Abstract
Complicating proteomic analysis of whole tissues is the obvious problem of cell heterogeneity in tissues, which often results in misleading or confusing molecular findings. Thus, the coupling of tissue microdissection for tumor cell enrichment with capillary isotachophoresis-based selective analyte concentration not only serves as a synergistic strategy to characterize low abundance proteins, but it can also be employed to conduct comparative proteomic studies of human astrocytomas.
A set of fresh frozen brain biopsies were selectively microdissected to provide an enriched, high quality, and reproducible sample of tumor cells. Despite sharing many common proteins, there are significant differences in the protein expression level among different grades of astrocytomas. A large number of proteins, such as plasma membrane proteins EGFR and Erbb2, are up-regulated in glioblastoma. Besides facilitating the prioritization of follow-on biomarker selection and validation, comparative proteomics involving measurements in changes of pathways are expected to reveal the molecular relationships among different pathological grades of gliomas and potential molecular mechanisms that drive gliomagenesis.
INTRODUCTION
Gliomas are the most common primary tumors of the brain, with an incidence of about 30,000 new cases per year in the United States1–2. Most gliomas (> 80%) appear to arise from astrocytes, the most abundant type of glial cells, and are known as astrocytomas. Astrocytomas are divided into four grades by the World Health Organization (WHO). Grade I tumors are histologically benign and may be cured by surgical resection; most are found in children.
The tumors seen in adults are infiltrative and are generally grade II-IV gliomas. Grade II tumors exhibit mild cellular proliferation, have moderate cellular pleomorphism, and infiltrate the surrounding brain which makes curative resection difficult. Grade III tumors are hypercellular, have a higher degree of proliferation, and are malignant and lead to death in two to five years. Grade IV tumors, the glioblastoma (GBM), are exceptionally-aggressive malignancies with increased mitotic activity, pronounced angiogenesis (microvascular proliferation), necrosis, and proliferative rates 3–5 times higher than grade III tumors1–3. Patients with GBM have a median survival of 12–15 months with aggressive therapy; fewer than 5% will survive more than five years1,4.
The majority of human gliomas have a set of genetic alterations that cause the loss of protein regulation5. Studies based on DNA microarray analyses have indicated that GBMs overexpress various gene families related to cell adhesion, motility, invasion, and angiogenesis6. Approximately 50% of all GBMs are primary in origin while the other half arises secondarily from lower grade tumors. Current models of gliomagenesis coincide with the two clinically-recognized forms of GBM, de novo or primary and secondary or progression. In primary GBMs, EGFR gene amplifications, often combined with gene rearrangements that lead to a constitutively active, truncated receptor, occur in GBMs that generally express wild type p531,4,7–8. In secondary GBMs, progression from a low grade to a high grade glioma involves the serial accumulation of genetic alterations that inactivate tumor suppressor genes such as p53, p16, Rb, PTEN, or activate oncogenes such as MDM2, CDKs 4 and 61,4.
While proteomic measurements provide a wealth of information complementary to the transcriptomic data, the inherent disadvantage of biomarker dilution in complex biological fluids such as cerebrospinal fluids9–10 and serum/plasma11 of GBM patients necessitates highly sensitive analytical approaches, often exceeding the dynamic range of currently available proteomic platforms. Thus, investigative studies directed at tissues obtained from the primary site of pathology currently afford the best opportunity for the discovery of disease biomarkers12–14. For example, Melchior and co-workers15 employed the shotgun-based multidimensional protein identification technique (MuDPIT)16 and a semi-top-down approach involving combined intact protein/peptide separations in proteomic studies of whole GBM tissues.
Still, biologically relevant proteomic data can only be generated if the tissue samples investigated consist of homogeneous cell populations, in which no unwanted cells of different types and/or development stages obscure the results. For example, SM22, a dominant protein in smooth muscle cells, has been widely reported to be abnormally expressed in many solid tumors. However, Li and co-workers found that the highly expressed SM22 was mainly found in smooth muscle layers, blood vessels, and myofibroblasts instead of gastric cancer cells17. While limited two-dimensional polyacrylamide gel electrophoresis analyses of microdissected GBM tissues have been attempted18–21, these studies required significant manual effort and time to extract sufficient levels of protein for analysis, while providing little information on protein expression beyond a relatively small number of high abundance proteins.
Besides sample amount constraints from microdissection-procured tissues22, the large variation of protein relative abundances having potential biological significance in mammalian systems presents a major analytical challenge for achieving comprehensive proteomic studies of clinical specimens. In contrast to universally enriching all analytes by a similar degree, the result of the capillary isotachophoresis (CITP) stacking process is that major components may be diluted, but trace compounds are concentrated23–25. Such selective enhancement toward low abundance proteins drastically reduces the range of relative protein abundances and greatly enhances the proteome coverage for the analysis of human saliva and mouse brain mitochondrial proteomes26–27. In this work, we used CITP proteomic technology coupled with the label-free spectral counting approach28–30 to compare protein expression profiles within microdissected astrocytoma tissues, including WHO grade II, III, and IV astrocytomas.
EXPERIMENTAL SECTION
Materials and Reagents
Fused-silica capillaries (50 μm i.d./365 μm o.d. and 100 μm i.d./365 μm o.d.) were acquired from Polymicro Technologies (Phoenix, AZ). Acetic acid, dithiothreitol (DTT), and iodoacetamide (IAM) were obtained from Sigma (St. Louis, MO). Acetonitrile, ammonium acetate, hydroxypropyl cellulose (average MW 100,000), tris(hydroxymethyl)aminomethane (Tris), and urea were purchased from Fisher Scientific (Pittsburgh, PA). Pharmalyte 3–10 was acquired from Amersham Pharmacia Biotech (Uppsala, Sweden). Sequencing grade trypsin was obtained from Promega (Madison, WI). All solutions were prepared using water purified by a Neu-Ion system (Baltimore, MD) equipped with a UV sterilizing lamp and a 0.05 μm membrane final filter.
