Abstract
It has been previously shown that unmyelinated afferent fibres in human skin are differentiated not only by their receptor characteristics, but also by their profiles of activity-dependent slowing. One type of profile, described originally as ‘type 3’, is different from that of nociceptors (type 1), cold afferents (type 2) and sympathetic efferents (type 4), in that these fibers display a minimal activity-dependent slowing (~1% at 2 Hz). However, their function remains to be determined. Here we describe one unit with a typical ‘type 3’ activity-dependent slowing profile recorded from an undamaged fascicle of the superficial peroneal nerve of a patient. Its conduction velocity was 1.8 m.s−1 and it slowed by 1.3% during the 2 Hz tetanus. This unit had a mechanical receptive field in the hairy skin and responded readily to weak mechanical stimuli, and not to cold. This suggests that the low threshold unmyelinated mechanoreceptors recently described in human hairy skin are probably endowed with a ‘type 3’ activity-dependent profile.
Keywords: unmyelinated fibres, microneurography, low threshold mechanoreceptors
Introduction
Raymond et al. [15] and Gee et al. [9] reported that unmyelinated afferents in cutaneous nerves in the rat display profiles of activity-dependent conduction slowing (ADS) which correlate with their sensory modalities. In the late 1990s, we further investigated ADS profiles of single unmyelinated fibres in human cutaneous nerves [18]. Each profile has correlated consistently with a discrete modality. Nociceptors display a progressive slowing during a 3-minute 2 Hz tetanus (>10% latency increase), were named ‘type 1’, and include polymodal (type 1A) and ‘silent’ nociceptors (type 1B) [17, 22]. Another profile, ‘type 2’, belongs to thermoreceptors, now named C2 units, that are responsive to innocuous cold, heat and menthol [3]. Axons with ‘type 2’ profile slow ~5% during a 3-min 2 Hz tetanus. A similar degree of slowing is also displayed by unmyelinated efferent fibres (‘type 4’) [5], which exhibit a very steep initial slowing, and respond to maneuvers that activate the sympathetic nervous system. Finally, another type of unmyelinated fibre, the ‘type 3’, displays minimal ADS (~1% during 2 Hz tetanus), but the functional modality of such units could not be identified [18].
Here we report on one unmyelinated fibre recorded from and unaffected fascicle of the superficial peroneal nerve of a patient. The unit displayed a ‘type 3’ profile of activity-dependent slowing and was characterized as a low threshold mechanoreceptor.
Methods
Case report
The study was approved by the Legacy Health System Institutional Review Board, and written informed consent was received. Recordings were obtained from the right superficial peroneal nerve of a 49-year-old woman with a painful fascicular mononeuropathy following acute trauma to the leg in 2006. There was persistent pain and mechanical hyperalgesia/allodynia in the dorsum of the fourth and fifth toes. There was no sensory loss and there were no motor signs. There was a Tinel sign projecting to the fifth toe. Standard motor and sensory nerve conduction study revealed no convincing deficits.
The fascicle sampled for microneurography projected to the dorsum of the foot on a different territory than the fascicle(s) affected by the traumatic injury.
Microneurography
Microneurography was performed using a standard method described in detail elsewhere [3]. In short, a 0.2 mm diameter lacquer-insulated tungsten microelectrode (MNG active/1MΩ FHC Inc., Bowdoinham, ME, USA), was inserted percutaneously into the nerve. A subcutaneous reference electrode was inserted into the skin 1–2 cm from the nerve trunk. Intraneural position of the recording electrode was ascertained by the recognition of the typical neural signals on the monitor and loud speaker and by the reported paresthesias projecting to a cutaneous receptive field (RF). Temperature of the skin was measured with a thermocouple placed on the skin adjacent to the RFs of the units under study and was maintained above 30°C using an infrared lamp.
The neural signals were amplified and filtered (band-pass 50–5000 Hz) with an isolated microelectrode amplifier (FHC Inc., Xcell 3+). Line interference was removed with an on-line noise eliminator (HumBug, Quest Scientific, North Vancouver, Canada). Signals were digitised at 20 kHz (PCI-6221 data acquisition board, National Instruments Corporation, Austin, Texas) and displayed and recorded by Qtrac software (written by H. Bostock, copyright University College London), which also controlled electrical stimulation.
