Violent trauma and road traffic injuries kill more than 2.5 million people in the world every year. It has been calculated by the World Health Organization that in 2002 there occurred 1.6 million violent deaths [1] and 1.2 million deaths from traffic injury [2], for a combined mortality of 48 deaths per 100,000 population per year.
Most trauma deaths occur at the scene or in the first hour after trauma, with a proportion from 34% to 50% occurring in hospitals [3]. These deaths could be prevented by optimization of trauma care. Preventability of trauma deaths has been reported as high as 76% [4] and as low as 1% in mature trauma systems [5,6].
Prehospitalization procedures, elapsed time to hospital arrival are, of course, vital to the whole trauma scenario, but errors made in the in-hospital phase of care are responsible for one third to two thirds of the reported by different authors [7,8]. Of these, intensive care unit (ICU) errors are among the most frequent and significant. Errors in the ICU management of trauma patients were studied by Duke and colleagues [9]. They reported 165 ICU trauma deaths. Two hundred fifty-eight errors occurred in 81 patients (52%), and 134 of them contributed to death in 52 patients (34%). ICU errors were classified as management errors (82%), diagnostic (9%), technique (5%), and system inadequacies (4%). Davis and colleagues [10] identified critical care errors in 30% of 125 trauma deaths with errors. These errors contributed to 48% of all preventable deaths.
The most common critical care errors are related to airway and respiratory management, fluid resuscitation, neurotrauma diagnosis and support, and delayed diagnosis of critical lesions [9,10].
It is imperative for the general surgeon who takes care of trauma patients to know how to deal with these critical aspects, to reduce preventable morbidity and mortality. In the next segment, the situations in which the participation of the surgeon is crucial, during the initial phases of reanimation and stabilization of the critically traumatized patient will be discussed.
Airway and ventilation management
Airway and respiratory management errors are the most common of those identified by several authors [9–11]. The mechanically ventilated trauma patient may experience alterations in oxygenation as a result of the trauma itself or because of complications of therapeutic maneuvers. The source must be identified and treated expeditiously, to avoid additional injury, particularly in patients with encephalic trauma.
The cardinal manifestation is a sudden or a rapidly progressing desaturation, frequently accompanied by tachycardia and arrhytmias, and occasionally by agitation. Hypertension announces the cardiovascular collapse, and bradicardia appears immediately after the total collapse [12]. The symptoms should not be attributed to agitation when it is present, and other possible causes must be ruled out before. Diagnosing the complication involves a directed physical examination, the analysis of the airway pressures and ventilator volumes, chest radiographs, and sometimes the measurement of arterial blood gases (ABG), and the urinary bladder pressure (Table 1).
Table 1.
Differentiation of airway and ventilation crises
| Condition | Peak pressure | Plateau pressure | Clinical finding |
|---|---|---|---|
| Low or normal peak inspiratory pressure | |||
| Extubation/proximal tube migration |
Low | Low | Noises and saliva bubbles in the mouth |
| Disconnection | Low | Low | Disconnection from the ventilatory circuits |
| Negative pressure | Low | Low | Agytation. Low pressures alternating with high pressures |
| High peak inspiratory pressure | |||
| Artificial airway obstruction |
High | Normal | Cuff deflation test: minimal change |
| Patient’s airway obstruction |
High | Normal | Cuff deflation test: scape and pressures reduction |
| Atelectasis | High | High | Asymmetry. Chest X-rays confirmation |
| Acute lung injury | High | High | Chest X-rays confirmation |
| Tension pneumothorax | High | High | Asymmetry. Needle confirmation |
| Massive diaphragmatic hernia |
High | High | Asymmetry. Chest X-rays confirmation |
| Intraabdominal hypertension |
High | High | Symmetric diminished breath sounds. Elevated intravesical pressure |
The emergency conditions in which the access to the airways must be gained, the displacement to diagnosis areas or operating room and the agitation, often present, make the critical trauma patient prone to airway complications [13–16]. Accidents of the airway cause preventable deaths and increase morbidity, length of stay, and costs [17–20]. Adequate staffing and protocolization could have avoided more than the half of them [20–22].
