Abstract
Intralipid is a fat emulsion that is regularly infused into humans and animals. Despite its routine use, Intralipid infusion can cause serious adverse reactions, including immunosuppression. Intralipid is a complex mix of proteins, lipids, and other small molecules, and the effect of its infusion on the human plasma metabolome is unknown. We hypothesized that untargeted metabolomics of human plasma after an Intralipid infusion would reveal novel insights into its effects. We infused Intralipid and saline into 10 healthy men in a double-blind, placebo-controlled experiment and used GC/MS, LC/MS, and NMR to profile the small-molecule composition of their plasma before and after infusion. Multivariate statistical analysis of the 40 resulting plasma samples revealed that after Intralipid infusion, a less-well-characterized pathway of linoleic acid metabolism had resulted in the appearance of (9Z)-12,13-dihydroxyoctadec-9-enoic acid (12,13-DHOME, P < 10−3), a leukotoxin that has powerful physiological effects and is known to inhibit the neutrophil respiratory burst. Intralipid infusion caused increased plasma 12,13-DHOME. Given that 12,13-DHOME is known to directly affect neutrophil function, we conclude that untargeted metabolomics may have revealed a hitherto-unknown mechanism of intralipid-induced immunosuppression.
Keywords: fatty acid/metabolism; mass spectrometry; triglycerides; (9Z)-12,13-dihydroxyoctadec-9-enoic acid
Intravenous fat emulsions (IVFEs) are regularly infused into both patients and healthy humans in a range of settings. Of these IVFEs, perhaps the most common is Intralipid, a lipid emulsion made from soybean oil and egg yolk phospholipids (1). In clinical practice, Intralipid is used as a component of parenteral feeding because it contains a rich blend of diet-mimicking triacylglycerols, composed mostly of the 18-carbon unsaturated FAs linoleic (∼46%) and oleic (∼20%) acid (2). In addition, clinical infusion of Intralipid is an effective therapy for cardiac poisoning by local anesthetic (3) and as a drug delivery vehicle (4). In a research setting, acute Intralipid infusion is often used to study the effects of increased triacylglycerol and nonesterified FA concentrations on metabolism and physiological function (5–7).
Despite its wide application, Intralipid infusion is not without its hazards. Particularly in the very young, it can lead to a number of adverse events, including hypertriglyceridemia, liver disease (8), pancreatitis (9), and immunosuppression that leaves vulnerable patients at an increased risk of infection (4, 10, 11). It has been suggested that Intralipid infusion may inhibit the mononuclear phagocyte system function (4), inasmuch as this system not only contributes to the immune response but is also responsible for the clearance of fat emulsions from plasma (12). It has further been suggested that the high linoleic acid content of Intralipid (via metabolic conversion of linoleic to arachidonic acid) may lead to an increase in the eicosanoids (4), 20-carbon lipids that play a critical role in modulating human immune function (13).
The chemistry of Intralipid is far from simple. Its soybean and egg yolk constituents are a complex mixture of proteins, lipids, and small molecules, including phospholipids, sterols, tocopherols, hydrocarbons, and flavones (2), many of which are known to be biologically active. Thus, the reasons for both Intralipid's clinical effectiveness and its occasional toxicity may be more obscure than is currently thought. By extension, the chemical complexity of Intralipid may explain some of the contradictions in the literature with regard to effects of acute increases in circulating lipids (and lipid metabolites); for example, see recent work from this laboratory (14). Given that untargeted small-molecule profiling of human plasma or serum in response to Intralipid infusion has, to our knowledge, never been carried out, the complex interaction between Intralipid and physiology, both in the clinic and elsewhere, remains unclear.
The aim of this study was to measure changes in the human plasma metabolome in response to a standard Intralipid infusion, by using untargeted metabolomic profiling. We used GC/MS, high-resolution LC/MS, and 1H-NMR and aimed to profile novel compounds that were significantly increased in human plasma due either to the infusion or to secondary metabolism of the infusate. Our aim was to shed light on the possible contribution of these small molecules to both the beneficial, as well as the adverse, effects of Intralipid infusion in humans.
METHODS
Subjects
Ten healthy men were recruited from the community by local advertisement. The subjects’ ages, weights, heights, and body mass index (BMI) were measured during an initial assessment visit. Subjects were deemed eligible for participation if they were aged over 18 years, had a normal BMI (18.5–25), were not on any cardiac or metabolic medications, and had no adverse reaction to egg, nuts, soy, heparin, or Intralipid. They were screened twice for possible complicating conditions (for example coronary heart disease, diabetes mellitus, aortic valve stenosis, and diseases of the liver, pancreas, or kidney). Subjects were informed of the nature and possible risks associated with each experiment and were required to complete both a health screening questionnaire and a statement of informed consent. All protocols were approved by the human research ethics committee of the University of Queensland and conformed to the Declaration of Helsinki.
Study design and sample collection
The study was a randomized, double-blind, placebo-controlled experiment. Subjects attended the laboratory on two separate occasions separated by approximately 1 week (alternate randomization was implemented during the second visit). On each visit, the subjects arrived in the morning following an overnight fast. After at least 10 min supine rest in a temperature-controlled room, an intravenous cannula was inserted in the cephalic vein of each arm; the right for infusion and the left to obtain blood samples. A 20 ml baseline blood sample was taken, immediately after which a saline infusion was initiated (90 ml/h). After a 500 U bolus of heparin, subjects were randomized to one of two groups: 20% Intralipid or saline. Both were infused at 90 ml/h and coinfused with heparin. After 60 min of infusion, another blood sample was taken.
Clinical biochemistry and metabolomics sample preparation
Blood sampling yielded a total of 40 samples. Samples were collected in lithium heparin (gel) tubes for clinical biochemistry and EDTA tubes for subsequent metabolomic analysis. Clinical biochemistry was performed using a clinical pathology system (SYNCHRON LX System, Beckman Coulter). For metabolomics analysis, 1 ml aliquots of plasma were separated at 4°C using a methanol-water-chloroform 1:1:1 (v/v/v) extraction. The water and chloroform fractions were evaporated to dryness in a vacuum centrifuge and stored at −80°C. Just prior to analysis, the lyophilized water fraction was reconstituted in either 500 μl of HPLC-grade water (for mass spectrometry) or aqueous NMR buffer [100% D2O + 2 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS)].