RFs in the skin were stimulated electrically using a pair of needle electrodes resting on the surface of the skin, delivering 0.25 ms rectangular pulses (Grass S48, stimulus isolation unit SIU 5) at a rate of 0.25 Hz. Multiple units recorded at the same site could be recognized by the formation of horizontal dotted lines on a raster plot of latency against time.
Responses to natural stimulation were detected as deviations from the steady-state latency, reflecting activity-dependent conduction slowing caused by nerve impulse traffic during the continuous 0.25 Hz electrical stimulation. On average only 85% of the action potentials were recorded since Qtrac only allowed 850 ms sweeps to be recorded every second.
Results
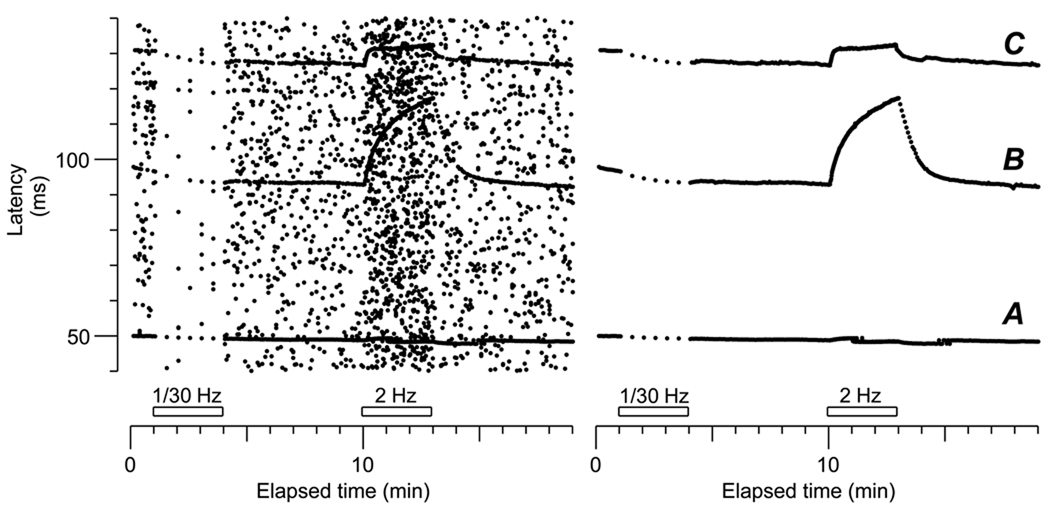
Figure 1 shows a 19-minute recording from the superficial peroneal nerve of the patient, plotted as a raster of latency measurements against time. Each dot represents a peak in the neurogram, which may be due to noise or an action potential. Action potentials excited by the electrical stimuli and conducting at constant velocity appear as horizontal lines. Three different units can be distinguished in the raster plot on the left, and by remeasuring the peaks at appropriate latencies, the noise was removed in the modified raster plot on the right. These 3 units all conducted within the C fibre range (< 2 m.s−1) and displayed very different ADS profiles during a 2 Hz tetanus lasting 3 minutes. The fibre labeled C displayed a total slowing of 4.9% with a fast initial ADS eventually reaching a plateau, a feature typical of sympathetic efferents (i.e. ‘type 4’ fibres; [5]). Fibre B had a more pronounced ADS of 26%, which was within the range of slowing of polymodal nociceptors (‘type 1’, [18]). Finally, fibre labeled A, with the highest signal-to-noise ratio, slowed by only 1.3% during the tetanus, and thus could be classified as ‘type 3’, according to previous observations [18].
Figure 1.
