Abstract
Previous studies of CRH-induced status epilepticus in infant rats demonstrated neuronal loss in several limbic structures, including the CA3 region of the hippocampus. The goal of the present study was to identify the neurons affected by CRH-induced seizures and determine whether they formed synapses with afferent axon terminals. Clusters of neurons in the CA3 region of the hippocampus were osmiophilic when viewed in thick sections. Semi-thin 2-εm sections of the pyramidal cell layer contained dark, shrunken neurons with apical and basal dendrites among normal appearing pyramidal cells. Electron microscopy revealed degenerating pyramidal cells with intact cell membranes and electron dense nuclei and cytoplasm. The shrunken dendrites of these cells had spines and were postsynaptic to large immature-appearing mossy fibers. Thus, CA3 pyramidal neurons that are linked via mossy fibers to the tri-synaptic excitatory hippocampal circuit die subsequent to CRH-induced status epilepticus. The shrunken appearance and selective loss of these neurons are incompatible with necrosis as the mechanism of degeneration.
Keywords: Epilepsy, Infant, Rat, Hippocampus
1. Introduction
In the rat, seizures during the first 3 postnatal weeks are often more severe than in the adult [14]. However, such seizures do not result in neuronal death [26], unlike that found in adults. For example, limbic status epilepticus (SE) induced by systemic kainate or by perforant path stimulation in the adult, induces death of hippocampal neurons in the CA3 and CA1 regions of Ammon’s horn [8,16] and in the hilus of the dentate gyrus [22]. Several studies show that neurons of the infant rat are resistant to seizure-induced neuronal death following kainate [12,17,25].
We have developed a model of peptide induced SE using corticotropin releasing hormone (CRH). Synthetic CRH given into the cerebral ventricles (i.c.v.) of infant rats during the second postnatal week rapidly causes severe seizures. Seizures occur with doses 200 fold lower than in adults (7.5 × 10−l2 mol); they originate in the amygdala and spread to the hippocampus and neocortex [4,5]. Smith and Dudek [24], using in vitro techniques, have demonstrated increased excitatory effects of CRH in the immature hippocampus in comparison to the adult. Higher CRH doses (150 × 10−12 mol) result in status epilepticus lasting 4–6 h [3,5].
Unlike other convulsants that do not cause neuronal death at this age, CRH-induced SE results in selective death of neurons in the hippocampal CA3 region, lateral amygdala nucleus and piriform cortex [3]. Previous experiments also showed mossy fiber sprouting of the granule cells of the dentate gyrus into the inner molecular layer [3]. This effect was dose dependent, and far more robust after repetitive infusions of CRH [3,6]. However, using Gallyas-stained preparations, it was difficult to identify the type of degenerating neurons [3]. Furthermore, in that light microscopic study, it could not be determined whether synaptic connections were made with these neurons. This paper focuses on the identification of hippocampal neurons that are vulnerable to CRH-induced SE. Synaptic connections of these neurons were also investigated to determine whether they participate in a CRH-mediated excitatory limbic circuit.
2. Materials and methods
Ten- to 12-day-old rats were used in these experiments, and were offspring of time-pregnant, Sprague–Dawley rats. The infant rats were born in our federally-approved animal facility, kept on a 12-h light/dark cycle and given access to unlimited food and water. The time of birth of pups was determined every 12 h, and the day of birth was considered day 0. Litters were culled to 12 pups and mixed among experimental groups. Cages were maintained in a quiet, non-crowded room, and were undisturbed for 24 h prior to experiments.
Pups were implanted with cannulae 24 h prior to experiments, and their position was verified in all cases. Briefly, stainless steel cannulae were implanted into the lateral ventricles under halothane anesthesia, using an infant-rat stereotaxic apparatus [4,29]. Infant rats (n = 7) were injected with CRH i.c.v. two times a day on postnatal days 10 and 11. These infusions were made while the pups were freely moving in a heated Plexiglas chamber. CRH (750 × 10−12 mol in 1–2 µl per dose) was infused via the chronic cannula using a micro-infusion pump. Cannula-carrying control animals (n = 4) were given saline/dye vehicle [4]. Brain growth during the 4 days of these experiments should result in some (~ 0.12 mm) anterior-posterior shift of cannulae relative to bregma [21]. This has not been problematic because both lateral ventricles and amygdala extend for > 1 mm on each side of the cannula in the anterior-posterior axis.
