Abstract
Toll-like receptor 4 (TLR4) and TLR2 were shown to be activated by saturated fatty acids (SFAs) but inhibited by docosahexaenoic acid (DHA). However, one report suggested that SFA-induced TLR activation in cell culture systems is due to contaminants in BSA used for solubilizing fatty acids. This report raised doubt about proinflammatory effects of SFAs. Our studies herein demonstrate that sodium palmitate (C16:0) or laurate (C12:0) without BSA solubilization induced phosphorylation of inhibitor of nuclear factor-κB α, c-Jun N-terminal kinase (JNK), p44/42 mitogen-activated-kinase (ERK), and nuclear factor-κB subunit p65, and TLR target gene expression in THP1 monocytes or RAW264.7 macrophages, respectively, when cultured in low FBS (0.25%) medium. C12:0 induced NFκB activation through TLR2 dimerized with TLR1 or TLR6, and through TLR4. Because BSA was not used in these experiments, contaminants in BSA have no relevance. Unlike in suspension cells (THP-1), BSA-solubilized C16:0 instead of sodium C16:0 is required to induce TLR target gene expression in adherent cells (RAW264.7). C16:0-BSA transactivated TLR2 dimerized with TLR1 or TLR6 and through TLR4 as seen with C12:0. These results and additional studies with the LPS sequester polymixin B and in MyD88−/− macrophages indicated that SFA-induced activation of TLR2 or TLR4 is a fatty acid-specific effect, but not due to contaminants in BSA or fatty acid preparations.
Keywords: docosahexaenoic acid, Toll-like receptors, reactive oxygen species
Pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain protein (NOD) like receptors detect invading pathogens by recognizing conserved pathogen-associated molecular patterns (PAMPs) and activate innate immune responses for host defense. However, PRRs can be activated by a wide variety of endogenous damage associated molecular patterns (DAMPs) derived from tissue injury or stress and induce sterile inflammation to initiate wound healing processes. Emerging evidence suggests that PRRs can also sense metabolic disturbance and link immune responses to metabolic homeostasis (1, 2). Such a functional diversity of PRRs may be achieved by their ability to recognize a wide variety of structurally unrelated molecules. However, such a broad specificity of PRRs in recognizing agonists can make them susceptible to dysregulation leading to development of chronic inflammation, which in turn can promote development and progression of many chronic diseases including atherosclerosis, insulin resistance, Alzheimer's disease, and cancer. Recent studies revealed that dietary components and metabolic intermediates can alter the activity and expression of PRRs, suggesting that PRR-mediated inflammation and its functional consequence are dynamically modulated by what we eat (3, 4).
High saturated fat diets have been used for diet-induced obesity and insulin resistance in many animal studies. Both in vitro and in vivo studies suggest that saturated fatty acids (SFAs) can activate proinflammatory signaling pathways leading to insulin resistance (5). The molecular mechanism by which SFAs activate proinflammatory signaling pathways remains obscure. Our previous studies revealed that SFAs activate but n-3 PUFA docosahexaenoic acid (DHA) inhibits TLR4- and TLR2-mediated signaling pathways leading to expression of proinflammatory marker gene products (6–9). Numerous studies with cells in culture and in animal models of mutated or deleted TLR4 or TLR2 subsequently demonstrated that SFAs indeed can activate TLR4- and TLR2-mediated proinflammatory signaling pathways and consequently, increase risk of insulin resistance (10–19). However, one report (20) suggested that SFA-induced TLR activation is due to contaminants in BSA used for solubilizing fatty acids. This report casted doubt upon the proinflammatory effects of SFAs.
TLRs are activated by various microbial components (i.e., endotoxins) that are ubiquitously present in our environment. Therefore, potential contamination of microbial components in reagents used for in vitro and in vivo studies is not a trivial technical issue for investigations focusing on the role of endogenous molecules in modulating TLRs and other PRRs. Such caution is particularly important if the testing materials are prepared in recombinant bacterial systems. This issue was exemplified in the controversy regarding whether the activation of TLR4 by HSP60 prepared as a bacterial recombinant protein is a bona fide effect of HSP60 or due to bacterial contaminants (21, 22).
It is not known whether commercial assay kits for the quantification of endotoxin (TLR4 ligand) can also be used to detect and quantify agonists for other TLRs. Therefore, we used cell-based biological assays to assess potential contamination of TLR agonists in reagents. In this report, multiple lines of evidence are presented that the activation of TLR-mediated proinflammatory signaling pathways by SFAs is a fatty acid-specific effect and not due to contaminants in the reagents. Experimental conditions optimizing responsiveness of cells to fatty acids revealed further mechanistic insight that the responsiveness of cells to TLR agonists is largely dependent on the tone of reactive oxygen species (ROS) in cell culture systems.
METHODS
Reagents
Lipopolysaccharide (LPS) (Cat #: 421), Pam3CSK4 (Cat #: tlrl-pms) and MDP (Cat #: G-1055) were purchased from List Biological Laboratories, Inc. (Campbell, CA), Invivogen (San Diego, CA), and BACHEM Bioscience, Inc. (King of Prussia, PA), respectively. Endotoxin free water, polymixin B, and antibody for β -actin were purchased from Sigma (Saint Louis, MO). Antibodies for c-Jun N-terminal kinase (JNK) (Cat #: 9252), phospho-JNK (Cat #: 9251), p44/42 mitogen-activated-kinase (ERK) (Cat #: 9102), phospho-ERK(Cat #: 9101), phospho-inhibitor of nuclear factor- κ B (I κ B α ) (Cat#: 2859), nuclear factor- κ B (NF κ B) p65 (Cat#: 3034), and phospho-NF κ B p65 (Cat#: 3033) were purchased from Cell Signaling Technology (Danvers, MA). I κ B α antibody (Cat#: SC-371) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-COX-2 immune serum was prepared using a synthetic COX-2 polypeptide as immunogen. FBSs were purchased as follows: premium select FBS (Catalog #: S11595 and S11550, Lot #: K0109) and Optima FBS (Catalog #: S12495, Lot #: L0019) from Atlanta Biologicals (Lawrenceville, GA), Hyclone FBS (Catalog #: SH30071, Lot #: ATD31956) from HyClone (Logan, UT), Gibco FBS (Catalog #: 10082, Lot #: 544122) from Invitrogen (Carlsbad, CA), and Atlas FBS (Catalog #: F-0500-A, Lot #: 80814) from Atlas Biologicals (Fort Collins, CO). BSA (Cat #: 30-AB79, Lot #: A10072001) was purchased from Fitzgerald Industries International (Acton, MA). Sodium laurate was purchased from Nu-Chek Prep (Elysian, MN). Palmitic acid (Cat #: P5585, Lot #:020M15811), sodium palmitate (Cat #: P9767, Lot # 079K1444), sodium salt DHA (Cat #: D8768, Lot #: 077K5215) and BSA (Catalog #: A8806, Lot #: 118K7405, 040M7715 and 034K7605) were purchased from Sigma. Apocynin (Cat #: 178385) was purchased from EMD Chemicals (Gibbstown, NJ). TLR4 inhibitor TAK-242 (Cat #: tlrl-cli95) was obtained from Invivogen.
Cell culture
RAW 264.7 (murine monocytic cell line), purchased from ATCC (Manassas, VA), immortalized MyD88 − / − macrophages (provided by Dr. Kate Fitzgerald, University of Massachusetts) and HEK293T (provided by Sam Lee, Beth Israel Hospital, MA) were cultured in DMEM containing 10% (v/v) FBS (premium select FBS, Catalog #: S11550, Lot #: K0109, Atlanta Biologicals), 100 units/ml penicillin, and 100 μ g/ml streptomycin. THP-1 cells (human monocytic cell line, ATCC TIB-202) were cultured in RPMI-1640 medium containing 10% (v/v) FBS, 0.05 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μ g/ml streptomycin. The immortalized MyD88 − / − macrophages were originally prepared using bone marrow-derived macrophages from MyD88 − / − mice with J2 recombinant retrovirus (23). All cell cultures were maintained at 37°C in a 5% CO2 atmosphere.
Plasmids
Expression vectors pDisplay, pDisplay-TLR1, pDisplay-TLR2, pDispay-TLR3, pDisplay-TLR4, pDisplay-TLR5, and pDisplay-TLR6 were obtained from Adeline Hajjar (University of Washington, Seattle, WA). MD2 was provided by Kensuke Miyake (Tokyo University, Japan). (2×)-NF- κ B-luciferase reporter construct was provided by Frank Mercurio (Signal Pharmaceuticals, San Diego, CA). pRSV- β -galatosidase plasmid was from Jongdae Lee (University of California, San Diego, CA). The plasmid DNA from these expression vectors was prepared in large scale for transfection using the EndoFree Plasmid Maxi kit (Qiagen, Valencia, CA).
