Abstract
Introduction
Postmortem redistribution (PMR), a well-described phenomenon in forensic toxicology for certain drugs, can result in increased central blood concentrations relative to peripheral blood concentrations. Δ9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis or marijuana, is the illicit substance most commonly implicated in driving under the influence of drugs (DUID) cases and fatally-injured drivers. No investigation of PMR of THC in human blood has been reported to date.
Methods
Matched heart and iliac postmortem blood specimens were collected from 19 medical examiner cases (16 Males, 3 Females) with positive cannabinoid urine immunoassay screens. THC, its equipotent metabolite 11-hydroxy-THC (11-OH-THC) and non-psychoactive metabolite 11-nor-9-carboxy-THC (THCCOOH) were quantified by two-dimensional gas chromatography-mass spectrometry with cryofocusing, with 0.5 ng/mL limits of quantification (LOQ) for all analytes.
Results
10 cases had quantifiable THC and 11-OH-THC; THCCOOH was present in all 19. Median (range) heart:iliac blood ratios were 1.5 for THC (range: 0.3–3.1); 1.6 for 11-OH-THC (range: 0.3–2.7); and 1.8 for THCCOOH (range: 0.5–3.0).
Discussion
Cannabinoids, in general, exhibited a mean and median central: peripheral (C: P) concentration ratio of less than 2 following death. A trend was observed for greater PMR with increasing postmortem interval between death and sampling. To our knowledge, these are the first data on THC PMR in humans, providing important scientific data to aid in the interpretation of postmortem cannabinoid concentrations in medico-legal investigations.
Keywords: Postmortem redistribution, THC, tetrahydrocannabinol, cannabinoids, cannabis, marijuana
Introduction
Postmortem redistribution (PMR) is a phenomenon in postmortem toxicology that has been recognized for over two decades. PMR is a process whereby drugs can diffuse from contiguous tissues with higher drug concentrations into the surrounding blood, and the concentration of drugs at certain anatomic sites (usually cardiac or central blood) can differ significantly from their antemortem concentrations. {[1][2][3], [4], [5], [6]}. While the likelihood of a specific drug exhibiting PMR is dependent on many factors, detecting PMR requires investigators to sample post-mortem blood from both central and peripheral sites. For drugs demonstrating PMR, diffusion occurs from tissue stores in cardiac, lung, and liver tissue; as well as gastric contents, along the concentration gradient into contiguous central blood in the heart or large vessels (vena cava, aorta, subclavian, etc). Drug properties, including lipophilicity (octanol:water partition co-efficient or Log P), charge, pKa and volume of distribution (Vd), influence the degree of PMR, as do host factors such as body fat composition, age and nutritional status {[1][2][3], [4], [6]}. Body temperature and degree of decomposition, time interval since death, body position, and body handling (causing movement of central blood) also may affect the degree of PMR {[5][3], [4]}.
Multiple drug classes including tricyclic antidepressants [7], cardiovascular drugs[3], and some drugs of abuse such as cocaine [2] display well-documented PMR, although reports are often hindered by small sample sizes (often limited to only 3–5 cases) and/or high variability. Many drugs subject to PMR are basic (pKa > 7) and highly lipophilic, with Log P >0.5, and with large apparent Vd{[1][2][3], [4], [5],[6]}. For those drugs that exhibit PMR, central blood samples (obtained from cardiac chambers) can have 3–5 times greater drug concentrations than simultaneously obtained peripheral (femoral, iliac,) blood samples. The heart:femoral or central:peripheral (C:P) ratio is a common PMR quantifier; when this ratio is substantially greater than unity, the drug is considered to exhibit PMR.
Δ9-tetrahydrocannabinol (THC), the primary psychoactive component of cannabis or marijuana, with a pKa of 10.6, is actually an acidic phenolic compound, but is quite lipophilic, with a high Log P of 5.648 [8] and a high Vd (4–14 L/kg) [7] and would be expected to demonstrate detectable PMR. Surprisingly, even though cannabis is the most commonly abused illicit drug and is often found in postmortem examinations, there are currently no published reports describing THC PMR (or lack thereof) in humans. As THC overdoses are exceedingly rare and almost never a direct cause of drug-overdose deaths, no “lethal” THC concentration in humans is described. Except in the setting of trauma resulting from drug-induced impairment, THC measurement in post-mortem specimens has little impact on the ultimate determination of cause and manner of death by medical examiners, and this fact is likely responsible for this relative scarcity of information.
