Abstract
Acrylodan-labeled rat-intestinal fatty acid binding protein, ADIFAB, binds both of lysophosphatidylcholines (LPC) and FA. Binding displaces Acrylodan and its fluorescence peak shifts from 432 to 505 nm. A fluorescence assay that relies on this shift is presented for quantitating LPC, FA, and phospholipase A2 (PLA2) activity in phospholipid bilayers in absolute units of μM/min/mg of enzyme. This is a development over an earlier assay that took into account only FA binding. Activities of bee venom PLA2 on dipalmitoylphosphatidylcholine (DPPC) and dioleylphosphatidylcholine (DOPC) bilayers were measured. Standard pH-Stat assays validated the present assay. Products increase linearly with time for about one minute in DOPC and five minutes in DPPC corresponding to completion of 5 to 8% hydrolysis in DOPC and 20% in DPPC. Membrane polarity and microviscosity measured using electron spin resonance (ESR) exhibited discontinuities at compositions that mimicked similar percentages of hydrolysis products in the respective bilayers. The observed hydrolysis rate decrease following the initial linear period thus correlates to changes in membrane polarity. The ability of the assay to yield actual product concentrations, reveal structure in the reaction progress curves, and interpretation in light of the ESR data bring insight into the shape of the reaction curve.
Keywords: lysophospholipids, lipid hydrolysis, fluorescence assay
Acrylodan-labeled rat-intestinal fatty acid binding protein (ADIFAB) exhibits a fluorescence emission peak at 432 nm when excited by light of wavelength 386 nm (1). The emission peak shifts to 505 nm upon binding an FA. The ADIFAB fluorescence response depends on the type of FA. The ratio of the intensities at 505 nm to that at 432 nm is a measure of the concentration of FA present (2, 3). This property, useful for detection of low levels of FAs, led to the development of a sensitive fluorescence assay for phospholipase A2 (PLA2) activity (4).
ADIFAB also binds lysophospholipids, anionic and some zwitterionic detergents. This fact, although intrinsically useful for quantitating not just FAs but lysophosphatidylcholines (LPCs) as well, calls into question the earlier PLA2 assay that took into account only FA binding. The goals of this work were to establish the quantitative and linear nature of ADIFAB fluorescence response to FA and LPC, develop quantitative assays for detecting both FA and LPC in aqueous solutions as well as for continuous real time monitoring of PLA2 hydrolysis at membrane interfaces expressing activity in absolute units of μM of product/minute/mg of enzyme, and extend the applicability of the ADIFAB PLA2 assay to more lipids and lipid bilayers.
Phospholipase catalyzed hydrolysis of phospholipids at membrane interfaces produces FAs and lysophospholipids (5). The rate of hydrolysis depends on membrane physicochemical properties like membrane curvature, lipid composition, lipid chain length, and headgroup charge (6, 7). It is typically quite low at membrane interfaces and can be well below the precision levels of the standard pH-Stat method (8). The importance of PLA2 activity to several physiological functions and membrane biochemistry demands sensitive methods not only to detect membrane hydrolysis but also to quantitatively and continuously monitor PLA2 activity in real time in absolute units (9, 10). In this context, the fluorescence response of the probe ADIFAB to the presence of hydrolysis products offers just such a method (1).
The ADIFAB fluorescence assay, first reported by Richieri and Kleinfeld, relies on the shift in fluorescence emission peak that results when the FA products that partition into the aqueous phase from the membrane following hydrolysis bind to ADIFAB (4). The ratio, R, of the fluorescence at 505 nm to that at 432 nm, being a measure of the amount of FA present, serves also as a measure of enzyme activity. However, there are several problems with the assay described. ADIFAB is introduced as an FA binding protein and the entire fluorescence response is attributed to FA binding. Present experiments show that ADIFAB also binds lysophospholipids, the other hydrolysis product with a response that is significant but different from that of FA binding. The partition coefficients for lysophospholipids and FAs between membrane and aqueous phase are not the same. Equimolar amounts are produced by hydrolysis but the amount of lysophospholipids released into the aqueous medium is about twice that of the FA in the case of dipalmitoylphosphatidylcholine (DPPC) vesicles, for example. The equilibrium binding constants for the two products differ from each other. These pose serious problems when using the equations formulated to quantify activity based on the binding of only the FA. The calibration equation used to fit the fluorescence data of ADIFAB and FA in aqueous solutions to determine the amount FA present involves the equilibrium binding constant, instrument parameters, and fluorescence properties of the maximum and minimum values of R[1]. Occurrence of too many fitting parameters in a rather cumbersome equation combined with the lack of a good range of FA concentrations, before aggregation, over which to gather data, complicates fitting. The problem becomes intractable when mixtures of two types of binding species are present. Under such conditions, reliably quantifying the activity in absolute units is challenging. In the light of the problems stated with the use of ADIFAB, the ability of ADIFAB as an analytical, quantitative probe to determine PLA2 activity compels further investigation.
In the present work, investigations of ADIFAB as a probe to quantitatively detect lysolipids and FAs and their mixtures in aqueous solutions were first conducted. The results prompted a new way of performing the ADIFAB assay that overcomes the stated problems. The observed structure in the PLA2-lipid membrane reaction progress curves follow physicochemical changes in the membrane measured using electron spin resonance (ESR). ADIFAB can indeed be a viable candidate for a sensitive, convenient assay. Assays were conducted on DPPC as well as dioleylphosphatdylcholine (DOPC) vesicles using bee venom PLA2. The initial work of Richieri and Kleiffeld was on DOPC vesicles (4). The activity values of 1800 and 2500 μM/min/mg reported for some of the enzymes are rather high for bilayers. The modified assay presented here yield values for the activity that are in the ballpark expected for bilayers. Standard pH-Stat assays were also conducted to validate the fluorescence assay.
Surface tension measurements were conducted to determine the consequences of lipid aggregation to the performance of ADIFAB as a probe. Complementary ESR experiments were conducted for a comprehensive understanding of the consequences of product accumulation, in the course of hydrolysis, to membrane physicochemical properties and the rate of hydrolysis.