Recording from the right superficial peroneal nerve. Every dot displayed in the raster on the left represents one of the highest 60 peaks in every 850 ms sweep. Dots that maintain a stable latency in consecutive sweeps are single action potentials of a given fibre. The raster on the right has been cleaned from all the random noise leaving only stable responses. Three fibres are recognized by their stable latency, all conducting below 2 m.s−1 (conduction distance 90 mm). A corresponds to a fibre conducting at 1.8 m.s−1. This fibre displays a minor slowing during repetitive stimulation at 2 Hz, of 1.3%, thus classified as a type 3 (see Serra et al., 1999). B represents a fibre conducting at 0.96 m.s−1 and has the typical slowing profile of a polymodal nociceptor (27%). In C another unit with an initial fast slowing reaching a plateau, with a total slowing during the 3 min 2 Hz tetanus of 4.5% (conduction velocity 0.71 m.s−1). This profile corresponds to our type 4 (Campero et al., 2004), a sympathetic efferent, slightly different form type 2, not found in the current patient.
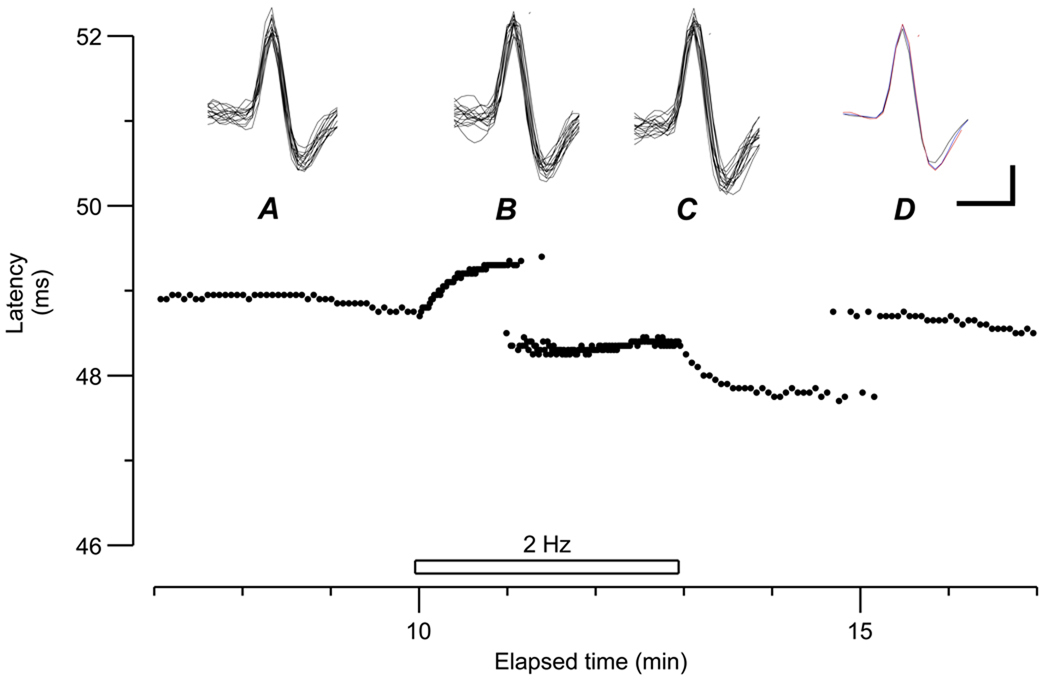
The pattern of ADS of the ‘type 3’ afferent is illustrated in more detail in Figure 2. This fibre had the fastest conduction velocity of 1.8 m.s−1 (baseline latency 49 ms; conduction distance 90 mm). Like the ‘type 4’ (fibre labeled C in Fig 1), and similar to the ‘type 2’ [6] this ‘type 3’ fibre displayed an initial slowing reaching a plateau at ~50 s after the start of the tetanus, but the degree of slowing was much less. The latency also underwent ‘jumps’ during the tetanus, initially fluctuating between two values 1 ms apart. After the end of the tetanus, the latency again jumped between two values before settling at its longer (baseline) value.
Figure 2.