The major reference textbook in forensic toxicology regarding postmortem blood levels does not discuss the possible PMR of THC as it does for most other drugs [7]. Karch presents only a theoretical discussion of THC PMR based on its physical properties and the difficulties with interpretation of postmortem THC concentrations [9]. Drummer estimates THC PMR as most likely “low to moderate,” although no supportive data are presented[2].
While little information is available regarding THC PMR in humans, some recent studies investigated this phenomenon in animals. Brunet, et al. studied postmortem redistribution of THC utilizing the Large White Pig model[10]. Fifteen pigs were administered intravenous THC and euthanized two hours later, with blood and tissue samples collected at autopsy (0, 6, 15, 24 and 48 h post-mortem). Cardiac (central) and suprarenal inferior vena cava (authors considered peripheral) blood specimens were analyzed for THC (LOQ 0.5 ng/mL). The authors concluded that THC in this species is subject to PMR as C:P ratios increased with increasing postmortem interval. They also concluded that no collection site adequately reflects perimortem THC concentrations.
Another study conducted by Hilberg, et al. studied PMR of a number of different drugs and compared human cases to experimental animal models[11]. In two human cases where THC was examined, postmortem femoral (obtained one day postmortem) to perimortem heart blood (obtained within one hour of death) ratio (i.e. peripheral to central ratio) for THC of 2.8 in one case and postmortem femoral (2 days postmortem) to perimortem neck blood (one hour postmortem) ratio of 0.4 was obtained in a second case. In the animal portion of the study, the authors reported THC concentrations in rats administered 30 mg THC by gastric tube. They compared antemortem heart blood THC concentrations to postmortem heart blood, postmortem suprarenal vena cava blood and postmortem vitreous humor THC concentrations. Mean (SEM) ratios of postmortem heart blood concentrations to postmortem vena cava blood concentrations were 1.9 (0.4).
Although no fatalities from cannabis (or THC) overdose have been reported, cannabis use is an important public health issue. Numerous studies indicate THC is the most common substance found in DUID cases worldwide [12] [13]. Determining impairment due to THC is more complex than for ethanol, since impairment cannot be inferred by THC blood concentrations alone, especially for chronic cannabis smokers who may have detectable THC in whole blood for up to 7 days after last cannabis intake, when no longer impaired [14] However, given the degree of THC involvement in DUID and its implications on traffic accidents and fatalities, assigning culpability is increasingly important in many jurisdictions [13,15,16]. Therefore, this research was initiated to determine whether THC exhibits PMR in humans. We postulate that the parent drug THC, being the most lipid soluble, would exhibit the most PMR. Since more water soluble drugs and metabolites generally have smaller Vd and generally exhibit less PMR, we also postulate that the more polar THC metabolites 11-OH-THC and THCCOOH would exhibit less PMR than the parent THC molecule.
Materials and Methods
Specimen Collection
Nineteen cases were selected from consecutive adult deaths falling under the jurisdiction of the Onondaga County Medical Examiner’s Office (OCMEO), Syracuse, NY, from July 2009 through October 2009. Cases were chosen if an autopsy examination was to be performed and if a postmortem urine immunoassay (Status DS, LifeSign, LLC, Somerset, NJ) was positive for cannabinoids. During autopsy, 10–15 mL cardiac (central) and iliac vein (peripheral) blood was collected in appropriately-labeled Vacutainer® sodium fluoride blood tubes (BD, Franklin Lakes, NJ). Bloods were frozen at −60 °C and shipped frozen to Chemistry and Drug Metabolism, National Institute on Drug Abuse IRP (Baltimore, MD) for analysis. 20 control cases were selected in which a postmortem urine drug screen was negative for cannabinoids; these were utilized for analytical method validation of cannabinoids in postmortem blood. Central and peripheral control samples were collected, stored and transported similarly to study samples. In three cases, antemortem whole blood specimens (K2EDTA preserved) were available from referring hospitals. The dates and times of collection for these specimens were recorded.