Tissue Microdissection and Proteomic Sample Preparation
A set of fresh frozen brain biopsies, including grade II astrocytoma (n = 4), grade III anaplastic astrocytoma (n = 3), and grade IV GBM (n = 5), were obtained under an Institutional Review Board-approved protocol at the Cleveland Clinic Foundation. By following the procedures described in our previous studies31–32, these tissue sections (1 cm × 1 cm × 10 μm) were microdissected using the Arcturus Laser Capture Microdissection (LCM) System of Applied Biosystems (Foster City, CA) to gather approximately 100,000 tumor cells from each clinical specimen (Fig. 1).
Figure 1.

Demonstration of selective tissue microdissection of a relatively pure population of tumor cells. Left, a hypercellular focus of GBM cells, abutting the acellular space to the left of the slide, surrounded on the right by white matter (brain) infiltrated by tumor cells, but at a substantially lower cellular density; middle, the tumor cells selectively removed by LCM; and, right, the tumor cells isolated on the LCM cap.
The microdissected cells obtained from each specimen were individually processed and placed directly into a microcentrifuge tube containing 8 M urea and 20 mM Tris-HCl at pH 8.0. The soluble proteins were collected in the supernatant by centrifugation at 20,000 g for 30 min. Proteins in the supernatant were reduced and alkylated by sequentially adding DTT and IAM with final concentrations of 10 mg/mL and 20 mg/mL, respectively. The solution was incubated at 37 °C for 1 hr in the dark and then diluted 8-fold with 100 mM ammonium acetate at pH 8.0. Trypsin was added at a 1:40 (w/w) enzyme to substrate ratio and the solution was incubated at 37 °C overnight. Tryptic digests were desalted using a Peptide MacroTrap column (Michrom Bioresources, Auburn, CA), lyophilized to dryness using a SpeedVac (Thermo, San Jose, CA), and then stored at −80 °C.
CITP-Based Tissue Proteomic Analysis
A total of 12 proteomic samples, containing proteolytic digests processed from 4 grade II, 3 grade III, and 5 grade IV astrocytoma tissue specimens, were prepared using a 2% pharmalyte solution with a final total protein concentration of approximately 1 μg/μL. Each proteomic sample was analyzed in duplicate. Similar to the procedures described in our previous studies26–27,33, a 80-cm long CITP capillary (100 μm i.d./365 μm o.d.) coated with hydroxypropyl cellulose was initially filled with a background electrophoresis buffer of 0.1 M acetic acid at pH 2.8. A 50-cm long sample plug, corresponding to a 4.0 μL sample volume or 4 μg total protein loading, was hydrodynamically injected into the capillary.
A positive electric voltage of 24 kV was applied to the inlet reservoir, which was filled with a 0.1 M acetic acid solution. The cathodic end of the capillary was housed inside a stainless steel tube (460 μm i.d./785 μm o.d.) using a coaxial liquid sheath flow configuration34. A sheath liquid composed of 0.1 M acetic acid was delivered at a flow rate of 1 μL/min using a Harvard Apparatus 22 syringe pump (South Natick, MA). The stacked and resolved peptides in the CITP capillary were sequentially fractionated and loaded into individual wells on a moving microtiter plate. The entire capillary content was separated and sampled into 18 individual fractions in less than 2 hr. The CITP separation and fractionation apparatus was operated on an in-house built robotic platform controlled by LabView (National Instruments, Austin, TX).
To perform combined CITP/nano-reversed phase liquid chromatography (nano-RPLC) separations, peptides collected in individual wells were sequentially injected into dual trap columns (3 cm × 100 μm i.d. × 365 μm o.d.) packed with 5 μm porous C18 particles. Each peptide fraction was subsequently analyzed by nano-RPLC equipped with an Ultimate dual-quaternary pump (Dionex, Sunnyvale, CA) and a dual nano-flow splitter connected to two pulled-tip fused-silica capillaries (50 μm i.d. × 365 μm o.d.). These two 15-cm long capillaries were packed with 3.5-μm Zorbax Stable Bond (Agilent, Palo Alto, CA) C18 particles.
Nano-RPLC separations were performed in parallel in which a dual-quaternary pump delivered two identical 2-hr organic solvent gradients with an offset of 1 hr. Peptides were eluted at a flow rate of 200 nL/min using a 5–45% linear acetonitrile gradient over 100 min with the remaining 20 min for column regeneration and equilibration. The peptide eluants were monitored using a linear ion-trap mass spectrometer (LTQ, Thermo, San Jose, CA) equipped with an electrospray ionization interface and operated in a data dependent mode. Full scans were collected from 400 - 1400 m/z and 5 data dependent MS/MS scans were collected with dynamic exclusion set to 30 sec.
MS Data Analysis
Raw LTQ data were converted to peak list files by msn_extract.exe (Thermo). Open Mass Spectrometry Search Algorithm (OMSSA)35 developed at the National Center for Biotechnology Information was used to search the peak list files against the SwissProt sequence library with decoyed sequences appended. This decoyed database was constructed by reversing all sequences and appending them to the end of the sequence library. Searches were performed using the following parameters: fully tryptic, 1.5 Da precursor ion mass tolerance, 0.4 Da fragment ion mass tolerance, 1 missed cleavage, alkylated Cys as a fixed modification and variable modification of Met oxidation. Searches were run in parallel on a 14 node, 28 CPU Linux cluster (Linux Networx, Bluffdale, UT).
