosclerosis-prone sites in humans exceeds this critical diffusion distance of oxygen, and during atherogenesis, the thickness of the intima may increase to more than 3,000 µm (12). Under hypoxia, cells switch to anaerobic metabolism, which leads to the production of lactate and thus the secretion of excess H+ ions. Indeed, increased extracellular lactate concentrations are found particularly in the hypoxic areas of atherosclerotic lesions (14). Also, decreased extracellular pH values have been determined (15). Thus, already at a distance of 200 µm from the lumen, pH values as low as 6.8 have been measured, and visualization of human carotid plaques with pH-sensitive fluorescent dyes has indicated that the pH may reach values which are even below pH 6.0. A recent report indicates that macrophages generate pericellular pH gradients, with their lowest values reaching 5.0 (16). In addition, the acidity of the extracellular fluid may be increased in the vicinity of the sulfate and carboxylic acid groups of proteoglycan side chains. Interestingly, accumulation of lipids in the atherosclerotic lesions begins in the deep areas of the diffuse intimal thickening of arterial intima (17), where the pH is more acidic than in the superficial intima. In the acidic areas of the atherosclerotic lesions, secretory SMase is located in an extracellular milieu with an optimal pH.
In the present study, we examined the effect of pH on SMase-induced LDL aggregation. The lower the pH, the higher was the degree of aggregation and the larger were the aggregates formed. Thus, when LDL particles identically lipolyzed with bacterial SMase were incubated at acidic pH, micron-sized LDL aggregates were formed, while aggregates formed at neutral pH had sizes averaging 100–200 nm. SMase-induced LDL aggregation was found to be mediated by apoB-100 and required conformational changes in the secondary structure of the apolipoprotein.
EXPERIMENTAL PROCEDURES
Isolation and modifications of LDL
Human LDL (d = 1.019–1.050 g/ml) and HDL3 (d = 1.125–1.210 g/ml) were isolated from the plasma of healthy volunteers (Finnish Red Cross) by sequential ultracentrifugation in the presence of 3 mM EDTA (18, 19). The amounts of LDL were expressed in terms of their protein concentrations, which were determined by the method of Lowry et al. (20), with BSA as the standard. Each experiment was performed with LDL from at least two donors. 3H- and 35S-radiolabeled LDL was prepared by labeling the protein component of LDL by the procedure of Bolton and Hunter (21), with 3H-labeling reagent (t-butoxycarbonyl-l-[3H]methionine N-hydroxy-succinimidyl ester [Amersham Biosciences]) or with 35S-labeling reagent (t-butoxycarbonyl-l-[35S]methionine N-hydroxysuccinimidyl ester [Amersham Biosciences]).
Bacterial SMase-treated LDL was prepared by incubating LDL (1.25 mg/ml) in Dulbecco's PBS (BioWhittaker; Verviers, Belgium) containing 200 mU/ml of Bacillus cereus SMase (bcSMase) (Sigma-Aldrich). After 30 min incubation, lipolysis was stopped by adding EDTA, to give a final concentration of 10 mM. The degree of lipolysis was determined by a measurement of phosphorylcholine using a modification of the Amplex Red SMase Assay Kit (Molecular Probes; Invitrogen, UK).
LDL particles were also modified with hrSMase (a kind gift from Genzyme) by incubating LDL (1.0 mg/ml) with 20 µg/ml hrSMase in a buffer containing 150 mM NaCl and 50 µM ZnCl2 in either 20 mM MES (pH 5.5 or pH 6.0), 20 mM PIPES (pH 6.5), or 20 mM HEPES (pH 7.0 or 7.5) for the indicated times, at 37°C.
Preparation of lipid microemulsions from LDL lipids
Lipids were extracted from 5–10 mg of LDL by the method of Bligh and Dyer (22) and evaporated under N2. 3H-cholesteryl linoleate was added to the lipid mixtures for radiolabeling. Microemulsion particles were prepared essentially as described by Ginsburg et al. (23), and the dried lipids were resuspended in 2 ml PBS and sonicated under N2 at 50°C for 6 × 5 min using a Branson Sonifier 250 sonicator, followed by centrifugation for 5 min at 14,000 g. The amount of total cholesterol in the lipid particles was determined using a commercial kit (Roche; Indianapolis, IN).
Aggregation of LDL particles
Native or bcSMase-treated LDL particles (100 µl, 1 mg/ml) were incubated in a microtiter well plate in a buffer containing 150 mM NaCl, and either 20 mM MES (pH 5.5 or 6.0), 20 mM PIPES (pH 6.5 or 7.0), or 20 mM HEPES (pH 7.5). In some experiments, LDL was proteolyzed with α-chymotrypsin (Sigma-Aldrich) or pronase E (Sigma-Aldrich) either before or after bcSMase treatment (10). In addition, in some experiments, the incubation mixture contained monoclonal antibodies (Bsol 2, Bsol 14: 0.36 mg/ml). Aggregation of the LDL samples was followed by measuring the absorbance of the LDL samples at 405 nm. The sizes of the aggregated particles were determined by asymmetrical flow-field-flow fractionation (AsFlFFF) as described (24) or by dynamic light scattering (DLS) (Zetasizer Nano, Malvern).
Aggregation of LDL particles with lipid microemulsions
35S-radiolabeled LDL particles and 3H-radiolabeled lipid microemulsions, either unmodified or bcSMase-modified, were incubated together in the pH buffers as described above. After the incubation, the amounts of aggregated LDL particles and microemulsions were determined by separating the large aggregates by centrifugation at 15,000 g for 15 min. After centrifugation, the supernatants and the sedimented particles were collected separately and their radioactivities were measured.
Circular dichroism analyses
Circular dichroism (CD) analysis of LDL samples was performed using 50 μg/ml LDL, native or bcSMase-modified. CD spectra were recorded on a JASCO J-715 spectropolarimeter (Japan Spectroscopic Co.; Tokyo, Japan) using a 0.1 cm quartz cuvette in the region of 190–250 nm with a step size of 0.5 nm, scan speed of 50 nm per min, band width of 1 nm, and 1 s response. The cell holder compartment was thermostatically maintained at 37 ± 0.1°C. Five spectra for each sample were averaged, and blank measurements were subtracted.