GC-MS
Lyophilized chloroform fractions were reconstituted in 20 μl chloroform, vibrated for 15 s, then removed with a micropipette into a 150 μl glass insert inside a Waters 2 ml HPLC vial. Twenty microliters of BSTFA were added; the sample was mixed and then heated to 60°C for 15 min. These samples were then analyzed by GC/MS on a Varian 3800 GC coupled to a Bruker 300 triple quadrupole mass spectrometer, using helium as carrier gas at 1.2 ml/min in constant-flow mode. The column was a Varian “Factor Four” VF-5ms (30 m × 0.25 mm internal diameter and 0.25 μm film). Injections of 1 μl were made using a Varian CP-8400 autosampler and a Varian 1177 split/splitless injector in split mode using a 10:1 split. The injector temperature was 260°C. The column oven was programmed at 100°C to 150°C at 30 degrees per min, then to 290°C at 8°C per min. The ion source was held at 220°C, and the transfer line at 290°C.
Full-scan MS data were acquired using electron ionization over the range m/z 35 to 500 in 0.2 s. Selected ion channels for the characteristic ions at m/z 285 (14:0), 311 (16:1), 313 (16:0), 335 (18:3), 337 (18:2), 339 (18:1), 341 (18:0), and 361 (20:4) with dwell times of 20 ms per channel were also included as a precaution in case of any weak full-scan data. Peaks were identified by the use of reference standards. Data were quantified on the total ion current (TIC) for each FA TMS ester peak, with the exception of linolenic acid, which partially coeluted with oleic acid under these conditions. For this minor FA, the extracted ion chromatogram at m/z 335 was measured, and this was scaled up to the full TIC value after examining a pure spectrum of linolenic acid to determine this correction factor. All FAs were then expressed as their percentage of the total FAs determined. Data were processed using Bruker Workstation software.
LC/MS
LC/MS analysis was conducted using a hybrid linear trap quadrupole/Orbitrap high-resolution mass spectrometer (Thermo Fisher Scientific; Bremen, Germany). The samples were separated using a Waters 2690 separations module employing a Waters Nova-Pak 4.0 μm C18 column (3.91 × 150 mm) at a flow rate of 0.8 ml/min-1 using HPLC-grade solvents (0.1% formic acid in water for buffer A and 0.1% formic acid in methanol for buffer B). A 10 μl aliquot of each sample was injected onto the column, and compounds were eluted over a step gradient of 10–50% buffer B over 3 min, then 50–80% buffer B over 8 min, and 80–100% buffer B over 1 min. After holding at 100% buffer B for 2 min, the column was reequilibrated in 10% buffer B for 0.5 min. The column was operated at ambient temperature (20°C), and the samples were maintained at 10°C.
Centroid mass spectra were acquired in the mass/charge (m/z) range of 50–1,000 at a target resolution of 30,000, operating according to the following parameters: capillary temperature of 300°C; sheath gas and auxiliary gas flow rates set to 30 au and 5 au, respectively. A capillary voltage of 7 V was used for positive-ion acquisition and −44 V was used for negative-ion acquisition. Raw MS files were exported and analyzed offline using XCMS (15) running in the R environment.
NMR spectral acquisition and processing
One-dimensional 1H nuclear Overhauser effect spectroscopy (NOESY) spectra were acquired from the reconstituted water fractions using an Agilent/Varian Inova 400 MHz spectrometer (Varian Medical Systems; Palo Alto, CA). Spectra were acquired with a 90° pulse, pulse length 9.7 μs with a sweep width of 4,299 ppm at 25°C. A total of 128 transients were collected into 32,000 datum points. The water signal was set to the center of the transmitter offset, and a presaturation pulse of 2 s was applied. Presaturation occurred during the relaxation delay and also during the mixing time (100 ms) of the NOESY pulse sequence.
The resulting free induction decays were Fourier transformed, line broadened (1 Hz), and baseline corrected in NMR Manager 12.0 (Advanced Chemistry Development; Toronto, Canada). All chemical shifts were referenced relative to DSS at 0 ppm. The spectra were normalized and bucketed using probabilistic-quotient normalization (16) and adaptive intelligent binning (17), respectively; both routines were custom-written in Matlab. Peaks due to EDTA were excluded from further analysis (18).
Metabolite identification
This experiment presented unusual challenges with regard to feature annotation, given that the small molecules comprising the metabolic profile could have come from any of four sources: human metabolism, soy metabolism (from the soybean oil), chicken metabolism (due to the egg yolk content of Intralipid), and possible synthetic chemicals (including plasticizers). To aid in distinguishing human metabolites from those compounds that were components of Intralipid (and thus directly infused), we added 30 mM Intralipid directly to a sample of whole blood, treated it identically to the experimental samples, and used this as a control. Peaks that were not present in the control sample, but only in the postinfusion sample, were likely to be the products of secondary (human) metabolism.
For LC/MS, features were putatively annotated by searching public databases by accurate m/z alone. Once candidates had been selected, we used any orthogonal data that were available (including isotope patterns and retention times) for either exclusion or increased confidence. For example, the positive identification of (3R)-3-hydroxybutanoic acid in the 1H-NMR data was used to confirm the annotation of the corresponding LC/MS chromatograms. Furthermore, we used retention times to exclude putative annotations, even though their molecular mass was consistent with the formula. Our guiding principle was to not annotate rather than mis-annotate. Finally, a number of compounds of interest were further characterized using tandem mass spectrometry (MS/MS). These compounds [monobenzyl phthalate, dihydroxy-stearic acid, (3R)-3-hydroxybutanoic acid (3HB), 12,13-dihydroxy-9Z-octadecenoic acid (12,13-DHOME), 9S-hydroperoxy-10E,12Z-octadecadienoic acid (9(S)-HPODE), 12R-hydroxy-9Z-octadecenoic acid (Ricinoleic acid), and glycerol] were fragmented, and the resulting daughter ions were compared with library spectra. For example, the [M-H]− ion that was believed to correspond to 12,13-DHOME was fragmented using collision-induced dissociation at 25 eV; the resultant diagnostic masses at both 183.14 and 129.09 were entirely consistent with an equivalent spectrum for 12,13-DHOME in the METLIN database. When library MS/MS spectra were not available, fragments were compared with results generated using the in silico fragmentation prediction tool, MetFrag (19). However, no important compounds were annotated in this way.