Unit A from Fig. 1, displayed in an expanded latency scale, before, during and after the 2 Hz tetanus. Baseline stimulation rate 0.25 Hz. At the beginning of the tetanus there is a rapid increase in latency, reaching a plateau of slowing at 50 s. During the tetanus, there is an abrupt change in latency, jumping to a faster branch 2 ms shorter. Two min after the end of the tetanus, the latency jumped again to its original value. Insets show 16 superimposed traces between min 13 and 14 (A), 16 traces during the first part of the tetanus (15.6–15.9 min; B) and 16 superimposed traces during the late tetanus (17.6–17.9 min). In D, averages of the three previous waveforms are superimposed. Vertical bar 5 µV. Horizontal bar 0.5 ms
This fibre had a large signal-to-noise ratio which allowed unambiguous recording of the action potential. To demonstrate that the same fibre was recorded during the entire electrical stimulus period, the action potentials were superimposed at 3 different intervals: before the tetanus (A), during the first 30 s of the tetanus (before the latency jump, B) and at the end of the tetanus (within the shorter latency, C). In all instances, the action potentials were identical, as shown by the superimposed averaged responses in D.
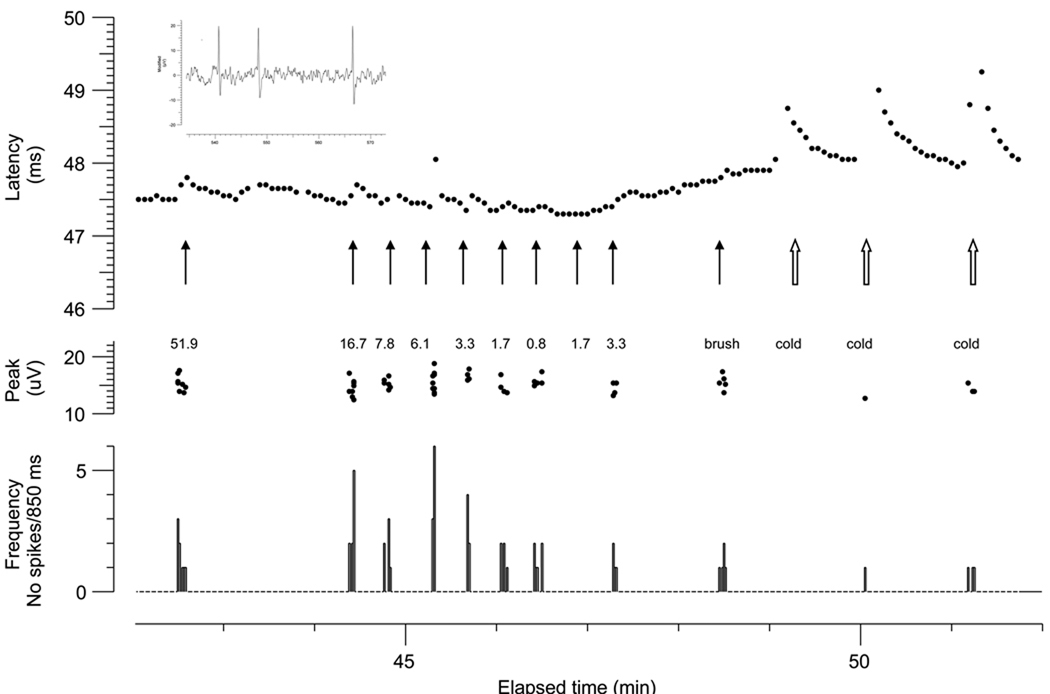
The receptive field of this unit was a single spot, ~1 mm diameter. Light mechanical stimuli applied to the RF under repetitive electrical stimulation excited this ‘type 3’ unit, as shown by the ADS illustrated in Figure 3. Mechanical stimulation with calibrated von Frey monofilaments excited the unit with a threshold of 0.3 mN. The number of action potentials evoked by stronger mechanical stimuli ranged from 1 to 6 per 850 ms sweep (i.e. maximal frequency of 6.1 imp.s−1). However, instantaneous frequencies of up to 250 imp.s−1 were reached during mechanical stimulation, as shown in the inset of Figure 3. The unit was also activated by light touch with a loose cotton thread.
Figure 3.
