Abstract
Proper regulation of immune homeostasis is necessary to limit inflammation and prevent autoimmune and chronic inflammatory diseases. Many autoimmune diseases, such as psoriasis, are driven by vicious cycles of activated T cells that are unable to be suppressed by regulatory T cells. Effective suppression of auto-reactive T cells by regulatory T cells (Treg) is critical for the prevention of spontaneous autoimmune disease. Psoriatic Treg cells have been observed to a defect in their capacity to regulate, which clearly contributes to psoriasis pathogenesis. A challenge for translational research is the development of novel therapeutic interventions for autoimmune diseases that will result in durable remissions. Understanding the mechanism(s) of dysregulated T cell responses in autoimmune disease will allow for the development of future therapeutic strategies that may be employed to specifically target pathogenic, proinflammatory cells. Several reports have demonstrated a pathogenic role for Th1 and Th17 cells in psoriasis as well as other autoimmune diseases. Similarly, several laboratories have independently demonstrated functional defects in regulatory T cells isolated from patients with numerous divergent autoimmune diseases. One primary challenge of research in autoimmune diseases is therefore to restore the balance between chronic T cell activation and impairment of Treg suppressor mechanisms. To this end, it is critical to develop an understanding of the many suppressive mechanisms employed by Treg cells in hopes of developing more targeted therapeutic strategies for Treg-mediated autoimmune diseases.
Keywords: Regulatory T cell, cytokine signaling, antigen presenting cell, autoimmunity
I. INTRODUCTION
Regulatory T cells (Treg) are a subset of CD4+ lymphocytes that were originally termed “suppressor cells” when they were first described in the 1970s.1 The concept of suppressor T cells re-emerged two decades later when a distinct phenotype of regulatory T cells (Treg) was identified.2,3 CD4+ cells that constitutively express the IL-2 receptor (IL-2R) a-chain (CD25) have been identified as Treg cells in mice.4–6 In human peripheral blood, approximately 5% of the CD4+ T-cell population consists of naturally occurring regulatory T cells (nTreg) cells, which are characterized by their constitutively high expression of CD25, diminished expression of the IL-7Ra chain,7,8 and expression of the forkhead/winged helix transcription factor Foxp3.9
The functional hallmark of CD4+CD25+ Treg cells is their remarkable capacity to suppress T effector/memory (Teff/mem) cell activation both in vitro and in vivo. Recent reviews have updated the idea that Tregs can regulate self-reactive T cells to maintain peripheral tolerance (non-autoimmune recognition of organ antigens).10,11 Impaired capacity or removal of Tregs allows unrestrained proliferative responses of pathogenic, autoreactive T cells. Adoptive transfer of Treg cells reduces the pathology of experimentally induced autoimmune diseases such as gastritis, insulin-dependent diabetes mellitus, and colitis,12–14 whereas depletion of CD4+CD25+ Treg cells results in the development of systemic autoimmune diseases.3,12,15 Both the suppressive capacity16 as well as the frequency of Treg cells17 are diminished in patients with autoimmune diseases such as psoriasis and pemphigus vulgaris.
Foxp3 is considered to be a “master regulator” of the Treg lineage, and mutations or absence of the gene lead to a fatal, autoimmune lymphoproliferative disease in both mice and humans.18 The activation of Foxp3 itself is mediated by both acetylation and phosphorylation events,19,20 and the protein acts in complex with other transcription factors, including the nuclear factor of activated T cells (NFAT), to control gene expression.21 The interaction of Foxp3 with other transcription factors, such as NFAT, serves to sequester these factors and thereby down-modulate expression of genes involved in T-cell activation and effector functions.21 Although Foxp3 is a unique marker of murine Treg cells, its expression in human CD4+ T cells is not restricted to Treg cells.18 The identification of proteins uniquely expressed in human Tregs remains a major challenge in the field, although recent reports have shown that the transcription factor Helios, a member of the Ikaros family,22,23 may be a specific marker of human Treg cells.
