Abstract
Objective
Many scientific studies have shown that Asparagus officinalis has an antitumour effect and enhances human immunity, but the active components and the antitumour mechanisms are unclear. We investigated the effects of saponins isolated from Asparagus on proliferation and apoptosis in the human hepatoma cell line HepG2.
Methods
HepG2 cells were treated with varying concentrations of Asparagus saponins at various times. Using mtt and flow cytometry assays, we evaluated the effects of Asparagus saponins on the growth and apoptosis of HepG2 cells. Transmission electron microscopy was used to observe the morphology of cell apoptosis. Confocal laser scanning microscopy was used to analyze intracellular calcium ion concentration, mitochondrial permeability transition pore (mptp), and mitochondrial membrane potential (mmp). Spectrophotometry was applied to quantify the activity of caspase-9 and caspase-3. Flow cytometry was used to investigate the levels of reactive oxygen species (ros) and pH, and the expressions of Bcl2, Bax, CytC, and caspase-3, in HepG2 cells.
Results
Asparagus saponins inhibited the growth of HepG2 cells in a dose-dependent manner. The median inhibitory concentration (IC50) was 101.15 mg/L at 72 hours. The apoptosis morphology at 72 hours of treatment was obvious, showing cell protuberance, concentrated cytoplasm, and apoptotic bodies. The apoptotic rates at 72 hours were 30.9%, 51.7%, and 62.1% (for saponin concentrations of 50 mg/L, 100 mg/L, 200 mg/L). Treatment with Asparagus saponins for 24 hours increased the intracellular level of ros and Ca2+, lowered the pH, activated intracellular mptp, and decreased mmp in a dose-dependent manner. Treatment also increased the activity of caspase-9 and caspase-3, downregulated the expression of Bcl2, upregulated the expression of Bax, and induced release of CytC and activation of caspase-3.
Conclusions
Asparagus saponins induce apoptosis in HepG2 cells through a mitochondrial-mediated and caspase-dependent pathway, suggesting that they may be a potent agent for the treatment of hepatocellular carcinoma.
Keywords: Asparagus, saponins, hepatocellular carcinoma, apoptosis, mitochondrial pathway, caspase, laser scanning confocal microscope, flow cytometry
1. INTRODUCTION
Hepatocellular carcinoma is one of the most common malignancies worldwide, accounting for nearly 600,000 deaths each year. Despite surgical management and the use of nonsurgical therapeutic modalities, the incidence of hepatocellular carcinoma is still on the rise. In recent years, antitumour drugs from plants have drawn attention because of their strong effects and low toxicities.
Asparagus officinalis is a perennial plant and a popular vegetable used in salads, cooked dishes, and soups. It has many active components and abundant nutritional value. Steroidal saponins, polysaccharides, flavonoids, tissue protein, and trace elements have been isolated from its roots and buds 1. Many scientific studies have shown that Asparagus officinalis has antitumour effects and enhances human immunity 2,3. Research on Asparagus is gradually increasing around the world, especially with respect to cancer cure and prevention, but that research is focusing mainly on Asparagus officinalis juice or extracts 4,5, and so the exact active antitumour components and antitumour mechanisms of Asparagus officinalis are unclear. Given that steroidal saponins are the major components responsible for biologic activity 6–8, we choose to study Asparagus saponins with the goal of identifying their antitumour effects and mechanisms.
2. METHODS
2.1. Biochemical Reagents
Asparagus saponins were prepared by the Center of Research and Development on Life Sciences and Environmental Sciences of Harbin University of Commerce, and the following additional compounds were obtained: hydroxycamptothecin (Harbin Shengtai Pharmaceutical, Harbin, PR China); rpmi 1640 culture medium (Hyclone: Thermo Scientific, Waltham, MA, U.S.A.); fetal bovine serum (Hangzhou Sijiqing Biological Engineering Materials, Hangzhou, PR China); pancreatin (Gibco, Rockville, MD, U.S.A.); mtt (Sigma–Aldrich, St. Louis, MO, U.S.A.); Annexin V/PI apoptosis kit, reactive oxygen species (ros), and pH probe (Beyotime Institute of Biotechnology, Haimen, PR China); Fluo-3/AM (Molecular Probes, Eugene, OR, U.S.A.); mitochondrial permeability transition pore (mptp) kit (Genmed Scientifics, Wilmington, DE, U.S.A.); rhodamine 123 (Sigma–Aldrich); mouse anti-Bcl2 and mouse anti-Bax (Santa Cruz Biotechnology, CA, U.S.A.); rabbit anti-caspase-9 (Cell Signaling Technology, Danvers, MA, U.S.A.); mouse anti–Cyt-C and rabbit anti-activated–caspase-3 (Beyotime Institute of Biotechnology); fitc-goat anti-mouse-antibody and fitc-goat anti-rabbit-antibody (Beyotime Institute of Biotechnology); and Caspase-9,3 Activity kit (Beyotime Institute of Biotechnology).