Materials and Methods
Materials
The phospholipids investigated in this work were DOPC and DPPC and the products resulting from their hydrolysis lysopalmitoylphosphatdylcholine (LPPC), palmitic acid (PA), oleic acid (OA), and lysooleylphosphatdylcholine (LOPC). The DOPC, DPPC, LPPC, and LOPC, were obtained from Avanti Polar Lipids as lyophilized powders. Sodium salts of OA and PA were obtained from TCI America. The spin probe employed in the ESR experiments was 16-doxyl stearic acid methyl ester (16 DSE; 99% Sigma). The enzyme phospholipase A2 (PLA2) from honey bee venom was obtained from Sigma as a lyophilized powder. It was purified by dialysis against 0.05M sodium phosphate buffer at pH 8.0 for 3 days, changing the buffer every 8 h (11). Protein concentration was determined by the extinction coefficient method (11). The purified enzyme was stored at pH 8.0 in 0.05M sodium phosphate buffer at 4°C. The fluorescence probe, ADIFAB, was obtained from FFA Sciences (San Diego, CA).
Methods
The ADIFAB fluorescence spectra were measured with a Horiba Scientific Fluoromax - 4 spectrometer. The excitation was at 386 nm. The fluorescence response to the presence of binding entities represented by the generalized polarization (GP) defined by (12, 13)
was calculated where I505 and I432 are the fluorescence emission intensities at 505 nm and 432 nm respectively. The GP is a normalized representation of the concentration of the binding species.
In the first part of the experiments, ADIFAB fluorescence spectra in Hepes buffer solutions in the absence of any additives and in the presence individually of LPC, FA, mixtures of these in known proportions, and dispersions of LPC, FA, and their mixtures in PC bilayers were studied. These experiments were conducted primarily to understand ADIFAB fluorescence response with respect to the concentrations of the different binding entities. The properties determined were the partition coefficients of the LPC and FA between the model membrane vesicles and the aqueous medium, their proportions in the aqueous phase when both are dispersed in lipid vesicles, effects of micellar or other types of aggregation of LPC and FA on the fluorescence response, and the limits of linearity of GP with respect to the concentration of additives.
Complementary investigations of the aggregation behavior of LPPC and LPPC / PA mixtures in aqueous media were conducted by surface tension measurements. A Krüss K12 Surface Tensiometer with a Wilhelmy plate was employed to determine surface tension of the aqueous solutions as a function of concentration. The platinum plate was thoroughly cleaned with ethanol followed by exposure to flame before each measurement. In solutions of micelle forming surfactants, the surface tension decreases rapidly as concentration is increased until about the beginning of micelle formation. The surface tension then decreases more slowly, reaches its lowest value, and remains constant for further increase in concentration (14). The critical micelle concentration (cmc) is defined by the concentration at the intersection of the lines through the data points in the initial rapidly decreasing region and the postmicelle region.
The next part of this work deals with the assay for PLA2 activity. The method involves development and use of calibration curves. The ADIFAB fluorescence spectra were recorded for mixed vesicles of various proportions of phospholipids and hydrolysis products in Hepes buffer, each representing a certain level of hydrolysis. For example, a mixture containing 90 μM/L phospholipids and 10 μM/L each of LPC and FA represents the condition at 10% hydrolysis. The ADIFAB detects the amount of products that partition into the aqueous phase and responds with a change in its fluorescence emission features. The observed GP is the GP value for 10 μM/L of the product FA or LPC formed. A calibration curve of GP versus μM/L of either product formed was thus determined at compositions mimicking 0.1% to 40% hydrolysis of 100 μM/L of phospholipids.
Enzymatic activity measurements were conducted on small unilamellar vesicles (SUVs) and multilamellar vesicles (MLVs). The lipid concentration in all samples was 100 μM/L. The vesicles for enzymatic activity as well as for calibration and ESR were prepared by first dissolving calculated amounts of phospholipids (and the spin probe 16 DSE in the case of ESR) in ethanol. The ethanol solution was vortexed thoroughly to produce a clear solution, which was then dried under dry N2 flux to produce a film of lipid. Thereafter, the required amounts of the Hepes buffer (pH = 7.4) was added to the dry film to achieve the final concentration of 100 μM/L. The solutions were vortexed for 5 min to produce MLVs. The MLV solution was then sonicated for 20–25 min to produce SUVs. Appropriate amounts of 100 μM/L LPC and FA solutions were added to the phospholipid vesicles to prepare the samples for calibration consisting of mixtures of phospholipids, LPC, and FA. These samples were sonicated for 10 min to ensure homogeneous distribution. Calcium is a required cofactor in PLA2 hydrolysis (15). Calcium chloride was added to all samples at a concentration of 1 mM. The calibration and activity measurements were conducted at sample temperatures of 37°C.
The continuous time course of hydrolysis was determined by recording the fluorescence emission intensities at 432 nm and 505 nm at 12 s intervals, using the Multi option of the Fluoromax-4. In this mode of operation, the spectrometer grating swivels between positions to measure the intensities at user selected wavelengths at appropriately specified slew rates. In the present study, the two wavelengths were 432 nm and 505 nm. The recorded intensities at each of these two wavelengths were displayed as a function of time. The GP was then calculated and the rate of GP variation with time was obtained from the slope of the initial duration of hydrolysis. This slope of GP units/min was converted to μM/L /min of FA (or lysolipid) product formation rate using the calibration curve. Dividing by the concentration of enzyme in the sample in units of mg/L yields the enzyme activity in the standard units of μM of FA formed per min per mg of enzyme. The sample volume was 2 ml, to which 2 μl of 0.04 mg/ml of enzyme solution was added just before start of the experiment.