Whole Blood Cannabinoid Analysis
A previously validated method[17] was utilized with slight modifications to complete postmortem blood analyses. Blank postmortem blood (0.5 mL) and blank whole blood (0.5 mL) were pipetted into culture tubes to which working calibrator solutions were added to produce final calibrator concentrations of 0.5, 1.0, 2.5, 5, 10, 25, and 50 ng/mL of all three analytes (THC, 11-OH-THC and THCCOOH). Working quality control (QC) solutions were added to produce final QC samples of 0.7, 2.0, 20 and 40 ng/mL that were analyzed in duplicate in each batch. 25 μL deuterated THC, 11-OH-THC and THCCOOH internal standards (5 ng) were added to all calibrators, controls and authentic postmortem specimens, and proteins precipitated with 2 mL cold acetonitrile added in 0.5-mL increments while vortexing. After centrifugation (10 min, 3000 rpm), supernatants were decanted, solid phase extraction performed (United Chemical Technologies (UCT) Clean Screen® ZSTHC020) and analytes quantified by 2-dimensional gas chromatography-mass spectrometry (2D GCMS) as previously described [17]
Method Validation
Whole blood cannabinoid method development and validation were previously reported [17]. The current postmortem method was validated for linearity, limit of detection (LOD) and limit of quantification (LOQ), analytical recovery and intra- and inter-assay imprecision. Four replicate QC samples were analyzed during 4 separate validation runs (prior to analyzing case specimens) for a total of 16 replicates. During actual case specimen batches (after validation was completed), duplicate QC samples were included to monitor ongoing assay accuracy and precision.
Analytical recovery (acceptable within 80%–120% of target concentration) and intra- and inter-assay imprecision (acceptable if ± 20% CV) from 16 replicates of quality control samples at 0.7, 2, 20 and 40 ng/mL for all three analytes, assayed in 4 separate batches. Transition peak area ratios for QC and authentic specimens were required to be within ± 20% of the mean peak area ratios for calibrators of each respective analyte to be considered acceptable.
Coefficients of determination (r2values for the calibration curve; utilized to evaluate the fit of the linear regression to the empirical data.) were ≥ 0.990. LOD and LOQ were empirically determined by assaying decreasing analyte concentrations. LOD was the lowest calibrator concentration, with retention time within 2% of calibrator mean, acceptable chromatography, acceptable qualifier ion ratios, and signal-to-noise ratio ≥ 3:1. LOQ was the lowest calibrator meeting LOD criteria and quantifying within 20% of target. LOD and LOQ were 0.25 ng/mL and 0.5 ng/mL, respectively, for all analytes. Analytical recovery and inter- and intra-assay imprecision were within a priori specifications (± 20%). Potential endogenous matrix interference was investigated by analysis of 20 matched central and peripheral blank postmortem whole blood specimens.
Postmortem Validation
Dilution integrity, a measure of quantification accuracy after diluting a high-concentration specimen with blank matrix, was investigated for postmortem specimens up to a 1:4 dilution for THCCOOH (n = 6 each). Median (range) difference from undiluted specimens was −3.0% (−13.2%–7.3%), indicating acceptable dilution integrity up to a 1:4 dilution with blank blood for this analyte. Other analytes could not be investigated due to low initial concentrations and/or insufficient specimen volume. Reanalysis of some positive PMR specimens (central THC n = 4, peripheral THC n = 5, central 11-OH-THC n = 10, peripheral 11-OH-THC n = 6, central THCCOOH n = 19, peripheral THCCOOH n = 8) was performed to assess reproducibility. Individual quantifications differed by a median (range) of 3.6% (0.3%–9.4%) THC, 2.8% (0.0%–26.7%) 11-OH-THC and 1.4% (0.1%–11.0%) THCCOOH, indicating a lack of bias and acceptable analytical precision. All repeated specimens originally quantifying below LOQ (n = 12 across all analytes) quantified below LOQ during repeat analyses.
Statistical Analyses
Statistical calculations were performed with SPSS® 15.0 for Windows (SPSS Inc, Chicago, IL). A Wilcoxon signed-rank statistical test (median values) was performed for cases where there are both valid central and peripheral concentrations to determine potential differences (P < 0.05) between central and peripheral blood concentrations (n = 10, 10 and 19 for THC, 11-OH-THC and THCCOOH, respectively). As data were non-normal, linear regression analyses were performed to characterize the relationships between postmortem interval and C:P ratios. P values < 0.05 were required for statistical significance.