2.2. Apparatuses
We used a CO2 incubator (CO-150: New Brunswick Scientific, Edison, NJ, U.S.A.), a fluorescence inverted microscope (CKX 41: Olympus, Tokyo, Japan), a microplate reader (Bio-Rad, Hercules, CA, U.S.A.), a transmission electronic microscope (Carl Zeiss, Oberkochen, Germany), a confocal laser scanning microscope (SP2: Leica, Solms, Germany), and a flow cytometer (EPICS XL: Beckman Coulter, Brea, CA, U.S.A.).
2.3. Cell Line
Human hepatoma cell line HepG2 was provided by the Institute of Materia Medica of the Center of Research and Development on Life Sciences and Environmental Sciences of Harbin University of Commerce.
2.4. Cell Culture
HepG2 cells were grown in complete rpmi 1640 medium containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
2.5. MTT Assay
Exponentially growing cells were washed and re-suspended in complete rpmi 1640 medium to a concentration of 1×105 cells per millilitre. In a 96-well-plate, 100 μL of cells per well were cultured for the first 24 hours. They were then incubated with varying concentrations of the Asparagus saponins (10−1 mg/L to 103 mg/L) for a further 72 hours. After incubation, mtt was dissolved in phosphate-buffered saline (pbs) and added to the culture media at a final concentration of 0.5 mg/mL. After incubation at 37°C for 4 hours, all media were removed, and 150 μL dimethyl sulfoxide was added to each well to dissolve purple formazan crystals. The plate was shaken for 10 minutes, and then spectrophotometric absorbance at 570 nm was read using the microplate reader. The inhibition rate and the median inhibitory concentration (IC50) were used to evaluate cytotoxicity.
2.6. Apoptosis Morphology Observation
HepG2 cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 72 hours. The treated cells were digested with pancreatin and fixed in 3% glutaraldehyde at 4°C for 2 hours. To make ultrathin copper sections, cells were washed with pbs, fixed in 1% osmic acid for an additional hour, dehydrated in acetone, and embedded in epoxide resin. After staining with uranyl acetate and lead citrate, the sections were examined under the transmission electron microscope.
2.7. Apoptosis Rate Assay
To quantify the apoptotic cells, the occurrence of apoptosis was determined by staining cells with both Annexin V–fitc and propidium iodide. Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 72 hours, digested with pancreatin, rinsed twice with pbs, and then re-suspended in binding buffer at a concentration of 5×104 cells per millilitre. To those cells, 5 μL Annexin V–fitc and 10 μL propidium iodide were added, and the cells were then analyzed by flow cytometry.
2.8. Effect of Asparagus Saponins on ROS
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 hours. They were then digested with pancreatin, rinsed twice with pbs, and loaded with 5 μmol/L dcfh-da for 20 minutes at room temperature. The samples were rinsed once with pbs, and the cells were measured using flow cytometry.
2.9. Effect of Asparagus Saponins on Calcium Ions
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 hours. They were then digested with pancreatin, rinsed twice with pbs, and loaded with 4 μg/mL Fluo-3/am for 1 hour at 37°C. Samples were rinsed once with pbs, and the cells were measured using confocal laser scanning microscopy.
2.10. Effect of Asparagus Saponins on pH
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 hours, digested with pancreatin, rinsed twice with pbs, and then loaded with 10 μmol/L bcecf-am for 2 hours at 37°C. Samples were rinsed once with pbs, and the cells were measured using flow cytometry.