Disconnection, accidental extubation, and proximal orotracheal tube migration
Disconnection and extubation are easily recognized by the rapid deterioration of the patient, and the ventilator alarms of low pressure and gas leak. Reintubating the patient, after oxygenating him or her with mask-bag manual ventilation, is the treatment for the accidental extubation.
Migration of the tube to the pharynx produces a subtler clinical picture. The most remarkable physiopathologic alteration is alveolar hypoventilation due to gas leak. Occasionally, the loss of positive pressure produces hypoxemia in patients who need positive end expiratory pressure (PEEP). The appearance of saliva bubbles in the mouth must cause suspicion. The ABG will show hypercarbia, and the ventilator will indicate low airways pressure and low expired gas volume. The diagnosis is confirmed by direct laryngoscopy, and the complication is treated by replacing the tube. Proto-cols of endotracheal tube securing and ventilator circuit checking should avoid these complications [21,23,24].
Airway obstruction
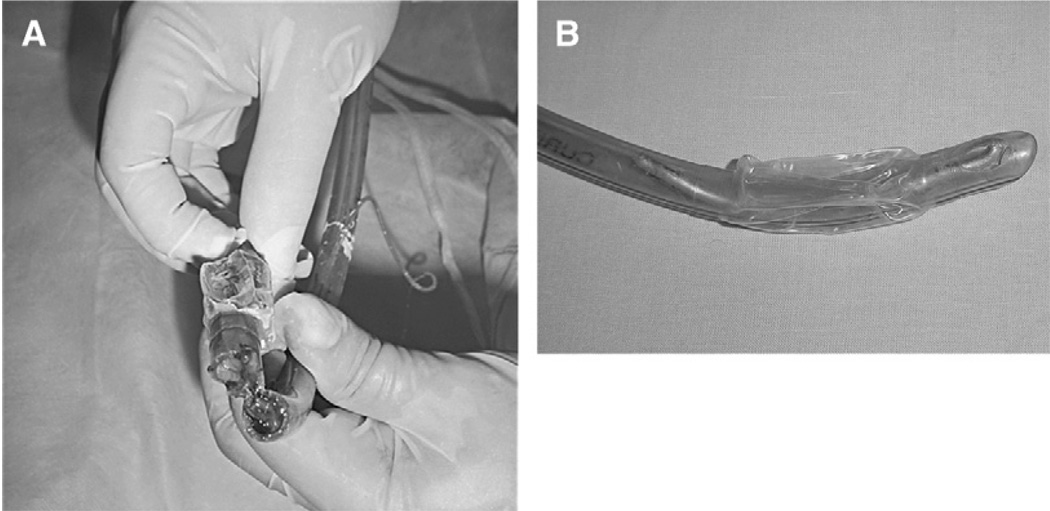
Acute airway obstruction can develop in the trauma mechanically ventilated patient at any level, from different causes: artificial airway may occlude by kinking, biting, impacted secretions, or clots; trachea and bronchii tubes by hematoma, clots, or secretions; and small airways by secretions or bron-chospasm [25,26]. Foreign bodies can obstruct the airways at any level [27] (Fig. 1). Manifestations include inspiratory effort, elevated peak inspiratory pressure, low level of plateau pressure (this difference results from the increased resistance to airflow), and decreased tidal volume. Oxygenation is compromised as well as CO2 excretion [28]. Chest radiographs may not reveal any acute abnormality, unless clots or secretions have caused an atelectasis.
Fig. 1.
(A) Orotracheal tube occluded by clots in a patient with lung contusion and hemoptysis. He developed sudden dyspnea, agitation, and desaturation. The peak airway pressure and plateau pressure was 38 and 28 cm H2O, respectively. A suction catheter passed easily. The patient improved after changing the occluded tube. (B) Orotracheal tube occluded by a segment of a broken guide, in a BTI intubated in the emergency room. The mechanical ventilation was difficult and airway obstruction was suspected because of high peak airway pressure and normal plateau pressure. The patient improved after changing the tube.