For 1H-NMR, features were putatively annotated from a custom database based on chemical shift only. Annotation was then confirmed using 2-dimensional spectroscopy and/or statistical correlation spectroscopy. As part of our in-house standard operating procedure, we use an annotation confidence scoring system similar to that recommended by the Metabolomics Standards Initiative and others (20).
Data analysis and statistics
For NMR data, post-Intralipid and postsaline spectra were compared using projection onto latent spaces discriminant analysis (PLSDA), using the SIMPLS algorithm as implemented in PLS Toolbox 6.5 (Eigenvector Research, Inc.; Wenatchee, WA). Prior to analysis, data were mean-centered and orthogonal signal-corrected (for ease of interpretation). To protect against overfitting, we performed permutation testing (21) as implemented in PLS Toolbox. A PLSDA model was considered significant if the estimated P value was <.05. However, PLSDA performed poorly when analyzing the LC/MS data (probably due to a relative lack of collinearity); therefore, we instead performed repeated-measures ANOVA on each feature/peak with correction of P values using the Benjamini and Hochberg False Discovery Rate correction (22). Features were accepted as significant if the corrected P value was <.05. Clinical biochemistry measurements and FA profiles were compared (Intralipid vs. saline) using a repeated-measures ANOVA, as implemented in SPSS 18.0 (IBM Corporation; Armonk, NY).
Data processing and analysis were conducted using R 2.14 or higher (http://cran.r-project.org/), Matlab R2011b (including the statistics toolbox) (Mathworks; Natick, MA), SPSS Statistics 18 or above (IBM Corporation), Microsoft Excel, Sirius2 [for isotope pattern matching (23)] and MZmine 2 [for offline visualization (24)], all running under Mac OS 10.6 or greater. All Matlab and R scripts are available from the corresponding author on request.
RESULTS
Clinical biochemistry
Subjects’ triglycerides and FAs were within normal ranges at baseline (Table 1). As expected, the infusion of Intralipid resulted in a significant increase in triglycerides. An increase in circulating free FAs was also seen both postsaline and post-Intralipid, with the increase postsaline most probably due to the action of heparin on lipoprotein lipase. There was no change in total cholesterol in either group, although there was a significant decrease in VLDL postsaline and post-Intralipid and a decrease in LDL post-Intralipid only.
TABLE 1.
Summary of plasma clinical biochemistry results
| Saline | Intralipid | ||||
| Pre | Post | Pre | Post | Exact Pa | |
| Glucose (mM) | 5.1 ± 0.1 | 5.0 ± 0.9 | 5.2 ± 0.1 | 5.1 ± 0.1 | 1.00 |
| Triglycerides (mM) | 1.1 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 2.4 ± 0.6 | .014 |
| Free FAs (mM) | 0.3 ± 0.04 | 0.7 ± 0.1 | 0.4 ± .1 | 1.5 ± 0.2 | .004 |
| Cholesterol | |||||
| Total (mM) | 4.8 ± 0.3 | 4.7 ± 0.3 | 5.1 ± 0.3 | 5.0 ± 0.3 | .664 |
| HDL (mM) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | .193 |
| LDL (mM) | 3.2 ± 0.3 | 3.2 ± 0.3 | 3.5 ± 0.3 | 2.8 ± 0.4 | .006 |
| VLDL (mM) | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 1.0 ± 0.2 | .003 |
| Total/HDL | 4.6 ± 0.5 | 4.4 ± 0.4 | 4.7 ± 0.5 | 4.7 ± 0.5 | .104 |
Repeated-measures ANOVA.
FA profiling (GC/MS)
The 3- to 4-fold increase in circulating free FAs was accompanied by a significant shift in the FA profile (Table 2). The most distinctive feature was a dramatic rise in the contribution to the total plasma FA pool from linoleic acid (from 12% to 35%), a major constituent of Intralipid. There was also a 4-fold increase in the contribution from linolenic acid, from ∼0.5% to >2.0%. The percentage contribution of other FAs either diminished or stayed the same, although this should be taken in the context of the large increase in total free FAs. Thus, although the contribution from oleic acid was unchanged, oleic acid concentration itself must have risen substantially for this to be the case (subsequently confirmed by LC/MS).
TABLE 2.
FA profiles, measured by GC/MS.
| Saline | Intralipid | ||||
| Pre | Post | Pre | Post | Exact P a | |
| Myristic (C14:0) % | 3.1 ± 0.2 | 3.1 ± 0.3 | 3.1 ± 0.3 | 1.6 ± 0.3 | <.001 |
| Palmitoleic (C16:1) % | 2.1 ± 0.2 | 2.7 ± 0.2 | 1.9 ± 0.3 | 0.8 ± 0.2 | .001 |
| Palmitic (C16:0) % | 29.6 ± 1.0 | 28.0 ± 0.7 | 30.1 ± 0.6 | 21.0 ± 2.3 | .004 |
| Linolenic (C18:3) % | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 00 | 2.3 ± 0.3 | <.001 |
| Linoleic (C18:2) % | 11.6 ± 0.5 | 12.1 ± 0.8 | 11.6 ± 0.7 | 34.5 ± 4.1 | <.001 |
| Oleic (C18:1) % | 29.2 ± 1.6 | 31.8 ± 2.1 | 25.7 ± 1.9 | 22.5 ± 1.3 | .155 |
| Stearic (C18:0) % | 24.0 ± 1.3 | 21.7 ± 1.7 | 27.2 ± 2.0 | 17.2 ± 2.1 | .037 |
Values given are % of total detected FAs.
Repeated-measures ANOVA.
LC/MS
A total of 6,914 features of interest in the mass spectra were identified using XCMS: 4,254 features from spectra acquired in positive-ion mode and a further 2,660 features in negative-ion mode. Of these, 101 features were significantly altered by Intralipid infusion (P < .05 after the Benjamini and Hochberg False Discovery Rate correction). These 101 features were hand-checked for artifacts (for example, Fourier transform “shoulders” from centroiding), which were removed. After isotopes and adducts were also removed, 52 features remained. Of the 52, 24 (46%) could be identified through the use of public databases and subsequent MS/MS experiments. Of these 24, three were identified as synthetics (monobenzyl phthalate, benzyl butyl phthalate, and polyethylene glycol) and excluded from further analysis. Table 3 is a complete list of annotated features. A spreadsheet of all the significant LC/MS features is available in the supplementary materials.