Transfections and luciferase assays
Transient transfections were carried out using SuperFect transfection reagent (Qiagen) according to the manufacturers’ recommendations. HEK293T cells were seeded at 2 × 105 per well of 24-well plates and cotransfected the following day with 10 ng each of pDisplay-TLR4 and MD2, or pDisplay-TLR2 and pDisplay-TLR1, or pDisplay-TLR2 and pDisplay-TLR6, in addition to 50 ng (2×)-NF- κ B-luciferase reporter and 10 ng pRSV- β -galatosidase expression vectors. Twenty nanograms of pDisplay empty vector was used in addition to the above amounts of (2x)-NF- κ B-luciferase and pRSV-b-galactosidase expression vectors for transfection as controls. Twenty-four hours after transfection, the cells were serum-starved in 0.25% FBS/DMEM medium for 6 h followed by 12 h treatment with fatty acids in the same low serum medium. The cells were lysed. Luciferase and β -galactosidase enzyme activities were determined from the lysate supernatants using the luciferase and β -galactosidase enzyme assay system (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase activity was normalized by β -galactosidase activity to correct differences in transfection efficiency among samples. Each of the experiments was repeated at least three times.
BSA solubilization of palmitic acid
Palmitic acid (C16:0) is water insoluble and needs to be complexed with BSA to solublize. The solubilization process was carried out as previously described (24) with modifications. Briefly, C16:0 was dissolved in 100% ethanol (250 mM or 500 mM) then mixed with BSA in a 10:1 molar ratio in 0.25% FBS/DMEM or 0.25% FBS/RPMI medium. The mixture was sonicated in a water bath for 15 min followed by incubation on a shaker in an oven at 55 Co for 15 min. The solubilized C16:0 was filtered through a 0.22 μ m filter before use.
Solubilization of sodium salt fatty acid
A stock solution of 100 mM C12 sodium salt was prepared in endotoxin free water. A stock solution of 100 mM C16 sodium salt was prepared in 70% ethanol in a glass vial and heated to 60°C. C16 sodium salt was reheated to 60°C and quickly vortexed before cells were treated to give appropriate final concentration indicated in figures. A stock solution of 10 mM DHA sodium salt was prepared in endotoxin free water, flushed with nitrogen, then sealed in an air tight tube and stored at − 20°C until use.
Immunoblot and ELISA assays
RAW 264.7 and MyD88 − / − macrophage cells were seeded at 1.2-1.5 × 106 per well of 6-well plates. The next day, the cells were serum-starved in 0.25% FBS/DMEM medium for 6 h and then treated with fatty acids in the same low-serum medium. THP-1 cells were seeded at 1 × 106 cells/ml in T-25 flasks and serum-starved in 0.25% FBS/PRMI medium for 12 h. The cells were then treated with fatty acids in the same low serum medium. After the treatments, the medium supernatants were collected. The cells were rinsed with cold PBS twice and then lysed by sonication in cell lysis buffer (Cell Signaling Technology) containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β -glycerophosphate, 1 mM Na3VO4, and 1 µg/ml leupeptin. The lysate supernatants were collected by centrifugation and subjected to 10% SDS-PAGE followed by transfer of the proteins to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked in TBST buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.05% (v/v) Tween 20] containing 5% nonfat milk. The membrane was probed with primary antibody for 1 h at room temperature or overnight at 4°C followed by incubation with horse radish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) for 1 h at room temperature. The proteins were detected by the ECL Western blot detection reagents (Amersham Biosciences) followed by exposure to an X-ray film (BioExpress, Kaysville, UT). ELISAs were carried out for tumor necrosis factor (TNF)- α and interleukin (IL)-8 in the cell culture medium supernatants using a TNF- α ELISA kit (eBioscience, San Diego, CA) and an IL-8 ELISA kit (BD Biosciences, San Diego, CA), respectively, and a Synergy 2 plate reader (BioTek, Winooski, VT) following the manufacturers’ instructions.
ROS analysis
RAW264.7 cells were seeded at 1 × 106 per well of 6-well plates in 10% FBS/DMEM medium. The next day, the cells were serum-starved in 0.25% FBS/DMEM for 6 h followed by treatment with 0.2 ng/ml LPS, 100 µM C12:0, 50 µM BSA, or 500 µM BSA-solubilized C16:0 for 18 h. The cells were washed two times with warm PBS and stained with 5 µM 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) for 30 min at 37°C. The stained cells were washed with cold PBS three times and resuspended by scraping in PBS containing 2% FBS. The levels of ROS production were determined from the gated living cells by flow cytometry with a laser excitation at 488 nm using a FACSCalibur flow cytometer (BD Biosciences, Sparks, MD). The levels of ROS production were also examined by confocal microscopy. Briefly, RAW 264.7 cells were seeded at 1 × 105 onto a coverglass placed in a well of 24-well plates in DMEM medium containing 10% FBS. The cells were serum-starved for 6 h and then treated with 10 μ M CM-H2DCFDA in PBS for 30 min at 37°C. After washing three times with warm PBS, the cells were treated with 100 ng/ml LPS, 150 μ M C12:0, 50 μ M BSA, or 500 μ M BSA-solubilized C16:0 for 30 min in 0.25% FBS/DMEM. The cells were washed with cold PBS three times, fixed in 10% formalin for 30 min at 4°C, and washed again with cold PBS three times. The coverglasses were then mounted on glass slides. Confocal microscopy was performed with a Zeiss LSM 510 microscope with 40 × 1.3 oil objective lens using laser excitation at 488 nm.
LAL assay
The LAL (Limulus Amebocyte Lysate) assays were carried out using a recombinant factor C endotoxin detection system (Lonza, Walkersville, MD) following the manufacturers’ instructions. A 100 µl sample was mixed with 100 µl substrate/enzyme reagents and incubated at 37°C for 1 h. The resulting fluorescence was measured at 380 nm/440 nm. The LAL assays were also carried out using a ToxinSensor chromogenic LAL endotoxin assay kit (GeneScript, Piscataway, NJ) following the manufacturer's instructions. Briefly, 25 µl of samples were mixed with 25 µl LAL lysate and incubated at room temperature for 5 min. Twenty-five microliters of chromogenic substrate was then added to each of the reactions and incubated at 37°C for 5 min (for high endotoxin samples) or 30 min (for low endotoxin samples). The reactions were terminated by adding 125 µl each of the three color stabilizer solutions. The endotoxin levels were then measured by spectrophotometry at 545 nm using a Synergy 2 plate reader (BioTek) following the manufacturers’ instructions. The endotoxin concentrations of the samples were calculated from the standard curve obtained using the endotoxin standards provided by the kits.
Data analysis
Each of the experiments was repeated at least three times. Data from the reporter, ELISA, and ROS assays presented as mean ± SEM were analyzed by a 2-tailed Student's t-test.
RESULTS
Induction of TLR-mediated target gene expression #8232by SFAs
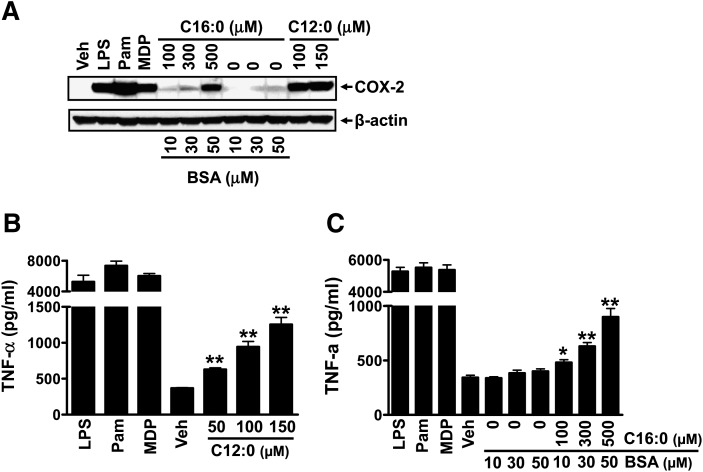
Our previous studies demonstrated that water-soluble sodium laurate (C12:0) (without BSA solubilization) activates PRRs (TLR2, TLR4, NODs) (7, 25). Therefore, potential contamination of PRR agonists in BSA was not an issue. Consistent with our previous results, treatment of RAW 264.7 cells with sodium laurate (C12:0) led to induction of COX-2 and TNF- α expression ( ) . However, palmitic acid (C16:0) is insoluble in aqueous solution and requires to be complexed with BSA to solubilize. To determine whether C16:0-induced expression of PRR target genes (COX-2, TNF- α ) is attributed to contaminants in BSA, we treated RAW 264.7 cells with BSA alone or BSA-solubilized C16:0 (C16:0-BSA). Similar to sodium salt C12:0, C16:0-BSA induced COX-2 and TNF- α expression whereas BSA alone at equivalent concentrations used to solubilize C16:0 had no effect (Fig. 1 A, C). Next, we determined whether C12:0 or C16:0-BSA-induced expression of COX-2 or TNF- α is due to contamination of LPS in BSA or fatty acid preparations. The LPS sequestering agent polymixin B did not attenuate C12:0 or C16:0-BSA-induced COX-2 and TNF- α expression indicating that the effects of the SFAs are not due to LPS contamination in BSA or fatty acid preparations ( ).
