Abstract
The effects of Therapeutic Lifestyle Change (TLC) diets, low and high in dietary fish, on apolipoprotein metabolism were examined. Subjects were provided with a Western diet for 6 weeks, followed by 24 weeks of either of two TLC diets (10/group). Apolipoprotein kinetics were determined in the fed state using stable isotope methods and compartmental modeling at the end of each phase. Only the high-fish diet decreased median triglyceride-rich lipoprotein (TRL) apoB-100 concentration (−23%), production rate (PR, −9%), and direct catabolism (−53%), and increased TRL-to-LDL apoB-100 conversion (+39%) as compared with the baseline diet (all P < 0.05). This diet also decreased TRL apoB-48 concentration (−24%), fractional catabolic rate (FCR, −20%), and PR (−50%) as compared with the baseline diet (all P < 0.05). The high-fish and low-fish diets decreased LDL apoB-100 concentration (−9%, −23%), increased LDL apoB-100 FCR (+44%, +48%), and decreased HDL apoA-I concentration (−15%, −14%) and PR (−11%, −12%) as compared with the baseline diet (all P < 0.05). On the high-fish diet, changes in TRL apoB-100 PR were negatively correlated with changes in plasma eicosapentaenoic and docosahexaenoic acids. In conclusion, the high-fish diet decreased TRL apoB-100 and TRL apoB-48 concentrations chiefly by decreasing their PR. Both diets decreased LDL apoB-100 concentration by increasing LDL apoB-100 FCR and decreased HDL apoA-I concentration by decreasing HDL apoA-I PR.
Keywords: fatty acids, hypercholesterolemia, apolipoproteins, dyslipidemias, fish oil, lipoproteins/linetics, lipoproteins/metabolism, nutrition, omega-3 fatty acids, mathematical modeling
Elevated LDL-cholesterol is an independent risk factor for coronary heart disease (CHD) (1). Nutritional and lifestyle modifications are the cornerstone of both the National Cholesterol Education Program (NCEP) and the American Heart Association (AHA) recommendations for the treatment of hyperlipidemia to prevent and reduce the risk of CHD (2, 3). Guidelines from both organizations have centered on reducing saturated fat and cholesterol intakes. These recommendations have evolved over time, based on epidemiological, clinical, and animal studies (4–8). The current dietary recommendations for hypercholesterolemic subjects are to consume a diet consisting of 25% to 35% of energy from fat, <7% of energy from saturated fat, and <200 mg cholesterol per day (2, 3). Of note, the consumption of diets consistent with the NCEP and AHA recommendations have been shown to lower plasma total cholesterol and LDL-cholesterol effectively, in the range of 14% to 20%, compared with diets commonly consumed within the United States (8–11). An understanding of the mechanisms that confer these benefits is, therefore, of clinical relevance.
Beyond traditional macronutrients, dietary supplements may play a role in decreasing CHD risk. Fish oils are a rich source of the long-chain n-3 FAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (12–14). Epidemiological and intervention studies suggest that fish oil consumption protects against CHD (12–14). Recent clinical outcome trials with n-3 FAs reported mixed findings, however (15–21). In particular, the Alpha Omega and the ORIGIN trials both failed to show a significant cardiovascular disease (CVD) benefit (16, 21). It is noteworthy that in these trials, n-3 FAs were taken against a background of optimal medical therapy for secondary prevention and the patients were at high risk of CVD events. Whether similar results would have been observed at higher doses of n-3 FAs and/or patients on suboptimal medical therapy is not known. Furthermore, the exact significance of these findings to dietary recommendations to consume more fish is unclear. Nonetheless, fish oils, provided as fish oil capsules, have been shown to decrease plasma triglyceride and atherogenic triglyceride-rich lipoprotein (TRL) particle concentrations by decreasing hepatic TRL apoB-100 synthesis and increasing TRL-to-LDL apoB-100 conversion (22–26). The modification of lipid and lipoprotein metabolism by fish oils appears to confer anti-atherogenic benefits (12–14).
In the present study, we compared the effects of two diets adhering to the dietary recommendations of the NCEP, one high and the other low in dietary fish-derived n-3 FAs, on lipoprotein metabolism in middle-aged and elderly subjects under controlled isoenergetic metabolic conditions. Of note, in the course of this work, the terminology changed from the NCEP Step-2 diet to the Therapeutic Lifestyle Change (TLC) diet. Therefore, the designation of TLC was applied. We hypothesized that the TLC diet high in dietary fish-derived n-3 FAs would have a more favorable effect on lipoprotein metabolism compared with the TLC diet low in dietary fish-derived n-3 FAs, chiefly by decreasing the production rates (PRs)of TRL particles. Although we have previously reported the effects of these diets on plasma lipid concentrations (9–11), the effects of these diets on apoB and apoA-I kinetics, however, are novel.
METHODS
Subjects
Twenty subjects (7 men and 13 women) aged >40 years participated in this study. All women were postmenopausal. Subjects had no evidence of any chronic illness including endocrine, hepatic, renal, thyroid, or cardiac dysfunction, and were not taking any medications known to affect lipid metabolism. All were nonsmokers and consumed no alcohol during the study. The study protocol was approved by the Tufts University Human Investigation Review Committee. All subjects provided written consent. The study was completed prior to the development of the clinicaltrials.gov registry.
Study design and clinical protocols
The compositions of the diets are shown in Table 1. The nutritional composition of the diets was determined by food analysis at the Hazelton Laboratories (Madison, WI), except for the individual type of PUFAs, which were calculated using food composition tables (GRAND database, release 867; USDA Human Nutrition Research Center, Grand Forks, ND) (9–11). The 20 subjects consumed a diet approximating that of a Western-type diet (35% of energy as total fat, 14% of energy as saturated fat, 35 mg cholesterol/MJ) as the baseline diet for 6 weeks. Ten subjects were then assigned to receive the TLC diet high in fish (TLC high-fish), while the remaining 10, the TLC diet low in fish and high in poultry (TLC low-fish) for 24 weeks. The two TLC diets were similar in composition (<30% of energy as total fat, <7% of energy as saturated fat, ≤15 mg cholesterol/MJ), differing primarily in the content of fish-derived n-3 FAs (TLC high-fish: 0.70% calories or 1.23 g/day in EPA and DHA equivalent to fish served 8 times per week vs TLC low-fish: 0.13% calories or 0.27 g/day in EPA and DHA equivalent to fish served twice per week). Of note, the types of fish provided were sole fillet, salmon, and tuna. The difference in energy derived from total fat between the baseline diet and the TLC diets was compensated for by an increase in energy derived primarily from complex carbohydrate (9–11). All meals (breakfast, lunch, dinner, after-lunch snack, and after-dinner snack) were prepared by the Metabolic Research Unit Kitchen of the Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging at Tufts University. Energy intake was adjusted to maintain body weight. Subjects were allowed to eat and drink only items provided by the Center, except for water and noncaloric beverages. Subjects were advised to maintain a constant level of physical activity.
TABLE 1.
Composition of the baseline and TLC diets, as assessed by chemical analysis of fooda
| Baseline | High-Fish Diet | Low-Fish Diet | |
| (% of energy) | |||
| Carbohydrate | 49.4 ± 2.2 | 56.1 ± 2.9 | 58.2 ± 1.8 |
| Protein | 15.0 ± 1.2 | 17.2 ± 0.9 | 16.3 ± 0.7 |
| Fat | 35.4 ± 2.3 | 26.4 ± 2.0 | 25.5 ± 1.8 |
| Saturated FAs | 14.1 ± 2.2 | 4.5 ± 0.7 | 4.0 ± 0.4 |
| 14:0 | 1.6 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| 16:0 | 7.1 ± 0.5 | 2.9 ± 0.6 | 2.2 ± 0.3 |
| 18:0 | 2.9 ± 0.2 | 1.3 ± 0.4 | 1.0 ± 0.1 |
| Monounsaturated FAs | 14.5 ± 1.0 | 11.6 ± 1.4 | 10.8 ± 0.9 |
| 18:1n-9 | 12.6 ± 1.1 | 10.5 ± 2.2 | 10.7 ± 2.7 |
| Polyunsaturated FAs | 6.9 ± 1.2 | 10.3 ± 0.2 | 10.5 ± 0.2 |
| 18:2n-6b | 4.1 ± 0.2 | 7.0 ± 0.4 | 7.1 ± 0.8 |
| 18:3n-3b | 0.7 ± 0.2 | 1.9 ± 0.6 | 2.0 ± 0.2 |
| 20:4n-6b | <0.01 | 0.1 ± 0.1 | <0.02 |
| 20:5n-3b | <0.01 | 0.2 ± 0.1 | <0.02 |
| 22:6n-3b | <0.01 | 0.5 ± 0.1 | 0.1 ± 0.1 |
| Cholesterol (mg/MJ) | 35 ± 6 | 15 ± 4 | 11 ± 4 |
Data are presented as mean ± SD of triplicate samples.