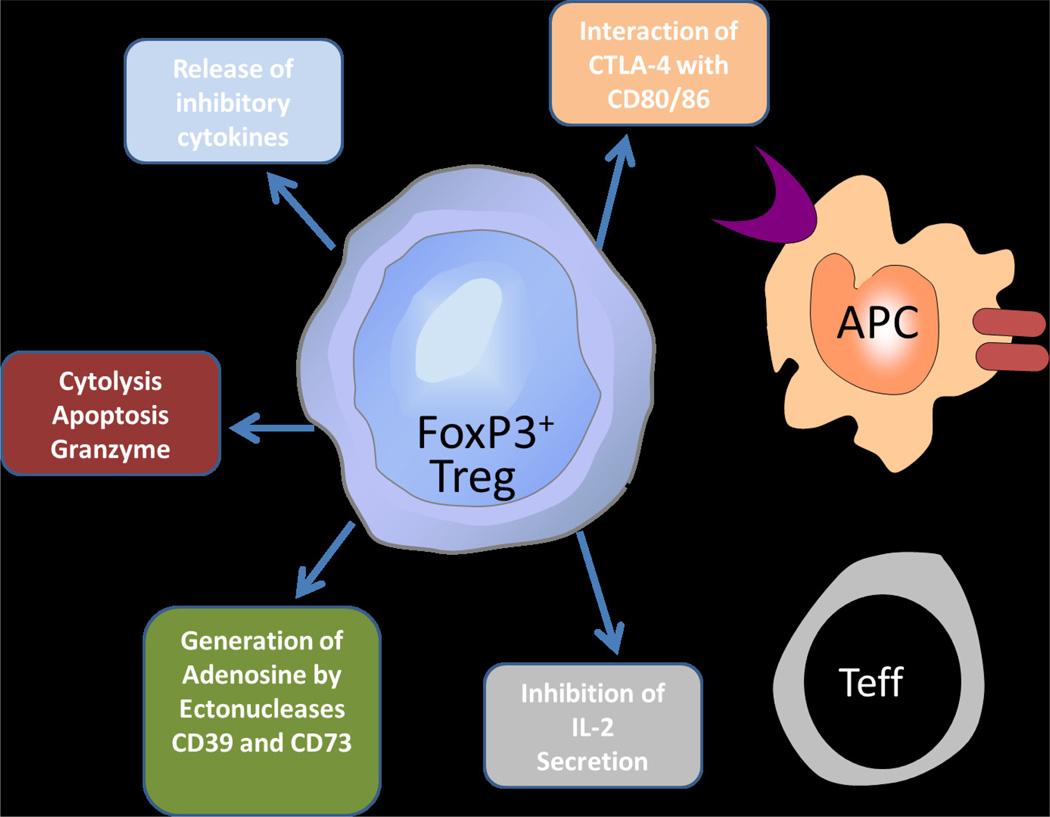
The identification of Foxp3+ Treg cell in human peripheral blood has re-energized the field to concentrate on the potential mechanism(s) of action of this class of cell.24–28 Indeed, regulatory T cells employ a variety of effector mechanisms to suppress immune responses,29–31 through both contact dependent mechanisms as well as the secretion of soluble factors. Several specific mechanisms have been described, including the inhibition of IL-2 secretion; release of inhibitory cytokines; perforin- or granzyme- dependent cytolysis of APCs or responder T cells; synthesis of immunosuppressive adenosine; and down-regulation of APC function via co-stimulation with cytotoxic T-lymphocyte antigen 4 (CTLA-4, Figure 1). This review discusses current knowledge regarding suppressive functions of human Treg cells and how these mechanisms work in concert to maintain immune tolerance.
Figure 1.
Human regulatory T cells use several diverse mechanisms to maintain immune tolerance. Shown in the figure are functional mechanisms used by human Treg cells to maintain suppression of target cells. Mechanisms include the release of secreted cytokines, such as TGFβ, IL-10 and IL-35; cytolysis of target cells; generation of immunosuppressive adenosine; and effects on dendritic cell co-stimulation.
II. INHIBITION OF IL-2 SECRETION
Of the multitude of suppressive mechanisms employed by human Treg cells, one of the better characterized is these cells’ inhibition of transcription of interleukin 2 (IL-2) within CD4+ Teff cells.5,32,33 When activated through the T cell receptor (TCR), CD4+ Teff cells rapidly synthesize IL-2 mRNA as well as other pro-inflammatory mRNAs including IFNγ, TNFα, and IL-6. IL-2 serves critical functions in the survival and activation of both Foxp3-Teff cells, as well as Foxp3+ Treg cells. By limiting Teff cells’ induction of IL-2 mRNA, as well as their transcription of other proinflammatory genes, Treg cells limit the activation of Teff cells in the periphery as well as their survival.