2.11. Effect of Asparagus Saponins on MPTP and MMP
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 hours. The cells were then digested with pancreatin and rinsed twice with pbs. Preheated Reagent A 500 μL was added to the cells, mixed, and kept for 1 minute. The supernatant obtained by centrifugation for 10 minutes at 1600 rpm was then discarded. Reagent B was added to the cells and incubated for 20 minutes at 37°C in no-light conditions, and the supernatant obtained by centrifugation for 10 minutes at 1600 rpm was discarded. Preheated Reagent A 500 μL was added to the cells and mixed. The cells were then measured using confocal laser scanning microscopy.
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 hours, digested with pancreatin, rinsed twice with pbs, and then loaded with 5 μg/mL rhodamine 123 for 30 minutes at 37°C. The change in fluorescence intensity of mmp was observed using confocal laser scanning microscopy.
2.12. Effect of Asparagus Saponins on Caspase-9 and Caspase-3 Activity
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 48 hours. Caspase activity was determined by colorimetric assay using caspase-3 and caspase-9 activation kits according to the manufacturer’s protocol. The optical density of the reaction mixture was quantified spectrophotometrically at a wavelength of 405 nm.
2.13. Effect of Asparagus Saponins on the Expression of Apoptosis-Related Proteins
Cells were treated with varying concentrations of Asparagus saponins (50 mg/L, 100 mg/L, 200 mg/L) for 24 and 48 hours. The cells were then digested with pancreatin and rinsed twice with pbs. To fix the cells, 2 mL paraformaldehyde (40 g/L) was added for 40 minutes. The fixation liquid was then removed, and the cells were rinsed with pbs twice. TritonX-100 0.1% (1 mL) was added to punch cells for 15 minutes and then removed. The cells were rinsed with pbs twice, and 1 mL 1% bovine serum albumin was added for 1 hour to seal the cells. The sealing liquid was then removed, and mouse–anti-human Bcl2, Bax, Cyt-C, and activated caspase-3 antibody were respectively added and incubated for 1 hour at 37°C. The supernatant was then removed, the cells were rinsed with pbs, and fitc–anti-mouse antibody was added. The cells were incubated for 30 minutes at room temperature, after which the supernatant was discarded. After the addition of 800 μL pbs, the cells were measured using flow cytometry.
2.14. Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical comparisons were performed using a one-way analysis of variance followed by the Fisher test. Significant differences between the groups were determined using an unpaired Student t-test.
3. RESULTS
3.1. MTT Assay
After treatment with various concentrations of Asparagus saponins (10−1 mg/L to 103 mg/L) for 72 hours, the mtt assay showed a concentration-dependent inhibitory effect on the proliferation of HepG2 cells. The optical displacements of the treated groups were statistically different from those of the control group (p < 0.01), and the IC50 was 101.15 mg/L.
3.2. Apoptosis Morphology Observation
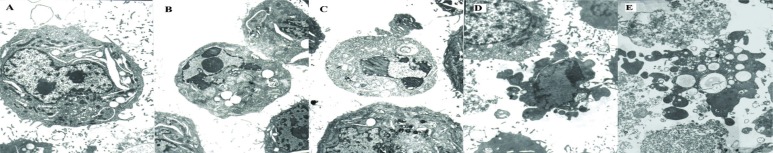
Observation with transmission electron microscopy showed that, after treatment with various concentrations of Asparagus saponins, the morphology of the HepG2 cells changed. Characteristic apoptotic morphology became evident gradually as the concentration of Asparagus saponins increased. In the control group, cells had nuclei and abundant organelles. In the low-dose group, cells showed shrinkage of their cellular and nuclear membranes and cytoplasmic vacuolation. In the mid-dose group, cells showed condensed heterochromatin around the nuclear periphery. In the high-dose group, cells showed chromosome fragmentation and apoptotic bodies (Figure 1).
FIGURE 1.