Kinking and biting are easily detected and corrected. Differentiating obstruction in the artificial airway or distal to it is critical, and often difficult to do. Passing a suction catheter into the endotracheal tube may detect an obstruction in it. Nevertheless, the distal advance does not exclude the endotracheal tube occlusion by clots or impacted secretions. The cuff deflation test [28] may help to find the obstruction site: the cuff of the endotracheal tube is deflated while the patient continues being ventilated. When the obstruction locates distal to the tube a dramatic reduction in the peak inspiratory pressure and a marked leak are observed. On the other hand, when the endotracheal tube is occluded, high peak inspiratory pressure persists and there is minimal leakage. Occasionally, the only way to be sure of the obstruction of the tube is by changing it. Broncoscopy may be required.
Acute reduction of respiratory system compliance
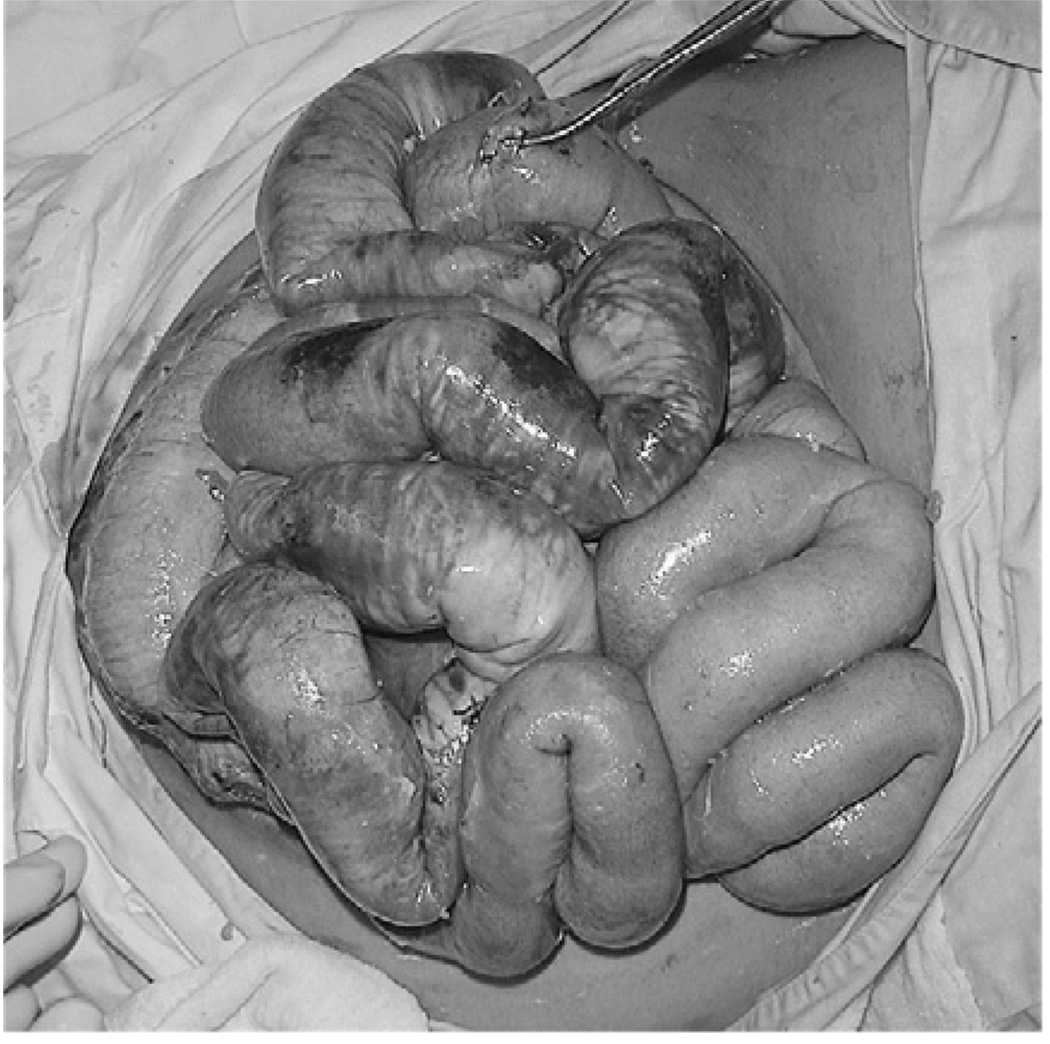
A group of mechanically ventilated trauma patients exhibit a picture of rapidly progressive elevation of the peak inspiratory pressure, with a parallel increase of the plateau pressure, reduction of the tidal volume, and variable hypoxemia and hypercarbia. This complex may be attributed to acute lung injury or atelectasis. Nevertheless, it may correspond to an extrapulmonary cause, that is, tension pneumothorax [29,30], massive diaphragmatic hernia [31–34], or intraabdominal hypertension [35–37]. Clearly, diagnosing these entities has critical transcendence, as they cause rapid hemodynamic deterioration and are susceptible to specific surgical treatment (Fig. 2).