Results
Specimens
Matrix interference from putrefactive or in vitro breakdown products markedly affected chromatography in some specimens analyzed 2–10 months after collection. It was necessary to repeat analyses or utilize reduced specimen volume to achieve acceptable results for some postmortem specimens. Two specimens could not be quantified for THC and one for 11-OH-THC due to matrix effects that resulted in poor internal standard recovery, even after multiple quantification attempts. Table 1 shows overall statistics for quantification results in each matrix.
Table 1.
Numbers and percentage of specimens having quantifiable Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) concentrations from the 19 postmortem specimens
| Central THC | Peripheral THC | Central 11-OH-THC | Peripheral 11-OH-THC | Central THCCOOH | Peripheral THCCOOH | |
|---|---|---|---|---|---|---|
| # of specimens > LOQ | 11 | 10 | 10 | 10 | 19 | 19 |
| # of specimens < LOQ | 6 | 7 | 8 | 8 | 0 | 0 |
| # of specimens NA | 2 | 2 | 1 | 1 | 0 | 0 |
| % of specimens > LOQ | 58% | 53% | 53% | 53% | 100% | 100% |
| % of specimens < LOQ | 32% | 37% | 42% | 42% | 0% | 0% |
| % of specimens NA | 11% | 11% | 5% | 5% | 0% | 0% |
#N/A – Matrix effects prevented quantification; LOQ: Limit of quantification
Cannabinoid PMR
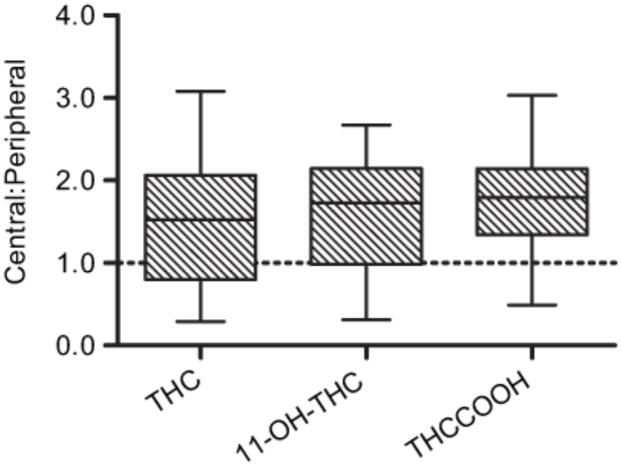
Median (range) C:P blood ratios were 1.5 (0.3–3.1), 1.7 (0.3–2.7) and 1.8 (0.5–3.0) for THC, 11-OH-THC and THCCOOH, respectively (Figure 1), suggesting modest post-mortem redistribution to the central blood for all three cannabinoids following death. Wilcoxon signed-rank testing yielded no significant difference between median central and peripheral blood concentrations for THC (T = 10.0, p > 0.164, r = −0.494) and 11-OH-THC (T = 9.5, p > 0.164, r = −0.487), although there was a significant difference between central and peripheral concentrations for THCCOOH (T = 39.0, p < 0.026, r = −0.517). Interestingly, cases #13 and #15 contained higher concentrations of all analytes in peripheral blood as compared to central blood, with case #13 C:P ratios 0.5 for THC and 0.8 for THCCOOH; and case #15 C:P ratios 0.3 for THC, 0.3 for 11-OH-THC, and 0.5 for THCCOOH. These cases contributed to the high variability observed in these data.
Figure 1.
Central: Peripheral Ratio
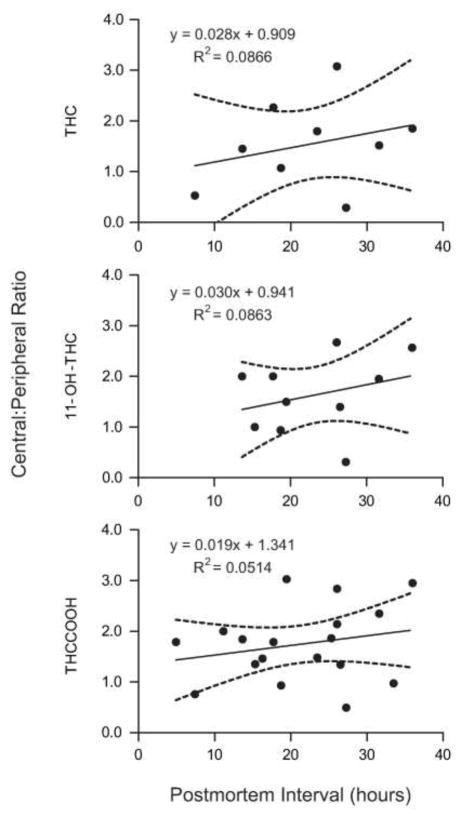
A trend toward positive correlation for time interval from death to blood collection and central: peripheral ratio for all analytes was observed (Figure 2). R2 values: THC, 0.0866; 11-OH-THC, 0.0863; THCCOOH, 0.0514. However, for all analytes, slopes were not significantly different than zero, with slopes (95% CI) of 0.028 (−0.054–0.110) for THC, 0.030 (−0.049–0.109) for 11-OH-THC and 0.019 (−0.024–0.062) for THCCOOH.