Fig. 1.
Sodium laurate (C12:0) and BSA-complexed palmitic acid (C16:0-BSA) but not BSA alone induced the expression of proinflammatory TLR target gene products COX-2 and TNF- α . RAW 264.7 cells were serum-starved (0.25% FBS) for 6 h in DMEM medium and then treated with C12:0, C16:0-BSA, or equivalent concentrations of BSA in the same low-serum medium for 18 h. The protein lysates were probed with anti-COX-2 or anti- β -actin antibody by immunobloting (A). Culture medium supernatants were assayed for TNF- α by ELISA (B, C). Controls: LPS (TLR4 agonist, 0.2 ng/ml), Pam (Pam3CSK4, TLR2 agonist, 2 ng/ml), MDP (NOD2 agonist, 100 µM). * P < 0.05, ** P < 0.01: significantly different from vehicle control (B) or from 50 µM BSA alone (C).
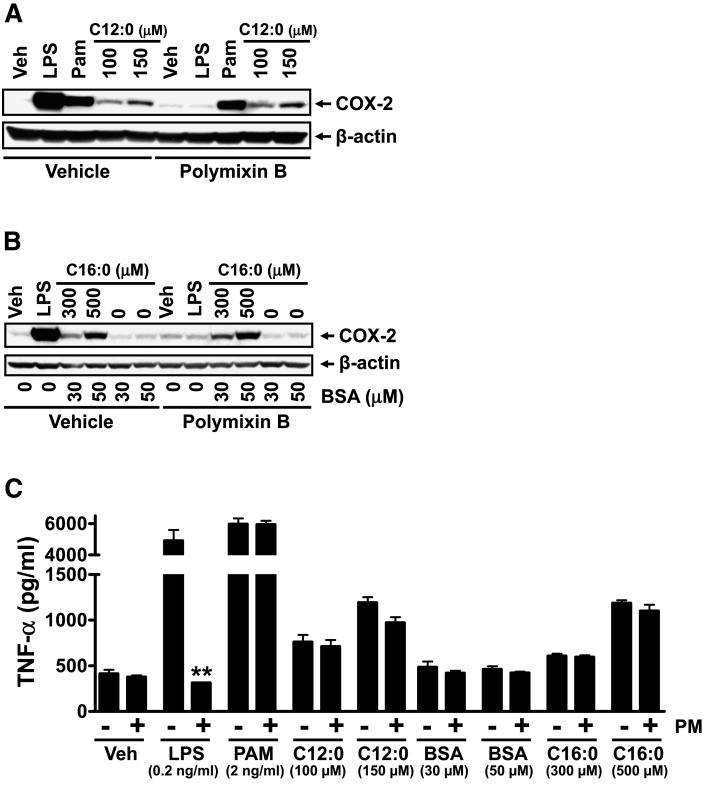
Fig. 2.
Polymixin B (LPS sequester) did not affect sodium laurate (C12:0)- or BSA-complexed palmitic acid (C16:0-BSA)-induced COX-2 expression indicating that the induction of COX-2 expression by these fatty acids is not due to LPS contamination in the fatty acid or BSA preparations. RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM medium for 6 h and then pretreated with 10 ug/ml polymixin B for 1 h followed by coincubation with C12:0, C16:0-BSA, or equivalent concentrations of BSA in the same low-serum medium for 18 h. The protein lysates were probed with anti-COX-2 or anti- β -actin antibody by immunobloting (A, B). Culture medium supernatants were assayed for TNF- α by ELISA (C). Controls: 0.2 ng/ml LPS, 2 ng/ml Pam3CSK4. PM, polymixin B. ** P < 0.01: significantly different from no polymix B control (C).
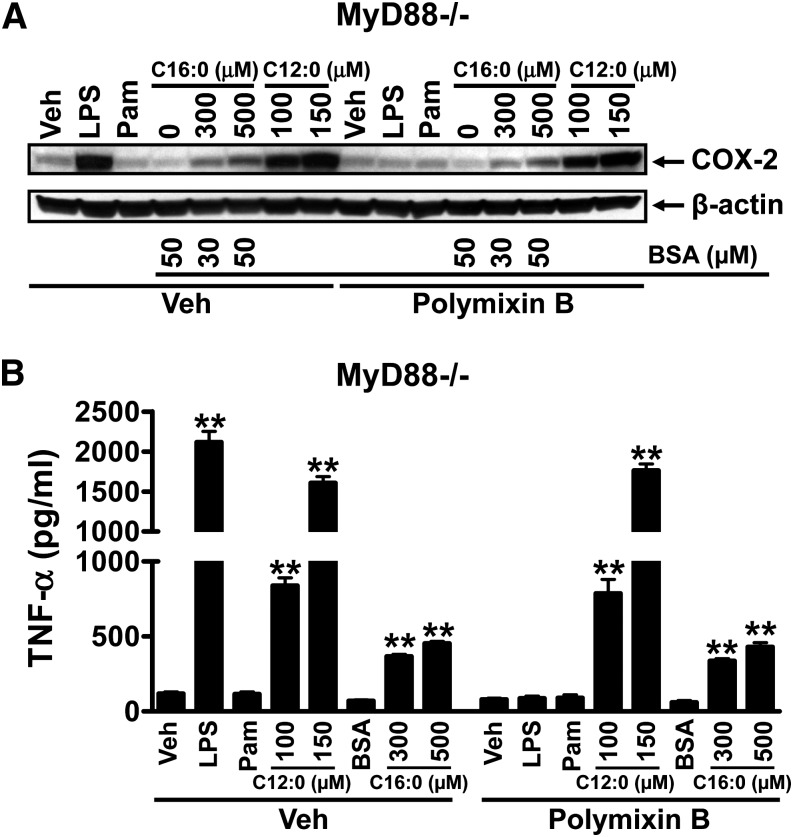
Erridge and Samani (20) suggested that SFA-induced cytokine expression in RAW 264.7 cells is due to contaminants present in BSA or fatty acid preparations that can activate TLR2. If that is the case, C12:0- or C16:0-BSA-induced COX-2 or TNF- α expression should be abolished in MyD88 − / − macrophages because TLR2 exclusively activates MyD88-dependent downstream signaling pathways. Therefore, we determined whether C12:0- or C16:0-BSA induced COX-2 or TNF- α expression is abolished in MyD88 − / − macrophages. The results showed that C12:0 or C16:0-BSA still induced COX-2 and TNF- α expression in MyD88 − / − macrophages ( ), eliminating the possibility that the induction of COX-2 or TNF- α expression by C12:0 or C16:0-BSA is due to contaminants in BSA or fatty acid preparations that can activate TLR2 or other MyD88-dependent TLRs or IL-1 receptor. Many studies have shown that SFAs can activate TLR4, TLR2, and NODs in macrophages (6, 7, 10, 13, 25). TLR4 activates both MyD88-dependent and independent pathways, and MyD88 is not the downstream component of NODs. Thus, SFAs should still induce the expression of COX-2 or TNF- α in MyD88 − / − cells as shown in Fig. 3. Additional evidence that proinflammatory effects of SFAs are not due to the potential contaminants in the reagents in in vitro systems is that whereas SFAs activate TLR-mediated proinflammatory signaling pathways, PUFAs, particularly n-3 fatty acid DHA, inhibit SFA-induced activation of TLR-mediated proinflammatory signaling pathways (3, 6–8). Similarly, C12:0 induced phosphorylation of ERK and JNK but DHA inhibited C12:0-induced ERK phosphorylation and COX-2 expression ( ). When MyD88 − / − cells were treated with fatty acids in the presence of polymixin B (LPS sequester), C12:0 or C16:0-BSA (Fitzgerald BSA) still induced COX-2 and TNF- α expression (Fig. 3). Under this condition, any effects of LPS and other contaminants that can activate MyD88 dependent TLRs are eliminated.
Fig. 3.