False discovery rates (FDRs) were determined using a target-decoy search strategy introduced by Elias and co-workers36 and employed in our previous study for a comparative evaluation among commonly used tandem MS identification search algorithms37. An E-value threshold corresponding to a 1% FDR in total protein identifications was used as a cutoff in this analysis as this correlates with the maximum sensitivity versus specificity in our previous work37. Furthermore, proteins were reported as being identified by at least two distinct peptides among which contain at least one unique peptide. For a protein family with shared redundancy peptides sequences, only one member of a protein family was reported which has the best scoring in the search results. After generation of search data, the OMSSA XML result files were parsed using a Java parser and loaded into an Oracle 10g database for analysis and reporting using in-house software
Protein Validation Using Immunohistochemistry (IHC)
One of highly confident and differentially expressed proteins among astrocytomas, IQGAP1, was chosen from MS-based proteomic measurements for validation using IHC. IHC was performed on tissue microarray (TMA) sections by removing three 1.5 mm diameter cores (> 90% tumor cells) of tissue from each block at the laboratory of US Biomax (Rockville, MD). Negative and positive controls were conducted using the normal cerebral tissue and the skin tissue of malignant melanoma, respectively.
The mouse Primary antibody against IQGAP1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and incubated with a dilution ratio of 1:150 at 4 °C overnight. The biotinylated anti-mouse immunoglobulin was employed as the link antibody and was incubated for 30 min. The avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA) was utilized as the labeling reagent and incubated for 30 min. A 10-min wash step with Tris buffered saline was carried out between each step. TMAs were counterstained by hematoxylin and mounted for examination by microscope.
RESULTS AND DISCUSSION
Besides selective analyte enrichment, CITP further provides high resolving power to analyze complex proteomic mixtures. As demonstrated in our previous studies of human saliva and mouse brain mitochondrial proteomes26–27, approximately 85–90% of distinct peptides are identified in only a single CITP fraction. For the investigation of microdissected astrocytoma tissues, about 80–85% of distinct peptides were identified in each of 18 CITP fractions. In contrast, a high degree of peptide overlapping in strong cation-exchange chromatography, as the first separation dimension of MuDPIT16, is generally observed with at least 40% of carryover of peptides that are identified in previous salt gradient fractions. The combination of selective analyte enrichment with excellent resolving power contributed to superior astrocytoma proteomic results with regard to the analytical reproducibility, the confidence in protein identification, and the overall tissue proteome coverage.
Overall Performance of Human Astrocytoma Proteomes
The overall tissue proteomic results are summarized in Table 1 with the complete protein lists provided in the Supporting Information. The number of proteins which are predicted to contain at least one or more transmembrane domains using TMHMM38 is also included in Table 1 and accounts for approximately 19% of total protein identifications. To the best of our knowledge, these data sets represent the most extensive tissue proteomics confidently identified so far in human astrocytomas. The coupling of tissue microdissection for tumor cell enrichment with CITP-based selective analyte concentration serves as a synergistic strategy to greatly increase tissue proteome coverage, including the characterization of low abundance proteins.
Table 1.
Number of Distinct Proteins Identified from Duplicated Runs for Each Microdissected Astrocytoma Tissue Specimen
| Specimen Number | Grade II | Grade III | Grade IV |
|---|---|---|---|
| 1 | 2,014 (403)* | 2,196 (396) | 2,663 (511) |
| 2 | 2,007 (454) | 2,314 (415) | 2,566 (440) |
| 3 | 2,483 (467) | 2,346 (455) | 2,327 (417) |
| 4 | 2,395 (478) | 2,540 (495) | |
| 5 | 2,207 (413) |
Number of identified membrane proteins is included in parentheses.
Identification reproducibility was evaluated and was determined to be around 83–88% in identified proteins among technical duplicates of the same tissue specimen. Furthermore, the average number of distinct peptides leading to each protein identification was around 6.8 and illustrated the broad sequence coverage of protein identification. The enhanced analytical reproducibility, together with the confidence of protein identification, ensures high impact inferences to be drawn from the proteomic investigations of archival astrocytoma tissues.
Comparisons among Different Grades of Astrocytomas
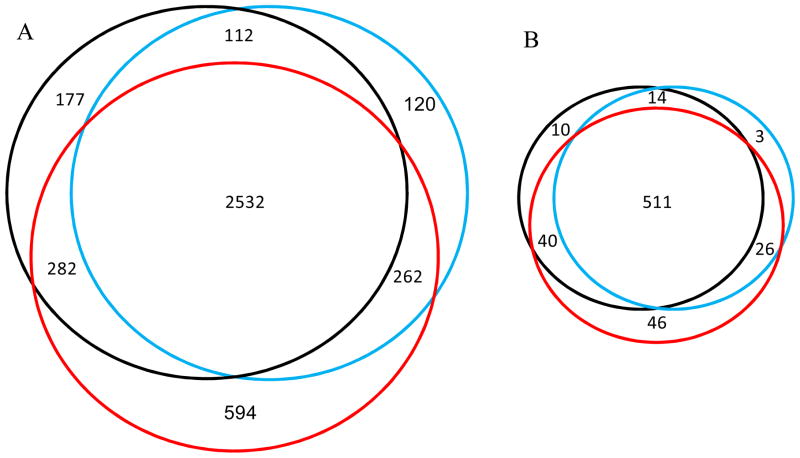
GBMs (grade IV) are aggressive malignancies with an increased mitotic activity, pronounced angiogenesis (microvascular proliferation), necrosis, and more rapid proliferative rates than grade III tumors1–3. GBMs, in general, exhibited the largest number of identified proteins than those of grades II and III (Table 1). Besides the identification of many common proteins among different grades of astrocytomas (Fig. 2A), only 177, 120, and 594 proteins were detected uniquely in grades II, III, and IV, respectively. Of the 650 membrane proteins identified, only 10, 3, and 46 membrane proteins were detected uniquely in grades II, III, and IV, respectively (Fig. 2B). Such high degree of overlapping in protein identification further supports the high confidence and the enhanced reproducibility of proteomic measurements performed with CITP.
Figure 2.
The Venn diagrams comparing (A) total proteins and (B) membrane proteins identified from grade II astrocytoma (black), grade III anaplastic astrocytoma (blue), and grade IV GBM (red).