Electron microscopy appearance of HepG2 cells at 72 hours. 5000× original magnification. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
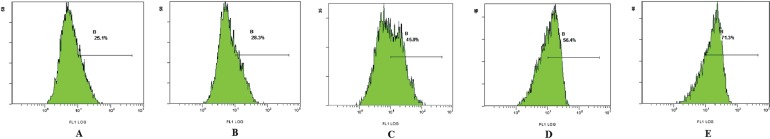
3.3. Apoptosis Rate Assay
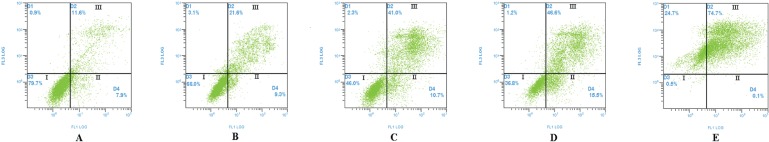
Analysis by flow cytometry showed that Asparagus saponins induced apoptosis in HepG2 cells. After treatment with various concentrations of Asparagus saponins, the early–to–mid-apoptotic cells (right lower section of the fluorocytogram, Figure 2) represented 9.3%, 10.7%, and 15.5% of the total cells respectively. By contrast, the control samples contained only 7.9% apoptotic cells. Meanwhile, late apoptotic and necrotic cells (right upper section of fluorocytogram, Figure 2) represented 21.6%, 41.0%, 46.6% of the total cells. By contrast, the control samples contained only 11.6% necrotic cells.
FIGURE 2.
Apoptosis rate of HepG2 cells induced by Asparagus saponins at 72 hours (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
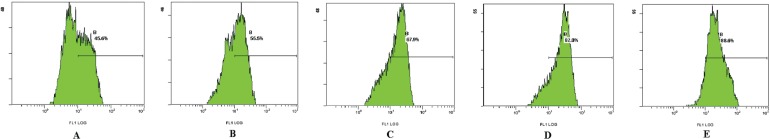
3.4. Effect of Asparagus Saponins on ROS
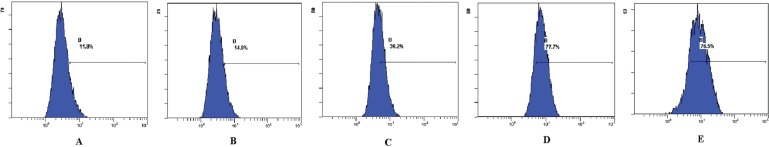
Analysis by flow cytometry showed that, after treatment with various concentrations of Asparagus saponins, the level of intracellular ros increased significantly. And as the concentration of saponins increased, the level of ros correspondingly increased—by 14.8%, 39.2%, and 77.7% respectively. In the control samples, the level was 11.8% (Table i and Figure 3).
TABLE I.
Effect of Asparagus saponins on reactive oxygen species in HepG2 (χ̄ ± s)
| Group | Dose (mg/L) | mfi |
|---|---|---|
| Control | — | 2.30±0.06 |
| Asparagus saponins | 50 | 3.02±0.13a |
| 100 | 5.45±0.20b | |
| 200 | 7.73±0.33b | |
| Hydroxycamptothecin | 10 | 10.10±0.41b |
p < 0.05 versus control group.
p < 0.01 versus control group.
mfi = mean fluorescence intensity.
FIGURE 3.
Effect of Asparagus saponins on reactive oxygen species at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
3.5. Effect of Asparagus Saponins on Calcium Ion Concentration
After treatment with various concentrations of Asparagus saponins, the fluorescence intensity of intracellular Ca2+ increased significantly compared with that in the control samples. Moreover, as the concentration of saponins increased, the Ca2+ concentration correspondingly increased (Table ii).
TABLE II.
Effect of Asparagus saponins on calcium ion concentration (χ̄ ± s)
| Group | Dose (mg/L) | mfi |
|---|---|---|
| Control | — | 20.91±2.85 |
| Asparagus saponins | 50 | 26.30±3.96 |
| 100 | 34.96±4.15a | |
| 200 | 53.37±5.47b | |
| Hydroxycamptothecin | 10 | 166.35±7.78b |
p < 0.05 versus control group.
p < 0.01 versus control group.
mfi = mean fluorescence intensity.