RESULTS
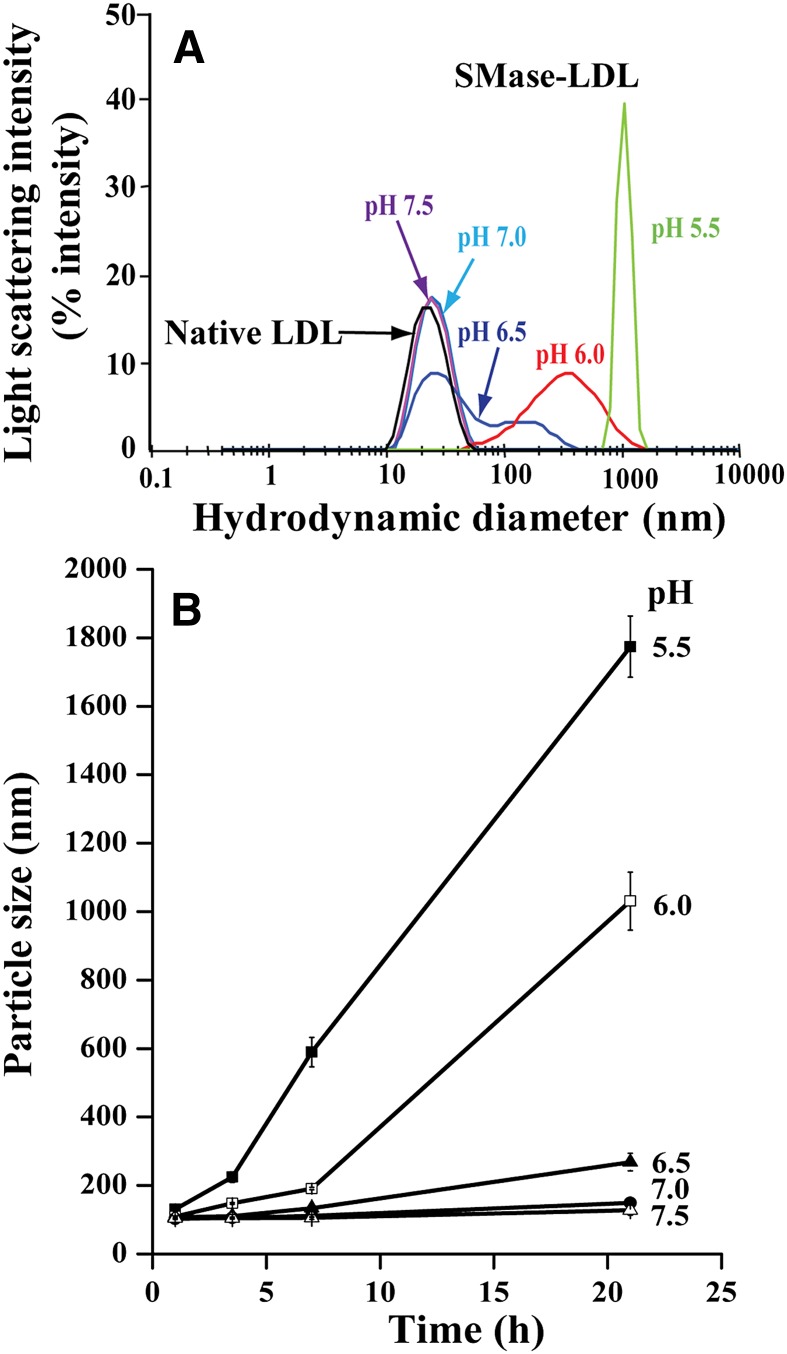
LDL was treated with hSMase at pH values 5.5–7.5. For this purpose, LDL was incubated in MES-, PIPES-, or HEPES-buffered saline containing 50 µM ZnCl2 with or without 20 µg/ml of hrSMase. Because the human enzyme has an acidic pH optimum, substantial hydrolysis of LDL was observed only at pH 6.0 and 5.5 (see supplementary Fig. IA). Similarly, substantial aggregation, measured as turbidity of the incubation mixtures, was observed only at these pH values (see supplementary Fig. IB). After incubation for 24 h, the actual sizes of LDL treated with hrSMase were determined with DLS. In accordance with the turbidity data, LDL aggregates were found only at pH 6.0 and 5.5, at which the average sizes of LDL aggregates were about 400 nm and 1,000 nm, respectively (Fig. 1A). Of note, the size of LDL aggregates varies among different LDL donors. Typically, the size of LDL treated with hrSMase for 20 h at pH 5.5 varied between 600 and 5,000 nm.
Fig. 1.
Aggregation of SMase-treated LDL at different pH values. A: LDL was incubated in the presence of 20 µg/ml hrSMase for 21 h at the indicated pH values. After the incubation, the sizes of native LDL particles (black) and SMase-treated LDL particles (pH 7.5, purple; pH 7.0, light blue; pH 6.5, dark blue; pH 6.0, red; pH 5.5, green) were determined by DLS. Please note the logarithmic scale in the x axis. B: LDL particles were treated with bcSMase for 30 min, after which the lipolysis was stopped by adding EDTA. Next, the identically lipolyzed LDL particles were incubated at 37°C at the indicated pH values, and at the indicated time points, particle size was determined by DLS.
Because the secretory SMase has an acidic pH optimum, the degrees of lipolysis in the above experiment were different. To examine the effect of pH on the aggregation process solely, LDL was first treated for 30 min with bcSMase at pH 7.5 to obtain an identical degree of lipolysis in all the samples. The use of bcSMase allowed us to use short incubation times for the lipolysis and so minimize particle aggregation during this initial phase. After the incubation, the activity of the enzyme was inhibited by the addition of EDTA. About 70% of LDL sphingomyelin was hydrolyzed under the conditions used here. The bcSMase-treated LDL was then divided into aliquots, the pH values of the aliquots were adjusted to 5.5, 6.0, 6.5, 7.0, or 7.5, and the samples were then incubated at the various pH values at 37°C for the indicated times. At each time point, the turbidity of the samples was determined as a measure of particle aggregation. As shown in supplementary Fig. II, the turbidity of each sample increased during the incubation, indicating progressive lipoprotein aggregation. The lower the pH of the incubation mixture, the higher the turbidity of the lipolyzed LDL samples, which indicates an increased tendency for aggregation at acidic pH values. Unmodified LDL did not aggregate at any pH, as exemplified by the extreme condition of pH 5.5 in the absence of bcSMase (see supplementary Fig. II). In accordance with the increased turbidity, the size of the bcSMase-treated LDL particles progressively increased, and the lower the pH was, the larger were the generated aggregates (Fig. 1B). Thus, the average size of bcSMase-treated LDL aggregates was 120 nm at pH 7.5, whereas it was 1,800 nm at pH 5.5. Again, the size of aggregates generated at pH 5.5 was found to vary significantly among different donors. Typically, the size of LDL aggregates after an overnight incubation was below 300 nm at pH 6.5−7.5, 400–1,500 nm at pH 6.0, and 500–5,000 nm at pH 5.5.