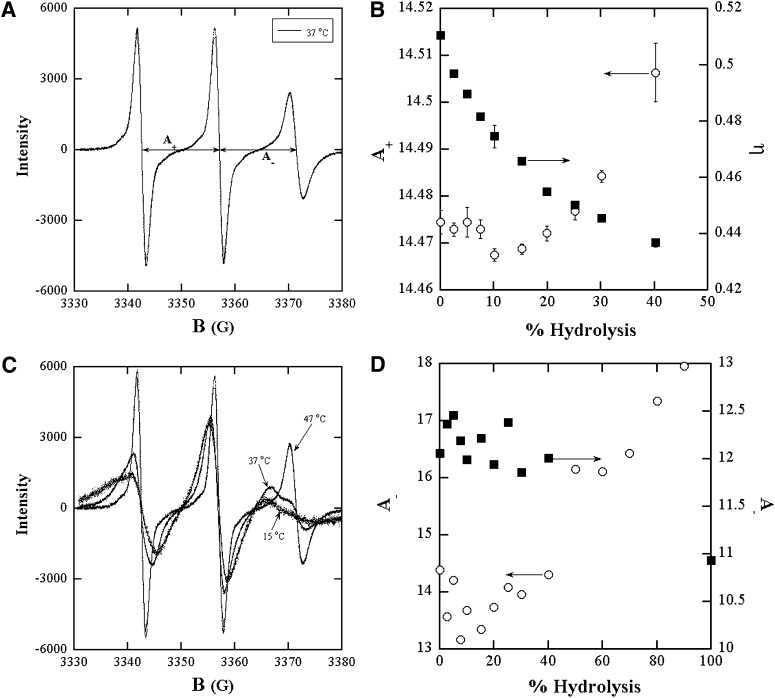
ESR spectra of spin probes incorporated in to bilayers of mixtures of the lipids and their hydrolysis products were used to probe the effect of accumulation of reaction products on the membrane physicochemical properties and on the course of the reaction itself. ESR spectra yield the polarity and microviscosity of the spin probe neighborhood in the form of the hyperfine splitting constant A+ and the rotational correlation time, respectively (16, 17). These properties depend on membrane composition and thus on the presence of hydrolysis products. The ESR spectra were taken at X-band using a Bruker ESP 300 E spectrometer equipped with a Bruker variable temperature unit (Model B-VT-2000). The details of sample configuration for ESR have been described previously (16, 17). Five ESR spectra were acquired for each sample. Fittings of the experimental ESR lines to a Lorentzian-Gaussian sum function return the position of the resonance fields of the three ESR lines with a precision of a few milligauss, and also separate the Lorentzian and Gaussian contributions of the spin label ESR lines. The spacing between hyperfine lines is defined as
where HMI denotes the resonance fields of the three lines: MI = +1, 0, and −1 corresponding to the low-, center-, and high-field lines, respectively (see Fig. 6A for example). In the case of slow motion spectrum such as in the gel phase of vesicles, an asymmetry is generally observed in the nitroxide spectrum (18). This asymmetry produces a shift of the MI = +1, −1 lines toward the center, that is greater for high field line (MI = −1). Thus changes around the neighborhood of spin probe in such rigid limit spectra are more clearly represented by changes in A_.
Fig. 6.
ESR spectra of 16 DSE in lipid bilayer dispersions of hydrolysis products in (A) LOPC (2.5 mM)+OA(2.5 mM)+DOPC(22.5 mM) MLV bilayers at 37°C; (C) LPPC (2.5 mM)+PA(2.5 mM)+DPPC(22.5 mM) MLV at 15°C, 37°C, 47°C. B: Hyperfine splitting constant A+ and microviscosity in DOPC +LOPC+OA and (D) Hyperfine splitting constants A_ of the gel (○) and liquid (■) phases in DPPC +LPPC+PA derived from spectral fitting as a function of the percent level of either of the hydrolysis products. Each of the compositions represents states of the bilayer during the progress of the hydrolysis reaction.
The ESR line widths yield the rotational correlation time τ of the spin probe (16, 18). This time depends on the viscosity of its environment referred to here as the microviscosity, η, according to the Debye-Stokes-Einstein equation;
where r is the hydrodynamic radius of the spin probe (= 3.75 Å for 16 DSE) (19, 20), k is the Boltzmann constant and T is the sample temperature.
The lipid concentration for the ESR experiments was 25 mM and the spin probe concentration was 0.25 mM.
In order to evaluate the new fluorescence assay, activity on the same samples was determined using the well-established technique of pH-Stat and the results of the two assays were compared. In this method, the change in pH induced by the FA released by the enzymatic reaction is sensed. The pH of the reaction mixture is maintained at 8.0 by automatic addition of 0.1 N NaOH. The amount of NaOH added is directly the amount of FA formed. The experiment gives the amount of NaOH added versus time and the slope of the curve is the rate of hydrolysis.
Results
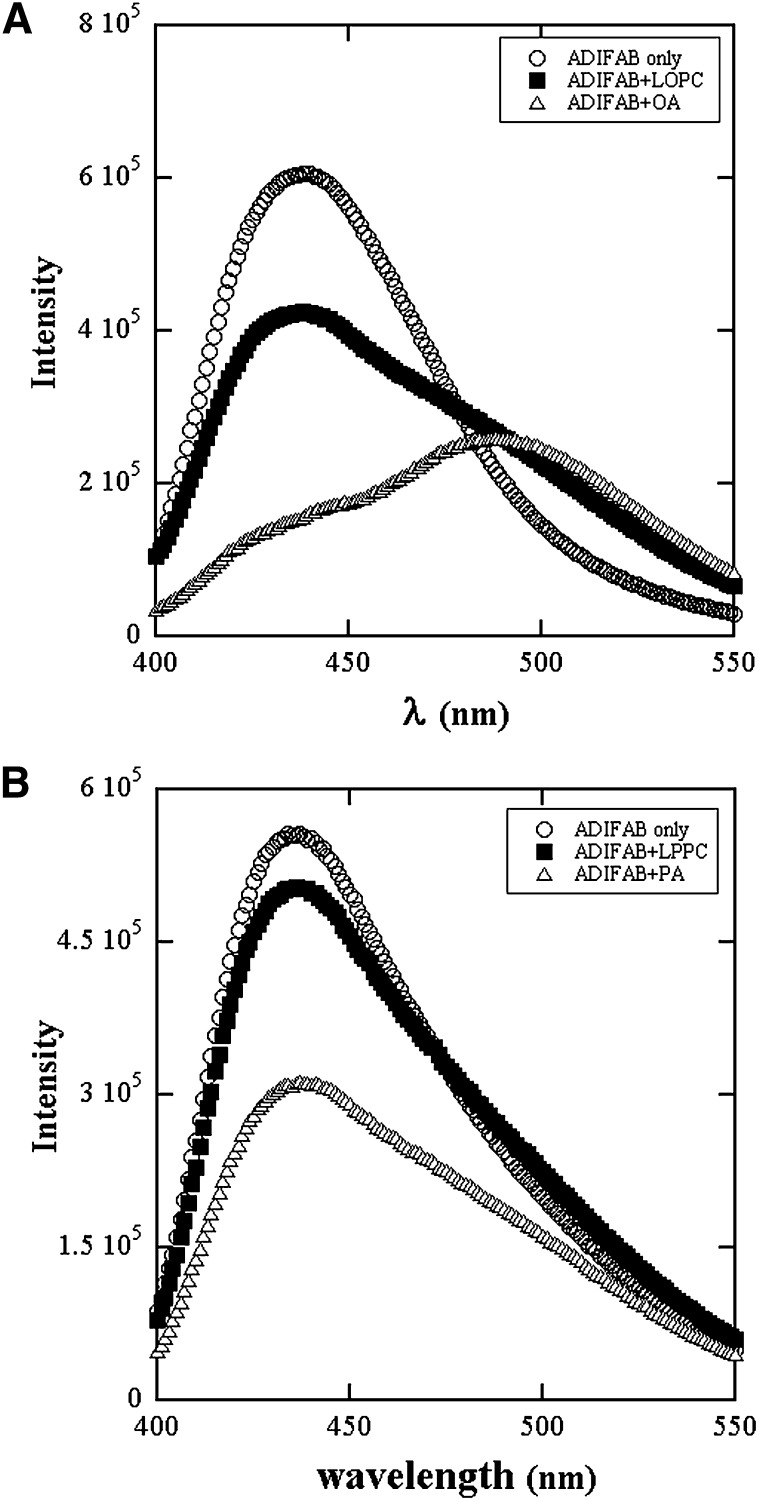
Fluorescence emission spectra of ADIFAB in Hepes buffer in the absence and presence of lysolipids and FAs are shown in Fig. 1A and B for concentrations of 1 μM/L. The increase in intensity at 505 nm demonstrates clearly that lysolipids as well as FAs bind to ADIFAB. The GP values calculated from the spectra increase linearly with concentration initially as shown in Fig. 2A. LPPC solutions exhibit a change to about a constant value at about the cmc, which was measured by surface tension. The GP value of ADIFAB in the buffer solutions obtained from blank runs performed before every experiment is -0.57 ± 0.025.