Sodium laurate (C12:0) or BSA-complexed palmitic acid (C16:0-BSA) induced COX-2 and TNF- α expression in MyD88 − / − macrophages even in the presence of polymixin B, eliminating the possibility that the fatty acid effects are due to the potential contaminants in BSA or fatty acid preparations that can activate TLR4 or MyD88-dependent TLRs or IL-1 receptor. MyD88 − / − cells were serum-starved in 0.25% FBS/DMEM medium for 6 h and then treated with C12:0, C16:0-BSA, or equivalent concentrations of BSA in the same low-serum medium for 18 h. The protein lysates were probed for COX-2 and β -actin (A) and the culture medium supernatants were assayed for TNF- α (B) as described in Fig 1. Controls: LPS 5 ng/ml, Pam3CSK4 5 ng/ml. ** P < 0.01: significantly different from vehicle control or from 50 µM BSA alone (B).
It should be noted that we have screened several commercially available BSA preparations for the presence of potential contaminants that can induce the expression of TLR or other PRR target gene products in RAW264.7 cells. Some commercially available BSA preparations induced the expression of TLR target gene product COX-2 (supplementary ), suggesting the presence of contaminants that can activate TLR or other PRRs in these BSA preparations. BSA-1 (Sigma Cat #: 8806, Lot 118K7405), BSA-2 (Sigma Catalog #: A8806, Lot 040M7715V), and BSA-3 (Sigma Catalog #: A8806, Lot 034K7605), which induced COX-2 expression in RAW264.7 cells even in the presence of polymixin B (supplementary ), did not induce COX-2 expression in MyD88 − / − cells (supplementary ) suggesting that the contaminants in these BSA preparations are compounds that can activate primarily TLR2 or other MyD88-dependent proinflammatory pathways. Taken together, these results demonstrate that fatty acid-induced COX-2 or TNF- α expression is not due to LPS or other contaminants that can activate TLR2 or other MyD88-dependent TLRs in BSA or fatty acid preparations.
LAL assays showed that these BSA preparations contained significant levels of endotoxins (supplementary Table I). Among these BSA preparations tested, the one from Fitzgerald Industries International (Cat #: 30-AB79, Lot #: A10072001) alone did not show significant induction of COX-2 and TNF- α expression (Fig. 1). An LAL assay also showed that this BSA preparation contained an undetectable level of endotoxin (supplementary Table I). Therefore, this BSA preparation was used throughout the current studies. In addition, the responsiveness of RAW264.7 cells to SFAs varied significantly with the source of FBS (supplementary ). This variation in the responsiveness of RAW264.7 cells to C12:0 was not correlated with the fatty acid composition of lipids in FBS (data not shown).
Activation of PRR signaling pathways by SFAs
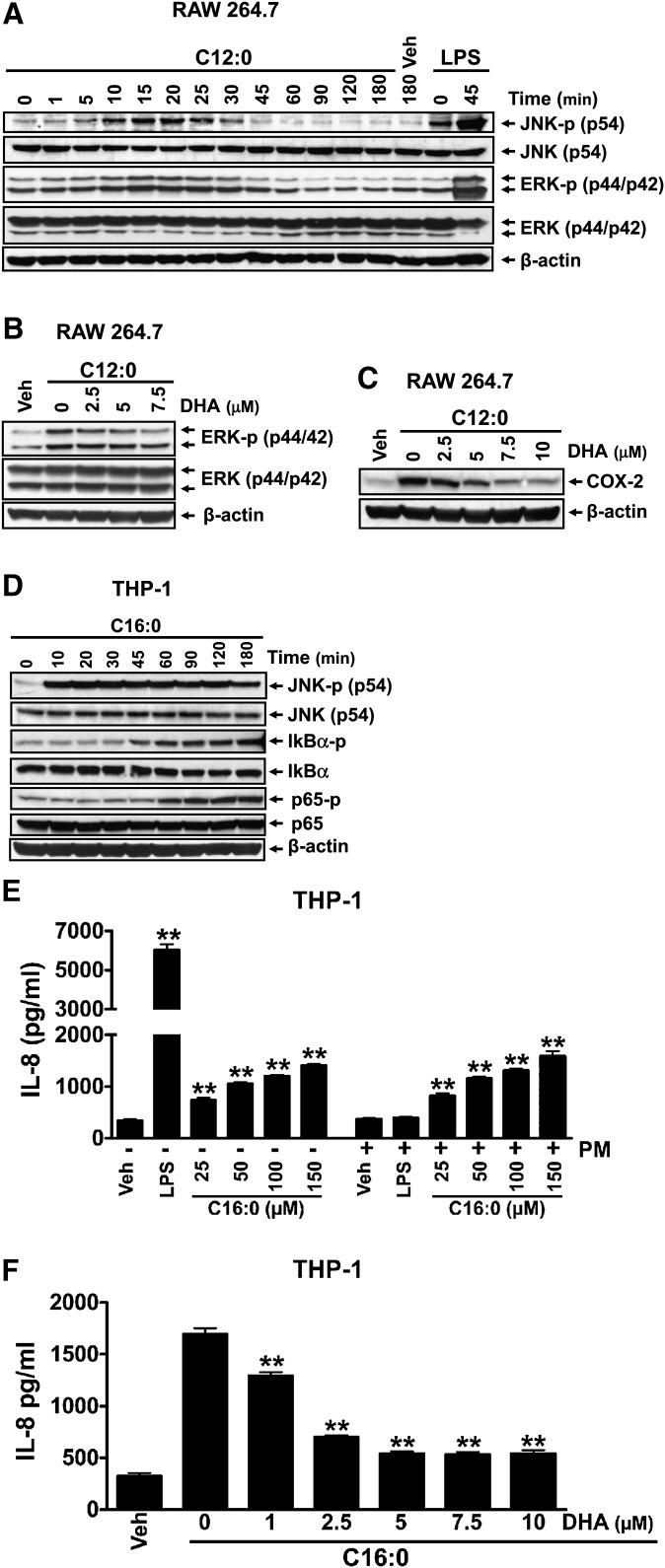
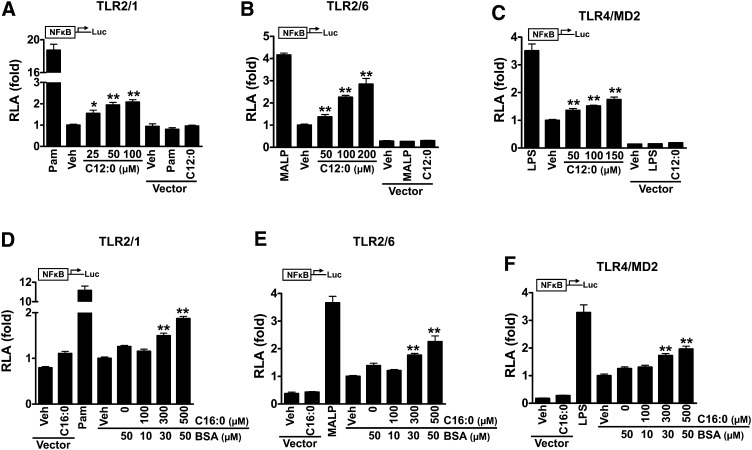
Sodium laurate (C12:0) without BSA solubilization activated the downstream signaling pathways of TLRs in adherent RAW 264.7 cells, leading to increased phosphorylation of JNK and ERK (Fig. 4A). C12:0-induced ERK phosphorylation was attenuated by DHA (Fig. 4B), leading to suppression of C12:0-induced COX-2 expression (Fig. 4C). Treatment of THP-1 cells (a monocytic suspension cell line) with sodium palmitate (C16:0) without BSA solubilization activated the downstream signaling pathways of TLRs, leading to enhanced phosphorylation of JNK, I κ B α , and NF κ B subunit p65 (Fig. 4D). The activation of the TLR pathways by C16:0 led to enhanced expression of IL-8 (Fig. 4E). Similar to C12:0, C16:0-induced IL-8 expression was also suppressed by DHA (Fig. 4F) whereas polymixin B had no effect (Fig. 4E). In addition, C16:0-BSA but not BSA alone also strongly induced JNK phosphorylation and IL-8 expression in THP-1 cells (supplementary ). IL-8 expression induced by C16:0-BSA was inhibited by DHA but was not affected by polymixin B treatment (supplementary ). Because BSA was not used in studies with sodium laurate and sodium palmitate, potential contaminants in BSA preparation that can activate PRRs were not an issue. C12:0 transactivated NF κ B mediated through TLR2 dimerized with TLR1 or TLR6, or through TLR4 ( ). Similarly, C16:0-BSA also induced NF κ B activation mediated through these TLRs (Fig. 5 D–F). Taken together, these results indicate that SFA-induced activation of downstream signaling pathways of TLR4 and TLR2 is the specific effect of SFAs and not due to potential contaminants in fatty acid or BSA preparations.