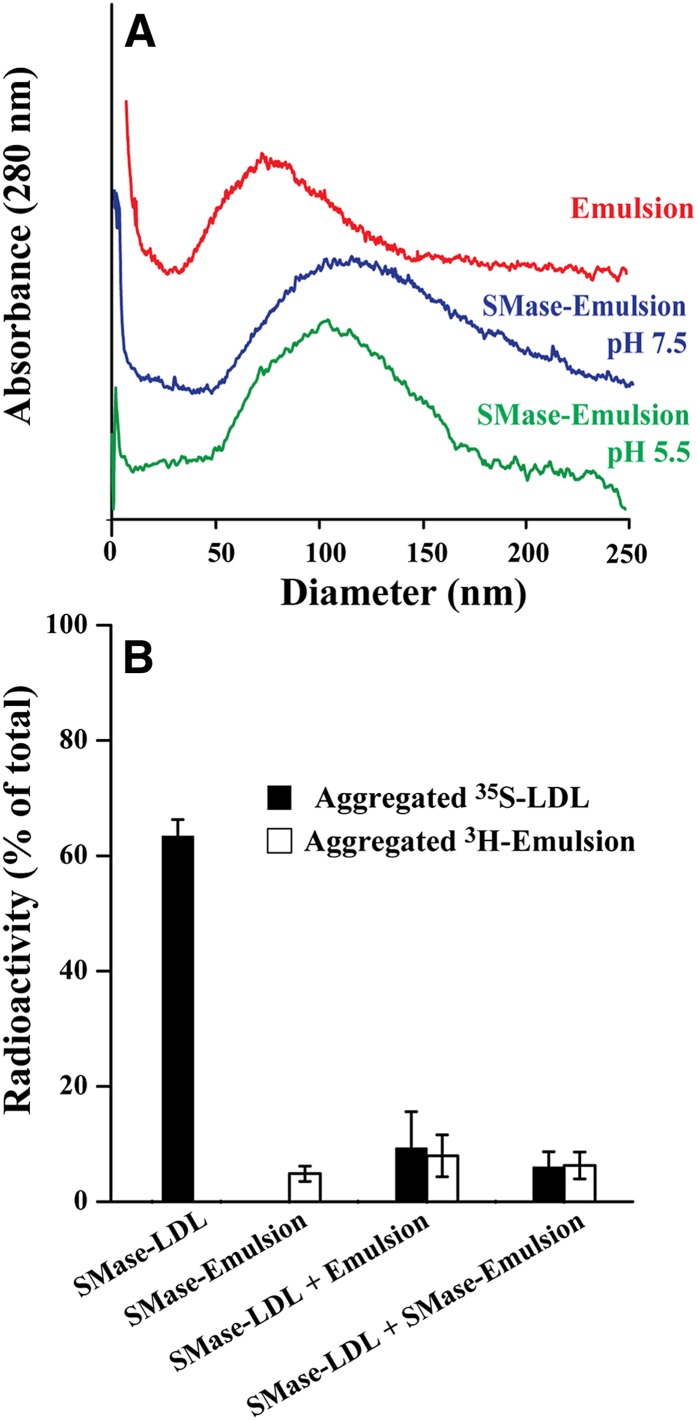
To examine the mechanisms by which the SMase-treated LDL particles aggregate, we next examined whether ceramide might induce aggregation more efficiently at acidic pH. For this purpose, we used lipids extracted from LDL particles to generate lipid microemulsions devoid of any protein. Because the turbidity of microemulsions is rather high, turbidity measurements could not be performed to follow particle aggregation. Instead, to estimate the sizes of unmodified and SMase-treated microemulsions, we applied AsFlFFF, a method that has been used to determine the sizes of modified LDL particles (24). We first treated the lipid microemulsions with bcSMase at neutral pH, stopped the activity of the enzyme, and divided the bcSMase-treated lipid microemulsions into two aliquots, whose pH was adjusted to 5.5 or 7.5. About 75% of the sphingomyelin molecules in the particles were lipolyzed. After incubation for 24 h, the sizes of the lipid particles were determined. The bcSMase-treatment only slightly increased the average size of the lipid microemulsions from 85 nm (unmodified microemulsions) to about 110 nm at both pH 5.5 and 7.5 (Fig. 2A). Thus, in striking contrast to the SMase-treated LDL particles, the pH of the incubation mixture had no effect on the aggregation of LDL-derived lipid microemulsions. This finding indicates that the protein component of the LDL particles was necessary for the increased aggregation of SMase-treated LDL at acidic pH.
Fig. 2.
Aggregation of bcSMase-treated lipid microemulsions. A: Lipid microemulsions were treated with 200 mU/ml of bcSMase for 30 min, after which lipolysis was stopped, and the bcSMase-treated lipid microemulsions were incubated at pH 5.5 or pH 7.5 overnight. Thereafter the lipolyzed samples and, as a control, untreated lipid microemulsions, were subjected to field-flow fractionation to determine the size of the particles. Please note that in contrast to Fig. 1, the scale of the x axis is linear. B: Radiolabeled LDL particles and lipid microemulsions, unmodified or bcSMase-modified, were incubated together in MES-buffered saline (pH 5.5). After an overnight incubation, the samples were centrifuged to separate the aggregates formed. The radioactivity of the pellets and supernatants was measured. Results shown are mean ± SD values of three independent experiments.
Next, we performed an experiment to determine whether the SMase-treated particles can aggregate via interactions between the protein component of one particle and the lipid component of another particle. For this purpose, nonlipolyzed or bcSMase-treated 35S-labeled LDL particles were incubated with nonlipolyzed or bcSMase-treated 3H-labeled lipid microemulsions in various combinations. After 20 h, the mixtures were centrifuged to sediment any large aggregated particles. Using 35S-labeled LDL and 3H-labeled lipid microemulsions, we could determine the amounts of LDL particles and lipid microemulsions in the sedimented aggregates. As shown in Fig. 2B, bcSMase-treated lipid microemulsions aggregated neither spontaneously nor after incubation with nonlipolyzed or bcSMase-treated LDL. In fact, the lipid microemulsions prevented the aggregation of bcSMase-treated LDL particles. Thus, the bcSMase-treated LDL particles aggregated less in the presence than in the absence of either nonlipolyzed or bcSMase-treated lipid microemulsions, so revealing an inhibitory effect of the emulsions on aggregation of bcSMase-treated LDL particles.