Values are derived from food composition table.
At the end of each diet phase, a primed, constant infusion of deuterated-leucine (5,5,5-2H3-l-leucine; Cambridge Isotope Laboratories, Andover, MA) was administered intravenously in the fed state to determine the kinetics of apolipoproteins, as previously described (9, 27, 28). In brief, following a 12 h overnight fast, subjects were provided with the experimental meal on an hourly basis for 20 h. The amount of food provided at each hour was 5% of the daily energy intake. The composition of the portioned meal approximated that of the diet that was provided prior to the infusion period. Five hours after the first meal, a bolus dose of deuterated leucine (10 µmol/kg) was administered, followed by a 15 h infusion of deuterated leucine (10 µmol/kg/hour). Blood samples were collected prior to the isotope infusion (time = 0 minute) and at 1, 2, 3, 4, 6, 8, 10, 12, and 15 h during the infusion.
Laboratory analyses
The protocol for plasma lipid and lipoprotein characterization, quantification, and isolation of the apolipoproteins, isotopic enrichment determinations, and kinetic analysis were performed as previously described (28, 29, 30). Plasma samples were stored at −80°C, and all laboratory analyses were completed within 1 year after each diet phase. In brief, fasting lipid and apolipoprotein values were averages of the blood samples collected at weeks 4, 5, and 6 for the baseline diet, and weeks 4, 8, 16, and 24 for the TLC diets. Nonfasting lipid and apolipoprotein values were averages of blood samples collected at 1, 4, 8, 12, and 15 h during the infusion study. ApoB within plasma, TRL, and IDL were measured using a noncompetitive ELISA (31). The proportion of apoB within the TRL fraction that was apoB-100 and apoB-48 was determined by densitometric scanning of Coomassie-stained gels (31, 32). Plasma apoA-I concentration was measured using an immunoturbidimetric assay, reagents, and calibrators from Wako Diagnostics (Richmond, VA).
Fasting plasma FA composition during different diets was measured as previously described (33, 34). In brief, FAs from lipid extracts of plasma were methyl-esterified and analyzed on a 5890 gas-liquid chromatograph (Hewlett-Packard; Palo Alto, CA) fitted with a 105 m fused silica capillary column, liquid-phase RTX 2330 (Restek Corp.; Port Matilda, PA) and a flame-ionization detector. Peak identification was obtained by chromatography of known FA methyl esters. Data were normalized by comparing the area of the FA peak with the area of the internal standard peak, heptadecanoic acid. The FA composition is expressed as a percentage of the total area of the identified FA peaks.
Kinetic analyses
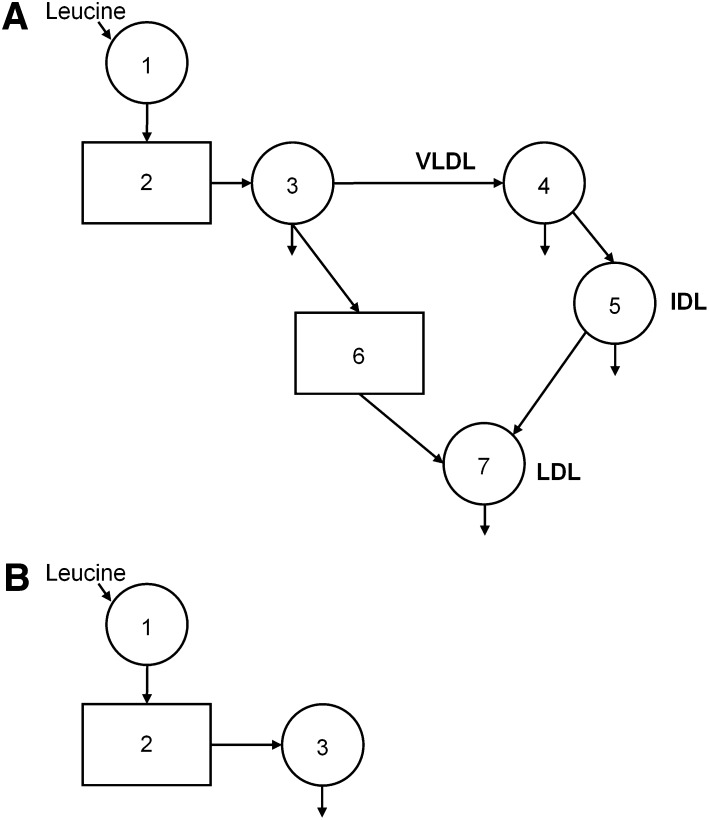
A multi-compartmental model (Fig. 1A) was used to describe TRL-, IDL- and LDL apoB-100 kinetics. The SAAM II program (University of Washington, Seattle, WA) was used for modeling the data. The details and assumptions of the model have been described previously (29, 30). The model consists of seven compartments. Compartment 1 represents the precursor compartment, the plasma leucine pool. Compartment 2 is an intracellular delay compartment that accounts for the synthesis and secretion of apoB-100 into the TRL pool (compartment 3). Compartments 3 and 4 account for the kinetics of apoB-100 in the TRL fraction. Compartment 5 accounts for the kinetics of IDL apoB-100. The kinetics of LDL apoB-100 are described by a plasma compartment (compartment 7). In order to fit the LDL apoB-100 tracer data, a delay compartment (compartment 6) between the TRL (compartment 3) and LDL compartments was required. The presence of a delay between TRL- and LDL apoB-100 has been previously reported, with studies suggesting that VLDL may leave plasma and reappear later in LDL (35, 36). The delay time for compartment 6 was (mean ± SEM) 0.40 ± 0.08 h for the TLC high-fish diet group and 0.46 ± 0.10 h for the TLC low-fish group. There was no significant difference in the delay time between the diet groups at baseline. No significant effect of diet on this delay time was observed.
Fig. 1.
A: Compartment model describing TRL, IDL and LDL apoB-100 kinetics The apoB-100 model consisted of seven compartments. Compartment 1 represents the precursor compartment, the plasma leucine pool. Compartment 2 is an intracellular delay compartment that accounts for the synthesis and secretion of apoB-100 into the TRL pool (compartment 3). Compartments 3 and 4 account for the kinetics of apoB-100 in the TRL fraction. Compartment 5 accounts for the kinetics of IDL apoB-100. The kinetics of LDL apoB-100 are described by a plasma compartment (compartment 7). In order to fit the LDL apoB-100 tracer data, a delay compartment (compartment 6) between the TRL and LDL compartments was required. B: Compartment model describing TRL apoB-48 and HDL apoA-I kinetics The kinetics of TRL apoB-48 and HDL apoA-I were described by a three-compartment model. Compartment 1 represents the precursor compartment, which is the plasma leucine pool. Compartment 2 is an intracellular delay compartment that accounts for the synthesis and secretion of apoB-48 or apoA-I into the TRL or the HDL pool, respectively (compartment 3). Compartment 3 accounts for the kinetics of apoB-48 or apoA-I in the TRL or HDL fraction, respectively. Of note, the TRL apoB-100 plateau was used as the tracer plateau for HDL apoA-I kinetic analysis.
The kinetics of TRL apoB-48 and HDL apoA-I were described by a three-compartment model (Fig. 1B). Compartment 1 represents the precursor compartment, the plasma leucine pool. Compartment 2 is an intracellular delay compartment that accounts for the synthesis and secretion of apoB-48 or apoA-I into the TRL or the HDL pool, respectively (compartment 3). Compartment 3 accounts for the kinetics of apoB-48 or apoA-I in the TRL or HDL fraction, respectively. Of note, the TRL apoB-100 plateau was used as the tracer plateau for HDL apoA-I kinetic analysis.