Fig. 1.
Fluorescence emission spectra of ADIFAB excited at 386 nm in (A) Hepes buffer without (○) and with 1 μM each of LOPC(■) and OA(△). B: Hepes buffer without (○) and with 1 μM each of LPPC(■) and PA(△). Acrylodan is displaced and its fluorescence emission shifts to the red region of the spectrum upon binding of LPC and FA to the ADIFAB protein.
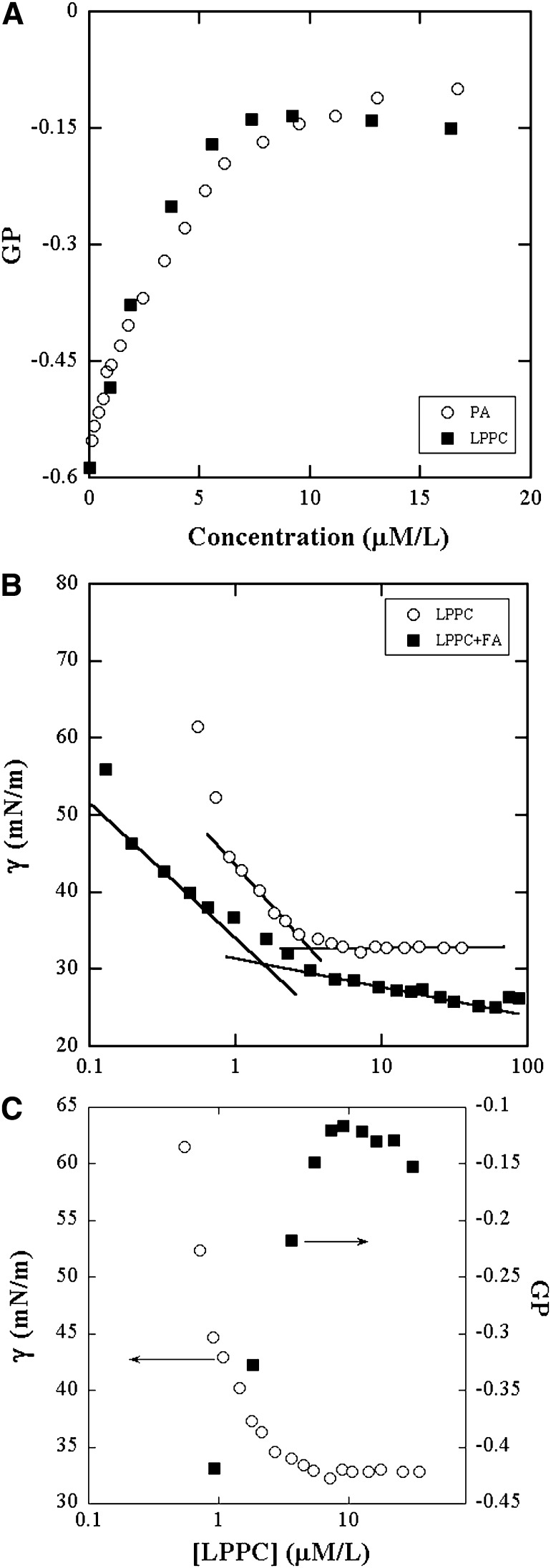
Fig. 2.
A:Variation of GP of ADIFAB fluorescence in Hepes buffer with concentration of LPPC (■) and PA (○). The flattening of the curve in LPPC solutions is due to micelle formation. B: cmc determination of LPPC (○) and 1.7:1 of LPPC:PA (■) by surface tension (γ) measurements. The surface tension decreases with concentration and cmc is the concentration at the intersection of the two lines shown. C: Comparison of GP (■) and surface tension (○) to show their similarities in behavior and the effect of micelle formation on GP.
The surface tension of aqueous solutions of LPPC, shown in Fig. 2B, decreases initially with concentration and then remains constant. This behavior is typical of micelle forming surfactants (21). The breakpoint denotes onset of the formation of micelles whereupon the surface monomer concentration stays constant. The cmc was determined to be 2.82 μM/L. The GP, like surface tension, remains constant in the post micelle region where the monomer concentration remains about constant while the micelle concentration increases. Fig. 2C illustrates this similarity in the behaviors of GP and surface tension. Fig. 2A and C demonstrate that micellar aggregation gives rise to the apparent saturation in GP and, furthermore, that ADIFAB binds only to the free unassociated monomers. The data in Fig. 2A establishes the linear region for use in calibration. Fig. 2B also shows the surface tension data in mixtures of 1:1.7 PA to LPPC. This is the proportion predicted by the experiments on PC bilayers described later to determine the partition coefficients of PA and LPPC. The surface tension decreases with concentration more slowly than in neat LPPC solutions, as expected for mixtures of surfactants and hydrophobic additives (22). The data show that micelles begin to form when the LPPC concentration is 1.01 μM/L. The cmc data of the mixtures provide information on the concentration of hydrolysis products at which micelle formation and bilayer dissolution may occur.
Similar experiments were conducted on LPPC and PA dispersed individually in DPPC bilayer vesicles. The observed GP values are due to binding of the monomers present in the aqueous phase. Using Fig. 2A for monomers in solution as the calibration, the concentrations of the monomers that partition into the aqueous phase were determined. In these experiments, the concentration of DPPC was constant at 100 μM/L. For dispersions of up to 4 μM/L LPPC or PA, 0.01% of PA and 0.017% of LPPC are present in the aqueous phase.