Fig. 4.
Sodium salt of lauric acid (C12:0) or sodium salt of palmitic acid (C16:0) without being complexed with BSA activated the downstream signaling pathways of pattern recognition receptors (PRRs). A: RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM for 6 h and then treated with 300 µM C12:0 in the same low-serum medium for indicated times. B, C: RAW 264.7 cells were serum-starved as in A. The cells were then pretreated with indicated concentrations of DHA for 1 h followed by coincubation with 300 µM C12:0 for 15 min (B) or 100 µM C12:0 for 18 h (C). (D) THP-1 cells were serum-starved in 0.25% FBS/RPMI1640 medium for 12 h and then treated with 150 µM C16:0 in the same low-serum medium for indicated times. Protein lysates from A to D were probed for phosphorylated JNK (JNK-p), JNK, phosphorylated ERK (ERK-p), ERK, phosphorylated I κ B α (I κ B α -p), I κ B α , phosphorylated NF κ B p65 (p65-p), NF κ B p65 (p65), COX-2, and β -actin by immunobloting. E, F: THP-1 cells were serum-starved as in D. The cells were then treated with indicated concentrations of C16:0 for 24 h (E) or pretreated with indicated concentrations of DHA for 1 h and then coincubated with 150 µM C16:0 for 24 h (F). The resulting culture medium supernatants were assayed for IL-8 by ELISA. ** P < 0.01: significantly different from vehicle control (E) or from C16:0 alone (F).
Fig. 5.
Sodium laurate (C12:0) or BSA-complexed palmitic acid (C16:0-BSA) induced NF κ B activation through TLR2 dimerized with TLR1 or TLR6 and through TLR4. HEK293T cells were cultured in 10% FBS/DMEM medium and cotransfected with TLR2 and TLR1 (A, D), TLR2 and TLR6 (B, E), TLR4 and MD2 (C, F), in addition to NF κ B-luciferase reporter and β –galactosidase expression vectors. After 24 h, the cells were serum-starved in 0.25% FBS/DMEM for 6 h and then treated with C12:0 (A–C) or C16:0-BSA (D–F) for 12 h. The cell lysates were assayed for luciferase and galactosidase activities. Values are expressed as RLA (relative luciferase activity). Controls: Pam (PamCSK4, TLR2/1 agonist, 10 ng/ml), MALP-2 (TLR2/6 agonist, 10 ng/ml), LPS (TLR4 agonist, 50 ng/ml). pDisplay empty vector was used as negative controls. * P < 0.05, ** P < 0.01: significantly different from vehicle control (A–C) or from 50 µM BSA (D–F).
Production of ROS is increased in cells cultured in a serum-poor medium and leads to enhanced propensity of cells to be activated by SFAs
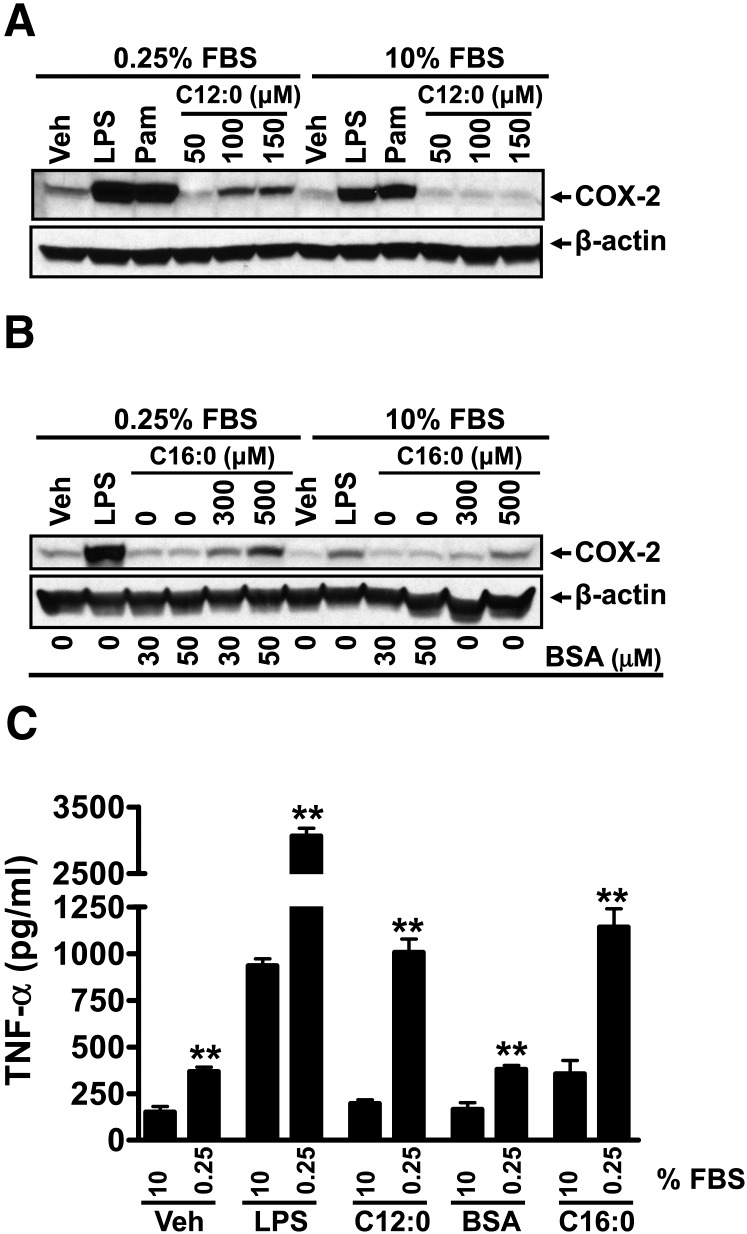
In our previous studies demonstrating that SFAs activate TLR2 and TLR4, cells were cultured in the serum-poor (0.25% FBS) medium. Thus, we examined how the responsiveness of cells to C12:0 or TLR agonists is affected by different serum concentrations in the culture media. The responsiveness of RAW 264.7 cells to C12:0, C16:0-BSA, or TLR agonists (LPS, a TLR4 agonist; Pam3CSK4, a TLR2 agonist) was greatly potentiated when the cells were cultured in the serum-poor (0.25%) medium compared with the cells cultured in 10% FBS ( ). The induction of COX-2 expression by C12:0 was almost completely abolished in the cells cultured in the medium with 10% FBS (Fig. 6A). Similar results were obtained with C16:0-BSA (Fig. 6B). The activation of TNF- α expression by LPS, C12:0, or C16:0-BSA was also significantly increased in cells cultured in the low-serum (0.25%) medium compared with cells cultured in the high-serum (10%) medium (Fig. 6C). It should be noted that the results reported by Erridge and Samani (20) showing that sodium laurate did not induce TNF- α expression or I κ B α degradation were obtained in RAW 264.7 cells cultured in the medium with 10% fetal calf serum.
Fig. 6.