In addition to protein identification, monitoring quantitative changes in identified peptides, and by inference, proteins, is essential for the discovery of cancer-associated biomarkers. Due to constraints requiring the study of proteins in a tissue environment with limited sample availability, the protein expression profiles within targeted tumor cells selectively procured from astrocytoma tissues were quantitatively compared by coupling the CITP technology with the label-free MS-based spectral counting measurements28–30. Even though major components may be diluted and trace compounds are concentrated as a result of selective analyte enrichment in the CITP process23–25, the bandwidth of individual peptides/proteins, however, is proportional to the amount injected into the CITP capillary and therefore the abundance in the original proteome sample39. Thus, the analyte bandwidth established during the CITP separation, correlated with the quantity of analyte, affects the distribution of each peptide within single or multiple CITP fractions, the peak width of peptide in subsequent nano-RPLC separation, and the number of total peptide hits (or tandem MS events) leading to individual protein identification and quantification through the spectral counting approach28–30.
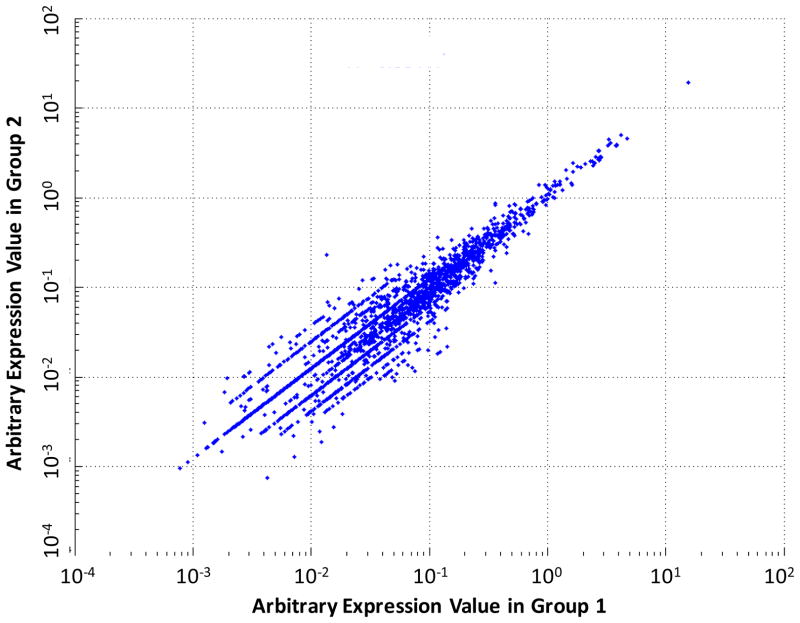
The Pearson correlation coefficient was employed to measure how well a linear equation describes the quantitative correlation between two proteomic runs to assess analytical reproducibility and allow for easy visual identification of outlier features (proteins). The calculation of the Pearson correlation coefficient was performed using the MATLAB Statistics Toolbox (MathWorks, Natick, MA). As shown in Fig. 3, technical replicates of two adjacent GBM sections of the same specimen give a Pearson coefficient of greater than 0.99 over a dynamic range of around 104.
Figure 3.
Pearson correlation coefficient plot of two proteomic runs analyzing adjacent GBM sections of the same tissue specimen.
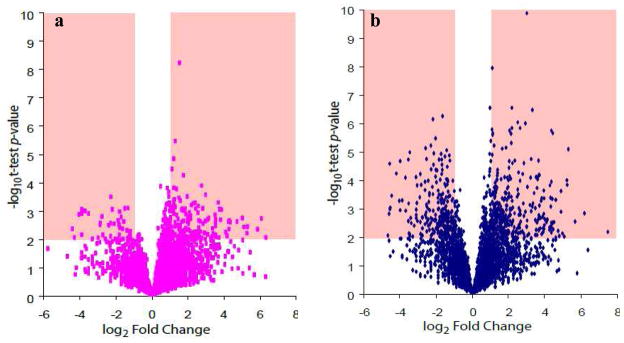
A Student’s t-test was performed to compare the protein expression profiles within microdissected tumor cells among different grades of gliomas. Despite sharing over 2,532 common proteins (Fig. 2A), highlighted areas shown in Figs. 4a and 4b specifically denote a large number of highly confident (p-value of less than 0.01) and differentially expressed (greater than 2-fold change) proteins discovered in comparative proteomic studies of astrocytomas.
Figure 4.
Volcano plots of (a) grade IV vs. grade III and (b) grade IV vs. grade II.
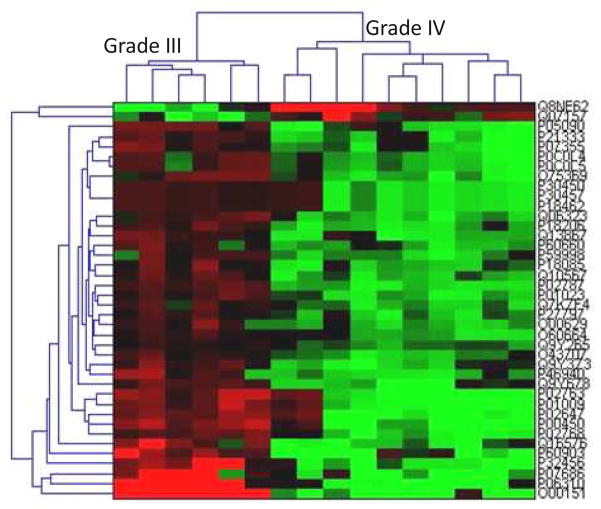
Unsupervised hierarchical clustering was conducted using Cluster 3.040. Arrays and genes were both clustered using the Euclidian distance similarity metric. The complete linkage method was applied for clustering grade III anaplastic astrocytoma with grade IV GBM. Forty proteins with the most significant p-values by t-test are shown in Fig. 5. This clustering analysis reveals significant and consistent expression differences between the two disease grades and provides a tractable set of proteins as potential candidates for follow-on validation studies and functional investigation. Furthermore, a list of top ten proteins selected from Fig. 5 exhibiting the highest spectral count ratios is provided in Table 1 in the Supporting Information.