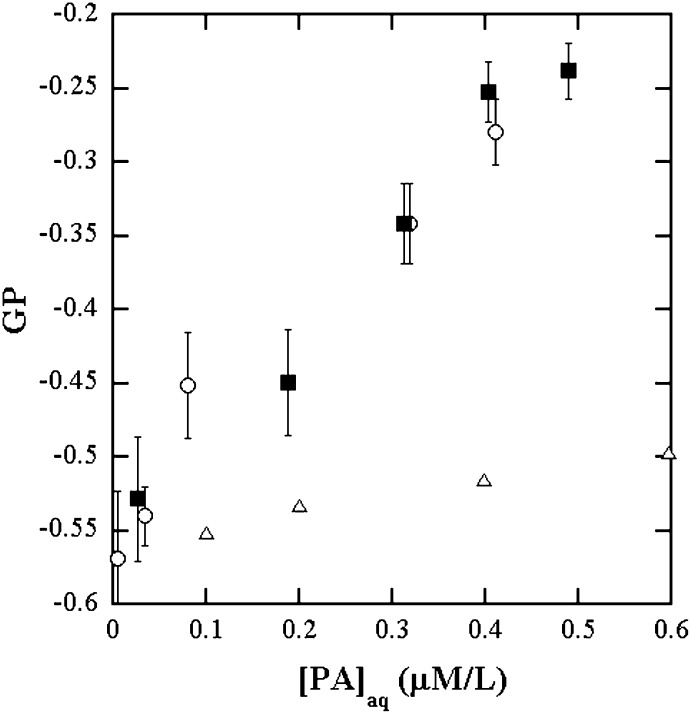
Fluorescence emission spectra in the presence of equimolar concentrations of LPPC and PA dispersed in DPPC bilayer vesicles were then measured. The LPPC and PA concentrations were each varied up to 40 μM/L. The DPPC + LPPC or PA was 100 μM/L. These samples represent the bilayer compositions present at various levels of hydrolysis up to 40%. The GP values in these samples are due to the LPPC and PA that partition into the aqueous phase. The composition ratio of LPPC to PA in the aqueous phase is predicted to be 1.7:1 from the partition experiments. The fluorescence emission of ADIFAB in 1.7:1 mixtures of LPPC to PA in Hepes buffer was then measured. The GP of the equimolar concentrations of LPPC and PA in DPPC is in quantitative agreement with mixtures in aqueous solutions at the ratio 1.7:1 of LPPC: PA predicted from the measured bilayer/aqueous phase partition coefficients up to about 20% each of LPPC and PA. Fig. 3 displays this agreement, demonstrating that when both of LPPC and PA are present in the bilayer dispersions, their ratio in the aqueous phase is 1.7:1. Also plotted in Fig. 3 are the GP data of ADIFAB in the presence of PA alone in the buffer solutions to illustrate that the increase in GP when LPPC is also present is not negligible. Results presented so far are those of investigations of the properties of ADIFAB for use in quantitating LPC and FA and their mixtures in bilayers. Of these, the data on the mixtures in bilayers are relevant for enzymatic activity measurements.
Fig. 3.
GP of ADIFAB fluorescence as a function of the concentration of PA in the aqueous phase for equimolar dispersions of LPPC and PA in DPPC SUV bilayers (■). The [PA]aq on the x axis are the aqueous phase concentrations calculated as 0.01% of [PA] present as predicted by the partition experiments; 1.7:1 mixtures of LPPC:PA in Hepes buffer (○); Hepes buffer (△). The agreement between A and B demonstrates that the partition coefficient from bilayer to aqueous phase of LPPC is 1.7 times that of PA.
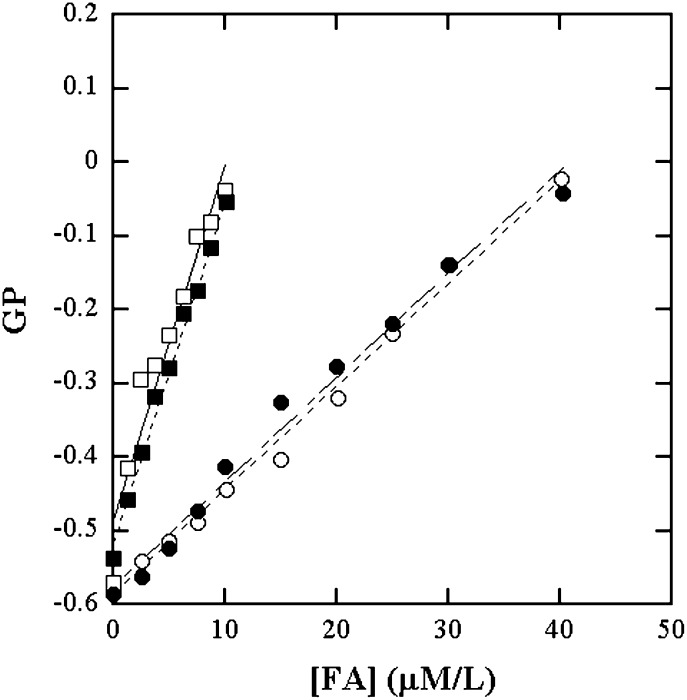
Phospholipid bilayer dispersions containing equimolar amounts of lysophospholipids and FAs are representations of the status at various levels of membrane hydrolysis. With this rationale, the ADIFAB fluorescence GP of these dispersions were viewed as functions of the concentrations of one of the constituents, namely the FA, as shown in Fig. 4. The curves in Fig. 4 for SUVs and MLVs of DPPC and DOPC were treated as calibrations to determine the amount of products formed in the hydrolysis experiments on these types of bilayers. The GP calibration curves are similar for SUVs and MLVs. The GP variation is linear until about 40% of products. At these concentrations, about 0.4 μM of PA and 0.68 μM of LPPC would be present in the aqueous phase. This is below the cmc of the LPPC/PA mixture at the ratio 1.7:1 (Fig. 2B). The linearity together with the cmc data on the mixtures suggest that micelle formation and membrane dissolution do not occur until at least about 40% hydrolysis.
Fig. 4.
Calibration curves of GP versus FA in equimolar dispersions of FA and LPC in lipid vesicles for use in PLA2 activity measurements on DPPC and DOPC. GP vedrsus [PA] in DPPC SUV (○); in DPPC MLV (●); [OA] in DOPC SUV (□) and in DOPC MLV (■).