Although it was initially thought that the major function of IL-2 was to provide survival and activation signals for peripheral CD4+ T cells, subsequent studies using IL-2- and IL-2R-knockout (KO) mice showed that IL-2 is critical for maintaining self-tolerance. Both IL-2-KO mice34,35 and IL-2R-KO mice36,37 develop systemic and lethal autoimmune disease. Given the central role of IL-2 in Treg cell function, it has been proposed that another IL-2-mediated mechanism of suppression may rely on Treg cells “competing” for IL-2, and thereby limiting the availability of the cytokine for Teff cells.38 Although this mechanism is likely not relevant for in vitro suppression assays, the competition for IL-2 between Treg and Teff cells may indeed play out in vivo. Whether or not Treg cells are able to act as efficient competitors for IL-2 may depend on their relative expression levels of IL-2 receptor chains. The high-affinity IL-2 receptor complex is comprised of three subunits: CD25, the IL-2Rα chain, as well as CD122 and CD132. Although Tregs are characterized by their constitutively high expression of CD25, their expression of CD122 and CD132 has not been characterized nor compared to the expression levels of these molecules on the surface of Teff cells.
III. RELEASE OF INHIBITORY CYTOKINES
Although potent Treg suppressive function relies on physical contact between Treg and Teff cells, third-party suppression can also be achieved through the release of soluble mediators;39 the suppressive function as well as peripheral homeostasis of Treg cells is now understood to be regulated by inhibitory cytokines including TGFβ, IL-10, and the newly described IL-12 family member, IL-35.40 The importance of these molecules to Treg cell suppression demonstrates that optimal Treg suppression is achieved through not only cell–cell contact but also through the actions of Treg-associated cytokines.41,42
Two major modes of action exist for inhibitory cytokines to promote Treg suppressive functions. First, these cytokines can directly inhibit activation and/or survival of Teff cells themselves, thereby dampening autoreactive Teff cell activation in the setting of autoimmunity. Secondly, soluble cytokines can also work to generate inducible Treg cells (iTreg) and contribute to the peripheral homeostasis and survival of these cells. TGFβ has a particularly strong association with the induction of iTreg cells (termed Th3) from naïve precursors in the periphery, as well as the maintenance of nTreg homeostasis.43,44
The importance of TGFβ and IL-10 to optimal Treg suppressive function has been clearly established in mouse models lacking these cytokines. TGFβ-deficient mice develop profound autoimmune disease at 4 to 5 weeks of age45,46 and display reduced numbers of CD4+CD25+ Treg cells.47 Further, inhibition of TGFβ by neutralizing antibodies attenuates both mouse and human Treg suppressive function in vivo.48, 49 Despite the established role of TGFβ in suppressing Teff cell activation, it is unclear whether its immunosuppressive effects are due to direct effects on Teff cells, or whether TGFβ functions primarily to maintain homeostasis within the peripheral Treg pool and induce the differentiation of iTreg cells from naïve precursors. Regardless of its primary function, it appears that TGFβ does not need to be produced by iTreg cells themselves in order to mediate suppression. Recent work showed that Treg-derived TGFβ was not required for protection against a mouse model of IBD,48,50 raising the possibility that cellular sources other than iTreg cells can produce sufficient TGFβ to protect against autoimmune colitis. It is therefore possible that TGFβ derived from other cellular sources may provide anti-inflammatory effects in vivo and may contribute to iTreg differentiation and homeostasis, without a direct immunosuppressive role.
TGFβ is also expressed as a transmembrane protein by murine51 and human52 Treg cells, and this membrane-bound cytokine is important for optimal Treg suppression. The membranous expression of TGFβ is coordinated by activation status of the Treg cell; upon activation, the Treg selectively upregulates expression of glycoprotein A receptor predominant (GARP) or leucine-rich repeat-cntaining (LRRC32), a member of the leucine-rich repeat family of proteins.53,54 GARP functions to tether latent TGFb, in complex with the latency-associated peptide (LAP), to the Treg cell membrane. A cleaved signal peptide site in LRRC32 is necessary for surface localization of native LRRC32 following activation of naturally occurring, freshly isolated regulatory T cells.55 Highlighting the importance of membrane-bound TGFb to Treg suppressive function, recent studies have shown that down-regulation of GARP expression on Treg cells leads to impairment of Treg function.53,54 Transmembrane TGFb is thought to be important not only for Treg-mediated suppression but also for infectious tolerance mechanisms; for example, transmembrane TGFb is required for the suppression of NK cell activation in vivo.52
Mechanistically, membrane-bound TGFβ may mediate its suppressive functions through interactions with NOTCH-expressing Teff cells. Membrane TGFβ has been shown to be necessary for the expression of NOTCH1 ligands on Treg cells;56 these Tregs can then interact with NOTCH-expressing Teff cells, leading to the initiation of intracellular signaling events. One such event is the expression of hairy and enhancer of split 1 (HES1), a repressive transcription factor. Activation of the NOTCH1-HES1 axis contributes to dampening of Teff activation and represents an important function of membrane-bound TGFβ56
The inhibitory role of IL-10 is also supported by numerous studies. Importantly, although IL-10 is not required for in vitro Treg suppression, mice lacking IL-10 are highly susceptible to experimental autoimmune disease, particularly colitis;57,58 this is in accordance with the known role for IL-10 in maintaining intestinal homeostasis. Neutralization of IL-10 in an adoptive transfer model results in rejection of allogeneic skin grafts,59 demonstrating the requirement for IL-10 in transplant immunity. In addition, local secretion of IL-10 is required for protection from experimental colitis and experimental allergic encephalomyelitis (EAE).60,61 Similarly to the Treg-inductive effects of TGFβ, IL-10 stimulation of naïve T cell precursors can also lead to the differentiation of iTreg cells (termed Tr1).