Subsequent to CRH infusion, seizure latency and duration were monitored: animals were scored for behavioral limbic seizures occurring within 5-min epochs [4]. The concordance of limbic automatisms and epileptic discharges induced by CRH was established in previous studies [4,5]. CRH doses used in this study resulted in a seizure duration of 4–6 h (status-epilepticus) per infusion. Vehicle-infused controls and CRH-infused rats were returned to cages together, and feeding was verified. All infusions were carried out at the same times daily, to eliminate the effects of circadian variability in endogenous CRH.
Following a 16-h survival time after the last injection, rats were perfused with a combined aldehyde solution containing 4% paraformaldehyde and 1% glutaraldehyde in 0.12 M phosphate buffer (pH 7.4). After overnight fixation in situ, the brains were removed and postfixed for a week in a fixative containing 4% paraformaldehyde and 2.5% glutaraldehyde in 0.12 M phosphate buffer (pH 7.4). Brains were then serially sectioned in the coronal plane with a Vibratome at a thickness of 60 µm. These sections were stored in 24 well petri dishes at 4°C. Every fifth section through each brain was selected for flat-embedding in Medcasi. These sections were washed in 0.12 M phosphate buffer and postfixed in 1% osmium tetroxide for 30 min at 4°C The sections were then washed with buffer, dehydrated through graded ethanols, and impregnated with Medcast resin. Then, the sections were flat-embedded with Medcast between silicon-coated slides (Sigma Cote, Sigma Chemicals) and coverslips. The flat- embedded sections were examined with a light microscope to determine the presence of degenerating neurons in the hippocampus.
All sections were examined blindly. Briefly, brains were coded by one investigator and analyzed without the knowledge of the treatment group. Regions with degenerating neurons were selected from the plastic sheet and re-embedded on the flat surface of a Medcast-filled and cured Beem capsule for thin sectioning with an ultramicrotome and a diamond knife. Serial thin sections were placed onto Formvar-coated slot grids (1 × 2 mm slot) and then stained with uranyl acetate and lead citrate prior to examination with a Philips CM 10 transmission electron microscope (see [20]).