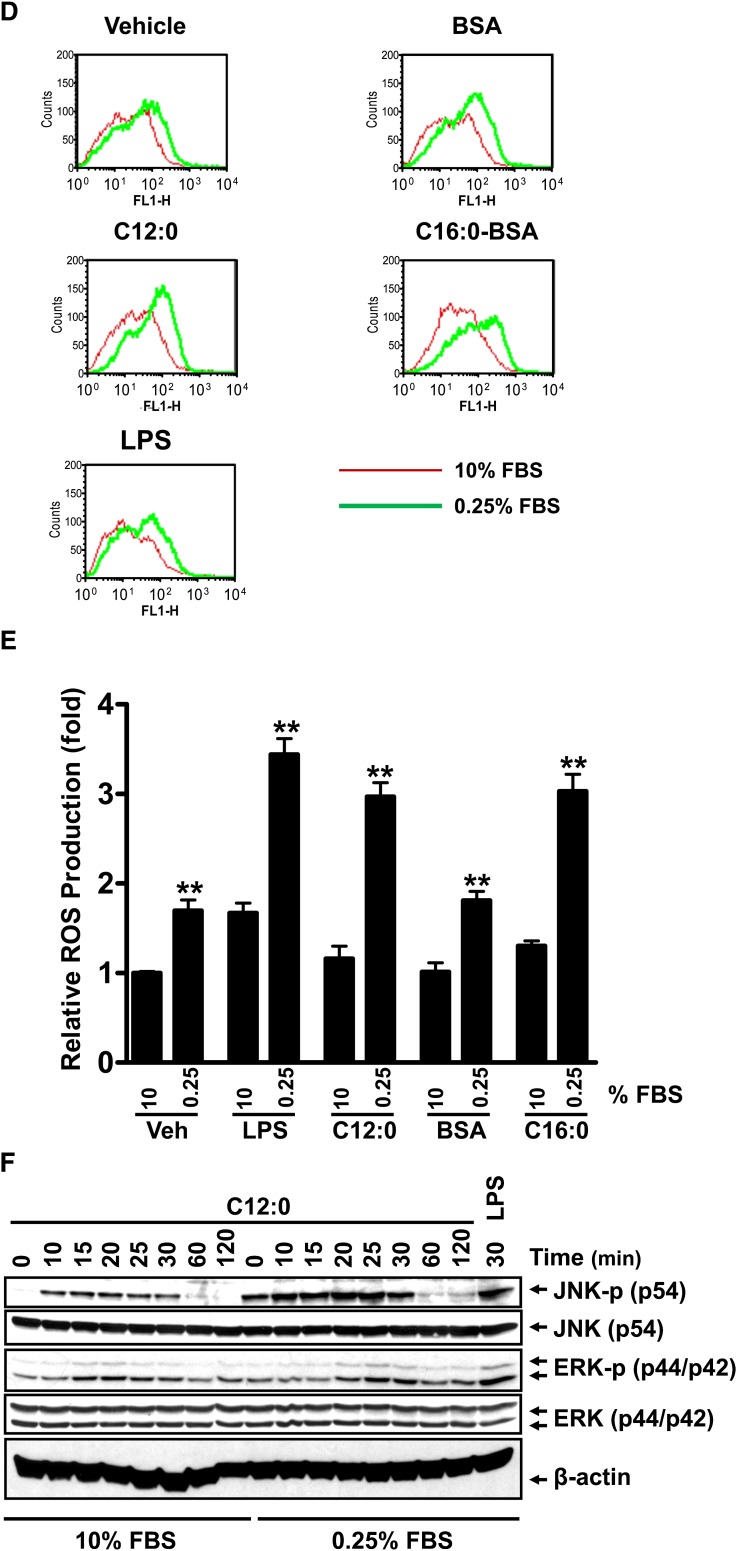
Sodium laurate (C12:0)-, C16:0-BSA-, or TLR agonist-induced COX-2 and TNF- α expression, reactive oxygen species (ROS) production, and phosphorylation of JNK and ERK were enhanced in the culture medium with low FBS (0.25%) compared with those with high FBS (10%). A–C: RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM medium for 6 h and then treated with C12:0 (A, C) or C16:0-BSA (B, C) for 16 h in the same low-serum medium or cultured continuously in 10% FBS/DMEM medium and treated with C12:0 (A, C) or C16:0-BSA (B, C) for 16 h. The protein lysates were probed for COX-2 and β –actin by immunobloting (A, B). The culture medium supernatants from vehicle, LPS, 100 µM C12:0, 50 µM BSA, and 500 µM C16:0-BSA treatments were assayed for TNF- α by ELISA (C). Controls: 0.2 ng/ml LPS, 2 ng/ml Pam3CSK4 (Pam). D: RAW 264.7 cells were cultured in DMEM medium containing 0.25% or 10% FBS for 6 h and then treated with vehicle, 0.2 ng/ml LPS, 100 µM C12:0, 50 µM BSA, or 500 µM C16:0-BSA for 16 h followed by CM-H2DCFDA staining for 30 min. ROS levels were measured by flow cytometry as described in Methods. E: Summary of the relative ROS production by the treatments in D as folds of geometric means. ** P < 0.01: significantly different from the value of 10% FBS for vehicle control, LPS, C12:0, BSA, and C16:0-BSA treatment samples. F: RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM for 6 h and then treated with 300 μ M C12:0 for indicated times or continuously cultured in 10% FBS/DMEM and treated with 300 µM C12:0 for the same time periods. The protein lysates were probed for phosphorylated JNK (JNK-p), JNK, phosphorylated ERK (ERK-p), ERK, and β -actin by immunobloting. Control: 10 ng/ml LPS.
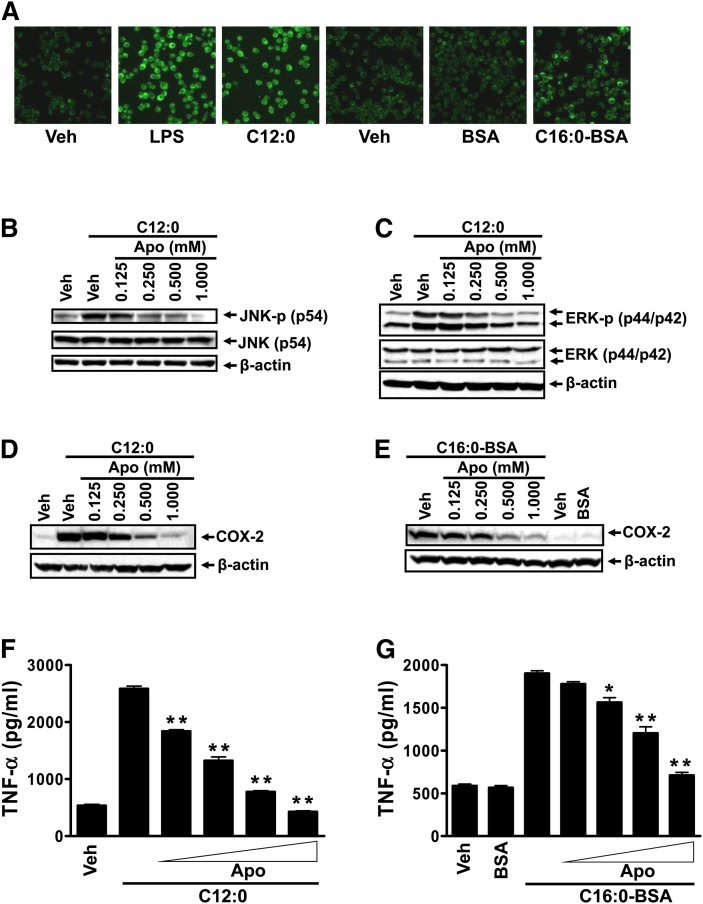
We further investigated what might contribute to the enhanced responsiveness of cells to fatty acids in the serum-poor medium. The production of ROS has been shown to be increased by serum deprivation in HEK293 or neuronal cells in culture (26–28). Ligand-induced activation of TLR4 requires recruitment of the receptor into lipid rafts in an ROS-dependent manner (29, 30). Our previous studies demonstrated that C12:0 induces dimerization and recruitment of TLR4 into lipid rafts in an ROS-dependent manner (8). Activation of TLR2 was also shown to stimulate the activity of NADPH oxidase (31, 32). Together, these results suggest that the responsiveness of cells to TLR4 agonists depends in part on ROS tone of cells in culture. Therefore, we examined the ROS levels in the cells cultured in the medium with high (10%) or low (0.25%) FBS concentrations. As shown in Fig. 6D and E, ROS production in vehicle control, LPS-, C12:0-, BSA-, or C16:0-BSA-treated cells was significantly increased by the culture medium with 0.25% FBS compared with that with 10% FBS, suggesting that ROS potentiates the agonist effects of SFAs. Consequently, C12:0-induced phosphorylation of JNK and ERK was significantly enhanced if the cells were cultured in low FBS (0.25%) compared with 10% FBS (Fig. 6F). These results suggest that low serum in culture medium increases ROS production, which, in turn, enhances the responsiveness of cells to LPS or SFAs in activating TLR-mediated signaling pathways. Consistent with this notion, treatment of RAW264.7 cells with sodium salt C12:0 or C16:0-BSA in the low-serum medium led to increased ROS production ( ). An inhibitor of NADPH oxidase, apocynin, suppressed the enhanced phosphorylation of JNK and ERK and the expression of COX-2 and TNF- α induced by C12:0 or C16:0-BSA in the low-serum medium (Fig. 7B–G). Taken together, these results demonstrate that ROS tone in cells in culture is one of the key conditions that can modulate the responsiveness of the cells to SFAs.
Fig. 7.