Although IL-10-expressing Treg cells do not express Foxp3, their ability to suppress Teff activation is comparable to that of nTreg cells,62 and they are thought to be critical for oral tolerance. Mechanistically, IL-10 is thought to exert its inhibitory effects via modulation of dendritic cell function. Tregs can induce the expression of immunosuppressive B7-H4 on the surface of DCs via IL-10 signaling.63
For many years, IL-10 and TGFβ occupied a unique niche as inhibitory, Treg-associated cytokines. The possibility that there may be many more such molecules was raised by the recent discovery of IL-35, an IL-12 family member which is also inhibitory and uniquely associated with Treg cells.40 A product of Foxp3+ Treg cells, IL-35 is a heterodimeric cytokine comprised of the IL-12a chain (p35) and EIB3, which is shared with IL-27. Treg cells deficient in either cytokine subunit are unable to control IBD and homeostatic T cell expansion,40 suggesting that IL-35 is necessary for optimal Treg suppressive function in vivo. Recent exciting work has shown that IL-35 is selectively expressed by activated human Treg cells and that contact-independent Treg function is dependent on IL-35.64 Most interestingly, this study goes on to show that suppression via IL-35 results in conversion of the suppressed Teff cell to an IL-35-expressing iTreg cell, termed iTr35.64 Thus, IL-35 plays an important role in human Treg function, not only at the level of Teff cell suppression but also in the context of infectious tolerance.
In addition to these Treg-associated cytokines, the galectin-1 protein is also selectively expressed by Treg cells and likely contributes to their suppressive functions in vivo. Galectin-1 is a highly conserved member of the B-galactoside binding protein family, and is secreted by Treg cells as a homodimer which can bind to glycoproteins including CD45, CD43 and CD7.65 Galectin-1 binding to receptors on APCs results in cell cycle arrest, apoptosis, and inhibition of proinflammatory cytokine release. Blocking Treg expression of Galectin-2 results in suboptimal suppressive capacity, further suggesting that this molecule contributes to functional suppression.65
IV. CYTOLYSIS/APOPTOSIS
Cytolysis of target cells via the perforin/granzyme pathway is an important way in which natural killer (NK) cells and CD8+ T cells kill virally infected cells and tumor cells. Inducible human Treg cells (iTreg) have been shown to express granzyme B and can mediate similar cytolysis of target cells in a perforin-dependent manner.66 Interestingly, the same study showed that Tr1-mediated cytolysis was independent of TCR activation or recognition of MHC on antigen presenting cells (APCs); further, the expression of granzymes was found to be markedly different in various lymphocyte subsets.66 More recently, human nTreg cells were shown to express granzyme A, but not granzyme B, upon TCR activation.67 Both nTreg and iTreg cells are therefore capable of killing autologous target cells, such as CD4+ and CD8+ T cells, monocytes, and DCs, in a perforin-dependent manner.67 In the case of nTreg-mediated cytolysis, adhesive interactions between the Treg and target cell are required and depend on CD18 expression, but not Fas/FasL.67 Similar mechanisms of granzyme-dependent cytolysis has been shown in murine Treg cells, which express higher levels of granzyme B upon activation and kill target cells in a perforin-independent, granzyme B-dependent manner.68
The significance of granzyme-dependent cytolysis mediated by Treg cells is demonstrated by studies examining Treg cells from mice lacking granzyme B. Granzyme B-KO Tregs displayed reduced suppressor functions,68 in contrast with suppression-competent Treg cells from perforin-KO animals. Although cytolysis mediated by Treg cells likely represents an important suppressor function, murine Treg suppression was recently shown to be independent of programmed-death pathways, such as apoptosis.69
V. GENERATION OF ADENOSINE
Several novel mechanisms of suppression have emerged in the last few years, one of which is the ability of Treg cells to synthesize immunosuppressive adenosine.70 Adenosine serves as a mechanism for cellular cross talk, as the engagement of adenosine receptors on Teff cells and APCs results in an inhibition of inflammatory cytokine gene expression in these target cells. Reduced cytokine secretion thereby mediates anti-inflammatory effects, contributing to the resolution of the inflammatory response.