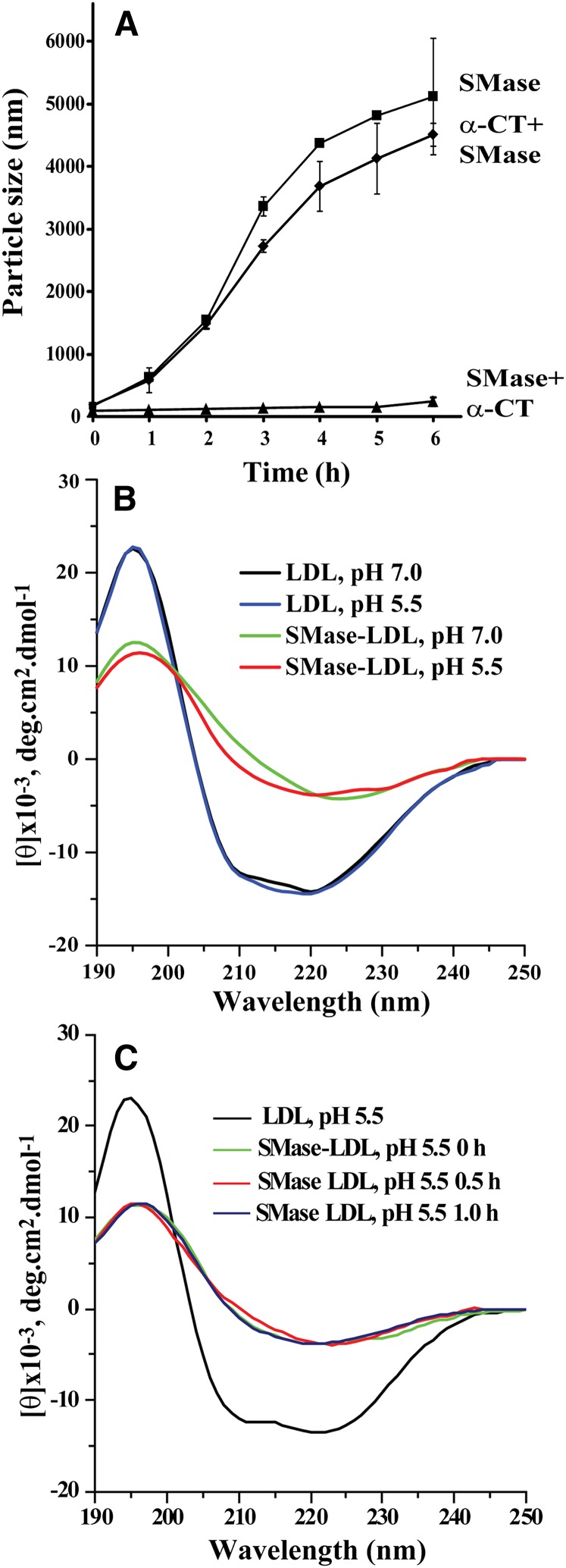
The above experiments indicate that at acidic pH, the lipid components of SMase-treated LDL particles are not responsible for LDL aggregation. Because many proteins are known to be prone to aggregation under acidic conditions, and, importantly, apoB-100 has been shown to be particularly susceptible to aggregation in various circumstances (25), we next explored the role of apoB-100 in SMase-induced aggregation of LDL at acidic pH. Proteolytic degradation of LDL particles by proteases having broad substrate specificity leads to release of peptide fragments from LDL. We reasoned that release of apoB-100 fragments could inhibit apoB-100-mediated LDL aggregation and followed the changes in the sizes of three different bcSMase-treated LDL preparations: 1) bcSMase-LDL, 2) preproteolyzed bcSMase-LDL, and 3) bcSMase-LDL that was proteolyzed after the bcSMase-treatment, i.e., postproteolyzed bcSMase-LDL. Thus, LDL was first treated with α-chymotrypsin, after which bcSMase was used to fully lipolyze the preproteolyzed LDL (α-CT + SMase) and unproteolyzed LDL particles (SMase). A fraction of the bcSMase-treated particles was treated with α-chymotrypsin after lipolysis (SMase + α-CT). All particles were fully lipolyzed and the degree of proteolysis was similar in the pre- and postlipolyzed particles (20% and 21% of trichloroacetic acid-soluble peptide fragments were generated, respectively). Particle aggregation was then followed at pH 5.5 by measuring the size of the particles. Preproteolysis had no effect on LDL aggregation (Fig. 3A). However, when the order of proteolysis and lipolysis was reversed, and LDL was first treated with bcSMase and only then subjected to proteolysis, postproteolysis completely blocked particle aggregation (Fig. 3A). These findings suggest that the segments of apoB-100 that mediate aggregation of SMase-treated LDL particles are not accessible to proteolytic cleavage in unmodified LDL particles, but are accessible to such cleavage in SMase-treated particles. Thus, SMase treatment appears to lead to exposure of aggregation-prone sites in apoB-100, and proteolytic cleavage of these sites can inhibit particle aggregation.
Fig. 3.
Changes in the secondary structure of apoB-100 upon bcSMase modification of LDL. A: LDL was proteolyzed by α-chymotrypsin (1 mg/ml) for 30 min, proteolysis was stopped by the addition of PMSF (1 mM), and the preproteolyzed LDL was treated with 500 mU/ml of bcSMase for 15 min, after which lipolysis was stopped (α-CT + SMase). Native LDL was treated similarly with bcSMase for 15 min, and the lipolysis was stopped (SMase). The nonproteolyzed and lipolyzed LDL was divided into two aliquots, one of which was treated for 30 min with α-chymotrypsin (1 mg/ml), after which the proteolysis was stopped as above (SMase + α-CT). The pH of the three incubation mixtures was adjusted to pH 5.5, and LDL aggregation was followed by measuring the size of the particles by DLS of the samples. B: LDL (1 mg/ml) was treated with bcSMase for 30 min, at which time aggregation of LDL particles was minimal, and lipolysis was stopped. The samples were diluted to give a concentration of 50 µg/ml and far-ultraviolet (UV) CD spectra were recorded at 37°C using a 0.1 cm quartz cuvette in the region of 190–250 nm at acidic and neutral pH. Five spectra for each sample were averaged, and blank measurements were subtracted. Data are representative of three independent experiments. C: The lipolyzed LDL was further incubated at pH 5.5 for 30 and 60 min to induce particle aggregation, after which the far-UV CD spectra of the samples were recorded.