Sodium laurate (C12:0)- or C16:0-BSA-induced JNK or ERK phosphorylation, and COX-2 or TNF- α expression in RAW 264.7 cells cultured in the low serum medium were attenuated by NADPH oxidase inhibitor apocynin. A: RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM for 6 h and then treated with 10 µM CM-H2DCFDA for 30 min followed by treatment with vehicle, 100 ng/ml LPS, 150 µM sodium salt C12:0, 500 µM BSA-complexed C16:0 (C16:0-BSA), or equivalent concentration of BSA for 30 min. ROS levels were analyzed by confocal fluorescent imaging as described in Methods. B, C: RAW 264.7 cells were serum-starved in 0.25% FBS/DMEM for 6 h. The cells were then pretreated with indicated concentrations of apocynin (Apo) for 1 h followed by coincubation with 300 µM C12:0 for 15 min. The protein lysates were probed for JNK-p, JNK, ERK-p, ERK, and β -actin by immunobloting. D–G: RAW 264.7 cells were serum-starved as in B, C. The cells were then pretreated with indicated concentrations of apocynin for 1 h followed by coincubation with 150 µM C12:0 (D, F) or 500 µM C16:0-BSA (E, G) in low 0.25% FBS serum medium for 18 h. The protein lysates were probed for COX-2 and β -actin by immunobloting (D, E) and the medium supernatants were assayed for TNF- α by ELISA (F, G). 50 µM BSA was used as a control for BSA-complexed C16:0 (C16:0-BSA). * P < 0.05, ** P < 0.01: significantly different from the C12:0 treated sample without apocynin treatment (F), or C16:0-BSA treated sample without apocynin treatment (G).
Discussion
Our results presented here demonstrate that SFAs without being bound to BSA can activate TLR-mediated proinflammatory signaling pathways in macrophages (RAW264.7) and monocytes (THP-1) in a low serum culture medium. However, DHA inhibits saturated fatty acid-induced activation of TLR-mediated signaling pathways. These results indicate that SFA-induced activation of TLR-mediated signaling pathways and target gene expression is not due to contaminants in BSA or fatty acid preparations. The results from LAL assays also showed that the fatty acids and other reagents used in these studies contained trace or undetectable amounts of endotoxins (supplementary Table I). LAL assay showed that sodium palmitate at 150 µM and palmitic acid at 500 µM (the maximum concentrations used in these studies) contain trace amounts of endotoxin (0.064 EU/ml and 0.046 EU/ml endotoxin, respectively; supplementary Table I), which is equivalent to 18 pg/ml LPS and 16 pg/ml LPS, respectively, as determined from the LPS titration standard curve (supplementary ). However, endotoxin levels in both sodium palmitate and palmitic acid as measured by LAL assay did not show a dose-dependent pattern (supplementary ), suggesting that LAL readings for palmitic acid may reflect assay interference caused by palmitic acid. It should be noted that a majority of the substances tested for endotoxin either inhibit or enhance the LAL test to some degree (33). Therefore, we have developed cell-based assays to assess endotoxin and other contaminants in our experimental reagents that can activate TLR2 or TLR4. In MyD88 − / − cells in the presence of polymixin B, any effects of endotoxin and potential contaminants that can activate TLR2 and other MyD88-dependent TLRs are eliminated. Therefore, if the activation of TLR2 or TLR4 by SFAs is due to contaminants that can activate TLR2 or TLR4, the SFAs should not be able to induce the expression of TLR target gene products in MyD88 − / − cells in the presence of polymixin B. The fact that the SFAs still induce the expression of the TLR target gene products under this condition (Fig. 3) indicates that the activation of TLR2 or TLR4 by the SFAs is not due to the presence of potential contaminants that can activate TLR2 or TLR4. Furthermore, even if we accept the trace amounts of endotoxin in palmitate as measured by LAL assay as valid, these levels of endotoxin (16-18 pg/ml LPS) in palmitate cannot account for the expression of COX-2, TNF- α , or IL-8 (supplementary ) induced by palmitate. In addition, DHA inhibited LPS- or SFA-induced TLR target gene expression whereas C12:0 or C16:0 consistently showed stimulatory effects on TLR activation in our studies (Fig. 4, supplementary ). As these fatty acids are prepared in an identical manner in the laboratory, it is highly improbable that SFAs are contaminated with something that can stimulate TLR activation, whereas DHA is contaminated with something that can inhibit TLR activation. Thus, these results further counter the speculation that the stimulatory effects of SFAs may be due to contaminants in fatty acid preparations. Furthermore, a pharmacological inhibitor (TAK-242) of TLR4 suppressed both LPS- and C12:0-induced COX-2 and TNF- α expression, suggesting that C12:0-induced activation of COX-2 and TNF- α expression is in part mediated through TLR4 signaling pathway (supplementary ). Our previous studies also showed that C12:0 induces dimerization and recruitment of TLR4 into lipid rafts (8).
The responsiveness of cells to SFAs is greatly potentiated in the culture medium with low FBS concentration (0.25%) compared with the culture medium with 10% FBS, which is correlated with enhanced ROS production by the cells cultured in the low-serum medium. These results provide mechanistic insight about the activation of TLR-derived signaling pathways induced by SFAs and further suggest that conditions that modulate ROS tone can affect the responsiveness of cells to SFAs in activating TLR-derived signaling pathways and its functional consequences.
How SFAs can activate TLR2 and TLR4 is an intriguing question. Ligand binding, receptor dimerization, translocation of the receptor into lipid rafts, and recruitment of the immediate downstream signaling molecules are the potential steps through which fatty acids can modulate activation of these receptors. The Lipid A moiety in LPS is acylated by medium-chain (C12-C14) SFAs (34). Removal of these SFAs from Lipid A results in complete loss of endotoxic activity (35). Lipid A acylated by unsaturated fatty acids instead of SFAs is nontoxic and acts as an antagonist against the wild-type endotoxin (36–38). Bacterial lipopeptides that activate TLR2 are also acylated by SFAs. These results suggest that the fatty acids acylated in lipid A or bacterial lipoproteins play a critical role in receptor activation for TLR2 and TLR4. The crystal structure for TLR4-MD2-LPS complex revealed that five out of six fatty acyl groups in LPS are completely buried inside the large hydrophobic ligand binding pocket in MD2 (39). Binding of LPS to MD2 induces dimerization with TLR4 through both hydrophobic and hydrophilic interaction mediated by the fatty acyl groups and two phosphate groups in Lipid A, respectively. It is an intriguing question whether free fatty acids (without the polar head group of Lipid A) resembling the SFAs acylated in Lipid A can still interact with the hydrophobic ligand binding site of MD2 and promotes dimerization of TLR4 with MD2 or MyD88.
The X-ray crystal structure for TLR1-TLR2 heterodimer revealed that two ester-bound fatty acyl chains of tri-acylated lipopeptide (Pam3CSK4) are inserted into the hydrophobic ligand binding pocket in TLR2, whereas the amide-bound fatty acyl group is inserted into a hydrophobic channel in TLR1 (40). Thus, binding of fatty acyl groups of Pam3CSK4 into TLR2 induces heterdimerization of TLR1-TLR2. It is again an interesting question whether saturated free fatty acids resembling the fatty acids in the lipopeptides can interact with the ligand binding site in TLR2 or TLR1 and promote the dimerization of the receptors or the dimerization of the receptors with the immediate downstream adaptor MyD88 through TIR domains. TLR2 and TLR1 or TLR6 can dimerize in a ligand independent manner when they are overexpressed (41). Interestingly, it was shown that SFA lauric acid can activate TLR4 and TLR2 dimerized with TLR1 or TLR6 but no other TLRs (6, 7). The X-ray crystal structure and sequence analysis indicated the presence of the lipid binding hydrophobic pocket or channel in MD2 or LRR domains of TLR1, TLR2, TLR4, and TLR6 but not in other TLRs (42). No ligands for TLRs other than TLR4 and TLR2 are known to be acylated by SFAs. Although the detailed molecular interaction between LPS or lipopetides, and TLR4 or TLR2, respectively, has been revealed using truncated receptors without cytoplasmic domains, potential ligand activity of various endogenous molecules containing fatty acyl chains remains to be determined.
Activation of TLR4 requires translocation of the receptor into lipid rafts within plasma membrane where it recruits downstream signaling molecules such as MyD88 and NADPH oxidase for the activation of downstream signaling pathways (8, 29, 30). Lipid rafts are liquid-ordered phase microdomains of plasma membrane, which serve as signaling platform to concentrate receptors, coreceptors, adaptors, and downstream signaling molecules to promote the transmission of extracellular signals to intracellular signaling pathways. Lipid rafts have a special lipid composition that is rich in cholesterol, sphingolipids, and glycolipids. The polar lipids in lipid rafts are predominantly acylated by SFAs unlike phospholipids in nonlipid raft membranes, which are acylated by PUFAs, suggesting that saturated fatty acyl chains favor lipid raft association. Both TLR4 and TLR2 ligands LPS and lipopeptides, respectively, are acylated by SFAs. An emerging paradigm for the regulation of lipid raft formation suggests that clustering of molecules with high affinity for lipid rafts (raftophilicity) can greatly increase the size and stability of lipid rafts (43). It was shown that SFA lauric acid induces but n-3 PUFA DHA inhibits dimerization and recruitment of TLR4 into lipid rafts in macrophages (RAW264.7) (8).