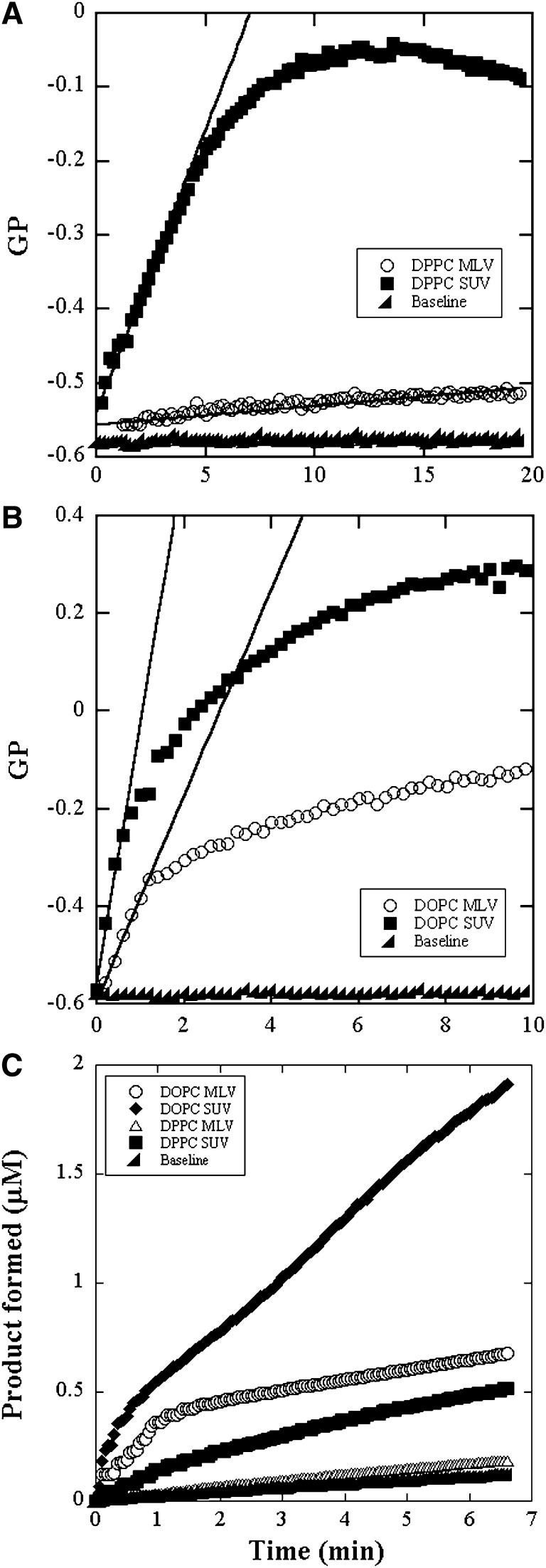
The reaction progress curves in the four different types of vesicles from GP of ADIFAB and from pH-Stat experiments are shown in Fig. 5A–C. Also shown in Fig. 5A and B is the background variation in GP over time measured for ADIFAB alone in buffer solutions. The initial linear period is about 1 min for DOPC SUVs and MLVs and about 5 min for DPPC SUVs and longer than 20 min for DPPC MLVs. The lines in Fig. 5A and B are the linear fits to the data from the first few minutes. The reaction exhibits a discontinuity at the end of this linear period and then appears to slow down continuously. The point of discontinuity is determined as that instant where the measured GP turns away from the fitted line by about 10%.
Fig. 5.
Reaction progress curves of bee venom PLA2 catalyzed hydrolysis of 100 μM DPPC and DOPC bilayers. GP of ADIFAB fluorescence in SUV and MLV of (A) DPPC and (B) DOPC. Two microliters of enzyme solution at a concentration of 0.04 mg/ml was added to the sample solution of volume 2 ml. The solid lines are linear fits to the initial linear region. The slopes of these lines yield the initial rate of hydrolysis. C: product formed measured by pH-Stat in the DPPC and DOPC bilayers. Three microliters of enzyme solution at a concentration of 0.8 mg/ml was added to the sample solution of volume 5 ml.
The slopes of the initial linear regions were converted to the rate of FA production using the appropriate GP calibration curve. The calculated activities for each of the vesicles in units of μM of FA/min/ mg of enzyme are presented in Table 1 along with the values obtained using pH-Stat. The ±8% uncertainty was the standard deviation obtained from five measurements on DPPC. From the activity values, the percent level of hydrolysis at which the discontinuity appears was calculated to be about 18.5% at the end of 5.2 min for DPPC and about 5 to 8% for DOPC at the end of about 1 min. At this point, in the case of DPPC, about 0.185 μM/L of PA and 0.315 μM/L of LPPC would be present in the aqueous phase according to the partition coefficients. The pH-Stat data for DPPC MLV is only barely resolved from the background variation obtained using aqueous solutions in atmosphere.
TABLE 1.
Activities of PLA2 from bee venom on phospholipids vesicles
| Phospholipid | Activity (ADIFAB)μM/mg/min | Activity (pH-Stat)μM/mg/min |
| DOPC-SUV | 241 ± 20 | 193 ± 83 |
| DOPC-MLV | 109 ± 9 | 125 ± 64 |
| DPPC-SUV | 89 ± 7 | 51 ± 50 |
| DPPC-MLV | 24 ± 2 | — |
Lipid concentration was 100 μM/L.
As reaction proceeds, there are physicochemical changes in the bilayers because of the accumulation of products. Membrane polarity and microviscosity derived from the ESR spectra of the spin probe, 16 DSE, in bilayer samples mimicking various levels of hydrolysis showed discontinuities at similar levels of hydrolysis as did the reaction progress curves. Figure 6 shows the ESR spectra obtained in DOPC and DPPC at conditions of 10% hydrolysis. The gel to liquid transition temperature of DOPC is −20°C and that of DPPC is 41°C (23). The three line spectrum, in Fig. 6A, of the mixed bilayers of DOPC and its hydrolysis products at 37°C signifies a homogeneous liquid phase. Bilayers of DPPC and its hydrolysis products exhibit three line spectra at 47°C and 15°C of homogeneous liquid and gel phases, respectively and a six line spectrum at 37°C signifying coexistence of liquid and gel phases. The membrane polarity represented by the hyperfine splitting constants, A+ and the microviscosity derived from fittings of the ESR spectra of DOPC/LOPC/OA samples, shown in Fig. 6B, show discontinuities at about 8% hydrolysis where the enzymatic reaction also shows a break. In DPPC/LPPC/PA, the lines in the liquid phase at 47°C are narrower and sharper than the lines in the gel phase at 15°C. Thus, the A+ and A_ are higher for the liquid phase implying also higher polarity. For the spectra of the coexisting phases of DPPC/LPPC/PA, the better resolution of the high field lines of the two phases (see section on Methods) makes A_ a better choice for study of variation with respect to composition, as in Fig. 6D. The higher A_ at low LPPC/PA content is taken to be that of the liquid phase. The A_ values in both phases are scattered until about 20% each of LPPC and PA. Thereafter the polarity of the gel phase increases. The fitting to the six lines was reasonable only for the lower A_ line. Further experiments using second harmonic generation are to be conducted to better resolve the lines (24). For the scope of this paper, the increase in A_ observed at about 20% hydrolysis is treated as evidence of a change in membrane polarity at that composition.