The fractional catabolic rates (FCRs) of TRL, IDL, and LDL apoB-100, TRL apoB-48, and HDL apoA-I were derived from the model parameters giving the best fit. The corresponding PRs were calculated as the product of FCR and pool size, which equals the plasma concentration multiplied by plasma volume estimated as 4.5% of body weight in kilograms.
Statistical analyses
Statistical analyses were performed using STATA (Version 11.1; StataCorp, College Station, TX). Data are presented as medians and inter-quartile ranges to account for the skewed nature of the parameters. Baseline values were different between the diet groups. As such, the change from baseline (change score) was used in all analyses to account for these differences. The change from baseline value of each variable was defined as the value of the variable on the TLC diet (high-fish or low-fish diets) minus the value of that same variable on the corresponding baseline diet. Wilcoxon signed-rank tests were performed to test for difference between each TLC diet group and their corresponding baseline diet. Mann-Whitney tests were performed to test for difference in the change from baseline in variables between the TLC high-fish diet and the TLC low-fish diet. Spearman correlation coefficients were determined to examine the statistical associations between changes from baseline in variables. The P values are reported, with statistical significance set at the 5% level.
RESULTS
The two groups of subjects (TLC high-fish vs. TLC low-fish) were similar with respect to age [67 (60–70) vs. 66 (44–71) years] and body mass index [26.0 (22.7–28.0) vs. 24.7 (23.5–28.8) kg/m2], and had, on average, mild to moderate hypercholesterolemia [fasting lipids and lipoprotein: total cholesterol, 5.16 (4.78–6.26) vs. 5.47 (4.81–6.81) mmol/l and LDL cholesterol: 3.37 (3.03–4.51) vs. 3.46 (3.25–4.70) mmol/l]. The TLC high-fish diet group consisted of four men and six women, whereas the TLC low-fish group, three men and seven women. No significant changes in body weight were observed in either TLC diet group compared with the baseline diet.
Effects on fasting plasma FA profile
The effects of the TLC diets on fasting plasma FA profile in the subjects are shown in Table 2. The decreases in oleic (18:1n-9) and arachidonic (20:4n-6) acids were significantly greater with the TLC high-fish compared with the low-fish diet (P < 0.02 and P = 0.02, respectively). The increases in EPA (20:5n-3) and DHA (22:6n-3) were, on average, 1.6- and 1.3-fold higher with the TLC high-fish diet compared with the TLC low-fish diet, respectively (P < 0.01 for both). Both TLC diets reduced saturated FAs (14:0, 16:0, and 18:0) and increased α-linolenic acid (18:3n-3) by a similar magnitude.
TABLE 2.
Fasting plasma FA profile of study subjects following baseline, TLC high-fish, and TLC low-fish diets
| FAs (mol %) | Baseline Diet | High-Fish Diet | Median % Change from Baseline Diet | Baseline Diet | Low-Fish Diet | Median % Change from Baseline Diet | High-Fish Diet vs. Low-Fish Diet aP |
| 14:00 | 1.00 (0.79, 1.54) | 0.75 (0.71, 1.08)b | −23 | 0.86 (0.72, 0.98) | 0.65 (0.59, 0.95)b | −18 | 0.41 |
| 16:00 | 20.0 (19.3, 21.8) | 18.8 (18.2, 20.3)b | −6 | 20.3 (18.8, 22.1) | 18.9 (16.5, 21.3) | −5 | 0.62 |
| 18:00 | 6.33 (6.19, 6.77) | 6.18 (5.94, 6.27)b | −5 | 6.28 (5.86, 7.55) | 6.10 (5.37, 7.25)b | −6 | 0.22 |
| 18:1n-9 | 17.3 (16.1, 18.6) | 15.0 (13.8, 16:1)b | −13 | 18.3 (16.7, 19.3) | 18.9 (17.5, 20.4) | 5 | <0.01 |
| 18:2n-6 | 32.0 (27.6, 33.9) | 34.4 (29.9, 35.8) | 7 | 36.9 (30.2, 40.8) | 36.7 (32.3, 43.6) | 2 | 0.12 |
| 18:3n-3 | 0.57 (0.52, 0.81) | 1.17 (1.04, 1.22)b | 106 | 0.47 (0.39, 0.65) | 1.03 (0.47, 1.38)b | 106 | 0.57 |
| 20:4n-6 | 7.87 (7.13, 8.53) | 5.92 (5.43, 6.51)b | −28 | 8.37 (7.70, 8.94) | 7.17 (5.39, 7.90)b | −17 | 0.02 |
| 20:5n-3 | 0.67 (0.55, 0.77) | 2.72 (1.82, 3.39)b | 287 | 0.47 (0.42, 0.62) | 0.75 (0.56, 0.92)b | 53 | <0.01 |
| 22:6n-3 | 2.97 (2.35, 3.70) | 5.15 (4.15, 6.23)b | 65 | 1.74 (1.70, 2.30) | 2.09 (1.84, 2.54)b | 10 | <0.01 |
Data are presented as median (IQR).
Comparison between change from baseline of high-fish vs. low-fish diet. n = 10.
P < 0.05 compared with corresponding baseline diet.
Effects on plasma lipids and apolipoproteins
The effects of the two TLC diets on nonfasting plasma lipoprotein and apolipoprotein concentrations are shown in Table 3. There was a 24% decrease in plasma triglycerides with the TLC high-fish diet compared with a 1% decrease with the TLC low-fish diet (P = 0.02). Although both diets decreased HDL cholesterol concentration, the decrease was significantly less in the TLC high-fish compared with the TLC low-fish diet (P = 0.04). Both TLC diets had comparable effects on total cholesterol, LDL cholesterol and plasma apoB and apoA-I concentrations.
TABLE 3.
Postprandial plasma lipid, lipoprotein, and apolipoprotein concentrations following baseline, TLC high-fish, and TLC low-fish diets
| Baseline Diet | High-Fish Diet | Median % Change from Baseline Diet | Baseline Diet | Low-Fish Diet | Median % Change from Baseline Diet | High-Fish vs. Low-FishaP | |
| Total cholesterol, mmol/l | 4.77 (4.29, 5.88) | 3.99 (3.72, 5.02)b | −16 | 5.02 (4.39, 6.24) | 3.97 (3.50, 4.96)b | −19 | 0.26 |
| Triglyceride, mmol/l | 1.93 (1.51, 2.87) | 1.46 (1.21, 1.79)b | −24 | 1.56 (1.38, 2.34) | 1.76 (1.30, 2.07) | −1 | 0.02 |
| LDL cholesterol, mmol/l | 3.24 (2.97, 3.71) | 2.50 (2.08, 2.88)b | −22 | 3.48 (3.11, 4.32) | 2.70 (2.31, 3.34)b | −24 | 0.82 |
| HDL cholesterol, mmol/l | 1.16 (0.83, 1.48) | 1.04 (0.71, 1.52) | −9 | 1.06 (0.93, 1.31) | 0.93 (0.71, 1.06)b | −19 | 0.04 |
| Apolipoprotein B, g/l | 0.97 (0.92, 1.15) | 0.82 (0.79, 0.97)b | −14 | 1.12 (0.99, 1.40) | 0.98 (0.73, 1.21)b | −20 | 0.11 |
| Apolipoprotein A-I, g/l | 1.24 (1.09, 1.39) | 1.04 (0.92, 1.14)b | −18 | 1.29 (1.14, 1.48) | 1.08 (0.93, 1.27)b | −21 | 0.94 |
Data are presented as median (IQR).
P < 0.05 compared with corresponding baseline diet. To convert cholesterol, HDL cholesterol, LDL cholesterol and non-HDL cholesterol in mmol/l to mg/dl, divide by 0.0259; triglycerides in mmol/l to mg/dl, divide by 0.0113.
Comparison between change from baseline of high-fish vs. low-fish diet, n = 10.