Another possible mechanism by which SFAs activate TLR2 and TLR4 includes palmitoylation (acylation) of endogenous signaling molecules or enhanced synthesis of complex lipids that can interact with ligand binding domains. However, no experimental evidence has been reported to support this hypothesis.
Summary
The results presented here demonstrate that SFA-induced activation of TLR2 and TLR4 is fatty acid specific effects but not due to contaminants in reagents. TLRs are involved not only in host defense but also wound healing and possibly linking metabolic disturbance to immune responses. Such diverse tasks of TLRs can be fulfilled by recognizing a variety of endogenous molecules. Although the detailed molecular interaction between TLR4 or TLR2 and their respective ligands (i.e., LPS and lipopetides) has been revealed by X-ray crystallographic studies using truncated receptors without cytoplasmic domains, the potential ligand activity of a variety of endogenous molecules containing fatty acyl chains remains to be determined. Revelation of the mechanism by which such endogenous molecules activate TLR-mediated signaling pathways will greatly advance our understanding of how TLR-mediated sterile inflammation is associated with the development and progression of many chronic diseases.
Supplementary Material
Footnotes
Abbreviations:
- DAMP
- endogenous damage associated molecular pattern
- DHA
- docosahexaenoic acid
- ERK
- p44/42 mitogen-activated-kinase
- IκB
- inhibitor of nuclear factor-κB
- IL
- interleukin
- JNK
- c-Jun N-terminal kinase
- LAL
- Limulus Amebocyte Lysate
- LPS
- lipopolysaccharide
- MDP
- muramyldipeptide MurNAc-L-Ala-D-isoGln
- NF-κB
- nuclear factor-κB
- NOD
- nucleotide-binding oligomerization domain family
- PAMP
- pathogen-associated molecular pattern
- PRR
- pattern recognition receptor
- ROS
- reactive oxygen species
- SFA
- saturated fatty acid
- TLR
- Toll-like receptor
This work was supported by grants from the National Institutes of Health R01 (DK064007, DK41868, DK078328S), the American Diabetes Association (1-12-BS-02), and program funds from the Western Human Nutrition Research Center/ARS/USDA (5306-51530-017-00D and 5306-51530-019-00). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies. USDA is an equal opportunity employer and provider.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of seven figures and one table.
REFERENCES
- 1.Hotamisligil G. S., Erbay E. 2008. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroder K., Zhou R., Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 327: 296–300. [DOI] [PubMed] [Google Scholar]
- 3.Lee J. Y., Zhao L., Hwang D. H. 2010. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr. Rev. 68: 38–61. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L., Lee J. Y., Hwang D. H. 2011. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr. Rev. 69: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass C. K., Olefsky J. M. 2012. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 15: 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J. Y., Sohn K. H., Rhee S. H., Hwang D. 2001. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276: 16683–16689. [DOI] [PubMed] [Google Scholar]
- 7.Lee J. Y., Zhao L., Youn H. S., Weatherill A. R., Tapping R., Feng L., Lee W. H., Fitzgerald K. A., Hwang D. H. 2004. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279: 16971–16979. [DOI] [PubMed] [Google Scholar]
- 8.Wong S. W., Kwon M. J., Choi A. M., Kim H. P., Nakahira K., Hwang D. H. 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284: 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. Y., Plakidas A., Lee W. H., Heikkinen A., Chanmugam P., Bray G., Hwang D. H. 2003. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 44: 479–486. [DOI] [PubMed] [Google Scholar]
- 10.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suganami T., Tanimoto-Koyama K., Nishida J., Itoh M., Yuan X., Mizuarai S., Kotani H., Yamaoka S., Miyake K., Aoe S., et al. 2007. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 27: 84–91. [DOI] [PubMed] [Google Scholar]
- 12.Kim F., Pham M., Luttrell I., Bannerman D. D., Tupper J., Thaler J., Hawn T. R., Raines E. W., Schwartz M. W. 2007. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 100: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen M. T., Favelyukis S., Nguyen A. K., Reichart D., Scott P. A., Jenn A., Liu-Bryan R., Glass C. K., Neels J. G., Olefsky J. M. 2007. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282: 35279–35292. [DOI] [PubMed] [Google Scholar]
- 14.Davis J. E., Gabler N. K., Walker-Daniels J., Spurlock M. E. 2008. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) . 16: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 15.Kleinridders A., Schenten D., Konner A. C., Belgardt B. F., Mauer J., Okamura T., Wunderlich F. T., Medzhitov R., Bruning J. C. 2009. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himes R. W., Smith C. W. 2010. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 24: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehses J. A., Meier D. T., Wueest S., Rytka J., Boller S., Wielinga P. Y., Schraenen A., Lemaire K., Debray S., Van Lommel L., et al. 2010. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia . 53: 1795–1806. [DOI] [PubMed] [Google Scholar]
- 19.Davis J. E., Braucher D. R., Walker-Daniels J., Spurlock M. E. 2011. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J. Nutr. Biochem. 22: 136–141. [DOI] [PubMed] [Google Scholar]
- 20.Erridge C., Samani N. J. 2009. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29: 1944–1949. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi K., Burkart V., Flohe S., Kolb H. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164: 558–561. [DOI] [PubMed] [Google Scholar]
- 22.Gao B., Tsan M. F. 2003. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J. Biol. Chem. 278: 22523–22529. [DOI] [PubMed] [Google Scholar]
- 23.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. 2003. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278: 10297–10303. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., Kwon M. J., Huang S., Lee J. Y., Fukase K., Inohara N., Hwang D. H. 2007. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J. Biol. Chem. 282: 11618–11628. [DOI] [PubMed] [Google Scholar]
- 26.Satoh T., Sakai N., Enokido Y., Uchiyama Y., Hatanaka H. 1996. Survival factor-insensitive generation of reactive oxygen species induced by serum deprivation in neuronal cells. Brain Res. 733: 9–14. [DOI] [PubMed] [Google Scholar]
- 27.Guo D., Han J., Adam B. L., Colburn N. H., Wang M. H., Dong Z., Eizirik D. L., She J. X., Wang C. Y. 2005. Proteomic analysis of SUMO4 substrates in HEK293 cells under serum starvation-induced stress. Biochem. Biophys. Res. Commun. 337: 1308–1318. [DOI] [PubMed] [Google Scholar]
- 28.Kuznetsov A. V., Kehrer I., Kozlov A. V., Haller M., Redl H., Hermann M., Grimm M., Troppmair J. 2011. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal. Bioanal. Chem. 400: 2383–2390. [DOI] [PubMed] [Google Scholar]
- 29.Powers K. A., Szaszi K., Khadaroo R. G., Tawadros P. S., Marshall J. C., Kapus A., Rotstein O. D. 2006. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 203: 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakahira K., Kim H. P., Geng X. H., Nakao A., Wang X., Murase N., Drain P. F., Sasidhar M., Nabel E. G., Takahashi T., et al. 2006. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 203: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C. S., Shin D. M., Kim K. H., Lee Z. W., Lee C. H., Park S. G., Bae Y. S., Jo E. K. 2009. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J. Immunol. 182: 3696–3705. [DOI] [PubMed] [Google Scholar]
- 32.Lee I. T., Wang S. W., Lee C. W., Chang C. C., Lin C. C., Luo S. F., Yang C. M. 2008. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J. Immunol. 181: 5098–5110. [DOI] [PubMed] [Google Scholar]
- 33.Dawson M. E. 2005. Interference with the LAL test and how to address it. Associates of CAPE COD Incorporated . 22: 1–5. [Google Scholar]
- 34.Raetz C. R. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59: 129–170. [DOI] [PubMed] [Google Scholar]
- 35.Munford R. S., Hall C. L. 1986. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science . 234: 203–205. [DOI] [PubMed] [Google Scholar]
- 36.Kitchens R. L., Ulevitch R. J., Munford R. S. 1992. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 176: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krauss J. H., Seydel U., Weckesser J., Mayer H. 1989. Structural analysis of the nontoxic lipid A of Rhodobacter capsulatus 37b4. Eur. J. Biochem. 180: 519–526. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi N., Takayama K., Kurtz R. 1991. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect. Immun. 59: 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature . 458: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 40.Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., Lee J. O. 2007. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell . 130: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 41.Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA . 97: 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang J. Y., Lee J. O. 2011. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 80: 917–941. [DOI] [PubMed] [Google Scholar]
- 43.Brown D. A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) . 21: 430–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.