Figure 5.
Unsupervised hierarchical clustering of forty proteins with the most significant t-test values resulting from the comparison of grade III and grade IV astrocytomas.
Function and Network Analysis of Proteins Up-regulated in GBM
Besides monitoring and comparing the protein expression profiles among different grades of astrocytomas, the approach of grouping individual expression changes into pathways of actions is not only useful in trying to interpret genome and proteome datasets, but also allows common biological themes to emerge from the comparative proteomic analyses41. Comparative proteomics involving measurements in changes of pathways or functional processes are expected to provide relevant tumor-associated markers and networks, molecular relationships among different stages of disease, and molecular mechanisms that drive the progression of cancer. From a practical perspective, the evaluation of comparative proteomic dataset within a biological context is essential for high-throughput data authentication, prioritization of follow-on biomarker selection, and validation.
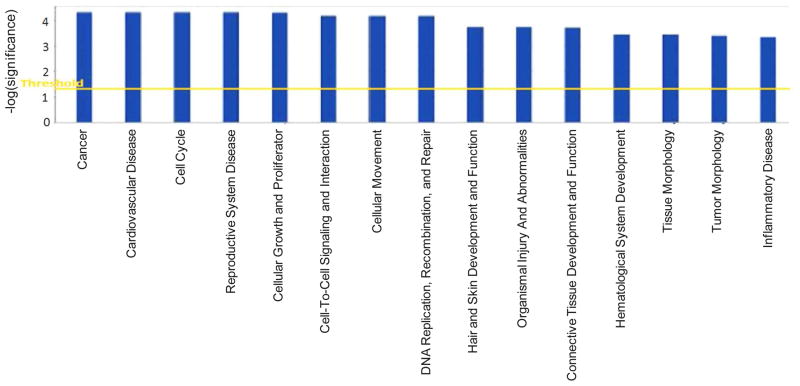
By employing the proteome dataset that is up-regulated in GBMs, the top functional pathways were mapped using the Ingenuity Pathway Analysis™ program (Ingenuity Systems, Redwood City, CA), as shown in Fig. 6. The significance value associated with the functional analysis for a dataset is a measure of how likely the proteins from the dataset under investigation participate in that function. The significance is expressed as a p-value, which is calculated using the right-tailed Fisher’s Exact Test. The p-value is calculated by comparing the number of user-specified proteins of interest that participate in a given function or pathway, relative to the total number of occurrences of these proteins in all functional/pathway annotations stored in the Ingenuity Pathway Knowledge Base. Table 2 lists the proteins that have been associated with the “cancer function” and that are uniquely up-regulated in GBM. Their spectral counting results among different grades of astrocytomas are also included in Table 2.
Figure 6.
Top functional pathways constructed from the up-regulated GBM proteome dataset using the Ingenuity Pathway Analysis™.
Table 2.
Proteins Related to Cancer Function and Up-Regulated in GBM
| Name | Description | Accession | Location | Family | Spectral Counts (Grades II/III/IV) |
|---|---|---|---|---|---|
| B4GALNT1 | β-1,4-N-acetyl-galactosaminyl Transferase 1 | Q00973 | cytoplasm | enzyme | (0/2/4) |
| BAG1 | BCL2-associated athanogene | Q99933 | cytoplasm | other | (0/0/2) |
| CASP1 | caspase 1, apoptosis-related cysteine peptidase (interleukin 1, β, convertase) | P29466 | cytoplasm | peptidase | (0/2/4) |
| CD97 | CD97 molecule | P48960 | plasma membrane | G-protein coupled receptor | (0/0/4) |
| COL6A3 | collagen, type VI, α 3 | P12111 | extracellular Space | other | (6/14/87) |
| CTSS | cathepsin S | P25774 | cytoplasm | peptidase | (0/0/5) |
| DCN | decorin | P07585 | extracellular Space | other | (2/2/9) |
| EGFR | epidermal growth factor receptor (erythroblastic (leukemia viral (v-erb-b) oncogene homolog, avian) | P00533 | plasma membrane | kinase | (5/9/47) |
| ERBB2 | v-erb-b2 erythroblastic eukemia viral oncogene homolog2, neuro/ glioblastoma derived oncogene homolog (avian) | P04626 | plasma membrane | kinase | (0/0/4) |
| GBP1 | guanylate binding protein 1, interferon- inducible, 67kDa | P32455 | cytoplasm | enzyme | (2/4/16) |
| IGFBP7 | insulin-like growth factor Binding protein 7 | Q16270 | extracellular space | transporter | (0/0/5) |
| IL18 | interleukin 18 (interferon-γ− inducing factor) | Q14116 | extracellular space | cytokine | (0/0/3) |
| IQGAP1 | Ras GTPase-activating-like protein IQGAP1 | P46940 | plasma membrane | other | (7/25/57) |