3.6. Effect of Asparagus Saponins on pH
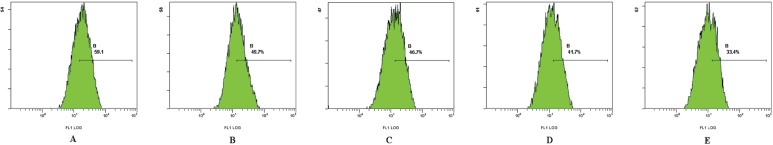
After treatment with various concentrations of Asparagus saponins, the fluorescence intensity of intracellular pH decreased significantly. And as the concentration of saponins increased, the pH intensity correspondingly decreased to 49.7%, 46.7%, and 4.7% respectively. By contrast, intensity in the control sample was 59.1% (Table iii, Figure 4).
TABLE III.
Effect of Asparagus saponins on pH in HepG2 (χ̄ ± s)
| Group | Dose (mg/L) | mfi |
|---|---|---|
| Control | — | 20.50±1.15 |
| Asparagus saponins | 50 | 15.95±0.76 |
| 100 | 13.63±0.74a | |
| 200 | 13.08±0.58a | |
| Hydroxycamptothecin | 10 | 11.44±0.45a |
p < 0.01 versus control group.
mfi = mean fluorescence intensity.
FIGURE 4.
Effect of Asparagus saponins on pH at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
3.7. Effect of Asparagus Saponins on MPTP and MMP
Observation with confocal laser scanning microscopy showed that, after treatment with Asparagus saponins, fluorescence intensity in the cells weakened, indicating increased activity of mptp. And as the concentration of saponins increased, the activity of mptp correspondingly increased (statistically significantly compared with the control samples).
After treatment with Asparagus saponins, the fluorescence intensity in cells weakened, indicating a decreased level of mmp. And as the concentration of saponins increased, the level of mmp correspondingly decreased (statistically significantly compared with the control samples; Table iv, Figures 5 and 6).
TABLE IV.
Effect of Asparagus saponins on mitochondrial permeability transition pore (mptp) and mitochondrial membrane potential (mmp) of HepG2 cells at 24 hours (χ̄ ± s)
| Group | Dose (mg/L) | Mean fluorescence intensity | |
|---|---|---|---|
| mptp | mmp | ||
| Control | — | 43.47±3.39 | 121.83±6.68 |
| Asparagus saponins | 50 | 36.09±2.87a | 105.36±7.92a |
| 100 | 30.21±3.41a | 86.96±5.68b | |
| 200 | 20.72±2.03b | 58.39±5.33b | |
| Hydroxycamptothecin | 10 | 21.33+2.56b | 50.74±3.72b |
p < 0.05 versus control group.
p < 0.01 versus control group.
FIGURE 5.
Effect of Asparagus saponins on mitochondrial permeability transition pore (mptp) of HepG2 cells at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
FIGURE 6.
Effect of Asparagus saponins on mitochondrial membrane potential (mmp) of HepG2 cells at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
3.8. Effect of Asparagus Saponins on Caspase-9 and Caspase-3 Activity
After treatment with various concentrations of Asparagus saponins, the peptide nucleic acid content of the samples increased, indicating increased caspase activity. According to the saponin concentration, caspase- 9 activity was 55.49%, 113.12%, and 150.39% higher than in the control group, Caspase-3 activity was 79.99%, 169.49%, and 218.66% higher than in the control group, which was statistically significant (Table v).
TABLE V.
Effect of Asparagus saponins on caspase-9 and -3 activity of HepG2 cells at 48 hours (χ̄ ± s)
| Group | Dose (mg/L) | Peptide nucleic acid (μmol/L) | Caspase-9 activity (%) | Peptide nucleic acid (μmol/L) | Caspase-3 activity (%) |
|---|---|---|---|---|---|
| Control | — | 20.73±1.21 | — | 11.34±0.91 | — |
| Asparagus saponins | 50 | 32.24±2.74a | 155.49 | 20.43±1.82a | 179.99 |
| 100 | 44.18±3.68b | 213.12 | 30.56±2.42b | 269.49 | |
| 200 | 51.90±2.42b | 250.39 | 36.17±0.91b | 318.66 | |
| Hydroxycamptothecin | 10 | 55.54±3.94b | 267.91 | 44.04±1.21b | 388.36 |
p < 0.05 versus control group.
p < 0.01 versus control group.