Figure 2.
C:P Ratio vs Post-Mortem Interval
Antemortem Specimens
Antemortem whole blood specimens were quantified for cases #5, #8 and #11 as shown in Table 2. In all cases, antemortem concentrations were greater than or equivalent to postmortem concentrations. In case #5 antemortem concentrations for THC and 11-OH-THC were quite low and similar to postmortem levels; however, THCCOOH (28.0 ng/mL) was more than twice the highest postmortem concentration (12.4 ng/mL). Conversely, in case #11 antemortem THC and 11-OH-THC were within 20% of postmortem concentrations, but THCCOOH was 49.5% higher (160 vs 107 ng/mL)
Table 2.
Demographics and postmortem and antemortem Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) concentrations.
| Case# | Demographics | THC | 11-OH-THC | THCCOOH | Antemortem Specimens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex1 | Race2 | PM Interval (hh:mm) | Central (ng/mL) | Peripheral (ng/mL) | C:P Ratio | Central (ng/mL) | Peripheral (ng/mL) | C:P Ratio | Central (ng/mL) | Peripheral (ng/mL) | C:P Ratio | THC(ng/mL) | 11-OH-THC(ng/mL) | THCCOOH (ng/mL) | |
| 1 | M | 3 | 17:43 | 5.9 | 2.6 | 2.27 | 1.0 | 0.5 | 2.00 | 7.0 | 3.9 | 1.79 | |||
| 2 | M | 4 | 13:39 | 1.6 | 1.1 | 1.45 | 1.6 | 0.8 | 2.00 | 20.4 | 11.1 | 1.84 | |||
| 3 | M | 4 | 2:05 | 11.4 | 3.7 | 3.08 | 4.0 | 1.5 | 2.67 | 47.4 | 16.7 | 2.84 | |||
| 4 | M | 4 | 4:55 | < LOQ | < LOQ | N/C | < LOQ | < LOQ | N/C | 3.4 | 1.9 | 1.79 | |||
| 5 | F | 4 | 23:28 | 1.8 | 1.0 | 1.80 | < LOQ | < LOQ | N/C | 12.4 | 8.4 | 1.48 | 1.4 | 1.0 | 27.7 |
| 6 | M | 4 | 15:19 | < LOQ | 1.0 | N/C | 0.6 | 0.6 | 1.00 | 21.5 | 15.9 | 1.35 | |||
| 7 | M | 4 | 19:27 | N/A | N/A | N/C | 2.1 | 1.4 | 1.50 | 23.6 | 7.8 | 3.03 | |||
| 8 | F | 4 | n/a | < LOQ | < LOQ | N/C | < LOQ | < LOQ | N/C | 1.6 | 1.0 | 1.60 | < LOQ | < LOQ | 1.4 |
| 9 | M | 4 | 2:05 | < LOQ | < LOQ | N/C | < LOQ | < LOQ | N/C | 1.5 | 0.7 | 2.14 | |||
| 10 | M | 1 | 2:31 | 0.6 | < LOQ | N/C | 0.7 | 0.5 | 1.40 | 30.5 | 22.7 | 1.34 | |||
| 11 | M | 1 | 18:44 | 50.5 | 47.1 | 1.07 | 10.4 | 11.1 | 0.94 | 100.2 | 107.6 | 0.93 | 59.3 | 13.6 | 161.7 |
| 12 | M | 4 | 11:09 | < LOQ | < LOQ | N/C | < LOQ | < LOQ | N/C | 1.0 | 0.5 | 2.00 | |||
| 13 | F | 1 | 7:25 | 0.8 | 1.5 | 0.53 | < LOQ | < LOQ | N/C | 3.4 | 4.5 | 0.76 | |||
| 14 | M | 1 | 11:59 | 7.4 | 4.0 | 1.85 | 3.6 | 1.4 | 2.57 | 81.1 | 27.5 | 2.95 | |||
| 15 | M | 4 | 3:16 | 2.2 | 7.5 | 0.29 | 0.9 | 2.9 | 0.31 | 20.5 | 41.9 | 0.49 | |||
| 16 | M | 4 | 7:37 | 9.4 | 6.2 | 1.52 | 4.3 | 2.2 | 1.95 | 48.2 | 20.5 | 2.35 | |||
| 17 | M | 4 | 1:20 | < LOQ | < LOQ | N/C | < LOQ | < LOQ | N/C | 3.9 | 2.1 | 1.86 | |||
| 18 | M | 4 | 9:30 | N/A | N/A | N/C | N/A | N/A | N/C | 32.4 | 33.4 | 0.97 | |||
| 19 | M | 2 | 16:17 | 0.5 | < LOQ | N/C | < LOQ | < LOQ | N/C | 1.9 | 1.3 | 1.46 | |||
M: Male; F: Female
1: African American; 2: Hispanic, Not White; 3: Native American; 4: White (Caucasian)
< LOQ: Not detected at the lower limit of quantification (0.5 ng/mL); N/A: not available, matrix effects prevented quantification; N/C: not calculable
Discussion