| ITGA2 | integrin, α 2 (CD49B, α 2 subunit of VLA-2 receptor) | P17301 | plasma membrane | other | (0/0/4) |
| ITGB2 | integrin, β 2 (complement component 3 receptor 3 and 4 subunit) | P05107 | plasma membrane | other | (0/2/5) |
| LGALS3 | lectin, galactoside-binding, soluble, 2 (galectin 3) | P17931 | extracellular space | other | (0/0/6) |
| LRPAP1 | low density lipoprotein receptor-related protein associated protein 1 | P30533 | plasma membrane | transmembrane receptor | (0/2/5) |
| MCM7 | MCM7 minichromosome Maintenance deficient 7 (S. cerevisiae) | P33993 | nucleus | enzyme | (0/0/6) |
| MMP2 | matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) | P08253 | extracellular space | peptidase | (0/0/3) |
| MRE11A | MRE11 meiotic recombination 11 homolog A (S. cerevisiae) | P49959 | nucleus | enzyme | (0/0/3) |
| NEK9 | NIMA (never in mitosis gene a)-related kinase 9 | Q8TD19 | nucleus | kinase | (0/2/5) |
| N QO1 | NAD(P)H dehydrogenase, quinone 1 | P15559 | cytoplasm | enzyme | (0/0/5) |
| OAS3 | 2'-5'-oligoadenylate synthetase 3, 100kDa | Q9Y6K5 | cytoplasm | enzyme | (0/2/8) |
| PML | promyelocytic leukemia | P29590 | nucleus | transcription regulator | (2/3/6) |
| S100A10 | S100 calcium binding protein A10 (annexin II ligand, calpactin I, light polypeptide (p11)) | P60903 | cytoplasm | other | (2/2/10) |
| SMARCA4 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 | P51532 | nucleus | transcription regulator | (0/0/3) |
| TBXAS | thromboxane A synthase 1 (platelet, cytochrome P450, family 5, subfamily A) | P24557 | plasma membrane | enzyme | (0/0/4) |
| TFRC | transferrin receptor (p90, CD71) | P02786 | plasma membrane | transporter | (2/4/9) |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | P01033 | extracellular space | other | (0/0/3) |
| TIMP2 | TIMP metallopeptidase inhibitor 2 | P16035 | extracellular space | other | (0/0/3) |
| TYMS | thymidylate synthetase | P04818 | nucleus | enzyme | (0/0/3) |
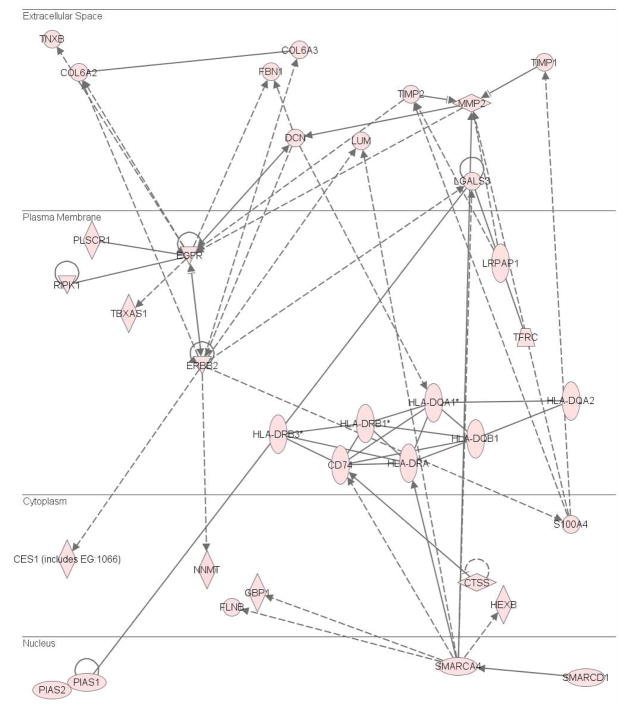
Since CITP’s selective analyte enrichment greatly increases proteomic coverage, several protein networks were partially or completely revealed in these gliomas. For example, one of the protein networks, containing tyrosine kinase receptors of EGFR and its ErbB family member of Her2 (Erbb2), shown in Fig. 7, is associated with the cell cycle dys-regulation and oncogenesis. Proteins shown in this network were up-regulated over 2 fold in GBM relative to lower grade astrocytomas. Previous studies have shown that EGFR amplification, over-expression and/or mutation are commonly observed in primary GBM. EGFR is involved in several major cellular signal transduction pathways such as the Ras, NF-κB, and PTEN pathways. It is believed that constantly activating these pathways contributes to cancer cell proliferation and survival. In addition to these well known cancer related plasma membrane proteins, two proteins expressed in the nucleus, PIAS1 and PIAS2, were over-expressed in GBM. These two proteins regulate STAT expression in the Jak-Stat signaling pathway, which can promote astrocyte differentiation through cross-talk between Notch and the BMP-Smad signaling pathways42.
Figure 7.
An up-regulated GBM protein network containing EGFR and Erbb2.
Selection and Validation of IQGAP1 Using IHC
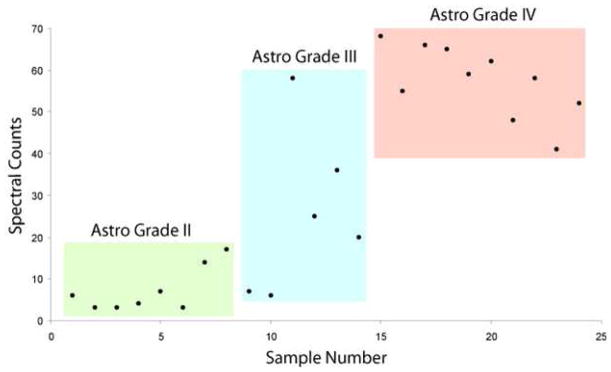
The cell membrane protein IQGAP1, which was differentially expressed between different grades of gliomas (Fig. 8) as measured by the spectral counting approach28–30, was selected for validation using IHC. IQGAP1 was chosen because of its role in key protein networks and pathways associated with the progression of brain tumors43–45. IHC was performed on TMA by removing three cores from each tissue block. For example, A1-A3 shown in Fig. 9 were the cores removed from the same tissue block of patient diagnosed with grade 1 astrocytoma.
Figure 8.
IQGAP1 expression relative to correlation with astrocytoma grade.