3. Results
Osmicated ovoid or fusiform neurons were observed in the CA3 hippocampal region, ipsilateral to the site of CRH infusion in thick sections derived from CRH-induced SE rat pups. Such osmiophilic neurons were not observed in sections obtained from control rats. The location of the dark neurons was within the pyramidal cell layer, mainly in the CA3b and CA3c subregions. No osmiophilic neurons were found in other layers of the hippocampus.
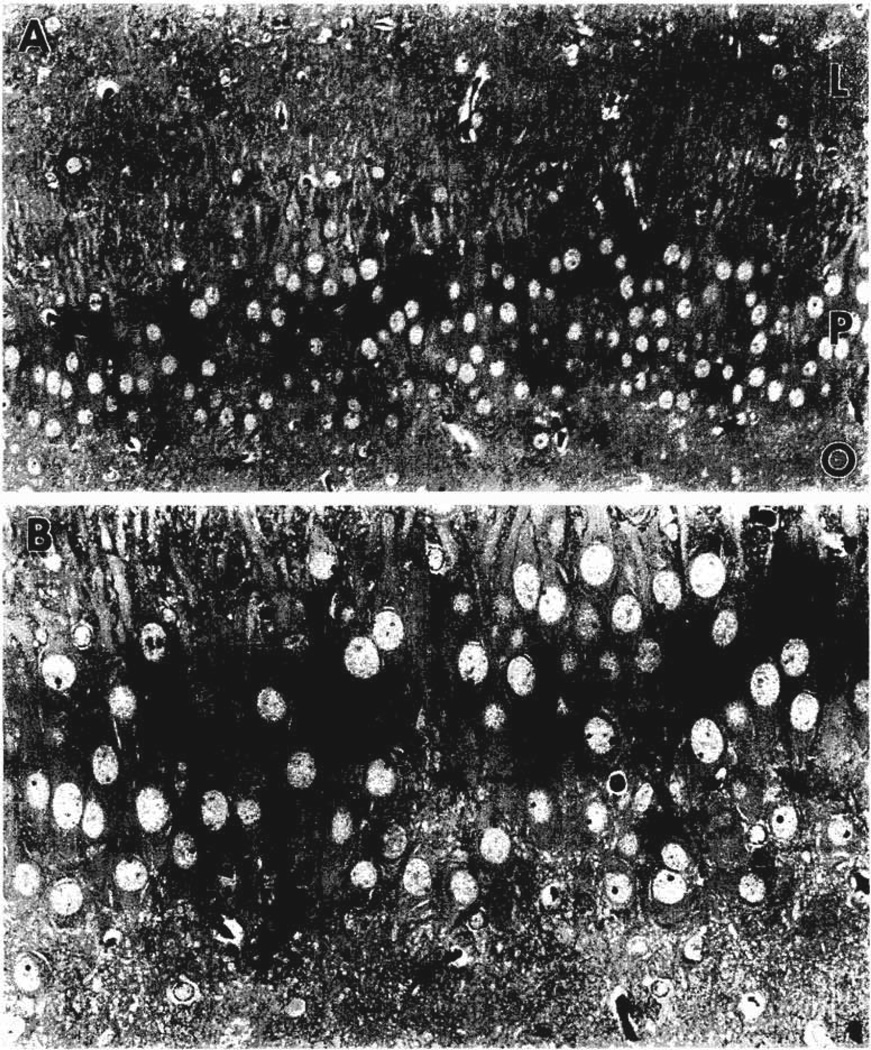
When regions containing these degenerating neurons were further sectioned into semi-thin, 2-µm sections, many degenerating neurons were found. The osmiophilic, dark neurons in the pyramidal cell layer of the CA3 region were interspersed among normal ‘plump-looking’ pyramidal cells and their dendrites (Fig. 1A and B). Some of the degenerating neurons had features characteristic of pyramidal cells in that they showed a single apical dendrite and a pair of basal dendrites (Fig. 1B).
Fig. 1.
Photomicrographs of a semi-thin 2-µm section of the hippocampus obtained from a 12-day-old rat following CRH-induced SE. A shows the CA3b region with several dark, shrunken neurons (large arrows) in the pyramidal cell layer (P). No degenerating neurons were observed in strata oriens (O) and lucidum (L). B shows an enlargement of the degenerating pyramidal cells (arrows) indicated in A. Note the normal looking adjacent pyramidal cells and the basal dendrites (arrowheads) that arise from some of the dark neurons. Magnifications: A = ×400, B = ×800.
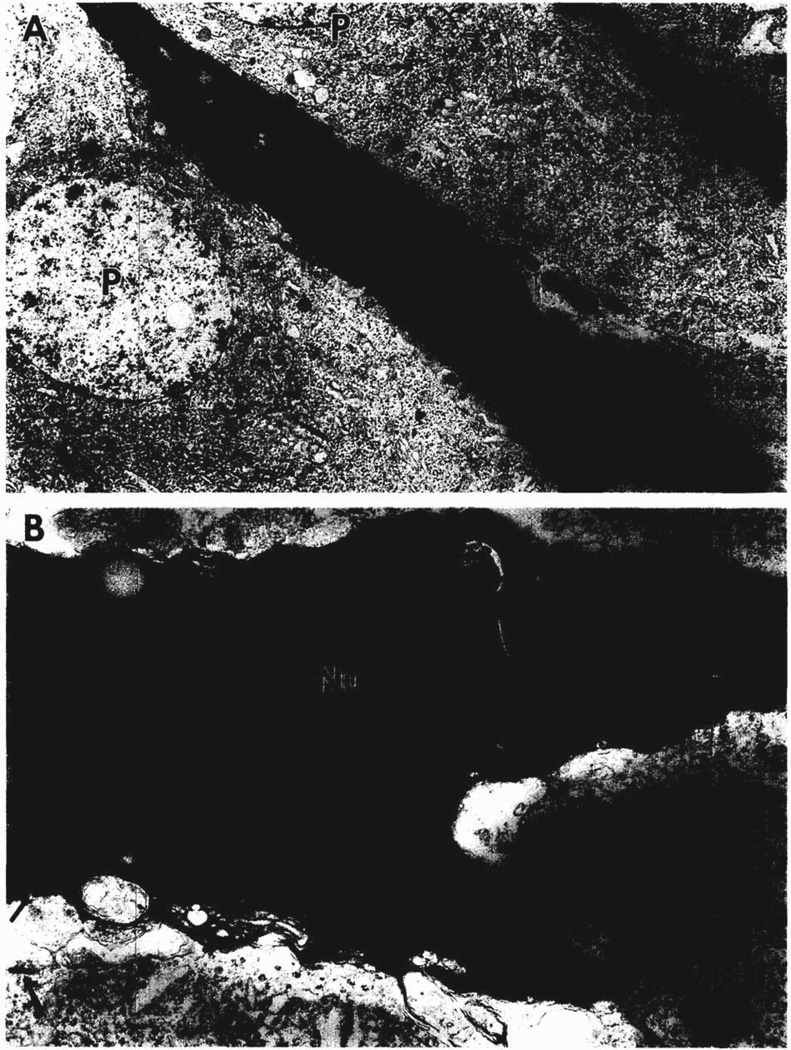