A drug is consider to exhibit post-mortem redistribution when its C:P ratio is substantially greater than unity. Here we report median C:P blood ratios between 1.5 and 2.0 for THC, 11-OH-THC and THCCOOH, suggesting modest PMR for all analytes and confirming the prediction for “low to moderate” redistribution as postulated by Drummer[2]. The relatively wide ranges and variability of C:P ratios is typical of PMR findings of many other drugs[7] and is illustrative of variables that can affect these results such as cause of death, postmortem interval and tissue breakdown. Other drugs subject to PMR, such as tricyclic antidepressants, cardiovascular drugs and cocaine, typically demonstrate increased redistribution with increased postmortem interval[18][19]. Here we report a similar trend for cannabinoids, consistent with these other drug classes. As the postmortem interval increases and decomposition progresses, additional drug is released from central cavity organs, resulting in C:P ratio increases. Although slopes here were not significantly different from zero for any analyte, we believe this results from the high variability inherent in C:P ratios. Further study is warranted to substantiate these preliminary findings.
We postulated that parent THC would exhibit increased PMR compared to its more polar metabolites. Our results did not support this hypothesis, as PMR was similar across all analytes. Of particular interest was the finding that the three antemortem specimens contained higher concentrations than post-mortem specimens for THC, 11-OH-THC and THCCOOH with the greatest difference observed for THCCOOH. The interval from antemortem specimen acquisition to time of death was approximately 4.5 and 5.5 h in cases #5 and #8, respectively. This would have allowed for further metabolism, tissue distribution or excretion in the time between blood collection and death, and could explain lower postmortem concentrations. However, in case #11, the antemortem specimen was obtained minutes prior to death, yet the antemortem THCCOOH concentration was still approximately 50% higher than the postmortem concentration. This case presented the highest postmortem (and antemortem) concentrations of any in the current study, with approximately 60 ng/mL antemortem THC. Concentrations of this magnitude indicate recent cannabis intake, likely within 0.1– 3.1 hours of blood collection according to predictive models[20,21]. In the two human cases described by Hilberg, perimortem and postmortem blood THC concentrations were assayed. In one case, perimortem heart blood was obtained < 1 hour after death, and was compared to autopsy femoral blood one day later, and the postmortem femoral concentration to perimortem heart blood concentration was 2.8, likely demonstrating PMR. However, the other case had a ration of perimortem THC neck vein blood concentration to femoral blood concentration (from autopsy two days later) of 0.4, indicating the post-mortem blood THC concentration was more than twice the peri-mortem value. In addition, the rat data from the same study showed a post-mortem vena cava blood THC to antemortem heart blood THC concentration ratio of 0.6, indicating antemortem blood THC nearly twice the concentration of post-mortem.[11]. If this trend of higher THC concentrations in ante-mortem blood than in post-mortem can be confirmed in future cases, it may have significant implications for future work in determining antemortem impairment using post-mortem blood concentrations and could have major implications in DUID cases: the possibility of falsely high post-mortem samples causing erroneous impairment conclusions would be negated. In addition, further studies could possibly focus on whether current formulae for estimating impairment and time of last cannabis use could be applied to post-mortem THC and THC-COOH levels. The low numbers of antemortem values (n = 3) render these results preliminary; further study and confirmation are needed. However, the feasibility of finding significant numbers of cases where antemortem blood is drawn moments before death is unlikely.