Same unit of Fig.2, excited electrically from the skin every 4 s (0.25 Hz). Each action potential is represented by a dot in the upper panel. The fluctuation in latency during the recording interval displayed is the consequence of ADS evoked by concomitant natural mechanical stimulation of its RF (black arrows). A larger latency change occurs when the cutaneous receptive field is cooled by a metal rod kept immersed in ice water. These changes in latency, however, are not related to ADS, since the cool metal rod barely activates the fibre mechanical or thermally, as shown by the peak dot displayed during the period between electrical stimulation (3 s) recorded on a different channel. In the lower panel the instantaneous frequency discharge registered in the non electrically stimulated channel is displayed. The inset (top left) shows the neurogram during the first mechanical stimulus (left filled arrow) recording 3 action potentials with interpotential intervals of 10 and 18 ms (i.e instantaneous frequencies of 250 and 111 imp.s−1, respectively).
Touching the RF with a metal rod pre-cooled by immersion in ice cold water caused slowing of conduction velocity. This was not due to an activity-dependent mechanism, but to cooling of the terminal segment of the axon. Touching with the cold metal rod evoked only few action potentials in some trials, the cooling effect being more prominent than the ADS. We did not attempt to activate this unit using heat or chemical stimuli, and we did not test for moving touch at different velocities.
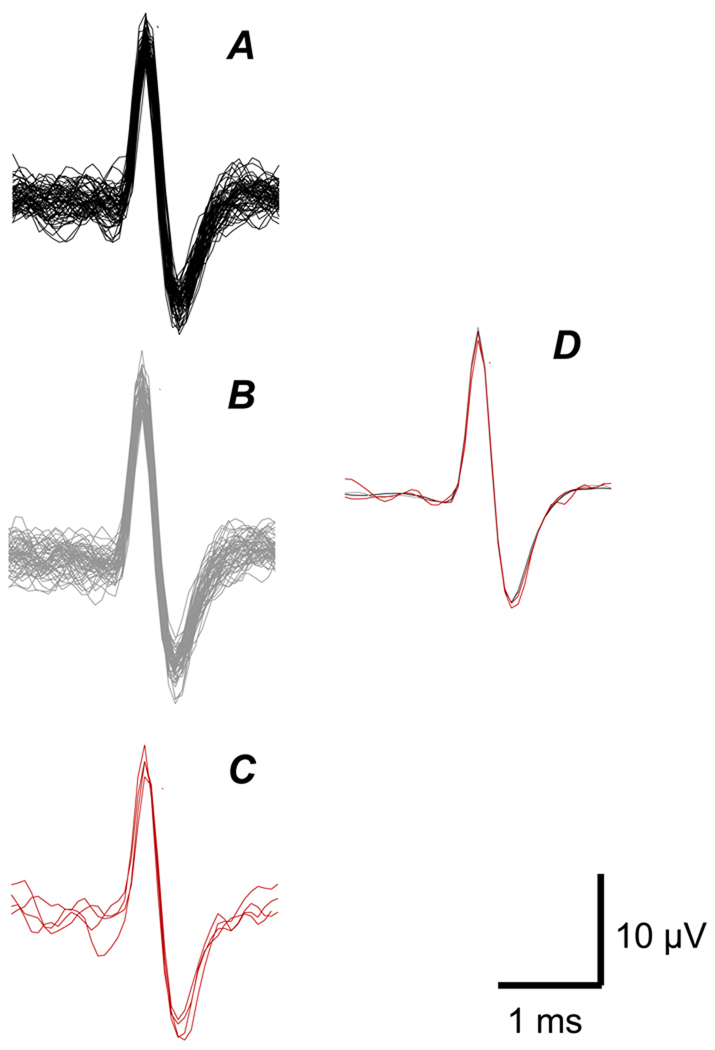
To demonstrate that the unit activated electrically was the same one that was activated mechanically by the cooled metal rod, 16 action potentials obtained from the channel that recorded the electrically-evoked response were superimposed and compared with a similar number of responses recorded on a different channel which registered 850 ms epochs without electrical stimulation (3 sweeps) (Figure 4). Likewise, all 4 action potentials evoked by the cold metal rod were also superimposed. The averages of each group of action potentials were superimposed and shown to have an almost identical shape (D).
Figure 4.