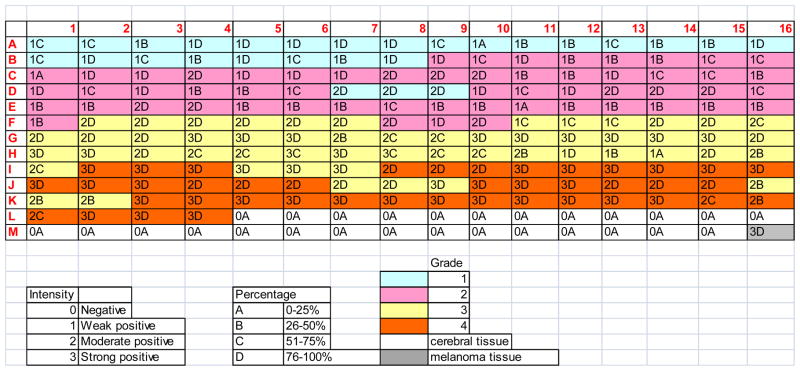
Figure 9.
Scoring of TMA with IHC staining of IQGAP1. “Intensity” refers to staining intensity using IHC; “percentage” refers to the percentage of tumor cells within each TMA that were positive by IHC; and “grade” refers to WHO grade I-IV gliomas. IHC results validate MS-based quantitative proteomic profiling of microdissected astrocytoma tissues.
A total of 60 tissue blocks, including 9 grade 1, 19 grade 2, 18 grade 3, and 14 grade 4, were employed for the validation studies of IQGAP1. Additionally, normal cerebral tissue (L5-M15) and the skin cancer, melanoma (M16), served as negative and positive controls, respectively. As shown in Fig. 9, both the staining intensity and the percentage score are reproducible among the cores removed from the same tissue block. Furthermore, the staining index, given for each case as the product by multiplying the percentage score with the staining intensity, increases with increasing tumor grade from grade 1 to grade 4 astrocytomas. Representative IHC staining images of IQGAP1 from the normal cerebral tissue, the grade II astrocytoma, the grade III anaplastic astrocytoma, and the grade IV GBM are shown in Fig. 1 in the Supporting Information.
CONCLUSION
Comprehensive and comparative proteomic profiling of relatively pure populations of glioma cells obtained from selectively microdissected tumors demonstrates the ability of this combined approach to accurately and reliably discover and validate proteins that may correlate directly with and predict prognosis, survival, and response to therapy in patients. This work represents the largest catalog of proteins indentified and quantified among different grades of astrocytomas reported to date. Our proteomic results, including an enhanced analytical reproducibility, a high confidence in protein identification, and the broad tissue proteome coverage, illustrate the utility of proteomic discovery using targeted tissue microdissection in combination with CITP’s selective analyte enrichment and its elevated resolving power prior to tandem MS measurements.
Despite sharing many common proteins, there are significant differences in protein expression among different grades of the infiltrative astrocytomas. The greatest number of differentially-expressed proteins was found in GBM, many of which are proteins frequently identified in other cancer types, including the plasma membrane proteins EGFR and Erbb2. Furthermore, two nuclear proteins up-regulated in GBM, PIAS1 and PIAS2, regulate STAT expression in the Jak-Stat signaling pathway, which can promote astrocyte differentiation through cross-talk between Notch and the BMP-Smad signaling pathways42.
IQGAP1, a cell membrane protein, was selected from comparative proteomic studies for validation using IHC. IQGAP1 is a prominent member of several key protein networks and pathways that have been associated with the progression of brain tumors43–45. To facilitate high-throughput IHC measurements, analysis of IQGAP1 expression was performed in a large TMA, including 60 astrocytoma specimens and validated MS-based proteomic profiling of microdissected astrocytoma tissues.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA 143177 to CSL and RR 21239 to XF and BMB. We wish to thank the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment within the Burkhardt Brain Tumor and Neuro-oncology Center at the Cleveland Clinic Foundation for additional support and funding.
References
- 1.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. J Neurosurg. 2001;95:190. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.Ichimura K, Ohgaki H, Kleihues P, Collins VP. J Neurooncol. 2004;70:137. doi: 10.1007/s11060-004-2747-2. [DOI] [PubMed] [Google Scholar]
- 3.Holland EC. Nat Rev Genet. 2001;2:120. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 4.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Genes Dev. 2001;15:1311. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 5.Kitange GJ, Templeton KL, Jenkins RB. Curr Opin Oncol. 2003;15:197. doi: 10.1097/00001622-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sallinen SL, Sallinen PK, Haapasalo HK, Helin HJ, Helen PT, Schraml P, Kallioniemi OP, Kononen J. Cancer Res. 2000;60:6617. [PubMed] [Google Scholar]
- 7.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Cancer Cell. 2002;1:269. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 8.Maher EA, Brennan C, Wen PY, Durso L, Ligon KL, Richardson A, Khatry D, Feng B, Sinha R, Louis DN, Quackenbush J, Black PM, Chin L, DePinho RA. Cancer Res. 2006;66:11502. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi M, Matsumoto T, Nagashio R, Kageyama T, Utsuki S, Oka H, Okayasu I, Sato Y. Pathol Int. 2009;59:797. doi: 10.1111/j.1440-1827.2009.02447.x. [DOI] [PubMed] [Google Scholar]
- 10.Schuhmann MU, Zucht HD, Nassimi R, Heine G, Schneekloth CG, Stuerenburg HJ, Selle H. Eur J Surg Oncol. 2010;36:201. doi: 10.1016/j.ejso.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Kumar DM, Thota B, Shinde SV, Prasanna KV, Hegde AS, Arivazhagan A, Chandramouli BA, Santosh V, Somasundaram K. J Proteome Res. 2010;9:5557. doi: 10.1021/pr1001737. [DOI] [PubMed] [Google Scholar]