Another possible explanation for higher antemortem values is the potential difference in analyte stability between the K2EDTA-preserved antemortem blood specimens and the NaF-preserved postmortem blood specimens. In one study, THCCOOH and the ester-linked THCCOOH-glucuronide demonstrated time- and temperature-dependent concentration changes during fortified plasma and urine storage[22]. However, in another study, 1 month of either room temperature or 4°C storage yielded no concentration differences in fortified THCCOOH whole blood samples stored in various blood collection tubes (including NaF and EDTA) [23]. It should be noted these were fortified, not authentic, specimens and did not include THCCOOH-glucuronide. Further research regarding analyte stability, especially THCCOOH-glucuronide, in these matrices is warranted as matrix-induced stability differences could confound interpretation.
The detection window of cannabis impairment is poorly defined. After acute cannabis consumption in occasional cannabis users (less than daily use), impairment is suggested for at least 4 to 8 h, with some indicating possible residual effects for as long as 24 h[24]. Specific concentrations are frequently meaningful when correlated with hours since last cannabis use. Extensive work by Huestis et al showed that in living volunteers who were less than daily cannabis smokers, time since last cannabis use is reliably predicted from plasma THC and THCCOOH concentrations. Predictive models were developed to estimate this time window and are often helpful in determining whether impaired driving had occurred.[25–27].
It is currently unknown whether these models would be valid for impairment determination when using postmortem THC and THCCOOH concentrations for several reasons. First, impairment studies correlating driving performance and cannabinoid blood concentrations are conducted with live participants. Second, performance studies typically utilize plasma or serum, not whole blood as is used for postmortem analyses. While it has been demonstrated that average plasma: whole blood ratios for THC are approximately 0.5 in antemortem specimens, because THC does not partition well into erythrocytes [7], [28], [29]; documenting this ratio in postmortem specimens is more problematic. Third, it was previously unknown how postmortem whole blood concentrations correlated with antemortem concentrations, and most importantly, prior to this study, it was unknown whether THC exhibited PMR.
Conclusions
THC and its metabolites 11-OH-THC and THCCOOH undergo only modest PMR, much less than expected based on the lipophilic nature and the high Vd of the cannabinoids. Average C:P ratios for all analytes were less than 2.0. There was a positive trend (albeit not statistically significant) of increasing PMR and postmortem interval between death and blood draw observed for all analytes.