TABLE 3.
Annotated small molecules (<1,000 Da) in human plasma, detected by LC/MS, that were significantly different after an Intralipid infusion.
| PI/NI | m/z | RT | Formula | Name | Adduct | Mass error | Pa |
| u/e | sec | ppm | |||||
| PI | 317.268 | 834 | C18H36O4 | Dihydroxy-stearic acid | [M+H]+ | −0.8 | 6×10−6 |
| PI | 315.253 | 801 | C18H34O4 | 12,13-DHOME | [M+H]+ | −1.0 | 2×10−4 |
| PI | 313.237 | 770 | C18H32O4 | 9(S)-HPODE | [M+H]+ | −1.2 | 6×10−4 |
| PI | 319.194 | 911 | C19H26O4 | Ubiquinone-2 | [M+H]+ | −2.9 | .001 |
| PI | 317.208 | 829 | C18H30O3 | Oxo-linolenic acid (9-OxoODE)b,c | [M+Na]+ | −1.2 | .002 |
| PI | 455.169 | 911 | C25H26O8 | Dimethylpyrano-flavone | [M+H]+ | −2.7 | .002 |
| NI | 297.243 | 853 | C18H34O3 | Ricinoleic acid | [M-H] | +1.1 | .003 |
| PI | 93.054 | 83 | C3H8O3 | Glycerol | [M+H]+ | −2.3 | .005 |
| PI | 370.331 | 851 | C22H43NO3 | Aplidiasphingosine | [M+H]+ | −0.7 | .005 |
| PI | 105.054 | 128 | C4H8O3 | 3-Hydroxybutyrate | [M+H]+ | −2.6 | .02 |
| NI | 279.233 | 909 | C18H32O2 | Linoleic acid | [M-H] | +1.6 | .02 |
| PI | 301.214 | 892 | C18H30O2 | Linolenic acid | [M+H]+ | −0.6 | .02 |
| NI | 295.228 | 841 | C18H32O3 | Hydroxy-octadecadienoic acid (9-HODE)b | [M-H] | +0.8 | .02 |
| PI | 500.274 | 880 | C23H44NO7P | Lysophosphatidylethanolaminec | [M+Na]+ | −1.9 | .03 |
| PI | 453.153 | 893 | C23H26O8 | Flavonoid | [M+Na]+ | −3.3 | .03 |
| NI | 201.113 | 471 | C10H18O4 | Sebacic acid | [M-H] | +0.9 | .04 |
| NI | 207.044 | 152 | C6H12N2O4S | Lanthionine | [M-H] | −1.8 | .04 |
| PI | 387.182 | 910 | C22H26O6 | Burseran | [M+H]+ | +3.4 | .04 |
| NI | 563.506 | 923 | C18H34O2 | Oleic acidc | [2M-H] | +1.1 | <.05 |
P value is for the most significant feature (e.g., if [M+Na]+ was most significant, then P for the most probable structure is given).
Classification is correct, but exact structure was not determined with certainty. Most probably, structure is in brackets.
[M±H] ion was also detected.
[M+Na]+ ion was also detected.
Of the 35 annotated features (in both positive- and negative-ion mode), some were FAs that had been released from the infused triacylglycerols by lipoprotein lipase, including linoleic acid, linolenic acid, and oleic acid. Several more features were due to metabolites of linoleic acid (the most-abundant FA in Intralipid), including hydroxy-linoleic acid, 12,13-dihydroxy-9Z-octadecenoic acid (12,13-DHOME), and 9S-hydroperoxy-10E,12Z-octadecadienoic acid (9(S)-HPODE). We also identified a chromatogram at m/z = 295.229 that may have corresponded to 12 (13)-EpOME (the 12,13-DHOME precursor). The signal at this mass and retention time was significantly higher after Intralipid infusion (uncorrected P = .0048), although the Benjamini and Hochberg correction (P = .0157) meant that this signal was not identified in our initial, unbiased analysis.
The ketoacid (3R)-3-hydroxybutanoic acid (3HB) was significantly raised post-Intralipid, thus independently confirming its identification as a compound of interest by 1H-NMR (see below). Also putatively annotated were several flavones, a lysophospholipid, and some unusual FAs such as ricinoleic acid and sebacic acid (Table 3).
NMR spectroscopy
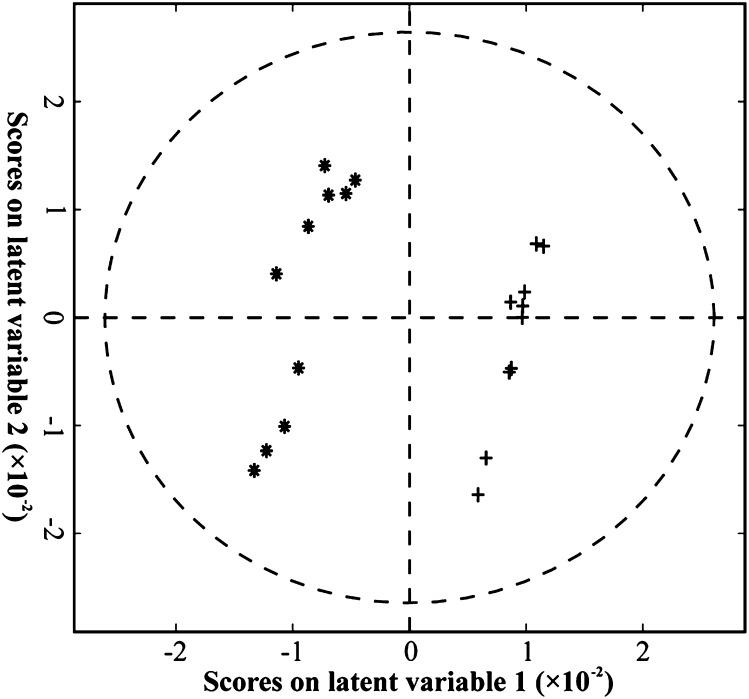
A two-latent-variable PLSDA model was fitted to the postintervention 1H-NMR data and accounted for 99% of the between-class variance (Fig. 1). The degree of fit was confirmed by permutation testing as being unlikely to be due to chance (P < .05). The first latent variable showed clear separation between the post-Intralipid and postsaline samples, and alone accounted for 94% of the between-class variation. Latent variable 1 was dominated by a pair of features, corresponding to a doublet in the NMR spectra at 1.20 ppm. These peaks were subsequently identified using correlation spectroscopy (25) as 3HB. Thus, the NMR data provided further confirmation of the LC/MS data that 3HB was raised in the post-Intralipid samples compared with the postsaline samples.