- 12.Nirmalan NJ, Harnden P, Selby PJ, Banks RE. Mol Biosyst. 2008;4:712. doi: 10.1039/b800098k. [DOI] [PubMed] [Google Scholar]
- 13.Rifai N, Gillette MA, Carr SA. Nat Biotechnol. 2006;24:971. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 14.Cottingham K. J Proteome Res. 2007;6:2052. doi: 10.1021/pr070753l. [DOI] [PubMed] [Google Scholar]
- 15.Melchior K, Tholey A, Heisel S, Keller A, Lenhof HP, Meese E, Huber CG. J Proteome Res. 2009;8:4604. doi: 10.1021/pr900420b. [DOI] [PubMed] [Google Scholar]
- 16.Wolters DA, Washburn MP, Yates JR. Anal Chem. 2001;73:5683. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Zhang J, Liang Y, Shao J, Peng F, Sun M, Xu N, Li X, Wang R, Liu S, Lu Y. J Proteome Res. 2007;6:3304. doi: 10.1021/pr0702363. [DOI] [PubMed] [Google Scholar]
- 18.Iwadate Y, Sakaida T, Hiwasa T, Nagai Y, Ishikura H, Takiguchi M, Yamaura A. Cancer Res. 2004;64:2496. doi: 10.1158/0008-5472.can-03-1254. [DOI] [PubMed] [Google Scholar]
- 19.Odreman F, Vindigni M, Gonzales ML, Niccolini B, Candiano G, Zanotti B, Skrap M, Pizzolitto S, Stanta G, Vindigni A. J Proteome Res. 2005;4:698. doi: 10.1021/pr0498180. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zhuang Z, Okamoto H, Vortmeyer AO, Park DM, Furuta M, Lee YS, Oldfield EH, Zeng W, Weil RJ. Neurology. 2006;66:733. doi: 10.1212/01.wnl.0000201270.90502.d0. [DOI] [PubMed] [Google Scholar]
- 21.Gimenez M, Souza VCdO, Izumi C, Barbieri MR, Chammas R, Oba-Shinjo SM, Uno M, Marie SKN, Rosa JC. Proteomics. 2010;10:2812. doi: 10.1002/pmic.200900722. [DOI] [PubMed] [Google Scholar]
- 22.Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA. Science. 1997;278:1481. doi: 10.1126/science.278.5342.1481. [DOI] [PubMed] [Google Scholar]
- 23.Gebauer P, Bocek P. Electrophoresis. 2000;21:3898. doi: 10.1002/1522-2683(200012)21:18<3898::AID-ELPS3898>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 24.Foret F, Szoko E, Karger BL. Electrophoresis. 1993;14:417. doi: 10.1002/elps.1150140167. [DOI] [PubMed] [Google Scholar]
- 25.Stegehuis DS, Irth H, Tjaden UR, Van der Greef J. J Chromatogr. 1991;538:393. doi: 10.1016/s0021-9673(01)88860-9. [DOI] [PubMed] [Google Scholar]
- 26.Fang X, Yang L, Wang W, Song T, Lee C, Devoe D, Balgley B. Anal Chem. 2007;79:5785. doi: 10.1021/ac070611a. [DOI] [PubMed] [Google Scholar]
- 27.Fang X, Wang W, Yang L, Chandrasekaran K, Kristian T, Balgley BM, Lee CS. Electrophoresis. 2008;29:2215. doi: 10.1002/elps.200700609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Sadygov RG, Yates JR. Anal Chem. 2004;76:4193. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 29.Rappsilber J, Ryder U, Lamond AI, Mann M. Genome Res. 2002;12:1231. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Mol Cell Proteomics. 2005;4:1265. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Furuta M, Weil RJ, Vortmeyer AO, Huang S, Lei J, Huang T-N, Lee Y-S, Bhowmick DA, Lubensky IA, Oldfield EH, Zhuang Z. Oncogene. 2004;23:6806. doi: 10.1038/sj.onc.1207770. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Z, Lee YS, Zeng W, Furuta M, Valyi-Nagy T, Johnson MD, Vnencak-Jones CL, Woltjer RL, Weil RJ. Neurology. 2004;62:2316. doi: 10.1212/wnl.62.12.2316. [DOI] [PubMed] [Google Scholar]
- 33.Fang X, Balgley BM, Wang W, Park DM, Lee CS. Electrophoresis. 2009;30:4063. doi: 10.1002/elps.200900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Lee CS, Hofstadler SA, Smith RD. Anal Chem. 1998;70:4945. doi: 10.1021/ac980223w. [DOI] [PubMed] [Google Scholar]
- 35.Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH. J Proteome Res. 2004;3:958. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 36.Elias JE, Haas W, Faherty BK, Gygi SP. Nat Methods. 2005;2:667. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 37.Balgley BM, Laudeman T, Yang L, Song T, Lee CS. Mol Cell Proteomics. 2007;6:1599. doi: 10.1074/mcp.M600469-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. J Mol Biol. 2001;305:567. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 39.An Y, Cooper JW, Balgley BM, Lee CS. Electrophoresis. 2006;27:3599. doi: 10.1002/elps.200600093. [DOI] [PubMed] [Google Scholar]
- 40.Eisen MB, Spellman PT, Brown PO, Botstein D. Proc Natl Acad Sci U S A. 1998;95:14863. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin B, White JT, Lu W, Xie T, Utleg AG, Yan X, Yi EC, Shannon P, Khrebtukova I, Lange PH, Goodlett DR, Zhou D, Vasicek TJ, Hood L. Cancer Res. 2005;65:3081. doi: 10.1158/0008-5472.CAN-04-3218. [DOI] [PubMed] [Google Scholar]
- 42.He F, Sun YE. Int J Biochem Cell Biol. 2007;39:661. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Hart MJ, Callow MG, Souza B, Polakis P. EMBO J. 1996;15:2997. [PMC free article] [PubMed] [Google Scholar]
- 44.Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R. J Biol Chem. 2004;279:48495. doi: 10.1074/jbc.M408113200. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, McNulty DE, Marler KJM, Lim L, Hall C, Annan RS, Sacks DB. J Biol Chem. 2005;280:13871. doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.