Electron microscopy was used to show the ultrastructural features of degenerating neurons in the pyramidal cell layer of the CA3 region. Shrunken, degenerating neurons had electron dense nuclei and nucleoli (Fig. 2A). In addition, the perikayal cytoplasm was electron dense, contained several cisternae of granular endoplasmic reticulum and showed many vacuolated bodies (Fig. 2B). Axosomatic symmetric synapses were present on the degenerating cell bodies as well as on adjacent normal pyramidal cells (Fig. 2B).
Fig. 2.
Electron micrographs of an electron dense, degenerating cell body of a neuron in the pyramidal cell layer of CA3b. This thin section was obtained from the same tissue block as Fig. 1. A shows the nucleus (N) of this neuron, its large apical dendrite oriented toward stratum lucidum, and two normal looking pyramidal cells (P). A portion of another degenerating neuron appears in the upper right-hand corner. B shows an enlargement of the nucleus in A with its prominent dark nucleolus (Nu) and dense heterochromatin. Granular endoplasmic reticulum (er) is evident in the electron dense cytoplasm of this neuron. Symmetric axosomatic synapses (large arrows) are found on adjacent pyramidal cells and on the degenerating cell body (small arrow). A = ×5000; B = 18,500.
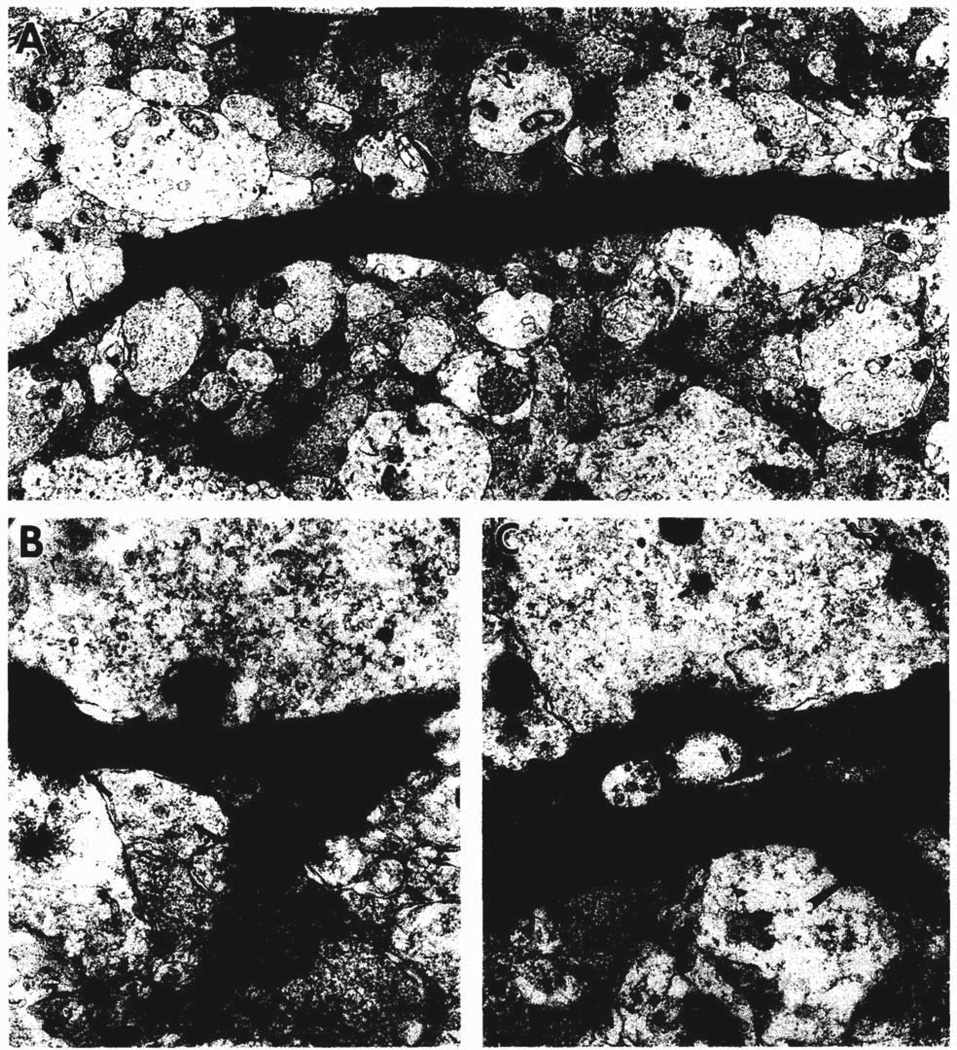
Fig. 3 focuses on the electron-dense dendrites of these degenerating cell bodies. In the stratum lucidum, the layer of mossy fiber termination, these dendrites had spines along their lengths (Fig. 3A). Some spines were stubby (Fig. 3B), whereas others were long and pedunculate (Fig. 3C). The axodendritic synapses on these dendrites were formed by large axon terminals with round synaptic vesicles and a few dense core vesicles (Fig. 3C). Axon terminals with this morphology were described by Amaral and Dent [2] as being immature mossy fiber terminals.
Fig. 3.
Electron micrographs of dendrites in stratum lucidum of CA3b that arise from degenerating neurons in the pyramidal cell layer. A shows two longitudinally-sectioned, degenerating dendrites (d and arrow) found in the neuropil that also contains many normal cross-sectioned dendrites and large axon terminals. B shows a stubby spine from the dendrite at the top of A. Two axon terminals with round synaptic