3.9. Effect of Asparagus Saponins on Expression of Apoptotic-Related Proteins
After treatment with various concentrations of Asparagus saponins for 24 hours, the expression of Bcl2 in HepG2 cells (in both percentage and mean fluorescence intensity) decreased, and the expression of Bax and Cyt-C increased in a concentration-dependent manner. After treatment for 48 hours, expression of caspase-3 increased gradually (Table vi, Figures 7–10).
TABLE VI.
Effects of Asparagus saponins on the expression of apoptosis-related proteins χ̄ ± s)
| Group | Dose (mg/L) | Mean fluorescence intensity | |||
|---|---|---|---|---|---|
| Bcl2 | Bax | Cyt-C | Caspase-3 | ||
| Control | — | 22.54±1.30 | 12.35±1.20 | 5.10±0.35 | 4.54±0.30 |
| Asparagus saponins | 50 | 19.39±1.57 | 17.39±1.51a | 5.63±0.32 | 5.17±0.34 |
| 100 | 14.75±1.03a | 25.41±1.28b | 9.80±0.45a | 6. 75±0.41a | |
| 200 | 9.83±0.87b | 36.65±1.87b | 14.35±0.76b | 9.83±0.46b | |
| Hydroxycamptothecin | 10 | 5.53±0.32b | 39.07±2.12b | 19.24±1.03b | 12.69±0.57b |
p < 0.05 versus control group.
p < 0.01 versus control group.
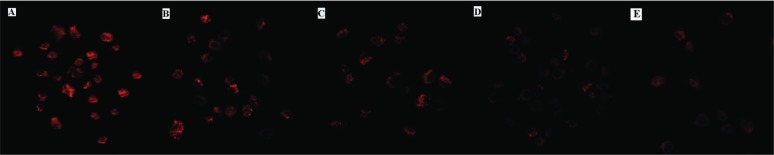
FIGURE 7.
Effect of Asparagus saponins on Bcl2 at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
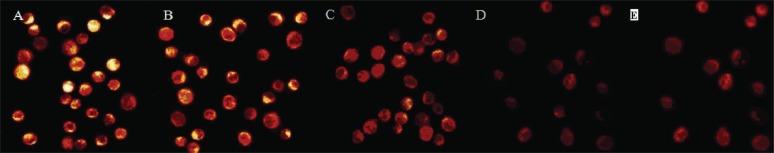
FIGURE 10.
Effect of Asparagus saponins on caspase-3 at 48 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
FIGURE 8.
Effect of Asparagus saponins on Bax at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
FIGURE 9.
Effect of Asparagus saponins on Cyt-C at 24 hours. (A) Control group. (B) HepG2 cells treated with 50 mg/L Asparagus saponins. (C) HepG2 cells treated with 100 mg/L Asparagus saponins. (D) HepG2 cells treated with 200 mg/L Asparagus saponins. (E) HepG2 cells treated with hydroxycamptothecin.
4. DISCUSSION
The occurrence and development of tumours is closely related to cell apoptosis 9. The potential to induce apoptosis has therefore become an important topic in the study of antitumour drugs.
Steroidal saponins have good antitumour effects, and the antitumour targets and pathways are extensive. Many studies have shown that steroidal saponins are the major components in Asparagus officinalis. In the present work, we studied apoptosis in the human hepatoma cell line HepG2 induced by Asparagus saponins. In the apoptosis pathway mediated by mitochondria, the antitumour mechanism of the saponins was clearly confirmed by various detection indices, including induction of apoptosis factors (ros, Ca2+, pH), induction of the life-or-death apoptosis switch (mptp, mmp), induction of the start molecules of apoptosis (Cyt-C), and induction of the effector molecules of apoptosis (caspase-9, caspase-3), among others.