Fig. 1.
Biplot showing results of PLSDA analysis of 1H-NMR data. Model is significant at P < .05. * Intralipid; + saline.
DISCUSSION
To study the effects of Intralipid infusion on the human plasma metabolome, we profiled the plasma of 10 healthy men, using GC/MS, high-resolution LC/MS, and 1H-NMR, before and after infusion with either Intralipid or saline. As expected, Intralipid infusion caused an increase in circulating triglycerides and nonesterified FAs. However, we also identified over 50 novel chemical and biochemical features that were increased by Intralipid infusion. The significance of these is discussed below.
IVFEs have been regularly infused into both patients and healthy humans in a range of settings for more than 50 years. Clinically, they are most commonly used for parenteral feeding, either on their own or as a component of total parenteral nutrition. They have also recently found a novel application as a drug delivery vector. In this case, they are used as a solvent for drugs that are poorly soluble in water (26). Finally, infusion of IVFE is an effective therapy for accidental cardiac poisoning by local anesthetic (3). Many biomedical researchers also infuse IVFEs into healthy subjects (human and animal) as an experimental model of increased plasma triacylglycerol and FA concentration. Given that chronically elevated plasma lipid concentrations (dyslipidemia) are associated with an increased risk of cardiovascular disease and increased mortality rates in humans, experiments using IVFEs have played an important role in advancing our understanding of the effect of acute elevations of plasma lipid.
Yet despite its seeming ubiquitousness, IVFE infusion can and does cause adverse reactions. These include liver disease, pancreatitis, hypertriglyceridemia, and immunosuppression, with infants being particularly susceptible. Although these adverse reactions are rare, they can be catastrophic when they do occur. Furthermore, given that the chemical profile of many IVFEs is complex (due to their biological origin), the exact mechanism whereby adverse events occur remains unclear.
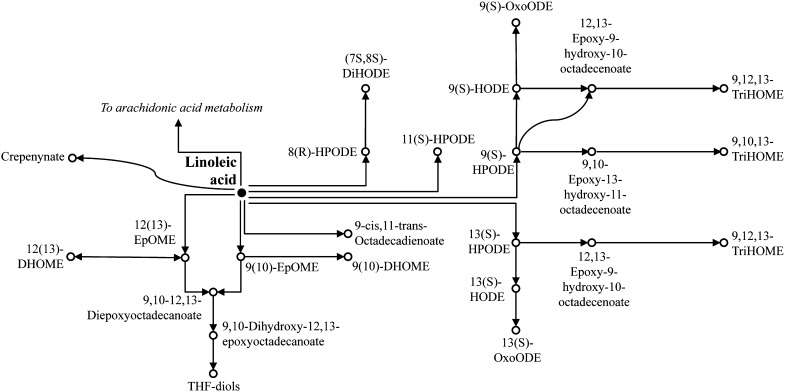
Therefore we used metabolomics methods to profile the plasma of 10 healthy men, before and after infusion with either saline or one of the most-common IVFEs, Intralipid. We identified a range of compounds that were previously not known to be increased in response to Intralipid infusion, including several linoleic acid epoxides and their diols that are thought to have toxic effects (discussed below). Although a few of these features were only putatively annotated, their copresence on the same metabolic pathway(s) (Fig. 2) significantly increased our confidence in these annotations. Indeed, such an approach (using existing knowledge about metabolic pathways as a bioinformatics tool for metabolome annotation) has already been suggested (27, 28).
Fig. 2.
Metabolism of linoleic acid in humans (adapted from KEGG).
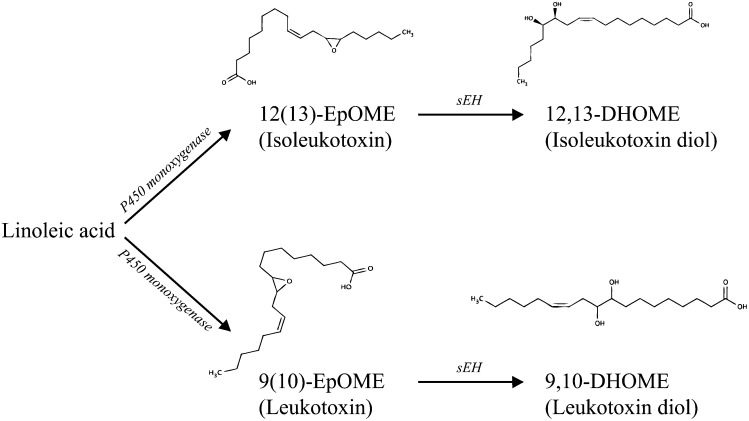
Of these metabolites, 12,13-DHOME (isoleukotoxin diol) (29) (Fig. 3) is of particular note. 12,13-DHOME is produced in humans from linoleic acid, via the protoxin 12(13)-EpOME (isoleukotoxin) by soluble epoxy hydrolase [sEH (EC: 3.3.2.10), (Fig. 3)] and forms part of a metabolic pathway that leads from linoleic acid via its oxidation (by P450 monoxygenase) to the leukotoxins (9(10)-EpOME and 12(13)-EpOME) and, potentially, the tetrahydrofurandiols (THF-diols, Fig. 2). Both 12,13-DHOME and 12(13)-EpOME are PPAR-γ ligands with potentially wide-ranging effects. The PPARs, or peroxisome proliferator-activated receptors, are lipid-activated transcription factors that regulate a host of genes related to energy balance and lipid metabolism (30). The effects of the PPARs are very wide-ranging (30). Thus 12,13-DHOME is known to directly affect cell differentiation (31) through its PPAR binding activity.