Discussion
The binding of lysolipids to ADIFAB results in weaker GP response than that of FA but it is not insignificant in general. It is truer for the products, LOPC and OA, of DOPC hydrolysis than for those of DPPC hydrolysis, namely LPPC and PA. However, the partitioning of lysolipids into the aqueous phase is stronger than for FAs, which means the presence of a higher concentration of the weaker binding lysolipids. Therefore, the fluorescence response is not exclusive to FAs only. The new method, developed to include the response to both products of hydrolysis, is more rigorous than the initially proposed assay.
Concurrence between the % levels of hydrolysis at which discontinuities in the reaction progress curves and in the membrane properties are observed imply the role of membrane polarity and microviscosity in the rate constants of the enzymatic reactions. Studies of PLA2 kinetics in the past have generally not paid enough attention to inflection points and changes in shape of the reaction progress curves. The ESR data included here show the correlation between the profiles of the reaction rates and membrane polarity of the bilayer as it is being digested. This work shows the potential of ESR in bringing insight more directly into the role of membrane physical properties. Extending the ESR experiments to investigate the phase behavior of phospholipid/lysolipid/FA systems is a point in its own right to consider for the future. More sensitive ESR methods involving second harmonic measurements of the ESR absorption spectra to resolve better the presence of different phases in mixed bilayers of phospholipids and its PLA2 catalyzed hydrolysis products are planned.
Discontinuities occur at similar % level of hydrolysis whether SUV or MLV for any given PC bilayer. Duration of the initial linear periods of the reaction progress curves are longer in MLVs than in SUVs because activities (∝ initial slope of GP vs. time) are higher in SUVs than in MLVs. This duration will also depend on the concentration of the enzyme. The occurrence of the discontinuity does depend on the PC forming the bilayer, suggesting that membrane properties due to the type of lipid rather than changes in substrate concentration may be responsible for the discontinuity in the slope of the time dependence of GP. Membrane polarity and microviscosity as well as the hydrolysis rate (∝ slope of GP vs. time) change continuously following the initial linear period. Substrate depletion and the changing membrane properties may together be contributing factors for this behavior. Determination of the activity in absolute units of μM of FA produced per min per mg of enzyme from which the actual amount of products can be calculated. This is useful for complementary experiments aimed at elucidating the effects of accumulation of hydrolytic products
When proposing new methods, comparisons and validations from other methods are needed. For this reason, pH-Stat assays were included in the present study. The activity values from the new ADIFAB assay and the well-established pH-Stat method are in excellent agreement. Discontinuities in the reaction progress curves were observed in both methods and at the same instants in the case of DOPC. It is significant that the shapes and features of the curves from the two methods are in remarkable agreement. Sudden slope change in the pH-Stat data on DPPC SUV could not be clearly identified, perhaps due to the lower precision of the method. The uncertainties in the activity values from the ADIFAB assay are due to variations between samples. Uncertainties due to GP fluctuations of about ±0.5% are negligible in comparison. In pH-Stat, the uncertainty is due to the resolution limit as well as sample variations and is much larger. In addition, background variations in pH over time give a systematic error. Therefore, fluorescence assays are in general more sensitive and have better precision than pH-Stat. This advantage of ADIFAB allows measurements of low levels of activity as, for example, the case of DPPC MLV where the GP data is clearly distinguishable from the background whereas the pH-Stat data are not (Fig. 5). The pH-Stat method has the advantage of being a direct measure of the FA formed whereas ADIFAB assays rely on calibration curves. ADIFAB assays yield absolute values for activity only when calibration can be obtained with the same type lipid and its hydrolysis products. Even for relative values of activity, comparisons can be made only between the same lipids to test for example different environments or additives because the fluorescence response is sensitive to the type of lipid.
In addition to binding lysophospholipids and FAs, ADIFAB also binds bile salts and other anionic detergents and to phosphates. The enzyme solution, prepared in phosphate buffers, contributes about 50 μM/L of phosphates in the samples. The effect could be a rapid increase of about 10% in GP upon addition of enzyme. This can contribute to the intercept of GP versus time in the activity experiments but the slope is not affected. No detectable response was found for calcium salts, nonionic detergents, and zwitterionic detergents like DPS where the charge sequence is reversed from that of lysophospholipids. This indicates that negative charge and access to it are required.
SUMMARY AND CONCLUSIONS
The nonexclusivity of the binding properties of ADIFAB obligated an investigation of its applicability to measuring PLA2 activity as proposed in previously reported assays. The problem was addressed in this work and a modified PLA2 activity assay was developed. Remarkable agreement between the activity values as well as the observed characteristics in the reaction progress curves measured by the present ADIFAB assay and pH-Stat methods validate the new assay. In addition to establishing a more rigorous ADIFAB assay, this work studied the kinetic evolution of the reaction. Complementary ESR experiments showed that changes in membrane polarity and microviscosity accompany the reaction. The correlation of these changes with the observed shape of the reaction progress curves brings insight that is significant to elucidating the mutual effects of membrane microstructure and PLA2 activity. In future work, a recently formulated novel surface dilution kinetic assay together with the present ADIFAB assay will be employed to determine lipid specificity of kinetic parameters and to better define the role of physicochemical properties (25).