The results show that Asparagus saponins can induce apoptosis in HepG2 cells, exerting their bioactivity by binding to cell-surface receptors. Such binding might stimulate an increase in ros levels. On one hand, an increase in ros might damage the mitochondrial membrane and cause mptp to open, resulting in the release of Ca2+ and Cyt-C. On the other hand, it might upregulate Bax expression, leading to the formation of Bax homodimers, acting on mptp and causing a decrease in mmp, promoting the release of apoptosis factor, and thus inducing apoptosis 10–12. The increase of cytoplasm Ca2+ might further promote mptp to open, which causes a decrease in mmp. In addition, it could activate a series of proteins related to the apoptosis mediated by mitochondria and then induce the apoptosis 13–15. At the same time, the increase of Ca2+ might also cause an increase in H+ and acidification in the cells, which, on one hand, could activate dnase ii to degrade dna, or, on the other hand, could promote release into the cytoplasm of Cyt-C from mitochondria, potentially activating caspase-9. The activated caspase-9 acts on the downstream target of caspase-3 enzymes, and the activated caspase-3 acts on the target cells as an effector molecule, damaging the cell structure and causing functional disorder by proteolysis, ultimately inducing apoptosis through the caspase-dependent mitochondria pathway 16,17. In addition, Asparagus saponins might decrease the expression of Bcl2 protein in HepG2 cells, increasing the expression of Bax protein and the Bax/Bcl2 ratio. Thus, the generation of Bcl2–Bax heterodimer might be decreased, promoting generation of the Bax–Bax homodimer and the formation and opening of mptp, leading to a decrease in membrane potential, promotion of Cyt-C release, and activation of the caspase cascade, finally inducing apoptosis 18,19. In addition, downstream regulation of Bcl2 protein expression by Asparagus saponins might reduce the inhibition of Bcl2 on Cyt- C release from the mitochondria and, at the same time, the formation of the Bcl2–Apaf1–caspase-9 complex, promoting the activation effect of caspase-9 on caspase-3, and finally promoting apoptosis 20,21.
5. CONCLUSIONS
Asparagus saponins can induce apoptosis in the human hepatoma cell line HepG2 through a mitochondrial-mediated and caspase-dependent pathway. Asparagus saponins can increase the intracellular level of ros, increase intracellular Ca2+, decrease intracellular pH, and so induce HepG2 apoptosis. Asparagus saponins can open mptp, turn on the death switch, and decrease mmp, thus inducing apoptosis by an irreversible mitochondrial pathway. Asparagus saponins can adjust apoptosis-related protein expression in HepG2 cells and thereby induce apoptosis. They also increase intracellular Cyt-C, activated caspase-3, and Bax expression and decrease intracellular Bcl2 expression. Asparagus saponins can increase caspase-9 and caspase-3 activity in HepG2 cells in a dose- and time-dependent manner, and induce cell apoptosis through a caspase-dependent mitochondrial pathway. Asparagus saponins therefore have the potential to be a potent drug for cancer prevention and treatment.
6. ACKNOWLEDGMENTS
This study was supported by a grant from the National Natural Science Foundation of China (no. 304005926). YJ proposed the study. CJ and LY performed the research and wrote the first draft. HX collected and analyzed the data. All authors contributed to the design and interpretation of the study and to the second and subsequent drafts of the manuscript.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest with respect to this manuscript.