Fig. 3.
Structure and metabolic production of leukotoxins and their diols from linoleic acid via P450 monoxygenase and soluble epoxide hydrolase (sEH).
In addition to its role as a PPAR ligand, 12,13-DHOME is also a potential toxin. The leukotoxin diols (including 12,13-DHOME) can exert a range of pathophysiological effects in mammals including inhibition of mitochondrial function (32) and increased oxidative stress (33), whereas their leukotoxin precursors have been implicated in cases of acute respiratory distress syndrome in burn patients (34, 35) and cardio-pulmonary toxicity (36–38). Although it was once thought that sEH acted to render the leukotoxins safe by converting them to their diols, it now appears that the diols (including 12,13-DHOME) are the more-potent cytotoxins (29). Thus, inhibition of sEH ameliorates many of the toxic effects of the leukotoxins (39), whereas 12,13-DHOME adversely affects the electrophysiology of cells including oligodendrocytes (40) and cardiac myocytes (41). In addition, both 12(13)- and 9(10)-EpOME can be metabolized to the THF-diols (Fig. 2), themselves (together with the leukotoxin diols) capable of acting as endocrine disruptors in rats (42, 43).
Given that a compromised immune system is a significant adverse effect of Intralipid infusion, particularly for the very young and the critically ill, both of whom receive Intralipid infusions and both of whom are especially vulnerable to infections, much interest is focused on potential mechanisms and appropriate prevention. Any mechanism that involves linoleic acid, the major FA constituent of Intralipid, is attractive. The more commonly considered route of linoleic acid metabolism is via the 20-carbon FA arachidonic acid to the eicosanoids, a class of lipids (including prostaglandins, leukotrienes, and thromboxanes) that are key mediators of inflammatory and immune function (13). Thus, the prevailing hypothesis is that Intralipid infusion leads to a spike in eicosanoid concentrations with attendant effects on inflammatory status and immune response. Yet data supporting this hypothesis are inconsistent (for a discussion, see Ref. 4).
Critically, 12,13-DHOME inhibits the neutrophil respiratory burst (44), thus impairing the ability of neutrophils to respond appropriately to pathogens such as Gram-negative bacteria. In other words, 12,13-DHOME is significantly raised after Intralipid infusion, and is known to directly inhibit immune function in a manner that is consistent with Intralipid-induced immunosuppression. Our data, therefore, suggest that metabolism of linoleic acid, via sEH to the protoxin 12(13)-EpOME and the potentially toxic 12(13)-DHOME, may directly inhibit neutrophil function and thus immune response. This mechanism raises the possibility for use of sEH inhibitors (e.g., see Ref. 45) as a potential therapy for patients suffering adverse effects (or a prophylactic for those at risk), thus adding prevention of Intralipid-induced toxicity to the already-impressive list of potential clinical applications of sEH inhibitors (39).
Additional analysis of the control samples (blood + 30 mM Intralipid) showed that no 12,13-DHOME was present in the Intralipid itself. However, although our Intralipid was stored in glass bottles, Intralipid is often stored in oxygen-permeable bags for extended periods of time in clinical environments, which may well lead to spontaneous oxidation of linoleic acid and the presence of 12,13-DHOME and other epoxides and epoxide diols prior to infusion. Further studies should establish whether Intralipid that has been stored under different conditions, and for different durations, contains leukotoxins or their diols. One potential limitation of our work is that only a single duration and infusion rate of Intralipid was studied. It is unclear whether lower doses (or different durations of infusion) may have reduced the flux into 12,13-DHOME, and future studies should also address this question.
Finally, although our study shows that a sharp increase in circulating linoleic acid (due to Intralipid infusion) leads to formation of leukotoxins and their diols, it remains a possibility that a sudden increase in dietary intake of linoleic acid could have similar effects. There is evidence that humans evolved with a relatively low dietary linoleic acid intake (approximately 1:1 with the omega-6 FA α-linolenic acid) (46). Yet this is no longer the case, and a modern Western diet contains prodigious amounts of linoleic acid from ingredients such as vegetable oil that were not components of our legacy diet (47). This may mean that leukotoxins and their diols are now produced ubiquitously at low levels in many humans; future research should address this important question.
Our finding that Intralipid infusion acutely increases 3HB is consistent with existing theory and earlier data in rats (48). The accumulation of 3HB suggests that mitochondrial FA transport and β-oxidation in the liver were rapid enough to exceed the rate at which acetyl-CoA was being used by the TCA cycle. This leads to an oversupply of acetyl-CoA and a redirection of acetyl-CoA through acetoacetyl CoA thiolase (EC:2.3.3.10) into ketogenesis. Although perhaps rather obvious, the accumulation of 3HB during Intralipid infusion may yet have profound physiological implications. Ketones are an unusual metabolic fuel in that they increase the free energy available from ATP hydrolysis above normal values (49). Thus, acute infusion of 3HB into Langendorff-perfused rat hearts leads to a 25% increase in hydraulic work efficiency of the heart (50) (36% when combined with insulin). Furthermore, very recent work shows that feeding mice a ketone ester diet affects both brown fat (51) and brain metabolism (52). Thus, the effects of Intralipid infusion that were previously ascribed to acute increases in lipids may in fact be due to increased ketone concentration. In the context of our other findings (that Intralipid infusion increases the concentration of a number of linoleic acid metabolites that could potentially have a detrimental effect on cardiac function), an attractive hypothesis arises, that the effect of Intralipid on, for example, cardiac function would depend on the metabolic balance between the production of positive inotropes (such as 3HB) and negative inotropes (such as leukotoxin diols). More work in this area is clearly warranted.
CONCLUSIONS
We infused Intralipid into 10 healthy men and studied the changes in their plasma metabolome using LC/MS and 1H-NMR. We found that several metabolites of linoleic acid were significantly raised after Intralipid infusion. Of these, 12,13-DHOME (isoleukotoxin diol) is known to have adverse effects on neutrophil function. Thus, we hypothesize that one of the known adverse effects of Intralipid infusion, immunosuppression, may in part be mediated by raised 12,13-DHOME.
Acknowledgments
The authors thank Dr. Tim De Meyer for his donation of Matlab scripts, and Dr. Helen Atherton for support and advice. They also acknowledge the input of two anonymous reviewers whose excellent suggestions during the review process significantly enhanced this paper.