Effects on TRL, IDL, and LDL apoB-100 metabolism
The effects of the TLC diets on TRL, IDL, and LDL apoB-100 concentrations and kinetics are shown in Table 4. There was a 23% decrease in TRL apoB-100 concentration with the TLC high-fish diet compared with a 1% increase with the TLC low-fish diet (P < 0.01). TRL apoB-100 PR was significantly decreased by 9% with the TLC high-fish diet as compared with a 1% increase with the TLC low-fish diet (P = 0.03). The direct catabolism of TRL apoB-100 was significantly decreased by 53% with the TLC high-fish diet compared with the 3% increase with the TLC low-fish diet (P < 0.01). Although both diets decreased LDL apoB-100 concentration, the decrease was significantly less with the TLC high-fish diet compared with the TLC low-fish diet (P = 0.01). There was a 39% increase in TRL-to-LDL apoB-100 conversion with the TLC high-fish diet compared with a 7% increase with the TLC low-fish diet, but this failed to reach statistical significance (P = 0.08). Both diets had comparable effects on IDL and LDL apoB-100 FCR and PR, and the conversion of TRL-to-IDL apoB-100 and IDL-to-LDL apoB-100.
TABLE 4.
Concentrations and kinetics of TRL, IDL and LDL apoB-100, TRL apoB-48, and HDL apoA-I following baseline, TLC high-fish, and TLC low-fish diets
| Baseline Diet | High-Fish Diet (HF) | Median % Change from Baseline Diet | Baseline Diet | Low-Fish Diet | Median % Change from Baseline Diet | High Fat vs. Low Fat aP | |
| Concentrations (mg/l) | |||||||
| TRL apoB-100 | 9.57 (5.26, 16.3) | 6.58 (4.12, 40.4)b | −23 | 5.52 (3.53, 8.63) | 5.63 (3.32, 8.10) | 1 | <0.01 |
| IDL apoB-100 | 2.27 (1.03, 2.45) | 1.77 (1.44, 2.19) | −3 | 1.59 (0.88, 3.21) | 1.3 (1.00, 2.66) | −17 | 0.97 |
| LDL apoB-100 | 85.1 (75.9, 96.7) | 73.0 (67.6, 91.1)b | −9 | 105 (91.9, 133) | 90.3 (67.1, 111)b | −23 | 0.01 |
| TRL apoB-48 | 0.48 (0.31, 0.9) | 0.42 (0.10, 0.56)b | −24 | 0.33 (0.31, 0.71) | 0.34 (0.27, 0.79) | 6 | 0.04 |
| HDL apoA-I | 120 (109, 139) | 104 (92, 114)b | −15 | 129 (114, 148) | 108 (93, 127)b | −14 | 0.77 |
| Fractional catabolic rates (pools/day) | |||||||
| TRL apoB-100 | 5.91 (4.18, 8.89) | 8.22 (5.44, 10.8)b | 16 | 7.71 (5.04, 11.5) | 7.1 (4.84, 16.3) | −9 | 0.50 |
| IDL apoB-100 | 2.92 (2.12, 3.39) | 3.79 (2.62, 5.27) | 37 | 2.93 (1.93, 5.64) | 3.82 (2.45, 5.50) | 12 | 0.50 |
| LDL apoB-100 | 0.25 (0.22, 0.38) | 0.43 (0.32, 0.46)b | 44 | 0.28 (0.22, 0.36) | 0.43 (0.35, 0.54)b | 48 | 0.76 |
| TRL apoB-48 | 4.93 (3.69, 5.79) | 3.37 (2.73 . 5.24)b | −20 | 4.58 (2.78, 5.10) | 3.71 (3.02, 5.14) | 8 | 0.04 |
| HDL apoA-I | 0.21 (0.19, 0.26) | 0.22 (0.18, 0.27) | 9 | 0.22 (0.18, 0.27) | 0.21 (0.18, 0.26) | 2 | 0.97 |
| Production rates (mg/kg/day) | |||||||
| TRL apoB-100 | 28.2 (20.4, 34.3) | 23.2 (18.1, 27.1)b | −9 | 22 (14.9, 23.2) | 22.9 (16.0, 26.5) | 1 | 0.03 |
| IDL apoB-100 | 3.41 (1.07, 3.63) | 2.75 (1.99, 4.00) | 20 | 2.01 (1.28, 7.40) | 2.97 (1.48, 5.23) | 10 | 0.55 |
| LDL apoB-100 | 9.45 (7.97, 14.3) | 14.3 (11.2, 17.5)b | 32 | 12.9 (10.9, 17.4) | 14.7 (12.0, 20.0) | 7 | 0.29 |
| TRL apoB-48 | 1.02 (0.68, 1.71) | 0.57 (0.23, 0.94)b | −50 | 0.74 (0.60, 0.96) | 0.80 (0.48, 1.13) | −9 | 0.04 |
| HDL apoA-I | 12.2 (10.9, 13.5) | 10.7 (7.71, 13.7)b | −11 | 12.0 (10.2, 15.6) | 9.47 (8.71, 14.2)b | −12 | 0.53 |
| Percent conversion (%) | |||||||
| TRL to IDL apoB-100 | 10.6 (7.92, 13.3) | 12.1 (9.32, 23.5) | 27 | 10.5 (6.65, 34.4) | 11.61 (6.13, 28.8) | −9 | 0.33 |
| IDL to LDL apoB-100 | 100 (100, 100) | 100 (100, 100) | … | 100 (100, 100) | 100 (100, 100) | … | … |
| TRL to LDL apoB-100 | 48.5 (26.6, 65.5) | 79.6 (45.8, 92.3)b | 39 | 70.1 (58.9, 83.8) | 75.63 (67.7, 88.3) | 7 | 0.08 |
| Absolute conversion rates (mg/kg/day) | |||||||
| TRL to IDL apoB-100 | 3.4 (1.1, 3.6) | 2.7 (2.0, 4.0) | 20 | 2.0 (1.3, 7.4) | 3.0 (1.5, 5.2) | 10 | 0.55 |
| IDL to LDL apoB-100 | 3.4 (1.1, 3.6) | 2.7 (2.0, 4.0) | 20 | 2.0 (1.3, 7.4) | 3.0 (1.5, 5.2) | 10 | 0.55 |
| TRL to LDL apoB-100 | 9.4 (8.0, 14.3) | 14.3 (11.2, 17.5)b | 32 | 12.9 (10.9, 17.4) | 14.7 (12.0, 20.0) | 6 | 0.29 |
| TRL apoB-100 direct catabolism | |||||||
| (mg/kg/day) | 17 (7.20, 23.2) | 4.4 (1.10, 15.9)b | −53 | 5.2 (1.90, 7.10) | 5.4 (3.20, 7.50) | 3 | <0.01 |
Data are presented as median (IQR).
Comparison between change from baseline of high-fish vs. low-fish diet, n = 10.
P < 0.05 compared with corresponding baseline diet.
Effects on TRL apoB-48 metabolism
The effects of the TLC diets on TRL apoB-48 concentration and kinetics are shown in Table 4. There were 27%, 32%, and 51% decreases in TRL apoB-48 concentration, FCR, and PR, respectively, with the TLC high-fish diet as compared with 6% and 8% increases in TRL apoB-48 concentration and FCR, and an 11% decrease in PR with the TLC low-fish diet (P = 0.04 for all).
Effects on HDL apoA-I metabolism
The TLC diets decreased HDL apoA-I concentration and PR by a comparable magnitude, and neither diet altered HDL apoA-I FCR significantly (Table 4).