Next, the effect of SMase treatment on the conformation of apoB-100 was examined. LDL was treated with bcSMase for 30 min, after which the secondary structure of apoB-100 was analyzed by CD. As shown in Fig. 3B, acidification alone did not have an effect on the conformation of apoB-100, but SMase treatment dramatically decreased the α-helix content of apoB-100, with an apparent concomitant increase in the β-strand content, as inferred from the large changes in the shapes of the curves. Importantly, the changes in the conformation of apoB-100 occurred before particle aggregation and were thus caused by the lipolytic modification alone. No further changes in apoB-100 secondary structure occurred, when the particles were further incubated for up to 1 h (Fig. 3C), at which point, particle aggregation is already in progress and the average size of the particles is typically 200–600 nm.
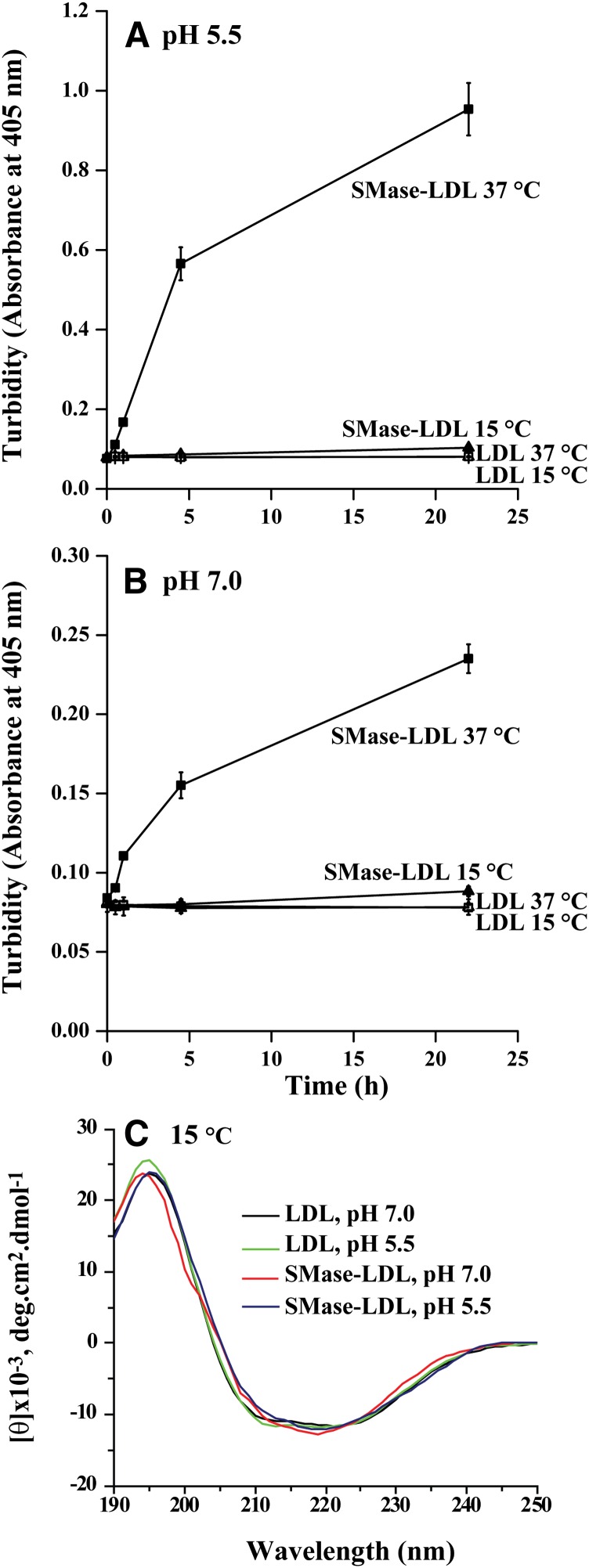
We have previously (26) shown that SMase-induced LDL aggregation at neutral pH does not occur below the transition temperature of LDL particles, which is 32–35°C (27, 28) and at which the core lipids of LDL particles are in an ordered state. Here, we first studied whether SMase-induced LDL aggregation would occur below the transition temperature of LDL particles at acidic pH. LDL was first treated with bcSMase at 15°C for 30 min; lipolysis was then stopped by adding EDTA, and the incubation of the prelipolyzed LDL particles was continued either at 15°C or at 37°C and at neutral or acidic pH. The treatment of LDL with bcSMase at 15°C resulted in hydrolysis of about 60% of the sphingomyelin molecules and, importantly, both sets of particles were identically lipolyzed. LDL aggregation was observed only at 37°C, but not at 15°C at both pH values (Figs. 4A, B). CD analysis of the samples revealed that at 15°C, no changes in the conformation of apoB-100 occurred at either neutral or acidic pH (Fig. 4C). This finding indicates that the conformational changes in apoB-100 are required for particle aggregation and implies that changes in LDL lipids are responsible for the conformational changes.
Fig. 4.
Aggregation of LDL at 15°C. LDL particles were incubated at pH 7.5 with 200 mU/ml of bcSMase for 30 min at 15°C. After the incubation, the particles were incubated at either 37°C or 15°C, and particle aggregation was followed by measuring the turbidity of the samples at pH 5.5 (A) or at pH 7.0 (B). The samples were diluted to give a concentration of 50 µg/ml, and far-UV CD spectra were recorded at 15°C using a 0.1 cm quartz cuvette in the region of 190–250 nm at acidic and neutral pH (C).