vesicles form synapses (probably asymmetric, arrows) with the electron dense dendrite (left) and a stubby spine (right). C shows a pedunculate spine (large arrow) and an axodendritic synapse (small arrow) formed by a large axon terminal with round synaptic vesicles and a few dense core vesicles, similar in appearance to a developing mossy fiber axon terminal. A = ×10,000; B = ×37,000; C = ×28,000.
4. Discussion
The two main findings of this study are that (1) neurons degenerating after CRH-induced SE are pyramidal cells, and (2) their dendrites are postsynaptic to mossy fiber axon terminals. These results are discussed in the context of the methodology used and the relevance of this model to epilepsy.
In a previous study [3], we used the Gallyas stain for the light microscopic identification of degenerating neurons. It is similar to other silver stains in that the results are somewhat unpredictable: certain sections from one animal may be labeled adequately, but adjacent sections on the same slide may not (personal observations). Therefore, this method was not adequate to provide a consistent mapping of all degenerating neurons in a brain. In addition, since it is used exclusively at the light microscopic level, the Gallyas stain is limited in its ability to identify the type of degenerating neurons.
The method used in this analysis involves a routine procedure for flat-embedding 60 µm thick sections for electron microscopy. The use of osmium tetroxide provides a dense staining of the degenerating neurons similar to the appearance of the dark neurons observed with the Gallyas stain [3]. However, the current method permits surveying sections from the entire brain of a treated animal, and selecting sections that show degenerating neurons for ultrastructural analysis. In addition, these same electron microscopic preparations can be used to determine whether degenerating neurons possess mature synapses. Both of these aspects were used to identify and characterize the degenerating neurons in the hippocampus following CRH-induced SE.