Associations between absolute changes in TRL apoB-100 concentrations and kinetics with fasting plasma FA concentrations
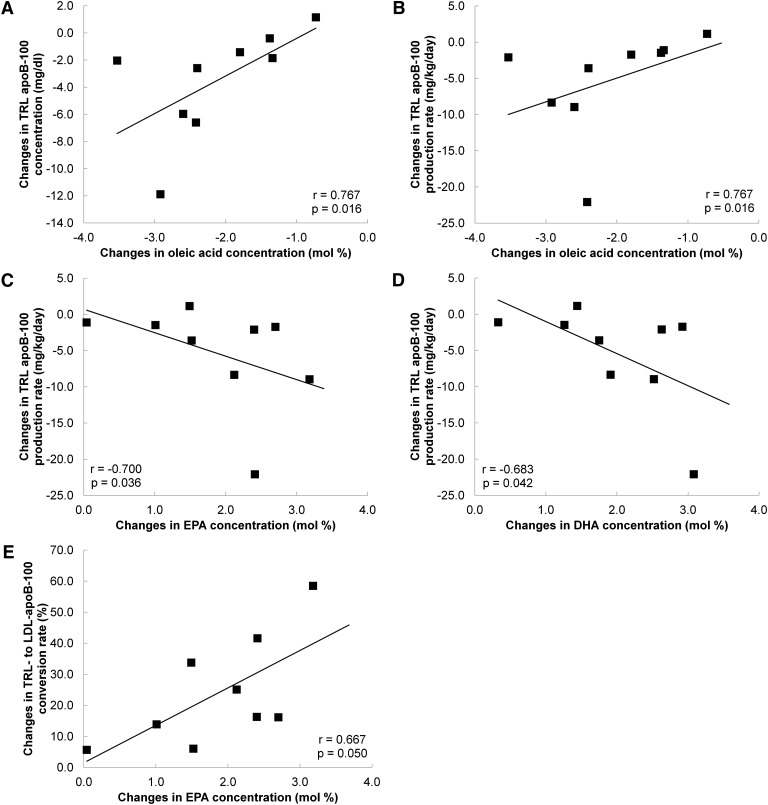
The associations between the changes in TRL apoB-100 concentration and kinetics with the changes in fasting plasma FA concentrations were explored (Fig. 2). With the TLC high-fish diet, the changes in TRL apoB-100 concentration were significantly correlated with the changes in plasma oleic acid (Fig. 2A). The changes in TRL apoB-100 PR were significantly correlated with the changes in plasma oleic acid (Fig. 2B), EPA (Fig. 2C), and DHA (Fig. 2D). The changes in TRL-to-LDL apoB-100 conversion were significantly correlated with the changes in plasma EPA (Fig. 2E). The changes in TRL apoB-100 FCR and TRL-toLDL apoB-100 conversion rate were correlated with the changes in plasma DHA, but these failed to reach statistical significance (r = 0.617, P = 0.07 and r = 0.57, P = 0.11, respectively). All of these associations were also observed with the TLC low-fish diet but were not statistically significant.
Fig. 2.
Correlations between the changes in TRL apoB-100 concentration with changes in plasma oleic acid (A), the changes in TRL apoB-100 production rate with changes in plasma oleic acid (B), EPA (C), and DHA (D), and the changes in TRL-to-LDL apoB-100 conversion rate with changes in plasma EPA (E) in the NCEP high-fish diet group (n = 9; the plasma FA profile of one subject was not measured during the study). Spearman correlation analyses were performed to examine the statistical associations between changes from baseline in variables.
DISCUSSION
We provide new information on the effects of two diets adhering to the TLC diet criteria, one high and the other low in dietary fish-derived n-3 FAs, on lipoprotein metabolism in middle-aged and elderly men and women. The TLC high-fish diet decreased postprandial plasma triglyceride concentration, with concomitant reductions in TRL apoB-100 concentration, PR, and direct catabolism, and TRL apoB-48 concentration, FCR, and PR. Both TLC diets had comparable effects on LDL apoB-100 and HDL apoA-I metabolism. These effects were achieved with no significant change in body weight.
Previous kinetics studies
To date, only three studies have examined the effects of decreasing dietary saturated fat together with increasing n-6 FAs on apoB-100 metabolism (37–39). These studies have yielded inconsistent results due, in part, to different study populations and/or the degree of saturated fat replacement with n-6 FAs (please see supplementary data). No studies have examined the effects of these dietary changes on TRL apoB-48 metabolism in humans. Furthermore, no studies have examined the effects of dietary fish-derived n-3 FAs as part of a therapeutic low total and saturated fat, and cholesterol diet on lipoprotein metabolism. Our study extends previous reports by examining the effects of two TLC diets, in which total fat, saturated fat, and cholesterol content were lowered, with one low and the other high in dietary fish-derived FAs, on TRL, IDL, and LDL apoB-100, TRL apoB-48, and HDL apoA-I using stable isotope methods.
Effects of TLC diets on apoB-100 metabolism
Studies in animals and humans have shown that the hypotriglyceridemic effect of n-3 FAs chiefly involves the suppression of TRL apoB-100 synthesis (22–26, 40, 41). This suppression is due to reduced triglyceride synthesis via inhibition of diacylglycerol acyltransferase, FA synthase, and acetyl CoA carboxylase enzyme activities (14). N-3 FAs also enhance FA β oxidation via a peroxisome proliferator-activated receptor-α-mediated pathway resulting in decreased substrate availability for triglyceride formation (14). In addition, n-3 FAs suppress transcription of the sterol regulatory element binding protein (SREBP)-1c gene and hence, inhibit de novo lipogenesis (14). Furthermore, n-3 FAs may stimulate the post-endoplasmic reticulum (ER) presecretory proteolysis pathway, thereby increasing the degradation of newly synthesized apoB (42). Our study concurred with earlier human studies that n-3 FAs decrease TRL apoB-100 hepatic secretion and concentration.
Of interest, the associations between changes in TRL apoB-100 concentrations and PRs, with fasting plasma FAs, particularly oleic acid, EPA, and DHA, are consistent with prior in vitro studies. These studies, utilizing either HepG2 cells and/or perfused rat liver, suggest that oleic acid stimulates apoB-100 secretion, without altering apoB-100 mRNA levels, whereas EPA and DHA have the opposite effects (43–45). These observations, together with our findings, support the concept of differential modes of action of individual FAs in regulating TRL apoB-100 secretion (43).
We observed that dietary fish-derived n-3 FAs decreased TRL apoB-100 direct catabolism, and, by implication, conversion of TRL to LDL was increased. Consistent with this observation, others (46) have shown that enrichment of n-3 FAs in TRL preferentially converts TRL to LDL. Furthermore, a positive association between changes in TRL-to-LDL conversion with changes in EPA, and a concomitant increase in LDL apoB-100 PR were observed. The mechanism for the accelerated conversion of TRL to LDL, however, is unknown. TRL particles enriched with n-3 FAs may be better substrates for lipolysis. Animal studies suggest that fish oil increased margination of TRL (binding to LPL at the endothelium) (47). If TRL binds more tightly to LPL, the period of interaction may be longer, resulting in the formation of smaller remnants. This hypothesis is supported by experimental data indicating that heparin injection in primates, which stimulates release of LPL from heparin sulfate proteoglycans, transiently increased LDL concentrations (48) and that fish oil supplementation in humans increased LPL activity (46). In addition, we cannot rule out the possibility that higher n-3 FA intake results in the secretion of smaller and less apoE-enriched TRL particles. Yamada et al. previously reported that apoE-poor TRL particles are preferentially converted to LDL (49). Of note, no change in TRL apoB-100 metabolism was observed with the TLC low-fish diet. This supports the notion that the reductions in TRL apoB-100 secretion and altered TRL apoB-100 catabolism were chiefly an effect of dietary fish-derived n-3 FAs.
Moderate fat and low cholesterol diets are recommended nutritional modifications to reduce CHD risk due, in part, to their hypocholesterolemic effects (4–8). In the present study, both TLC diets significantly reduced total, LDL cholesterol, and LDL apoB-100 concentrations. This was chiefly due to increased LDL apoB-100 FCR. Animal studies have reported increases in LDL apoB-100 FCR with reduced dietary saturated fat and cholesterol intake (50–53). In addition, fat and cholesterol feeding have been associated with a reduction in SREBP-2 and LDL receptor mRNA expression (51, 52, 54). Furthermore, postprandial enrichment of TRL particles with apoE after fat ingestion was recently shown to inhibit LDL binding and uptake by the LDL receptor in HepG2 cells (55). Collectively, these observations imply that reductions in dietary saturated fat and cholesterol, may upregulate hepatic expression of LDL receptors and hence, binding and clearance of LDL particles in vivo. Of note, the incorporation of dietary fish-derived n-3 FAs did not confer additional effect on LDL apoB-100 FCR. The decrease in LDL apoB-100 concentration, however, was of a smaller magnitude relative to the TLC low-fish diet, in spite of comparable increases in LDL apoB-100 FCR. This is probably due to the enhanced TRL-to-LDL apoB-100 conversion with the high-fish diet. Nonetheless, the decrease in total plasma apoB-100 concentration was similar with both TLC diets.