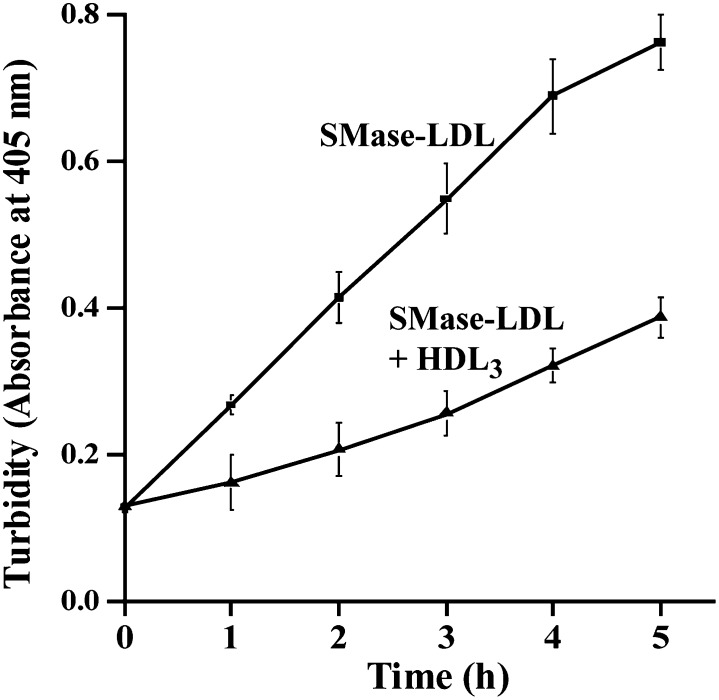
SMase-induced changes in LDL can be linked with an increase in the surface hydrophobicity of the modified particles (29). Hydrophobic interactions between modified LDL particles can be blocked by isolated and purified apoA-I and by the apoA-I-containing HDL3 particles (5, 30). Here, we examined the ability of HDL3 particles to inhibit SMase-induced aggregation of LDL at acidic pH. Indeed, as shown in Fig. 5, HDL3 particles effectively inhibited the aggregation of bcSMase-treated LDL particles.
Fig. 5.
HDL3 inhibits bcSMase-induced LDL aggregation. LDL was treated with 200 mU/ml of bcSMase, lipolysis was stopped, and the pH of the incubation mixture was adjusted to pH 5.5. LDL aggregation in the absence and presence of 100 µg/ml of HDL3 was followed by measuring the turbidity of the samples.
To examine the role of apoB-100 in the SMase-induced LDL aggregation at acidic pH in more detail, we decided to block specific regions of the peptide with available antibodies. Importantly, the N- and C-terminal regions of apoB-100 have been shown to become more accessible to certain monoclonal antibodies after the LDL particles have been treated with SMase (31). Therefore, we next tested whether the SMase-induced LDL aggregation would be blocked by the two monoclonal antibodies Bsol 14 or Bsol 2, which recognize the N- and C-terminal regions of apoB-100, respectively. As demonstrated in supplementary Fig. III, preincubation of LDL with either of the antibodies failed to prevent and actually enhanced the subsequent SMase-induced LDL aggregation.
DISCUSSION
In the present study, we show that the lower the pH, the higher the degree of aggregation of identically lipolyzed LDL particles and the greater is the size of the aggregates formed. The aggregates formed after SMase treatment in vitro had sizes bigger than 1,000 nm at pH ≤6.0, while the average size of the aggregates formed at neutral pH was much smaller, i.e., only up to about 200 nm. Interestingly, in human atherosclerotic lesions, the ceramide-containing LDL particles have been reported to be present in two different forms: as very large, micron-sized aggregates, and as smaller aggregates (5), so providing support for potential in vivo relevance of the present in vitro observations.
The long-standing inference has been that ceramide is responsible for aggregation of SMase-treated LDL particles (5). Indeed, at neutral pH, ceramide has been suggested to generate hydrophobic nanoenvironments on the surface of LDL particles, and particle aggregation is thought to arise from interactions between these areas (3). However, as shown in the present work, at acidic pH, the SMase-induced particle aggregation is apoB-100-dependent, the conformational changes in apoB-100 induced by sphingomyelin hydrolysis being pivotal for LDL aggregation. The SMase-induced changes in the lipid monolayer of LDL particles were found to lead to remarkable changes in the conformation of apoB-100, as reflected by significantly decreased α-helical and increased β-sheet content of the protein in the SMase-modified particles. Particle aggregation could be prevented by performing the SMase treatment at a temperature (15°C) at which the conformational changes of apoB-100 were prevented. Below the transition temperature of LDL particles (32−35°C) (27, 28), lateral diffusion of LDL surface phospholipids is inhibited by the ordered core lipids (32), which can at least partly explain the inhibited conformational changes. Lipolysis-induced changes in the conformation of apoB-100 have been reported to occur also when LDL is modified by phospholipase A2 (33, 34). However, although the aggregation of SMase-treated LDL increases dramatically at acidic pH, no pH-dependent increase in the aggregation of phospholipase A2-treated LDL is seen (35). Thus, the SMase-induced changes in the conformation of apoB-100 appear to lead to exposure of specific regions of the protein that are particularly prone to join LDL particles together at acidic pH.
The water-exposed parts of apoB-100 are accessible for proteases, and, accordingly, proteolytic enzymes cleave such regions of apoB-100 into fragments, some of which remain bound and some of which are released from the proteolyzed particles (3). Interestingly, we could strongly inhibit the SMase-induced LDL aggregation by proteolytic degradation of SMase-treated LDL particles, but only if proteolysis was performed after SMase treatment. Accordingly, if proteolysis was performed before SMase treatment, i.e., when native LDL particles were proteolyzed, subsequent SMase treatment resulted in LDL aggregation. Thus, we can infer that the SMase-induced conformational changes in apoB-100 must have led to exposure of protease-sensitive apoB-100 segments that are buried in native LDL, and that these newly exposed segments of apoB-100 are crucial for LDL aggregation. These findings illustrate both the importance of SMase-induced conformational changes in apoB-100 in triggering LDL aggregation and the indispensable role of apoB-100 in particle aggregation. Interestingly, plasmin treatment has been shown to disaggregate vortex-aggregated and phospholipase C-aggregated LDL particles (36), a finding pointing to the possibility that apoB-100 may have a more general role in governing LDL aggregation. In fact, it has been suggested that the unique susceptibility of apoB-100 to aggregation may serve as a quality control mechanism that blocks the secretion of potentially toxic lipoproteins by the liver, but may unfortunately lead to extracellular aggregation and retention in the arterial wall (25).
The SMase-induced conformational changes can be linked with an increase in the surface hydrophobicity of SMase-treated LDL particles, because SMase-treatment of LDL, but not of LDL-derived lipid microemulsions, increases the hydrophobicity of the particles (29). Therefore, a likely cause for particle aggregation is interaction between hydrophobic areas of the apoB-100 moieties of the particles. ApoA-I and HDL particles have been shown to block aggregation mediated by hydrophobic interactions between modified LDL particles, and, indeed HDL particles did inhibit SMase-induced LDL aggregation at acidic pH. SMase-induced LDL aggregation was also blocked by the presence of LDL-derived lipid microemulsions. Interestingly, emulsions have been shown to interact with LDL particles, and incubation of LDL together with emulsions can lead to transfer of lipids between emulsions and LDL and can even lead to fusion of the emulsions with LDL (37, 38). These changes have also been suggested to modify the conformation of apoB-100 (37) and could so interfere with SMase-induced aggregation of LDL particles.