A. Superimposed action potentials recorded on channel triggered by the electrical stimulation of the cutaneous receptive field of the unit. B. Superimposed action potentials evoked by mechanical stimulation of the receptive field, recorded on a different channel within the intervals between two electrical stimuli at a frequency of 0.25 Hz. C. Superimposed action potentials (4) recorded on the non-electrically stimulated channel during mechanical stimulation with a metal rod immersed in ice cold water. D. Averaged responses from A, B and C, superimposed. Horizontal bar = 1 ms; vertical bar = 10 µV.
Discussion
The basis for the differences in profiles of ADS is uncertain: intra-axonal sodium accumulation [8], electrogenic hyperpolarization [19] and sodium channel inactivation [7] have been implicated in different C-fibre preparations. In humans, the ADS of unmyelinated fibres was first utilized in the ‘marking’ technique [20]. In this method, an axon being excited by regular electrical stimuli, displays an increase in latency when simultaneously activated by natural stimuli, thus being ‘marked’ by the natural stimulus. Beyond the 3 profiles of ADS described by Serra et al (17), Weidner et al. [22] have further differentiated polymodal nociceptors from silent nociceptors since the latter display a significant slowing at very low stimulation frequency. Other C fibres slowing near 5% and reaching a plateau before the end of the tetanus, are either sympathetic efferents (‘type 4’ fibres; [5]), or afferents responsive to innocuous cold, heat and menthol (‘type 2’; [3]). Another distinct slowing profile, ADS close to 1% during the 3-min 2 Hz tetanus (‘type 3’), is rather uncommon, and remains to be characterized in terms of functional modality. Serra et al. [18] speculated that these ‘type 3’ afferents might be low-threshold mechanoreceptors, by comparison with the behaviour of C fibres of this type in the rat [9], but direct evidence was lacking. Here we describe one single fibre with a ‘type 3’ profile of ADS from an intact fascicle from a patient with a partial traumatic neuropathy that responded to very light mechanical stimulation from a punctiform RF.
Low threshold mechanosensitive afferent C fibres have been discovered in human cutaneous nerves, both in the hairy skin of the forehead [12] and the forearm [21, 23]. These ‘tactile C fibres’ have small RFs and respond readily to light skin indentation. Their instantaneous discharge frequencies have been described to reach ~60 imp.s−1 in response to a finger tip tap [23], although mechanical thresholds are significantly lower. In the present unit, the number of action potentials evoked by mechanical stimulation reached 6 per 850 ms sweep (i.e. ~6.3 imp.s−1). However, instantaneous rates of up to 250 imp.s−1 were registered, which is closer to the firing rates achieved by low threshold mechanoreceptive units reported previously [23]. These high firing rates are more in line with the very small ADS displayed by the present type 3 unit during the 2 Hz tetanus.
Recently, Obreja et al. [13] described tactile C fibres in the pig. Those fibres slowed ~10% during the 2 Hz tetanus and had mechanical thresholds between ~1–10 mN, except for one fibre with a lower threshold. Tactile C fibres in humans have significantly lower mechanical threshold (0.04–2.25 mN [11]). Nevertheless, the pig tactile C fibres might be equivalent to tactile C fibres in humans.
The functional role of low threshold fibres is speculative: while Nordin et al. [12] suspected that these fibres may mediate a tickle sensation from light touch of the forehead, others [11, 23] have intuited a role associated in affective touch, or in pain modulation, without necessarily evoking a conscious percept. It has been recently suggested that C-touch fibers projecting to wide dynamic range neurons in lamina I in the dorsal horn of the rat might have a role in allodynia in neuropathic pains [1]. Given that tactile allodynia is a pain sensation in response to normally nonnoxious mechanical stimuli, there must exist low threshold afferents involved in its primary signaling, and the novel C low threshold tactile afferent would certainly be eligible. Indeed, in experimental animals, mechanical allodynia seems to be mediated, at least in part, by low threshold tactile C fibres [16].