Effects of TLC diets on TRL apoB-48 metabolism
The assembly and secretion of chylomicrons by the small intestines in humans is a complex process that brings together the very large and highly amphipathic apoB polypeptide and four different classes of lipids in a fixed sequence. Microsomal transfer protein (MTP) plays a key role in the assembly and secretion of these particles by transferring lipids from the ER membrane to the lipid binding domains of nascent apoB-48, resulting in small, dense particles that are triglyceride poor (56, 57). In addition, MTP facilitates the production of small triglyceride droplets in the smooth ER. It is hypothesized that these droplets and small, dense apoB-48 fuse to form mature chylomicron particles (58).
We showed, for the first time, that dietary fish-derived n-3 FAs decreased TRL apoB-48 concentration by decreasing TRL apoB-48 PR in humans. Our finding is consistent with observations from in vitro and animal studies that showed that n-3 FAs suppressed TRL apoB-48 synthesis (59–62) either by decreasing apoB-48 mRNA expression (60) and/or increasing posttranslational degradation of newly synthesized apoB-48 (59, 61). It is also plausible that additional pathways, including altered MTP mRNA expression and activity, may explain changes in apoB-48 PR, although future studies are required. Of note, TRL apoB-48 FCR was significantly decreased with the TLC high-fish diet. The exact mechanism for the reduction in TRL apoB-48 FCR is, however, unclear. It is possible that these particles were less triglyceride-enriched and were, therefore, poorer substrates for lipoprotein lipase.
Effects of TLC diets on HDL apoA-I metabolism
We showed that both TLC diets reduced HDL cholesterol and apoA-I concentrations by decreasing HDL apoA-I production to a similar extent. From these data, it can be inferred that these changes reflect the reductions in dietary saturated fat and cholesterol rather than fish intake per se. Animal studies have shown that dietary saturated fat and cholesterol increased HDL cholesterol and apoA-I concentrations between 36% and 67% (52, 63–65), comparable to what we observed in our studies in humans. Similarly, Vessby et al. reported reductions in HDL cholesterol (−15%) and apoA-I (−9%) concentrations with a diet low in total fat (35% energy from fat) in nine hypercholesterolemic subjects (66).
The decrease in HDL apoA-I PR may be due to downregulation of apoA-I mRNA expression with dietary fat and cholesterol reduction (64, 67). Studies have shown that dietary saturated fat and cholesterol intake upregulates posttranslational apoA-I expression, involving either increased translation of the apoA-I mRNA or less intracellular apoA-I degradation (64). Dietary fat has also been shown to increase the fraction of apoA-I mRNA in the translating pool (67). Hence, reductions in dietary fat and cholesterol may have decreased HDL apoA-I production. Whether the lower dietary fat and cholesterol translates to a need for less HDL-mediated cholesterol removal and hence, lower HDL apoA-I PR, needs further exploration (64, 68). Of note, the total cholesterol:HDL cholesterol ratio, a powerful predictor of CHD risk, was significantly decreased in a pooled analysis of both diets (−6.2%, P < 0.01). Hence, we propose that the reduction in HDL cholesterol with the TLC diets should not be viewed as a negative outcome with regard to CHD risk.
Limitations
Several limitations are noteworthy. We studied middle-aged and elderly subjects with mild-to-moderate hypercholesterolemia. Studies in hypertriglyceridemic and type 2 diabetic subjects are warranted. Our study examined the combined effects of decreasing dietary total fat, saturated fat, and cholesterol content. Future studies to distinguish the independent effects of these dietary macronutrients, as well as the impact of specific carbohydrate (69), are required. TRL subspecies and TRL triglycerides kinetics were not examined, but we anticipate that their production will also be reduced with n-3 FA consumption. ApoB-containing particles are subject to modifications by hepatic lipases and may exhibit kinetic and structural heterogeneity based on the content of regulatory apolipoproteins, including apoC-III and apoE (70). Measurements of lipase masses and activities, as well as the concentrations and kinetics of key regulatory apolipoproteins, may further elucidate our findings.
Implications and conclusions
Our study showed that the consumption of dietary fish-derived n-3 FAs against the background of the TLC therapeutic diet decreased plasma triglyceride and TRL concentrations. This is particularly relevant in light of recent evidence that nonfasting plasma triglycerides are risk factors for CHD (71–73). The reduction in TRL concentration with n-3 FA intake was, chiefly, a function of decreased hepatic and intestinal TRL particle production. Consumption of various TLC diets, low in fat, saturated fat, and cholesterol content, was associated with significant reductions in LDL cholesterol and LDL apoB-100 due to increased catabolism of LDL particles. These diets were also associated with decreases in HDL cholesterol, a function of decreased HDL apoA-I production. The changes in lipoprotein metabolism with the TLC diets may provide a mechanistic explanation for the CHD benefits reported in intervention studies (6, 11–13, 74). Our study supports the nutritional and lifestyle recommendations of the NCEP and AHA for the prevention of CHD to lower saturated fat and cholesterol to that of the TLC diet and increase the consumption of fish-derived FAs.
Supplementary Material
Footnotes
Abbreviations:
- AHA
- American Heart Association
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- ER
- endoplasmic reticulum
- FCR
- fractional catabolic rate
- MTP
- microsomal transfer protein
- NCEP
- National Cholesterol Education Program
- PR
- production rate
- SREBP
- sterol regulatory element binding protein
- TLC
- Therapeutic Lifestyle Change
- TRL
- triglyceride-rich lipoprotein
The study was supported by Grants HL-39326 and HL-56895 from the National Institute of Health and contract 1950-51000-072-02S from the U.S. Department of Agriculture Research Services. E.M.M.O. is supported by a National Health and Medical Research Council (NHMRC) of Australia Postdoctoral Research Fellowship. P.H.R.B. is an NHMRC Senior Research Fellow. All authors declare that there are no potential sources of conflict of interest.