The degenerating neurons were more electron dense than normal neurons (e.g. Fig. 2A). This was not considered to be an artifact of fixation because sections from control animals did not display such dark neurons and the brain was kept in situ for 12 h to prevent the appearance of dark neurons caused by handling of freshly perfused brains [10]. The dense labeling of the degenerating neurons did not prevent the identification of their synapses. However, the type of synapse was more difficult to identify within the electron dense cytoplasm of these neurons (see Fig. 3B,C). To distinguish asymmetric and symmetric synapses, we examined the synaptic vesicles in the presynaptic axon terminal. Round synaptic vesicles are associated with asymmetric synapses, whereas pleomorphic synaptic vesicles are found in axon terminals forming symmetric synapses [18].
Our data indicate that the degenerating neurons in the hippocampal CA3 region are pyramidal cells. This conclusion is based on both light and electron microscopic observations. Pyramidal cells have their cell bodies in the pyramidal cell layer and possess apical and basal dendrites with spines [7]. Virtually all of the degenerating neurons in these preparations were located in the pyramidal cell layer and possessed dendritic spines. These features are characteristic for pyramidal cells in CA3, but not for interneurons [13].
Further evidence that the degenerating neurons are pyramidal cells is derived from the distribution of synapses of these cells. Symmetric axosomatic synapses were found on the cell bodies (Fig. 2B), as is commonly observed for pyramidal cells [13]. Similarly, asymmetric synapses occurred on the dendritic spines and shafts of dendrites of these neurons in stratum lucidum as described previously for developing neurons by Amaral and Dent [2]. Thus, both the synapse distribution and type indicate that these neurons are CA3 pyramidal cells. The presence of asymmetric synapses formed by developing mossy fibers suggests that these degenerating pyramidal neurons in CA3 participate in the excitatory tri-synaptic circuit of the hippocampus [15].
We found a 16-h survival time optimal for the visualization of degenerating neurons. Longer survival periods may result in the cells undergoing active destruction [19,23]. The amount of neuronal loss is difficult to determine in these preparations. As a conservative estimate, 7–10% of CA3 neurons degenerate. This finding may explain why adults treated with CRH as infants did not show any significant decrease in neuronal density in CA3 as determined by counting neuronal cell bodies in Nissl preparations of 10-µm sections (unpublished observations). The location of degenerating neurons within CA3 in this study approximates the location that was reported for degenerating neurons in temporal lobe epilepsy [27].
The mechanism for the degeneration of these pyramidal cells is unlikely to be necrosis. The most important property of the death of these neurons is selectivity. The condensation of chromatin, the enhanced electron density of the cytoplasm and the shrinking of the somata and dendrites of these degenerating neurons are morphological criteria suggestive for apoptosis [23].
The age-related vulnerability of limbic neurons to seizure-induced death is clinically important. In the human, environmentally-induced (e.g. febrile) seizures are quite common, affecting 3–5% of infants and young children [1]. Whether these seizures lead to neuronal death is controversial: prospective studies of children with febrile seizures have failed to reveal a progression to limbic (temporal lobe) epilepsy with its associated hippocampal cell loss [9]. Conversely, in populations of adults with temporal lobe epilepsy and evidence of hippocampal neuronal death and synaptic reorganization (mesial temporal sclerosis), there is a high prevalence (30–50%) of prolonged febrile seizures in childhood [11]. These data are somewhat contradictory [28]. The present study shows that a neuropeptide, CRH, that is enhanced during stress causes status epilepticus and neuronal degeneration in the infant. The data therefore offer a potential mechanism for neuronal degeneration in certain cases of status epilepticus in human infants.
Acknowledgements
The authors are grateful for the excellent technical assistance of Yashoda Jhurani and Linda Schultz. This research was supported by NIH Grants NS 15669 and NS 28912.