Footnotes
Abbreviations:
- BMI
- body mass index
- IVFE
- intravenous fat emulsion
- NOESY
- nuclear Overhauser effect spectroscopy
- PLSDA
- projection onto latent spaces discriminant analysis
- PPAR
- peroxisome proliferator-activated receptor
- sEH
- soluble epoxy hydrolase
- TIC
- total ion current
REFERENCES
- 1.Anon 2011. Intralipid official FDA information, side effects and uses. In. Baxter Healthcare Corporation. [Google Scholar]
- 2.Hammond E. G., Johnson L. A., Su C., Wang T., White P. J. 2005. Soybean oil. In Bailey's Industrial Oil and Fat Products. F. Shahidi, editor. John Wiley and Sons, Inc. 577–653. [Google Scholar]
- 3.Manavi M. V. 2010. Lipid infusion as a treatment for local anesthetic toxicity: a literature review. AANA J. 78: 69–78. [PubMed] [Google Scholar]
- 4.Mirtallo J. M., Dasta J. F., Kleinschmidt K. C., Varon J. 2010. State of the art review: intravenous fat emulsions: current applications, safety profile, and clinical implications. Ann. Pharmacother. 44: 688–700. [DOI] [PubMed] [Google Scholar]
- 5.Minh T. C., Oliver S. R., Flores R. L., Ngo J., Meinardi S., Carlson M. K., Midyett J., Rowland F. S., Blake D. R., Galassetti P. R. 2012. Noninvasive measurement of plasma triglycerides and free fatty acids from exhaled breath. J. Diabetes Sci. Technol. 6: 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sondermeijer B. M., Klein Twennaar C. F., Kastelein J. J., Franssen E. J., Hutten B. A., Dallinga-Thie G. M., Stroes E. S., Fliers E., Twickler M. T., Serlie M. J. 2012. Infusion of a lipid emulsion in healthy men decreases the serotonergic response. Neuroendocrinology. 95: 325–331. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shayji I. A., Caslake M. J., Gill J. M. 2012. Effects of moderate exercise on VLDL and Intralipid kinetics in overweight/obese middle-aged men. Am. J. Physiol. Endocrinol. Metab. 302: E349–E355. [DOI] [PubMed] [Google Scholar]
- 8.Postuma R., Trevenen C. L. 1979. Liver disease in infants receiving total parenteral nutrition. Pediatrics. 63: 110–115. [PubMed] [Google Scholar]
- 9.Lashner B. A., Kirsner J. B., Hanauer S. B. 1986. Acute pancreatitis associated with high-concentration lipid emulsion during total parenteral nutrition therapy for Crohn's disease. Gastroenterology. 90: 1039–1041. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y., Klein N. J., van Saene H. K., Webb G., Holzel H., Pierro A. 2000. Bactericidal activity against coagulase-negative staphylococci is impaired in infants receiving long-term parenteral nutrition. Ann. Surg. 231: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada Y., Papp E., Klein N. J., Pierro A. 1999. Total parenteral nutrition directly impairs cytokine production after bacterial challenge. J. Pediatr. Surg. 34: 277–280. [DOI] [PubMed] [Google Scholar]
- 12.Waddell W. R., Geyer R. P., Clarke E., Stare F. J. 1954. Function of the reticuloendothelial system in removal of emulsified fat from blood. Am. J. Physiol. 177: 90–94. [DOI] [PubMed] [Google Scholar]
- 13.Mancini A. D., Di Battista J. A. 2011. The cardinal role of the phospholipase A(2)/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E(2) (PCPP) axis in inflammostasis. Inflamm. Res. 60: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 14.Holland D. J., Erne D., Kostner K., Leano R., Haluska B. A., Marwick T. H., Sharman J. E. 2011. Acute elevation of triglycerides increases left ventricular contractility and alters ventricular-vascular interaction. Am. J. Physiol. Heart Circ. Physiol. 301: H123–H128. [DOI] [PubMed] [Google Scholar]
- 15.Smith C. A., Want E. J., O'Maille G., Abagyan R., Siuzdak G. 2006. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78: 779–787. [DOI] [PubMed] [Google Scholar]
- 16.Dieterle F., Ross A., Schlotterbeck G., Senn H. 2006. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78: 4281–4290. [DOI] [PubMed] [Google Scholar]
- 17.De Meyer T., Sinnaeve D., Van Gasse B., Tsiporkova E., Rietzschel E. R., De Buyzere M. L., Gillebert T. C., Bekaert S., Martins J. C., Van Criekinge W. 2008. NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal. Chem. 80: 3783–3790. [DOI] [PubMed] [Google Scholar]
- 18.Barton R. H., Waterman D., Bonner F. W., Holmes E., Clarke R., Nicholson J. K., Lindon J. C. 2010. The influence of EDTA and citrate anticoagulant addition to human plasma on information recovery from NMR-based metabolic profiling studies. Mol. Biosyst. 6: 215–224. [DOI] [PubMed] [Google Scholar]
- 19.Wolf S., Schmidt S., Muller-Hannemann M., Neumann S. 2010. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinformatics. 11: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn W. B., Broadhurst D., Begley P., Zelena E., Francis-McIntyre S., Anderson N., Brown M., Knowles J. D., Halsall A., Haselden J. N., et al. 2011. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6: 1060–1083. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren F., Hansen B., Karcher W., Sjöström M., Eriksson L. 1996. Model validation by permutation tests: Applications to variable selection. J. Chemometr. 10: 521–532. [Google Scholar]
- 22.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 57: 289–300. [Google Scholar]
- 23.Böcker S., Letzel M. C., Liptak Z., Pervukhin A. 2009. SIRIUS: decomposing isotope patterns for metabolite identification. Bioinformatics. 25: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pluskal T., Castillo S., Villar-Briones A., Oresic M. 2010. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 11: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes E., Foxall P. J., Spraul M., Farrant R. D., Nicholson J. K., Lindon J. C. 1997. 750 MHz 1H NMR spectroscopy characterisation of the complex metabolic pattern of urine from patients with inborn errors of metabolism: 2-hydroxyglutaric aciduria and maple syrup urine disease. J. Pharm. Biomed. Anal. 15: 1647–1659. [DOI] [PubMed] [Google Scholar]