We also reasoned that it would be possible to interfere with LDL aggregation by using anti-apoB-100 antibodies so as to block the critical interactions leading to aggregation of SMase-modified LDL particles. Accordingly, the antibodies to test this possibility were chosen based on their increased immunoreactivity toward SMase-treated LDL (31). Unexpectedly, however, LDL aggregation could not be blocked by these antibodies. Because the selected antibodies bind to sequences close to the N- and C-termini of apoB-100 (39, 40), this finding may indicate that these areas of apoB-100 are not responsible for the particle-particle interactions leading to SMase-induced LDL aggregation at acidic pH.
The extracellular pH of atherosclerotic plaques has been shown to be spatially nonuniform: decreased pH values are detected in the deep areas of advanced human atherosclerotic lesions, whereas subendothelial areas are more likely to have a neutral pH (15). The extracellular matrix proteoglycans may also contribute to the generation of acidic microenvironments by increasing the concentration of H+ ions near the sulfate and carboxylic acid groups of proteoglycan side chains. Thus, SMase-induced LDL aggregation may be particularly accelerated in the vicinity of proteoglycans. Indeed, a synergistic action of SMase and lipoprotein lipase has been shown to lead to extensive LDL aggregation on smooth-muscle cell matrix (41). In this work, lipoprotein lipase was found to play a purely structural role in linking aggregated LDL to the matrix. Interestingly, arterial-wall matrix, lipoprotein lipase, and SMase have each been shown to contribute to retention of apoB-100-containing lipoproteins and atherogenesis in vivo (6, 42–44).
The present study shows that acidity dramatically enhances the aggregation of SMase-modified LDL particles and leads to formation of micron-sized LDL aggregates. In human atherogenesis, acidic environments are likely to be found in advanced atherosclerotic lesions (15). Importantly, hypoxic, and therefore also acidic, areas are frequently found in the deep layers of prelesional arteries, where lipids initially accumulate and where the formation of the extracellular lipid core is initiated (11, 14). Because acidity increases the ability of human secretory SMase to hydrolyze LDL (9, 10), increases the binding of LDL to the extracellular matrix proteoglycans (45, 46), and, as shown here, enhances the extent of aggregation of ceramide-enriched LDL, SMase-induced modification of LDL is likely to be particularly deleterious in the acidic extracellular microenvironments of prelesional arteries and atherosclerotic plaques.
Supplementary Material
Acknowledgments
The excellent technical assistance of Maija Atuegwu, Mari Jokinen, and Hanna Lähteenmäki is gratefully acknowledged. Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation.
Footnotes
Abbreviations:
- AsFlFFF
- asymmetrical flow-field-flow fractionation
- bcSMase
- Bacillus cereus SMase
- CD
- circular dichroism
- DLS
- dynamic light scattering
- hrSMase
- human recombinant secretory SMase
Financial support was provided by the Research Council for Natural Sciences and Engineering, the Academy of Finland, under Grant 1133184 (M-L.R., K.Ö.)
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Williams K. J., Tabas I. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I., Williams K. J., Boren J. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116: 1832–1844. [DOI] [PubMed] [Google Scholar]
- 3.Öörni K., Pentikäinen M. O., Ala-Korpela M., Kovanen P. T. 2000. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J. Lipid Res. 41: 1703–1714. [PubMed] [Google Scholar]
- 4.Öörni K., Posio P., Ala-Korpela M., Jauhiainen M., Kovanen P. T. 2005. Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arterioscler. Thromb. Vasc. Biol. 25: 1678–1683. [DOI] [PubMed] [Google Scholar]
- 5.Schissel S. L., Tweedie-Hardman J., Rapp J. H., Graham G., Williams K. J., Tabas I. 1996. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 98: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin C. M., Leventhal A. R., Kuriakose G., Schuchman E. H., Williams K. J., Tabas I. 2008. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 28: 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marathe S., Schissel S. L., Yellin M. J., Beatini N., Mintzer R., Williams K. J., Tabas I. 1998. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J. Biol. Chem. 273: 4081–4088. [DOI] [PubMed] [Google Scholar]
- 8.Marathe S., Kuriakose G., Williams K. J., Tabas I. 1999. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 19: 2648–2658. [DOI] [PubMed] [Google Scholar]
- 9.Schissel S. L., Jiang X., Tweedie-Hardman J., Jeong T., Camejo E. H., Najib J., Rapp J. H., Williams K. J., Tabas I. 1998. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 273: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 10.Plihtari R., Hurt-Camejo E., Öörni K., Kovanen P. T. 2010. Proteolysis sensitizes LDL particles to phospholipolysis by secretory phospholipase A2 group V and secretory sphingomyelinase. J. Lipid Res. 51: 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres Filho I. P., Leunig M., Yuan F., Intaglietta M., Jain R. K. 1994. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc. Natl. Acad. Sci. USA. 91: 2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sluimer J. C., Daemen M. J. 2009. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J. Pathol. 218: 7–29. [DOI] [PubMed] [Google Scholar]
- 13.Sluimer J. C., Gasc J. M., van Wanroij J. L., Kisters N., Groeneweg M., Sollewijn Gelpke M. D., Cleutjens J. P., van den Akker L. H., Corvol P., Wouters B. G., et al. 2008. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J. Am. Coll. Cardiol. 51: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 14.Leppänen O., Björnheden T., Evaldsson M., Boren J., Wiklund O., Levin M. 2006. ATP depletion in macrophages in the core of advanced rabbit atherosclerotic plaques in vivo. Atherosclerosis. 188: 323–330. [DOI] [PubMed] [Google Scholar]
- 15.Naghavi M., John R., Naguib S., Siadaty M. S., Grasu R., Kurian K. C., van Winkle W. B., Soller B., Litovsky S., Madjid M., et al. 2002. pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis. 164: 27–35. [DOI] [PubMed] [Google Scholar]