Although adenosine can be generated via multiple biochemical pathways,71 synthesis of extracellular adenosine from ATP and ADP is mediated by ecto-nucleases CD39 (ecto-NTPDase-1) and CD73 (ecto-5’-nucleotidase), expressed on the surface of Treg cells.72,73 Although CD73 is also expressed by other subsets of CD4+ T cells, the co-expression of both CD39 and CD73 on Tregs results in optimal synthesis of adenosine. CD39 expression positively correlates with Foxp3 expression in murine Tregs,73,74 and these cells have been demonstrated to actively synthesize adenosine from ATP.72,73 In addition to their expression on murine Treg cells, CD39 and CD73 have been demonstrated to co-localize on human tissue-derived Treg cells.73,75,76
Adenosine signals to at least four distinct receptor subtypes, termed A1, A2A, A2B, and A3.71 CD4+ T cells and APCs predominantly express the A2A receptor, which is strongly upregulated in response to inflammatory stimuli such as TCR or TLR4 activation.77–82 Studies demonstrating the functional significance of signaling to the A2A receptor show that Treg cells from A2A−/− mice are unable to suppress inflammation in an adoptive transfer model of colitis, and pathogenic Teff cells from A2A−/− mice are resistant to suppression by wild-type Treg cells.83 Furthermore, inhibitors of ectonuclease activity and antagonists of A2A signaling were shown to block Treg suppression of human Teff cells in in vitro suppression assays.84 Primary Tregs from multiple sclerosis patients were shown to express reduced levels of CD39, correlating with impaired suppressive function.85 Collectively, these findings suggest that the optimal suppressive function of Treg cells depends on the ability to convert ATP and ADP to adenosine, and that this adenosine signals to A2A receptors to mediate anti-inflammatory effects.
The mechanisms by which adenosine mediates its inhibitory effects have recently begun to be elucidated. Adenosine signaling to the A2A receptor on Teff cells and APCs leads to decreased production of proinflammatory cytokines, including IFNγ, TNFα, IL-2, IL-4, and IL-12.77,79,86–88 These effects are thought to be due to a loss of mRNA stability.83,89 Interestingly, although it inhibits the secretion of proinflammatory molecules, adenosine actively stimulates the production of anti-inflammatory IL-10.77
Adenosine receptors are coupled to members of the G-protein coupled receptor (GPCR) family, and these GPCRs mediate an increase in intracellular cAMP following adenosine signaling.71,90 Elevated cAMP mediates numerous biochemical events within the T cell, ultimately contributing to adenosine’s anti-inflammatory effects. One such mechanism is the activation of sensor proteins, such as cAMP-dependent protein kinase (PKA). Activation of PKA in neutrophils can prevent the oxidative burst,91 and PKA’s substrates include proteins such as cAMP response element modulator and activating transcription factor 1 (CREB), which regulates the expression of Foxp3.92
In addition to synthesizing adenosine, Treg cells also express the A2A receptor and respond functionally to adenosine signaling.83,93 This suggests that adenosine may function in an autocrine fashion to dampen inflammation by optimizing Treg activity. Indeed, Treg suppressive function is optimized upon elevations in intracellular cAMP, as would occur following A2A activation.94,95
VII. EFFECTS ON DC MATURATION AND CO-STIMULATORY FUNCTION
Because of their intimate association with T cells in the immune synapse, APCs represent a major cellular target on which Tregs exert their suppressive functions. Most of Treg cell-mediated suppression of APCs involves contact-dependent mechanisms that affect APCs’ costimulatory potential. The interaction of CTLA-4, a co-stimulatory molecule constitutively expressed by Treg cells, with CD80 and CD86 on APCs, represents a major mechanism of suppression. The importance of effective co-stimulation for T cell activation and proliferation has been shown by studies demonstrating T cell hypo-proliferation, decreased production of cytokines, and anergy following addition of reagents that blocked co-stimulation.96,97