8. REFERENCES
- 1.Potduang B, Meeploy M, Giwanon R, Benmart Y, Kaewduang M, Supatanakul W. Biological activities of Asparagus racemosus. Afr J Tradit Complement Altern Med. 2008;5:230–7. doi: 10.4314/ajtcam.v5i3.31278. [Erratum in: Afr J Tradit Complement Altern Med 2010;7:377] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang XF, Lin YY, Kong LY. Steroids from the roots of Asparagus officinalis and their cytotoxic activity. J Integr Plant Biol. 2008;50:717–22. doi: 10.1111/j.1744-7909.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 3.Gautam M, Saha S, Bani S, et al. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: implications for immunoadjuvant potential. J Ethnopharmacol. 2009;121:241–7. doi: 10.1016/j.jep.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Visavadiya NP, Soni B, Soni B, Madamwar D. Suppression of reactive oxygen species and nitric oxide by Asparagus racemosus root extract using in vitro studies. Cell Mol Biol (Noisy-le-grand) 2009;55(suppl):OL1083–95. [PubMed] [Google Scholar]
- 5.Sun T, Powers JR, Tang J. Enzyme catalyzed change of antioxidants content and antioxidant activity of asparagus juice. J Agric Food Chem. 2007;55:56–60. doi: 10.1021/jf062775i. [DOI] [PubMed] [Google Scholar]
- 6.Sharma U, Saini R, Kumar N, Singh B. Steroidal saponins from Asparagus racemosus. Chem Pharm Bull (Tokyo) 2009;57:890–3. doi: 10.1248/cpb.57.890. [DOI] [PubMed] [Google Scholar]
- 7.Hayes PY, Jahidin AH, Lehmann R, Penman K, Kitching W, De Voss JJ. Steroidal saponins from the roots of Asparagus racemosus. Phytochemistry. 2008;69:796–804. doi: 10.1016/j.phytochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Zhou LB, Chen TH, Bastow KF, Shibano M, Lee KH, Chen DF. Filiasparosides A–D, cytotoxic steroidal saponins from the roots of Asparagus filicinus. J Nat Prod. 2007;70:1263–7. doi: 10.1021/np070138w. [DOI] [PubMed] [Google Scholar]
- 9.Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–21. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Franke JC, Plötz M, Prokop A, Geilen CC, Schmalz HG, Eberle J. New caspase-independent but ros-dependent apoptosis pathways are targeted in melanoma cells by an iron-containing cytosine analogue. Biochem Pharmacol. 2010;79:575–86. doi: 10.1016/j.bcp.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Kim BM, Choi YJ, Han Y, Yun YS, Hong SH. N,N-Dimethyl phytosphingosine induces caspase-8–dependent cytochrome C release and apoptosis through ros generation in human leukemia cells. Toxicol Appl Pharmacol. 2009;239:87–97. doi: 10.1016/j.taap.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Liu H, Jin J, Zhu X, Lu L, Jiang H. The role of endogenous reactive oxygen species in oxymatrine-induced caspase-3–dependent apoptosis in human melanoma A375 cells. Anticancer Drugs. 2010;21:494–501. doi: 10.1097/CAD.0b013e328336e927. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Li YC, Ip SW, et al. The role of Ca2+ in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria- and caspase-3–dependent pathway. Anticancer Res. 2008;28:1701–11. [PubMed] [Google Scholar]
- 14.Chiu PY, Luk KF, Leung HY, Ng KM, Ko KM. Schisandrin B stereoisomers protect against hypoxia/reoxygenation-induced apoptosis and inhibit associated changes in Ca2+–induced mitochondrial permeability transition and mitochondrial membrane potential in H9c2 cardiomyocytes. Life Sci. 2008;82:1092–101. doi: 10.1016/j.lfs.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–74. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- 16.Babu US, Gaines DM, Wu Y, Westphal CD, Pereira M, Raybourne RB. Use of flow cytometry in an apoptosis assay to determine pH and temperature stability of Shiga-like toxin 1. J Microbiol Methods. 2008;75:167–71. doi: 10.1016/j.mimet.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Di Sario A, Bendia E, Omenetti A, et al. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Dig Liver Dis. 2007;39:60–9. doi: 10.1016/j.dld.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Yadav S, Shi Y, Wang F, Wang H. Arsenite induces apoptosis in human mesenchymal stem cells by altering Bcl-2 family proteins and by activating intrinsic pathway. Toxicol Appl Pharmacol. 2010;244:263–72. doi: 10.1016/j.taap.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Richardson A, Kaye SB. Pharmacological inhibition of the Bcl-2 family of apoptosis regulators as cancer therapy. Curr Mol Pharmacol. 2008;1:244–54. doi: 10.2174/1874467210801030244. [DOI] [PubMed] [Google Scholar]
- 20.Song R, Harris LD, Pettaway CA. Downmodulation of Bcl-2 sensitizes metastatic LNCaP–LN3 cells to undergo apoptosis via the intrinsic pathway. Prostate. 2010;70:571–83. doi: 10.1002/pros.21091. [DOI] [PubMed] [Google Scholar]
- 21.Deng L, Adachi T, Kitayama K, et al. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3–dependent pathway. J Virol. 2008;82:10375–85. doi: 10.1128/JVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]