References
- 1.Aicardi J. Epilepsy in Children. New York: Raven Press; 1986. [Google Scholar]
- 2.Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J. Comp. Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 3.Baram TZ, Ribak CE. Peplide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev. Brain Res. 1993;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baram TZ, Hirsch E, Snead OC, III, Schultz L. CRH-induced seizures in the infant brain originate in the amygdala. Ann. Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baram TZ, Sarco DP, Schultz L, Jhurani Y, Ribak CE. Mossy fiber sprouting and neuronal death induction by repetitive corticotropin releasing hormone (CRH)-status epilepticus in the infant rat. Epilepsia. 1994;35(Suppl. 8):136. [Google Scholar]
- 7.Bayer SA. Hippocampal region. In: Paxinos G, editor. The Rat Nervous System. Sydney: Academic Press; 1985. pp. 335–352. [Google Scholar]
- 8.Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 9.Berg AT, Shinnar S, Hauser WA, Alemany M, Shapiro ED, Salomon ME, Crain EF. A prospective study of recurrent febrile seizures. N. Engl. J. Med. 1992;327:1122–1127. doi: 10.1056/NEJM199210153271603. [DOI] [PubMed] [Google Scholar]
- 10.Cammermeyer J. An evaluation of the significance of the ‘dark neuron,’. Ergeb. Anat. Entwickl- Gesch. 1962;36:1–61. [PubMed] [Google Scholar]
- 11.Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-Cendes I. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial temporal structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 12.Chang D, Baram TZ. Status epilepticus results in reversible neuronal injury in infant rat hippocampus: novel use of a marker. Dev. Brain Res. 1994;77:133–136. doi: 10.1016/0165-3806(94)90220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frotscher M. Mossy fiber synapses on glutamate decarboxylase-immunoreactive neurons: evidence for feed-forward inhibition in the CA3 region of the hippocampus. Exp. Brain Res. 1989;75:441–445. doi: 10.1007/BF00247950. [DOI] [PubMed] [Google Scholar]
- 14.Haas KZ, Sperber EF, Moshé SL. Kindling in developing animals: expression of severe seizures and enhanced development of bilateral foci. Dev. Brain Res. 1990;56:275–280. doi: 10.1016/0165-3806(90)90093-e. [DOI] [PubMed] [Google Scholar]
- 15.Lothman EW. Seizure circuits in the hippocampus and associated structures. Hippocampus. 1994;4:286–290. doi: 10.1002/hipo.450040311. [DOI] [PubMed] [Google Scholar]
- 16.Nadler JV. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- 17.Nitecka L, Tremblay E, Charton G, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. Neuro-science. 1984;13:1073–1084. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- 18.Peters A, Palay SL, Webster H, De F. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. New York: Oxford University Press; 1991. [Google Scholar]
- 19.Pollard H, Charriaut C, Cantgrel S, Represa A, Robain O, Moreau J, Ben-Ari Y. Kainate induced apoplotic cell death in hippocampal neurons. Neuroscience. 1994;63:7–18. doi: 10.1016/0306-4522(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 20.Ribak CE, Navetta MS. An immature mossy fiber innervation of hilar neurons may explain their resistance to kainate-induced cell death in 15-day-old rats. Dev. Brain Res. 1994;79:47–62. doi: 10.1016/0165-3806(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley: Univ. Calif. Press; 1970. [Google Scholar]
- 22.Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci. Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- 23.Sloviter RS, Dean E, Neubort S. Electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat: apoptosis in the adult central nervous system. J. Comp. Neural. 1993;330:337–351. doi: 10.1002/cne.903300305. [DOI] [PubMed] [Google Scholar]
- 24.Smith BN, Dudek FE. Age-related cpileptogenic effects of corticotropin releasing hormone in the isolated CA1 region of rat hippocampal slices. J. Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- 25.Sperber EF, Haas KZ, Sianlon PK, Moshé SL. Resistance of the immature hippocampus to seizure induced synaptic reorganization. Dev. Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- 26.Sperber EF, Stanton PK, Haas K, Ackcrman RK, Moshé SL. Developmental differences in the neurobiology of epileptic brain damage. Epilepsy Res. Suppl. 1992;10:67–81. [PubMed] [Google Scholar]
- 27.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 28.Swann JW, Meldrum B, Moshé SL, Shinnar S, Wasterlain C. Do seizures in early life produce brain damage? Epilepsia. 1991;32(Suppl. 3):1. [Google Scholar]
- 29.Yi SJ, Baram TZ. Methods for implanting steroid-containing Cannulae into the paraventricular nucleus of neonatal rats. J. Pharmacol Taxicol. Method. 1993;3(1):97–102. doi: 10.1016/1056-8719(93)90012-4. [DOI] [PubMed] [Google Scholar]