Footnotes
Abbreviations:
- ADIFAB
- acrylodan-labeled rat-intestinal fatty acid binding protein
- cmc
- critical micelle concentration
- DOPC
- dioleylphosphatidylcholine
- DPPC
- dipalmitoylphosphatidylcholine
- DSE
- doxyl stearic acid methyl ester
- ESR
- electron spin resonance
- GP
- generalized polarization
- LOPC
- lysooleylphosphatdylcholine
- LPC
- lysophosphatidylcholine
- LPPC
- lysopalmitoylphosphatdylcholine
- MLV
- multilamellar vesicle
- OA
- oleic acid
- PA
- palmitic acid
- PLA2
- phospholipase A2
- SUV
- small unilamellar vesicle
The authors acknowledge support of this project through the grant 1 SC3GM096876 from the National Institutes of Health and awards from the Office of Research and Sponsored Programs and the College of Science and Math, California State University Northridge. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
References
- 1.Richieri G. V., Ogata R. T., Kleinfeld A. M. 1992. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J. Biol. Chem. 267: 23495–23501. [PubMed] [Google Scholar]
- 2.Richieri G. V., Ogata R. T., Kleinfeld A. M. 1994. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J. Biol. Chem. 269: 23918–23930. [PubMed] [Google Scholar]
- 3.Richieri G. V., Ogata R. T., Kleinfeld A. M. The measurement of free fatty acid concentration with the fluorescent probe ADIFAB: A practical guide for the use of the ADIFAB probe, in: Molecular and Cellular Biochemistry, vol. 192, Springer Netherlands, 1999, pp. 87–94. [PubMed]
- 4.Richieri G. V., Kleinfeld A. M. 1995. Continuous measurement of phospholipase A2 activity using the fluorescent probe ADIFAB. Anal. Biochem. 229: 256–263. [DOI] [PubMed] [Google Scholar]
- 5.Berg O. G., Yu B. Z., Rogers J., Jain M. K. 1991. Interfacial catalysis by phospholipase A2: determination of the interfacial kinetic rate constants. Biochemistry. 30: 7283–7297. [DOI] [PubMed] [Google Scholar]
- 6.Tatulian S. A. 2001. Toward understanding interfacial activation of secretory phospholipase A2: membrane surface properties and membrane-induced structural changes in the enzyme contribute synergestically to PLA2 activation. Biophys. J. 80: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinnunen P. K. J., Kõiv A., Lehtonen J. Y. A., Rytömaa M., Mustonen P. 1994. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem. Phys. Lipids. 73: 181–207. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman W. J., Vahey M., Hajdu J. 1983. Pancreatic porcine phospholipase A2 catalyzed hydrolysis of phosphatidycholine in lecithin-bile salt mixed micelles: kinetic studies in a lecithin-sodium cholate system. Arch. Biochem. Biophys. 221: 361–370. [DOI] [PubMed] [Google Scholar]
- 9.Dennis E. A. 1997. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem. Sci. 22: 1–2. [DOI] [PubMed] [Google Scholar]
- 10.Dennis E. A. 2000. Phospholipase in eicosanoid generation. Am. J. Respir. Crit. Care Med. 161: S32–S35. [DOI] [PubMed] [Google Scholar]
- 11.Singh J., Ranganathan R., Hajdu J. 2008. Kinetics of bacterial phospholipase C activity at micellar interfaces: effect of substrate aggregate microstructure and a model for the kinetic parameters. J. Phys. Chem. B. 112: 16741–16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson E. D., Nelson J., Griffith K., Nguyen T., Streeter M., Wilson-Ashworth H. A., Gelb M. H., Judd A. M., Bell J. D. 2010. Kinetic evaluation of cell membrane hydrolysis during apoptosis by human isoforms of secretory phospholipase A2. J. Biol. Chem. 285: 10993–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris F. M., Smith S. K., Bell J. D. 2001. Physical properties of erythrocyte that determine susceptibility to secretory phospholipase A2. J. Biol. Chem. 276: 22722–22731. [DOI] [PubMed] [Google Scholar]
- 14.Giongo C., Bakshi M. S., Singh J., Ranganathan R., Hajdu J., Bales B. L. 2005. Effects of interactions on the formation of mixed micelles of 1,2-diheptanoyl-sn-glycero-3-phosphocholine with SDS and DTAB. J. Colloid Interface Sci. 282: 149–155. [DOI] [PubMed] [Google Scholar]
- 15.Pattus F., Slotboom A. J., De Haas G. H. 1979. Regulation of the interaction of pancreatic phospholipase A2 with lipid-water interfaces by calcium(2+) ions: a monolayer study. Biochemistry. 18: 2698–2702. [DOI] [PubMed] [Google Scholar]
- 16.Bales B. L., Messina L., Vidal A., Peric M., Nascimento O. R. 1998. Precision relative aggregation number determinations of SDS micelles using a spin probe. A model of micelle surface hydration. J. Phys. Chem. 102: 10347–10358. [Google Scholar]
- 17.Singh J. S., Miller J., Ranganathan R. 2007. Physicochemical characterization of phospholipid solubilized mixed micelles and a hydrodynamic model of interfacial fluorescence quenching. J. Phys. Chem. B. 111: 9317–9324. [DOI] [PubMed] [Google Scholar]
- 18.Rao K. V. S., Polnaszek C. F., Freed J. H. 1977. Electron spin resonance studies of anisotropic ordering, spin relaxation, and slow tumbling in liquid crystalline solvents. 2. J. Phys. Chem. 81: 449–455. [Google Scholar]
- 19.Griffiths P. C., Cheung A. Y. F., Finney G. J., Farley C., Pitt A. R., Howe A. M., King S. M., Heenan K., Bales B. L. 2002. Electron paramagnetic resonance and small-angle neutron scattering studies of mixed sodium dodecyl sulfate and (tetradecylmalono)bis(N-methylglucamide) surfactant micelles. Langmuir. 18: 1065–1072. [Google Scholar]
- 20.Bales B. L., Ranganathan R., Griffiths P. C. 2001. Characterization of mixed micelles of SDS and a sugar based nonionic surfactant as a variable reaction medium. J. Phys. Chem. B. 105: 7465–7473. [Google Scholar]
- 21.Funasaki N., Hada S. 1979. Surface tension of aqueous solutions of surfactant mixtures. The composition of mixed micelles. J. Phys. Chem. 83: 2471–2475. [Google Scholar]
- 22.James J., Vellaichami S., Krishnan R. S. G., Samikannu S., Mandal A. B. 2005. Interaction of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) triblock copolymer of molecular weight 2800 with sodium dodecylsulfate (SDS) micelles: some physicochemical studies. Chem. Phys. 312: 275–287. [Google Scholar]
- 23.Silvius D. Thermotropic Phase Transitions of Pure Lipids in Model Membranes and Their Modifications by Membrane Proteins. John Wiley & Sons, Inc., New York, 1982.
- 24.Alves M., Bales B. L., Peric M. 2008. Effect of lysophosphatidylcholine on the surface hydration of phospholipid vesicles. Biochim. Biophys. Acta. 1778: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh J., Ranganathan R., Hajdu J. 2010. Surface dilution kinetics using substrate analog enantiomers as diluents: Enzymatic lipolysis by bee venom phospholipase A2. Anal. Biochem. 407: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]