- 26.Rane S. S., Anderson B. D. 2008. What determines drug solubility in lipid vehicles: is it predictable? Adv. Drug Deliv. Rev. 60: 638–656. [DOI] [PubMed] [Google Scholar]
- 27.Kind T., Fiehn O. 2006. Metabolomic database annotations via query of elemental compositions: mass accuracy is insufficient even at less than 1 ppm. BMC Bioinformatics. 7: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gipson G., Tatsuoka K., Sokhansanj B., Ball R., Connor S. 2008. Assignment of MS-based metabolomic datasets via compound interaction pair mapping. Metabolomics. 4: 94–103. [Google Scholar]
- 29.Moghaddam M. F., Grant D. F., Cheek J. M., Greene J. F., Williamson K. C., Hammock B. D. 1997. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 3: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina-Gomez G., Gray S., Vidal-Puig A. 2007. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Health Nutr. 10: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 31.Lecka-Czernik B., Moerman E. J., Grant D. F., Lehmann J. M., Manolagas S. C., Jilka R. L. 2002. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 143: 2376–2384. [DOI] [PubMed] [Google Scholar]
- 32.Sisemore M. F., Zheng J., Yang J. C., Thompson D. A., Plopper C. G., Cortopassi G. A., Hammock B. D. 2001. Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch. Biochem. Biophys. 392: 32–37. [DOI] [PubMed] [Google Scholar]
- 33.Viswanathan S., Hammock B. D., Newman J. W., Meerarani P., Toborek M., Hennig B. 2003. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J. Am. Coll. Nutr. 22: 502–510. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa M., Kosaka K., Sugiyama S., Yokoo K., Aoyama H., Izawa Y., Ozawa T. 1990. Proposal of leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin. Biochem. Int. 21: 573–579. [PubMed] [Google Scholar]
- 35.Kosaka K., Suzuki K., Hayakawa M., Sugiyama S., Ozawa T. 1994. Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol. Cell. Biochem. 139: 141–148. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima A., Hayakawa M., Sugiyama S., Ajioka M., Ito T., Satake T., Ozawa T. 1988. Cardiovascular effects of leukotoxin (9, 10-epoxy-12-octadecenoate) and free fatty acids in dogs. Cardiovasc. Res. 22: 213–218. [DOI] [PubMed] [Google Scholar]
- 37.Sakai T., Ishizaki T., Ohnishi T., Sasaki F., Ameshima S., Nakai T., Miyabo S., Matsukawa S., Hayakawa M., Ozawa T. 1995. Leukotoxin, 9,10-epoxy-12-octadecenoate inhibits mitochondrial respiration of isolated perfused rat lung. Am. J. Physiol. 269: L326–L331. [DOI] [PubMed] [Google Scholar]
- 38.Ishizaki T., Shigemori K., Nakai T., Miyabo S., Ozawa T., Chang S. W., Voelkel N. F. 1995. Leukotoxin, 9,10-epoxy-12-octadecenoate causes edematous lung injury via activation of vascular nitric oxide synthase. Am. J. Physiol. 269: L65–L70. [DOI] [PubMed] [Google Scholar]
- 39.Morisseau C., Hammock B. D. 2005. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 45: 311–333. [DOI] [PubMed] [Google Scholar]
- 40.Ha J., Dobretsov M., Kurten R. C., Grant D. F., Stimers J. R. 2002. Effect of linoleic acid metabolites on Na(+)/K(+) pump current in N20.1 oligodendrocytes: role of membrane fluidity. Toxicol. Appl. Pharmacol. 182: 76–83. [DOI] [PubMed] [Google Scholar]
- 41.Harrell M. D., Stimers J. R. 2002. Differential effects of linoleic acid metabolites on cardiac sodium current. J. Pharmacol. Exp. Ther. 303: 347–355. [DOI] [PubMed] [Google Scholar]
- 42.Markaverich B. M., Alejandro M., Thompson T., Mani S., Reyna A., Portillo W., Sharp J., Turk J., Crowley J. R. 2007. Tetrahydrofurandiols (THF-diols), leukotoxindiols (LTX-diols), and endocrine disruption in rats. Environ. Health Perspect. 115: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markaverich B. M., Crowley J. R., Alejandro M. A., Shoulars K., Casajuna N., Mani S., Reyna A., Sharp J. 2005. Leukotoxin diols from ground corncob bedding disrupt estrous cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environ. Health Perspect. 113: 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson D. A., Hammock B. D. 2007. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 32: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang S. H., Tsai H. J., Liu J. Y., Morisseau C., Hammock B. D. 2007. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 50: 3825–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simopoulos A. P. 1999. Evolutionary aspects of omega-3 fatty acids in the food supply. Prostaglandins Leukot. Essent. Fatty Acids. 60: 421–429. [DOI] [PubMed] [Google Scholar]
- 47.Kris-Etherton P. M., Taylor D. S., Yu-Poth S., Huth P., Moriarty K., Fishell V., Hargrove R. L., Zhao G., Etherton T. D. 2000. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 71 (Suppl.): 179–188. [DOI] [PubMed] [Google Scholar]
- 48.Lutz O., Meraihi Z., Mura J. L., Frey A., Riess G. H., Bach A. C. 1989. Fat emulsion particle size: influence on the clearance rate and the tissue lipolytic activity. Am. J. Clin. Nutr. 50: 1370–1381. [DOI] [PubMed] [Google Scholar]
- 49.Veech R. L. 2004. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 70: 309–319. [DOI] [PubMed] [Google Scholar]
- 50.Sato K., Kashiwaya Y., Keon C. A., Tsuchiya N., King M. T., Radda G. K., Chance B., Clarke K., Veech R. L. 1995. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 9: 651–658. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava S., Kashiwaya Y., King M. T., Baxa U., Tam J., Niu G., Chen X., Clarke K., Veech R. L. 2012. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26: 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kashiwaya Y., Pawlosky R., Markis W., King M. T., Bergman C., Srivastava S., Murray A., Clarke K., Veech R. L. 2010. A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J. Biol. Chem. 285: 25950–25956. [DOI] [PMC free article] [PubMed] [Google Scholar]