Our study has obvious limitations. It reports a single unit and it was obtained from a patient with a fascicular nerve injury. The first concern is partially offset by the fact that the signal-to-noise ratio was large enough to allow unambiguous functional characterization, completely different from other units previously described. The second concern is relieved because the intraneural recording was obtained from a fascicle projecting to non symptomatic skin. Moreover, the original report of this type of fibre by Serra et al. [18] also included some diabetic patients with no neurophysiological evidence of polyneuropathy. It is unlikely that in both studies, the infrequent ‘type 3’ fibre might have been abnormal units, because this type of fibre has not been described through microneurography in patients with diabetic neuropathy [14] nor in patients with painful neuropathy [2, 4]. Furthermore, the ‘type 3’ profile has also been described in normal rats, but like in humans, it has not been functionally characterized [10]. It is noted that the earlier observations by Gee et al. [9] on low threshold mechanoreceptors in the rat are not strictly comparable, since they used 20 Hz rather than 2 Hz stimulus trains.
In summary, we describe a single cutaneous unmyelinated afferent with a ‘type 3’ profile of ADS, i.e. less than 2% slowing in a sequence of 0.25-2-0.25 Hz, having the receptive properties of the recently described low threshold mechanoreceptor units with C fibres in the human skin. This report should alert investigators to search for this type of afferent to corroborate or challenge the present observation.
Acknowledgments
this work has been partially supported by NIH Grant 5R01NS048932.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrew D. Quantitative characterization of low-threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J Physiol. 2010;588:117–124. doi: 10.1113/jphysiol.2009.181511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostock H, Campero M, Serra J, Ochoa JL. Temperature-dependent double spikes in C-nociceptors of neuropathic pain patients. Brain. 2005;128:2154–2163. doi: 10.1093/brain/awh552. [DOI] [PubMed] [Google Scholar]
- 3.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campero M, Bostock H, Baumann TK, Ochoa JL. A search for activation of C nociceptors by sympathetic fibers in complex regional pain syndrome. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol Scand. 2004;182:305–311. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 6.Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Col R, Messlinger K, Carr RW. Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol. 2008;586:1089–1103. doi: 10.1113/jphysiol.2007.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endres W, Grafe P, Bostock H, ten Bruggencate G. Changes in extracellular pH during electrical stimulation of isolated rat vagus nerve. Neurosci Lett. 1986;64:201–205. doi: 10.1016/0304-3940(86)90100-x. [DOI] [PubMed] [Google Scholar]
- 9.Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- 10.George A, Serra J, Navarro X, Bostock H. Velocity recovery cycles of single C fibres innervating rat skin. J Physiol. 2007;578:213–232. doi: 10.1113/jphysiol.2006.116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 12.Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obreja O, Ringkamp M, Namer B, Forsch E, Klusch A, Rukwied R, Petersen M, Schmelz M. Patterns of activity-dependent conduction velocity changes differentiate classes of unmyelinated mechano-insensitive afferents including cold nociceptors, in pig and in human. Pain. 148:59–69. doi: 10.1016/j.pain.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Ørstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jorum E, Torebjörk HE. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci. 2006;26:11287–11294. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond SA, Thalhammer JG, Popitz-Bergez F, Strichartz GR. Changes in axonal impulse conduction correlate with sensory modality in primary afferent fibers in the rat. Brain Res. 1990;526:318–321. doi: 10.1016/0006-8993(90)91239-d. [DOI] [PubMed] [Google Scholar]
- 16.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–2781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- 18.Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515(Pt 3):799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takigawa T, Alzheimer C, Quasthoff S, Grafe P. A special blocker reveals the presence and function of the hyperpolarization-activated cation current IH in peripheral mammalian nerve fibres. Neuroscience. 1998;82:631–634. doi: 10.1016/s0306-4522(97)00383-7. [DOI] [PubMed] [Google Scholar]
- 20.Torebjörk HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatry. 1974;37:653–664. doi: 10.1136/jnnp.37.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- 22.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessberg J, Olausson H, Fernstrom KW, Vallbo AB. Receptive field properties of unmyelinated tactile afferents in the human skin. J Neurophysiol. 2003;89:1567–1575. doi: 10.1152/jn.00256.2002. [DOI] [PubMed] [Google Scholar]