REFERENCES
- 1.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr, Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr, Stone N. J. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110: 227–239. [DOI] [PubMed] [Google Scholar]
- 2.Adult Treatment Panel III. 2001. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. J. Am. Med. Assoc. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 3.Krauss R. M., Eckel R. H., Howard B., Appel L. J., Daniels S. R., Deckelbaum R. J., Erdman J. W., Jr, Kris-Etherton P., Goldberg I. J., Kotchen T. A., et al. 2000. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 102: 2284–2299. [DOI] [PubMed] [Google Scholar]
- 4.Berglund L., Lefevre M., Ginsberg H. N., Kris-Etherton P. M., Elmer P. J., Stewart P. W., Ershow A., Pearson T. A., Dennis B. H., Roheim P. S., et al. 2007. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am. J. Clin. Nutr. 86: 1611–1620. [DOI] [PubMed] [Google Scholar]
- 5.Vincent-Baudry S., Defoort C., Gerber M., Bernard M. C., Verger P., Helal O., Portugal H., Planells R., Grolier P., Amiot-Carlin M. J., et al. 2005. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am. J. Clin. Nutr. 82: 964–971. [DOI] [PubMed] [Google Scholar]
- 6.Obarzanek E., Sacks F. M., Vollmer W. M., Bray G. A., Miller E. R., III, Lin P. H., Karanja N. M., Most-Windhauser M. M., Moore T. J., Swain J. F., et al. 2001. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am. J. Clin. Nutr. 74: 80–89. [DOI] [PubMed] [Google Scholar]
- 7.Singh R. B., Dubnov G., Niaz M. A., Ghosh S., Singh R., Rastogi S. S., Manor O., Pella D., Berry E. M. 2002. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 360: 1455–1461. [DOI] [PubMed] [Google Scholar]
- 8.Knopp R. H., Retzlaff B., Walden C., Fish B., Buck B., McCann B. 2000. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc. Soc. Exp. Biol. Med. 225: 191–199. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z., Welty F. K., Dolnikowski G. G., Lichtenstein A. H., Schaefer E. J. 2001. Effects of a National Cholesterol Education Program Step II Diet on apolipoprotein A-IV metabolism within triacylglycerol-rich lipoproteins and plasma. Am. J. Clin. Nutr. 74: 308–314. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer E. J., Lamon-Fava S., Ausman L. M., Ordovas J. M., Clevidence B. A., Judd J. T., Goldin B. R., Woods M., Gorbach S., Lichtenstein A. H. 1997. Individual variability in lipoprotein cholesterol response to National Cholesterol Education Program Step 2 diets. Am. J. Clin. Nutr. 65: 823–830. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer E. J., Lichtenstein A. H., Lamon-Fava S., Contois J. H., Li Z., Goldin B. R., Rasmussen H., McNamara J. R., Ordovas J. M. 1996. Effects of National Cholesterol Education Program Step 2 diets relatively high or relatively low in fish-derived fatty acids on plasma lipoproteins in middle-aged and elderly subjects. Am. J. Clin. Nutr. 63: 234–241. [DOI] [PubMed] [Google Scholar]
- 12.Saravanan P., Davidson N. C., Schmidt E. B., Calder P. C. 2010. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 376: 540–550. [DOI] [PubMed] [Google Scholar]
- 13.Lavie C. J., Milani R. V., Mehra M. R., Ventura H. O. 2009. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54: 585–594. [DOI] [PubMed] [Google Scholar]
- 14.Bays H. E., Tighe A. P., Sadovsky R., Davidson M. H. 2008. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 6: 391–409. [DOI] [PubMed] [Google Scholar]
- 15.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 354: 447–455. [PubMed] [Google Scholar]
- 16.Kromhout D., Giltay E. J., Geleijnse J. M. 2010. N-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 363: 2015–2026. [DOI] [PubMed] [Google Scholar]
- 17.Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., Deadman N. M. 1989. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 2: 757–761. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 19.Rauch B., Schiele R., Schneider S., Diller F., Victor N., Gohlke H., Gottwik M., Steinbeck G., Del Castillo U., Sack R., et al. 2010. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 122: 2152–2159. [DOI] [PubMed] [Google Scholar]
- 20.Galan P., Kesse-Guyot E., Czernichow S., Briancon S., Blacher J., Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. Epub ahead of print. November 9, 2010; doi: 10.1136/bmj.c6273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ORIGIN Trial Investigators. 2012. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. Epub ahead of print, June 11, 2012. doi: 10.1056/NEJMoa1203859. [Google Scholar]
- 22.Nestel P. J., Connor W. E., Reardon M. F., Connor S., Wong S., Boston R. 1984. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J. Clin. Invest. 74: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bordin P., Bodamer O. A., Venkatesan S., Gray R. M., Bannister P. A., Halliday D. 1998. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur. J. Clin. Nutr. 52: 104–109. [DOI] [PubMed] [Google Scholar]
- 24.Huff M. W., Telford D. E. 1989. Dietary fish oil increases conversion of very low density lipoprotein apoprotein B to low density lipoprotein. Arteriosclerosis. 9: 58–66. [DOI] [PubMed] [Google Scholar]
- 25.Fisher W. R., Zech L. A., Stacpoole P. W. 1998. Apolipoprotein B metabolism in hypertriglyceridemic diabetic patients administered either a fish oil- or vegetable oil-enriched diet. J. Lipid Res. 39: 388–401. [PubMed] [Google Scholar]
- 26.Chan D. C., Watts G. F., Mori T. A., Barrett P. H., Redgrave T. G., Beilin L. J. 2003. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am. J. Clin. Nutr. 77: 300–307. [DOI] [PubMed] [Google Scholar]
- 27.Cohn J. S., Wagner D. A., Cohn S. D., Millar J. S., Schaefer E. J. 1990. Measurement of very low density and low density lipoprotein apolipoprotein (Apo) B-100 and high density lipoprotein Apo A-I production in human subjects using deuterated leucine. Effect of fasting and feeding. J. Clin. Invest. 85: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welty F. K., Lichtenstein A. H., Barrett P. H., Dolnikowski G. G., Schaefer E. J. 1999. Human apolipoprotein (Apo) B-48 and ApoB-100 kinetics with stable isotopes. Arterioscler. Thromb. Vasc. Biol. 19: 2966–2974. [DOI] [PubMed] [Google Scholar]
- 29.Matthan N. R., Jalbert S. M., Barrett P. H., Dolnikowski G. G., Schaefer E. J., Lichtenstein A. H. 2008. Gender-specific differences in the kinetics of nonfasting TRL, IDL, and LDL apolipoprotein B-100 in men and premenopausal women. Arterioscler. Thromb. Vasc. Biol. 28: 1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welty F. K., Lichtenstein A. H., Barrett P. H., Dolnikowski G. G., Ordovas J. M., Schaefer E. J. 1997. Decreased production and increased catabolism of apolipoprotein B-100 in apolipoprotein B-67/B-100 heterozygotes. Arterioscler. Thromb. Vasc. Biol. 17: 881–888. [DOI] [PubMed] [Google Scholar]
- 31.Ordovas J. M., Peterson J. P., Santaniello P., Cohn J. S., Wilson P. W., Schaefer E. J. 1987. Enzyme-linked immunosorbent assay for human plasma apolipoprotein B. J. Lipid Res. 28: 1216–1224. [PubMed] [Google Scholar]
- 32.Zilversmit D. B., Shea T. M. 1989. Quantitation of apoB-48 and apoB-100 by gel scanning or radio-iodination. J. Lipid Res. 30: 1639–1646. [PubMed] [Google Scholar]
- 33.Lepage G., Roy C. C. 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27: 114–120. [PubMed] [Google Scholar]
- 34.Lichtenstein A. H., Chobanian A. V. 1990. Effect of fish oil on atherogenesis in Watanabe heritable hyperlipidemic rabbit. Arteriosclerosis. 10: 597–606. [DOI] [PubMed] [Google Scholar]
- 35.Beltz W. F., Kesaniemi Y. A., Howard B. V., Grundy S. M. 1985. Development of an integrated model for analysis of the kinetics of apolipoprotein B in plasma very low density lipoproteins, intermediate density lipoproteins, and low density lipoproteins. J. Clin. Invest. 76: 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havel R. J. 1982. Approach to the patient with hyperlipidemia. Med. Clin. North Am. 66: 319–333. [DOI] [PubMed] [Google Scholar]
- 37.Cortese C., Levy Y., Janus E. D., Turner P. R., Rao S. N., Miller N. E., Lewis B. 1983. Modes of action of lipid-lowering diets in man: studies of apolipoprotein B kinetics in relation to fat consumption and dietary fatty acid composition. Eur. J. Clin. Invest. 13: 79–85. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd J., Packard C. J., Grundy S. M., Yeshurun D., Gotto A. M., Jr, Taunton O. D. 1980. Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J. Lipid Res. 21: 91–99. [PubMed] [Google Scholar]