Both human and mouse Treg cells are able to down-regulate CD80/86 expression on dendritic cells (DC) in vitro.98,99 In a study of human Treg/ DC interactions, DCs co-cultured with Treg cells were unable to present antigen effectively, despite being pre-treated with CD40L.98 These studies shed light on novel mechanisms by which Treg cells can exert their suppressive functions – not only via suppression of Teff cells but also of bystander APCs. Treg expression of CTLA-4 was shown to be critical for immune tolerance, because mice selectively lacking CTLA-4 in Treg cells develop systemic, fatal autoimmune disease in the first few weeks of life.100 In vitro, Treg cells lacking CTLA-4 demonstrate impaired suppressor function when co-cultured with DCs and are also unable to upregulate CD80/86 expression on DCs.100 Collectively, these findings suggest that CTLA-4 can interact with its ligands, CD80/86, on DCs to prevent optimal antigen presentation and co-stimulatory potential of these cells. Reduced expression of CD80 and CD86, as well as occupancy of these receptors by CTLA-4, may also prevent effective interactions with CD28, thereby limiting APCs ability to co-stimulate and activate naïve T cells. Interestingly, activated Foxp3-Teff cells have also been shown to express CD80 and CD86, and it is possible that Treg-expressed CTLA-4 may inhibit the activation of Teff cells via a similar mechanism to that of APCs.101
Other surface proteins selectively expressed by Treg cells may also play a role in down regulating target cell activation. Lymphocyte activation gene-3 (LAG-3) is a CD4 homolog expressed by Treg cells that binds MHC-II with high affinity.102 Interactions mediated by LAG-3 between Treg cells and MHC-II-expressing DCs result in an inhibition of DC activation, most likely through an ITAM-mediated signaling pathway resulting in SHP-1 recruitment and subsequent suppression of DC maturation and antigen presentation ability.102
VIII. TCR DIVERSITY OF T REGULATORY CELLS
An unconventional way in which Treg cells maintain effective suppressor function is through the diversification of their TCRs. Interestingly, Treg cells display nearly the same level of diversity within their TCRs as non-Treg-TCRs;103,104 there is minimal overlap between TCR specificities in Tregs and non-Tregs, suggesting that most Treg target peptides are not recognized by Teff cells. The few overlapping TCR repertoires are largely between Tregs and Teff cells recognizing self-antigens.105,106 Although TCR diversity is critical for shaping the selection and differentiation of Treg cells in the thymus, a broad repertoire of TCR specificities is now understood to also contribute to Treg-mediated maintenance of immune tolerance in the periphery. The importance of TCR diversity in this process is demonstrated studies of adoptively transferred, TCR-restricted Treg cells in a model of GVHD.107
IX. CD8+ T REGULATORY CELLS
Although the majority of studies in the Treg field to date have focused on the functions of Foxp3+, CD4+CD25+ T cells, a population of CD8+ T cells has been described that suppress autoreactive Th2 responses.108 Follow-up work has shown that a non-classical MHC-1 molecule, Qa-1, is expressed by a subpopulation of follicular helper Th cells (Tfh), and this molecule is recognized by CD8. Interactions between CD8 and Qa-1 results in the inhibition of Tfh cell “help” to B cells, thus inhibiting autoreactive Tfh cell responses.109,110 Recent work has shown that disruption of this interaction, by a mutation of Qa-1 on Tfh cells, results in the development of autoantibody-mediated disease similar to SLE.111 This mode of suppression was shown to depend on perforin and IL-15 release.111
X. MODULATION OF TREG ACTIVITY BY CYTOKINE SIGNALING
Given the central role of Treg cells in maintaining immune homeostasis, it is critical to understand the regulation of their function. Recent work has shown that cytokines, including TNFa and IL-6, modulate Treg function in vivo.112,113 TNFa signals directly to Tregs through the TNFRII, which is expressed constitutively on un-stimulated Tregs and is upregulated by TNFa signaling.112 As TNFRII expression increases, allowing for increased TNFa signaling to Tregs, there is a resultant decrease in Foxp3 mRNA and protein expression as well as loss of suppressive function. Antibodies targeting TNFa have been very effective in treating autoimmune diseases including rheumatoid arthritis and psoriasis, and it is possible that this success is, in part, due to a restoration of Treg function through blockade of TNFa signaling.