Acknowledgments
The authors would like to thank the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health and SUNY Upstate Medical University Department of Emergency Medicine and the Onondaga County Medical Examiner’s Office for supporting this research. Additionally, the authors would like to thank Allan Barnes and Karl Scheidweiler for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pounder DJ, Jones GR. Post-mortem drug redistribution--a toxicological nightmare. Forensic Sci Int. 1990;45:253–263. doi: 10.1016/0379-0738(90)90182-x. [DOI] [PubMed] [Google Scholar]
- 2.Drummer OH. Postmortem toxicology of drugs of abuse. Forensic Sci Int. 2004;142:101–113. doi: 10.1016/j.forsciint.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Leikin JB, Watson WA. Post-mortem toxicology: what the dead can and cannot tell us. J Toxicol Clin Toxicol. 2003;41:47–56. doi: 10.1081/clt-120018270. [DOI] [PubMed] [Google Scholar]
- 4.Yarema MC, Becker CE. Key concepts in postmortem drug redistribution. Clin Toxicol (Phila) 2005;43:235–241. [PubMed] [Google Scholar]
- 5.Pelissier-Alicot AL, Gaulier JM, Champsaur P, Marquet P. Mechanisms underlying postmortem redistribution of drugs: a review. J Anal Toxicol. 2003;27:533–544. doi: 10.1093/jat/27.8.533. [DOI] [PubMed] [Google Scholar]
- 6.Prouty RW, Anderson WH. The forensic science implications of site and temporal influences on postmortem blood-drug concentrations. J Forensic Sci. 1990;35:243–270. [PubMed] [Google Scholar]
- 7.Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. 8. Biomedical Publications; Foster City, CA: 2008. [Google Scholar]
- 8.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karch SB. Karch’s Pathology of Drug Abuse. 4. CRC Press; Boca Raton, FL: 2009. [Google Scholar]
- 10.Brunet B, Hauet T, Hebrard W, Papet Y, Mauco G, Mura P. Postmortem redistribution of THC in the pig. Int J Legal Med. 2010;124:543–549. doi: 10.1007/s00414-009-0403-2. [DOI] [PubMed] [Google Scholar]
- 11.Hilberg T, Rogde S, Morland J. Postmortem drug redistribution--human cases related to results in experimental animals. J Forensic Sci. 1999;44:3–9. [PubMed] [Google Scholar]
- 12.Raes E, Verstraete AG. Usefulness of roadside urine drug screening in drivers suspected of driving under the influence of drugs (DUID) J Anal Toxicol. 2005;29:632–636. doi: 10.1093/jat/29.7.632. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Wilhelm L. Prevalence of alcohol and illicit drugs in blood specimens from drivers involved in traffic law offenses. Systematic review of cross-sectional studies. Traffic inj prev. 2007;8:189–198. doi: 10.1080/15389580601188121. [DOI] [PubMed] [Google Scholar]
- 14.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, et al. Do Delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khiabani HZ, Bramness JG, Bjorneboe A, Morland J. Relationship between THC concentration in blood and impairment in apprehended drivers. Traffic Inj Prev. 2006;7:111–116. doi: 10.1080/15389580600550172. [DOI] [PubMed] [Google Scholar]
- 16.Papafotiou K, Carter JD, Stough C. The relationship between performance on the standardised field sobriety tests, driving performance and the level of Delta9-tetrahydrocannabinol (THC) in blood. Forensic Sci Int. 2005;155:172–178. doi: 10.1016/j.forsciint.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Schwilke EW, Karschner EL, Lowe RH, Gordon AM, Cadet JL, Herning RI, et al. Intra- and intersubject whole blood/plasma cannabinoid ratios determined by 2-dimensional, electron impact GC-MS with cryofocusing. Clin Chem. 2009;55:1188–1195. doi: 10.1373/clinchem.2008.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan RJ, Connally G, Evans JM. Analytical toxicology: guidelines for sample collection postmortem. Toxicol Rev. 2005;24:63–71. doi: 10.2165/00139709-200524010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Cook DS, Braithwaite RA, Hale KA. Estimating antemortem drug concentrations from postmortem blood samples: the influence of postmortem redistribution. J Clin Pathol. 2000;53:282–285. doi: 10.1136/jcp.53.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 21.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) J Anal Toxicol. 1992;16:283–290. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- 22.Skopp G, Potsch L. Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry. Clin Chem. 2002;48:301–306. [PubMed] [Google Scholar]
- 23.McCurdy HH, Callahan LS, Williams RD. Studies on the stability and detection of cocaine, benzoylecgonine, and 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid in whole blood using Abuscreen radioimmunoassay. J Forensic Sci. 1989;34:858–870. [PubMed] [Google Scholar]
- 24.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–565. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- 25.Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML. Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther Drug Monit. 2006;28:540–544. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005:657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 27.Huestis MA, Barnes A, Smith ML. Estimating the time of last cannabis use from plasma delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol concentrations. Clin Chem. 2005;51:2289–2295. doi: 10.1373/clinchem.2005.056838. [DOI] [PubMed] [Google Scholar]
- 28.Giroud C, Menetrey A, Augsburger M, Buclin T, Sanchez-Mazas P, Mangin P. Delta(9)-THC, 11-OH-Delta(9)-THC and Delta(9)-THCCOOH plasma or serum to whole blood concentrations distribution ratios in blood samples taken from living and dead people. Forensic Sci Int. 2001;123:159–164. doi: 10.1016/s0379-0738(01)00538-2. [DOI] [PubMed] [Google Scholar]
- 29.Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28:155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]