- 39.Turner J. D., Le N. A., Brown W. V. 1981. Effect of changing dietary fat saturation on low-density lipoprotein metabolism in man. Am. J. Physiol. 241: E57–E63. [DOI] [PubMed] [Google Scholar]
- 40.Ouguerram K., Maugeais C., Gardette J., Magot T., Krempf M. 2006. Effect of n-3 fatty acids on metabolism of apoB100-containing lipoprotein in type 2 diabetic subjects. Br. J. Nutr. 96: 100–106. [DOI] [PubMed] [Google Scholar]
- 41.Illingworth D. R., Harris W. S., Connor W. E. 1984. Inhibition of low density lipoprotein synthesis by dietary omega-3 fatty acids in humans. Arteriosclerosis. 4: 270–275. [DOI] [PubMed] [Google Scholar]
- 42.Fisher E. A., Pan M., Chen X., Wu X., Wang H., Jamil H., Sparks J. D., Williams K. J. 2001. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276: 27855–27863. [DOI] [PubMed] [Google Scholar]
- 43.Wong S. H., Fisher E. A., Marsh J. B. 1989. Effects of eicosapentaenoic and docosahexaenoic acids on apoprotein B mRNA and secretion of very low density lipoprotein in HepG2 cells. Arteriosclerosis. 9: 836–841. [DOI] [PubMed] [Google Scholar]
- 44.Dixon J. L., Furukawa S., Ginsberg H. N. 1991. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J. Biol. Chem. 266: 5080–5086. [PubMed] [Google Scholar]
- 45.Sakata N., Wu X., Dixon J. L., Ginsberg H. N. 1993. Proteolysis and lipid-facilitated translocation are distinct but competitive processes that regulate secretion of apolipoprotein B in Hep G2 cells. J. Biol. Chem. 268: 22967–22970. [PubMed] [Google Scholar]
- 46.Lu G., Windsor S. L., Harris W. S. 1999. Omega-3 fatty acids alter lipoprotein subfraction distributions and the in vitro conversion of very low density lipoproteins to low density lipoproteins. J. Nutr. Biochem. 10: 151–158. [DOI] [PubMed] [Google Scholar]
- 47.Harris W. S., Hustvedt B. E., Hagen E., Green M. H., Lu G., Drevon C. A. 1997. N-3 fatty acids and chylomicron metabolism in the rat. J. Lipid Res. 38: 503–515. [PubMed] [Google Scholar]
- 48.Goldberg I. J., Le N. A., Paterniti J. R., Jr, Ginsberg H. N., Lindgren F. T., Brown W. V. 1982. Lipoprotein metabolism during acute inhibition of hepatic triglyceride lipase in the cynomolgus monkey. J. Clin. Invest. 70: 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada N., Shames D. M., Stoudemire J. B., Havel R. J. 1986. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: heterogeneity related to the presence of apolipoprotein E. Proc. Natl. Acad. Sci. USA. 83: 3479–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicolosi R. J., Stucchi A. F., Kowala M. C., Hennessy L. K., Hegsted D. M., Schaefer E. J. 1990. Effect of dietary fat saturation and cholesterol on LDL composition and metabolism. In vivo studies of receptor and nonreceptor-mediated catabolism of LDL in cebus monkeys. Arteriosclerosis. 10: 119–128. [DOI] [PubMed] [Google Scholar]
- 51.Woollett L. A., Spady D. K., Dietschy J. M. 1992. Regulatory effects of the saturated fatty acids 6:0 through 18:0 on hepatic low density lipoprotein receptor activity in the hamster. J. Clin. Invest. 89: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennessy L. K., Osada J., Ordovas J. M., Nicolosi R. J., Stucchi A. F., Brousseau M. E., Schaefer E. J. 1992. Effects of dietary fats and cholesterol on liver lipid content and hepatic apolipoprotein A-I, B, and E and LDL receptor mRNA levels in cebus monkeys. J. Lipid Res. 33: 351–360. [PubMed] [Google Scholar]
- 53.Fernandez M. L., Lin E. C., McNamara D. J. 1992. Differential effects of saturated fatty acids on low density lipoprotein metabolism in the guinea pig. J. Lipid Res. 33: 1833–1842. [PubMed] [Google Scholar]
- 54.Vallim T., Salter A. M. 2010. Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 82: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson K. G., Maitin V., Leake D. S., Yaqoob P., Williams C. M. 2006. Saturated fat-induced changes in Sf 60–400 particle composition reduces uptake of LDL by HepG2 cells. J. Lipid Res. 47: 393–403. [DOI] [PubMed] [Google Scholar]
- 56.Wu X., Zhou M., Huang L. S., Wetterau J., Ginsberg H. N. 1996. Demonstration of a physical interaction between microsomal triglyceride transfer protein and apolipoprotein B during the assembly of ApoB-containing lipoproteins. J. Biol. Chem. 271: 10277–10281. [DOI] [PubMed] [Google Scholar]
- 57.Levy E., Stan S., Delvin E., Menard D., Shoulders C., Garofalo C., Slight I., Seidman E., Mayer G., Bendayan M. 2002. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J. Biol. Chem. 277: 16470–16477. [DOI] [PubMed] [Google Scholar]
- 58.Alexander C. A., Hamilton R. L., Havel R. J. 1976. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J. Cell Biol. 69: 241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown A. M., Baker P. W., Gibbons G. F. 1997. Changes in fatty acid metabolism in rat hepatocytes in response to dietary n-3 fatty acids are associated with changes in the intracellular metabolism and secretion of apolipoprotein B-48. J. Lipid Res. 38: 469–481. [PubMed] [Google Scholar]
- 60.Murthy S., Albright E., Mathur S. N., Davidson N. O., Field F. J. 1992. Apolipoprotein B mRNA abundance is decreased by eicosapentaenoic acid in CaCo-2 cells. Effect on the synthesis and secretion of apolipoprotein B. Arterioscler. Thromb. 12: 691–700. [DOI] [PubMed] [Google Scholar]
- 61.Levy E., Spahis S., Ziv E., Marette A., Elchebly M., Lambert M., Delvin E. 2006. Overproduction of intestinal lipoprotein containing apolipoprotein B-48 in Psammomys obesus: impact of dietary n-3 fatty acids. Diabetologia. 49: 1937–1945. [DOI] [PubMed] [Google Scholar]
- 62.Wong S. H., Marsh J. B. 1988. Inhibition of apolipoprotein secretion and phosphatidate phosphohydrolase activity by eicosapentaenoic and docosahexaenoic acids in the perfused rat liver. Metabolism. 37: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 63.Calleja L., Trallero M. C., Carrizosa C., Mendez M. T., Palacios-Alaiz E., Osada J. 2000. Effects of dietary fat amount and saturation on the regulation of hepatic mRNA and plasma apolipoprotein A-I in rats. Atherosclerosis. 152: 69–78. [DOI] [PubMed] [Google Scholar]
- 64.Hayek T., Ito Y., Azrolan N., Verdery R. B., Aalto-Setala K., Walsh A., Breslow J. L. 1993. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. J. Clin. Invest. 91: 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwab D. A., Rea T. J., Hanselman J. C., Bisgaier C. L., Krause B. R., Pape M. E. 2000. Elevated hepatic apolipoprotein A-I transcription is associated with diet-induced hyperalphalipoproteinemia in rabbits. Life Sci. 66: 1683–1694. [DOI] [PubMed] [Google Scholar]
- 66.Vessby B., Boberg J., Gustafsson I. B., Karlstrom B., Lithell H., Ostlund-Linqvist A. M. 1980. Reduction of high density lipoprotein cholesterol and apoliproprotein A-I concentrations by a lipid-lowering diet. Atherosclerosis. 35: 21–27. [DOI] [PubMed] [Google Scholar]
- 67.Azrolan N., Odaka H., Breslow J. L., Fisher E. A. 1995. Dietary fat elevates hepatic apoA-I production by increasing the fraction of apolipoprotein A-I mRNA in the translating pool. J. Biol. Chem. 270: 19833–19838. [DOI] [PubMed] [Google Scholar]
- 68.Vélez-Carrasco W., Lichtenstein A. H., Welty F. K., Li Z., Lamon-Fava S., Dolnikowski G. G., Schaefer E. J. 1999. Dietary restriction of saturated fat and cholesterol decreases HDL ApoA-I secretion. Arterioscler. Thromb. Vasc. Biol. 19: 918–924. [DOI] [PubMed] [Google Scholar]
- 69.Furtado J. D., Campos H., Appel L. J., Miller E. R., Laranjo N., Carey V. J., Sacks F. M. 2008. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am. J. Clin. Nutr. 87: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C., Khoo C., Furtado J., Sacks F. M. 2010. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 121: 1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langsted A., Freiberg J. J., Nordestgaard B. G. 2008. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 118: 2047–2056. [DOI] [PubMed] [Google Scholar]
- 72.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. J. Am. Med. Assoc. 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 73.Bansal S., Buring J. E., Rifai N., Mora S., Sacks F. M., Ridker P. M. 2007. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. J. Am. Med. Assoc. 298: 309–316. [DOI] [PubMed] [Google Scholar]
- 74.Sacks F. M., Katan M. 2002. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 113 (suppl.): 13–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.