IL-6 signaling to effector T cells also leads to the reversal of murine113 and human114 regulatory T cell function. The mechanism by which IL-6 signaling allows effector T cells to escape suppression is not well understood, but was shown to be dependent on soluble factors including IL-6 secreted by TLR4 activated dendritic cells113 in a murine system, and DC- or endothelial cell-derived IL-6 in studies using primary human cells.114 IL-6 signaling through the IL-6 receptor complex can lead to phosphorylation and activation of multiple transcription factors, including Stat1 and Stat3.115,116 Interestingly, the relative levels of phosphorylated Stat3 (pStat3) and pStat1 are critical for determining whether regulatory T cell suppression remains intact; strong Stat3 phosphorylation relative to pStat1 results in the release of Teff cells from suppression, whereas preferential phosphorylation of Stat1 results in sustained Treg suppression.117 In situations where Treg suppression is ineffective, the inhibition of Stat3 phosphorylation restores functional suppression, regardless of the presence of high concentrations of IL-6.117 Therefore, contextual signaling in inflammatory tissue microenvironments that modify the relative activation of Stat3 and Stat1 can directly alter the effectiveness of Treg suppressive functions.
Indeed, IL-6 is over-expressed at the mRNA and protein level in numerous autoimmune diseases, including psoriasis,118–121 and the functional receptor for IL-6 is present on 30–40% of peripheral blood CD4+ T cells;122 therefore, IL-6 signaling to lymphocytes is likely an important mechanism for loss of Treg suppression in the context of autoimmune disease. The importance of IL-6 signaling in autoimmunity is demonstrated by the clinical efficacy of tocalizumab, a monoclonal antibody against the IL-6 receptor approved in 2010 for the treatment of adult and juvenile rheumatoid arthritis.123–125
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health grants AR-051498, P30AR39750 and P50AR05508 (to KDC), a VA Merit Award (to KDC), and the Murdough Family Center for Psoriasis.
ABBREVIATIONS
- APC
antigen presenting cell
- DC
dendritic cell
- GVHD
graft versus host disease
- IBD
inflammatory bowel disease
- iTreg
induced regulatory T cell
- MHC
major histocompatibility complex
- nTreg
naturally occurring regulatory T cell
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- Teff
T effector cell
- Tfh
T follicular/helper cell
- Th1
T-helper type 1
- Th17
T-helper type 17
- TLR
Toll-like receptor
- Treg
regulatory T cell
REFERENCES
- 1.Gershon RK. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 6.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, de St Groth BF. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 10.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–924. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toubi E. The role of CD4+CD25+ T regulatory cells in autoimmune diseases. Clin Rev Allergy Immunol. 2008;34:338–344. doi: 10.1007/s12016-007-8043-0. [DOI] [PubMed] [Google Scholar]
- 12.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 16.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama H, Matsue H, Nagasaka A, Nakamura Y, Tsukamoto K, Shibagaki N, Kawamura T, Kitamura R, Ando N, Shimada S. CD4+CD25high regulatory T cells are markedly decreased in blood of patients with pemphigus vulgaris. Dermatology. 2007;214:210–220. doi: 10.1159/000099585. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Greene MI. Special regulatory T-cell review: FOXP3 biochemistry in regulatory T cells--how diverse signals regulate suppression. Immunology. 2008;123:17–19. doi: 10.1111/j.1365-2567.2007.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z, Song X, Li B, Greene MI. FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity. Immunol Res. 2008;42:19–28. doi: 10.1007/s12026-008-8029-x. [DOI] [PubMed] [Google Scholar]
- 21.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F, Pardoll DM, Drake CG. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47:1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. Cd4(+)cd25(high) regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 29.Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunology and Cell Biology. 2011;89:346–351. doi: 10.1038/icb.2010.137. [DOI] [PubMed] [Google Scholar]
- 30.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends in Molecular Medicine. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol. 2007;179:3578–3587. doi: 10.4049/jimmunol.179.6.3578. [DOI] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 35.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 38.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 39.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 42.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Li D, Tan Z. The expression of interleukin-17, interferon-gamma, and macrophage inflammatory protein-3 alpha mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technolog Med Sci. 2004;24:294–296. doi: 10.1007/BF02832018. [DOI] [PubMed] [Google Scholar]