- 16.Haka A. S., Grosheva I., Chiang E., Buxbaum A. R., Baird B. A., Pierini L. M., Maxfield F. R. 2009. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell. 20: 4932–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima Y., Fujii H., Sumiyoshi S., Wight T. N., Sueishi K. 2007. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 27: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 18.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radding C. M., Steinberg D. 1960. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J. Clin. Invest. 39: 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 21.Bolton A. E., Hunter W. M. 1973. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem. J. 133: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 23.Ginsburg G. S., Small D. M., Atkinson D. 1982. Microemulsions of phospholipids and cholesterol esters. Protein-free models of low density lipoprotein. J. Biol. Chem. 257: 8216–8227. [PubMed] [Google Scholar]
- 24.Yohannes G., Sneck M., Varjo S. J., Jussila M., Wiedmer S. K., Kovanen P. T., Öörni K., Riekkola M. L. 2006. Miniaturization of asymmetrical flow field-flow fractionation and application to studies on lipoprotein aggregation and fusion. Anal. Biochem. 354: 255–265. [DOI] [PubMed] [Google Scholar]
- 25.Pan M., Maitin V., Parathath S., Andreo U., Lin S. X., St Germain C., Yao Z., Maxfield F. R., Williams K. J., Fisher E. A. 2008. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc. Natl. Acad. Sci. USA. 105: 5862–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paananen K., Kovanen P. T. 1994. Proteolysis and fusion of low density lipoprotein particles independently strengthen their binding to exocytosed mast cell granules. J. Biol. Chem. 269: 2023–2031. [PubMed] [Google Scholar]
- 27.Deckelbaum R. J., Shipley G. G., Small D. M. 1977. Structure and interactions of lipids in human plasma low density lipoproteins. J. Biol. Chem. 252: 744–754. [PubMed] [Google Scholar]
- 28.Deckelbaum R. J., Shipley G. G., Small D. M., Lees R. S., George P. K. 1975. Thermal transitions in human plasma low density lipoproteins. Science. 190: 392–394. [DOI] [PubMed] [Google Scholar]
- 29.D'Ulivo L., Yohannes G., Öörni K., Kovanen P. T., Riekkola M. L. 2007. Open tubular capillary electrochromatography: a new technique for in situ enzymatic modification of low density lipoprotein particles and their protein-free derivatives. Analyst (Lond.). 132: 989–996. [DOI] [PubMed] [Google Scholar]
- 30.Khoo J. C., Miller E., McLoughlin P., Steinberg D. 1990. Prevention of low density lipoprotein aggregation by high density lipoprotein or apolipoprotein A-I. J. Lipid Res. 31: 645–652. [PubMed] [Google Scholar]
- 31.Bancells C., Benitez S., Ordonez-Llanos J., Öörni K., Kovanen P. T., Milne R. W., Sanchez-Quesada J. L. 2011. Immunochemical analysis of the electronegative LDL subfraction shows that abnormal N-terminal apolipoprotein B conformation is involved in increased binding to proteoglycans. J. Biol. Chem. 286: 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenske D. B., Chana R. S., Parmar Y. I., Treleaven W. D., Cushley R. J. 1990. Structure and motion of phospholipids in human plasma lipoproteins. A 31P NMR study. Biochemistry. 29: 3973–3981. [DOI] [PubMed] [Google Scholar]
- 33.Jayaraman S., Gantz D. L., Gursky O. 2011. Effects of phospholipase A(2) and its products on structural stability of human LDL: relevance to formation of LDL-derived lipid droplets. J. Lipid Res. 52: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asatryan L., Hamilton R. T., Isas J. M., Hwang J., Kayed R., Sevanian A. 2005. LDL phospholipid hydrolysis produces modified electronegative particles with an unfolded apoB-100 protein. J. Lipid Res. 46: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lähdesmäki K., Öörni K., Alanne-Kinnunen M., Jauhiainen M., Hurt-Camejo E., Kovanen P. T. 2012. Acidity and lipolysis by group V secreted phospholipase A(2) strongly increase the binding of apoB-100-containing lipoproteins to human aortic proteoglycans. Biochim. Biophys. Acta. 1821: 257–267. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W. Y., Ishii I., Kruth H. S. 2000. Plasmin-mediated macrophage reversal of low density lipoprotein aggregation. J. Biol. Chem. 275: 33176–33183. [DOI] [PubMed] [Google Scholar]
- 37.Chanson N. F., Lontie J. F., Gulik A., Ferezou J., Carpentier Y. A. 2002. LDL binding to lipid emulsion particles: effects of incubation duration, temperature, and addition of plasma subfractions. Lipids. 37: 573–580. [DOI] [PubMed] [Google Scholar]
- 38.Chun P. W., Brumbaugh E. E., Shiremann R. B. 1986. Interaction of human low density lipoprotein and apolipoprotein B with ternary lipid microemulsion. Physical and functional properties. Biophys. Chem. 25: 223–241. [DOI] [PubMed] [Google Scholar]
- 39.Pease R. J., Milne R. W., Jessup W. K., Law A., Provost P., Fruchart J. C., Dean R. T., Marcel Y. L., Scott J. 1990. Use of bacterial expression cloning to localize the epitopes for a series of monoclonal antibodies against apolipoprotein B100. J. Biol. Chem. 265: 553–568. [PubMed] [Google Scholar]
- 40.Wang X., Pease R., Bertinato J., Milne R. W. 2000. Well-defined regions of apolipoprotein B-100 undergo conformational change during its intravascular metabolism. Arterioscler. Thromb. Vasc. Biol. 20: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 41.Tabas I., Li Y., Brocia R. W., Xu S. W., Swenson T. L., Williams K. J. 1993. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J. Biol. Chem. 268: 20419–20432. [PubMed] [Google Scholar]
- 42.Huang F., Thompson J. C., Wilson P. G., Aung H. H., Rutledge J. C., Tannock L. R. 2008. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J. Lipid Res. 49: 521–530. [DOI] [PubMed] [Google Scholar]
- 43.Tran-Lundmark K., Tran P. K., Paulsson-Berne G., Friden V., Soininen R., Tryggvason K., Wight T. N., Kinsella M. G., Boren J., Hedin U. 2008. Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ. Res. 103: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gustafsson M., Levin M., Skalen K., Perman J., Friden V., Jirholt P., Olofsson S. O., Fazio S., Linton M. F., Semenkovich C. F., et al. 2007. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ. Res. 101: 777–783. [DOI] [PubMed] [Google Scholar]
- 45.Sneck M., Kovanen P. T., Öörni K. 2005. Decrease in pH strongly enhances binding of native, proteolyzed, lipolyzed, and oxidized low density lipoprotein particles to human aortic proteoglycans. J. Biol. Chem. 280: 37449–37454. [DOI] [PubMed] [Google Scholar]
- 46.Öörni K., Kovanen P. T. 2006. Enhanced extracellular lipid accumulation in acidic environments. Curr. Opin. Lipidol. 17: 534–540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.