- 44.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 48.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 50.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 52.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Probst-Kepper M, Geffers R, Kroger A, Viegas N, Erck C, Hecht HJ, Lunsdorf H, Roubin R, Moharregh-Khiabani D, Wagner K, Ocklenburg F, Jeron A, Garritsen H, Arstila TP, Kekalainen E, Balling R, Hauser H, Buer J, Weiss S. GARP: a key receptor controlling FOXP3 in human regulatory T cells. J Cell Mol Med. 2009;13:3343–3357. doi: 10.1111/j.1582-4934.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan DV, Somani AK, Young AB, Massari JV, Ohtola J, Sugiyama H, Garaczi E, Babineau D, Cooper KD, McCormick TS. Signal peptide cleavage is essential for surface expression of a regulatory T cell surface protein, leucine rich repeat containing 32 (LRRC32) BMC Biochemistry. 2011;12:27. doi: 10.1186/1471-2091-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 59.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 60.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 61.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 63.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 64.Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 66.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 67.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 69.Szymczak-Workman AL, Delgoffe GM, Green DR, Vignali DA. Cutting edge: Regulatory T cells do not mediate suppression via programmed cell death pathways. J Immunol. 2011;187:4416–4420. doi: 10.4049/jimmunol.1100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 72.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 74.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 75.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, Crowe SE, Ernst PB. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, Lambert J, Kupper TS. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 78.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 79.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 80.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 83.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 84.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 86.Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, Linden J, Ernst PB. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009;182:4616–4623. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fotheringham JA, Mayne MB, Grant JA, Geiger JD. Activation of adenosine receptors inhibits tumor necrosis factor-alpha release by decreasing TNF-alpha mRNA stability and p38 activity. Eur J Pharmacol. 2004;497:87–95. doi: 10.1016/j.ejphar.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 90.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer Immunol Immunother. 2012;61:283–288. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol. 2009;182:4017–4024. doi: 10.4049/jimmunol.0803310. [DOI] [PubMed] [Google Scholar]
- 96.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nunez G, Tang A, Sayegh M, Hancock WW, Strom TB, Turka LA. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 98.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 99.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 100.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 101.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 102.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 103.Kasow KA, Chen X, Knowles J, Wichlan D, Handgretinger R, Riberdy JM. Human CD4+CD25+ regulatory T cells share equally complex and comparable repertoires with CD4+CD25-counterparts. J Immunol. 2004;172:6123–6128. doi: 10.4049/jimmunol.172.10.6123. [DOI] [PubMed] [Google Scholar]
- 104.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 105.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 106.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 107.Fohse L, Suffner J, Suhre K, Wahl B, Lindner C, Lee CW, Schmitz S, Haas JD, Lamprecht S, Koenecke C, Bleich A, Hammerling GJ, Malissen B, Suerbaum S, Forster R, Prinz I. High TCR diversity ensures optimal function and homeostasis of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:3101–3113. doi: 10.1002/eji.201141986. [DOI] [PubMed] [Google Scholar]
- 108.Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160:566–571. [PubMed] [Google Scholar]
- 109.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 110.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 111.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF down-modulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 114.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goodman WA, Young AB, McCormick TS, Cooper KD, Levine AD. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol. 2011;186:3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neuner P, Urbanski A, Trautinger F, Moller A, Kirnbauer R, Kapp A, Schopf E, Schwarz T, Luger TA. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J Invest Dermatol. 1991;97:27–33. doi: 10.1111/1523-1747.ep12477880. [DOI] [PubMed] [Google Scholar]
- 119.Castells-Rodellas A, Castell JV, Ramirez-Bosca A, Nicolas JF, Valcuende-Cavero F, Thivolet J. Interleukin-6 in normal skin and psoriasis. Acta Derm Venereol. 1992;72:165–168. [PubMed] [Google Scholar]
- 120.Krueger JG, Krane JF, Carter DM, Gottlieb AB. Role of growth factors, cytokines, and their receptors in the pathogenesis of psoriasis. J Invest Dermatol. 1990;94:135S–140S. doi: 10.1111/1523-1747.ep12876121. [DOI] [PubMed] [Google Scholar]
- 121.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB, Gottlieb AB. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Betz UA, Muller W. Regulated expression of gp130 and IL-6 receptor alpha chain in T cell maturation and activation. Int Immunol. 1998;10:1175–1184. doi: 10.1093/intimm/10.8.1175. [DOI] [PubMed] [Google Scholar]
- 123.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 124.Woo P, Wilkinson N, Prieur AM, Southwood T, Leone V, Livermore P, Wythe H, Thomson D, Kishimoto T. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–R1288. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]