Fig. 2.
Segmental intestine necrosis in a patient with peritonitis after penetrating abdominal trauma and late surgical decompression of the abdominal cavity. The respiratory manifestations were managed with oxygen 100% and high PEEP levels; the hemodynamic instability with IV fluids and norepinefrine infusion. As a consequence, the intraabdominal hypertension syndrome was diagnosed and treated late.
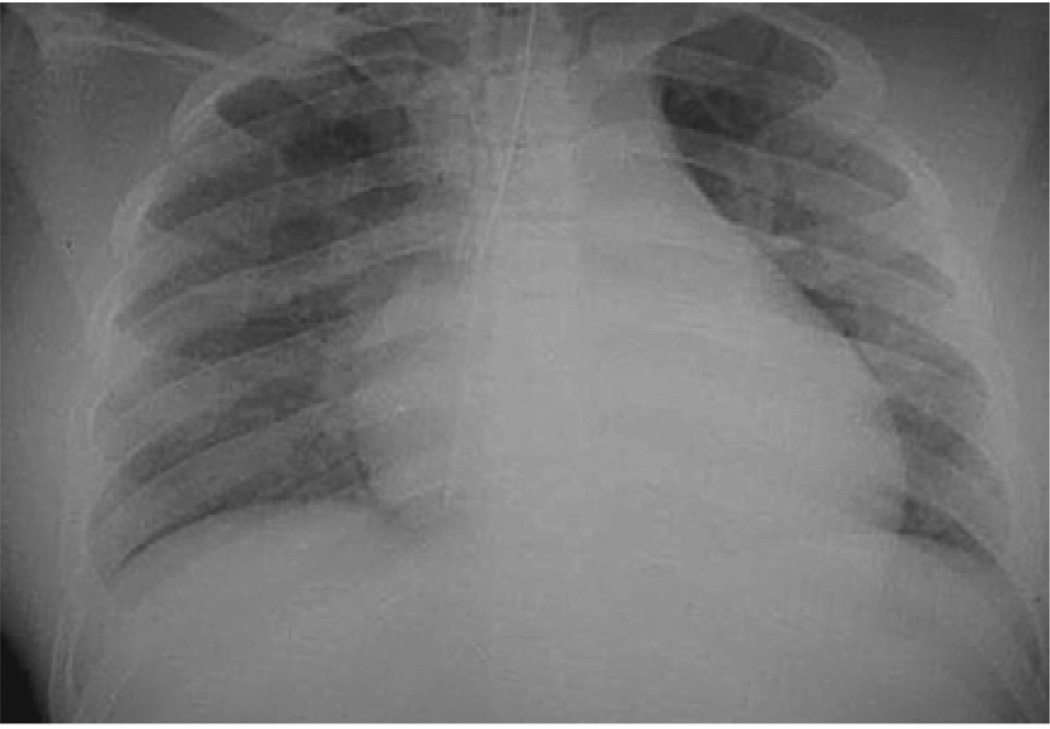
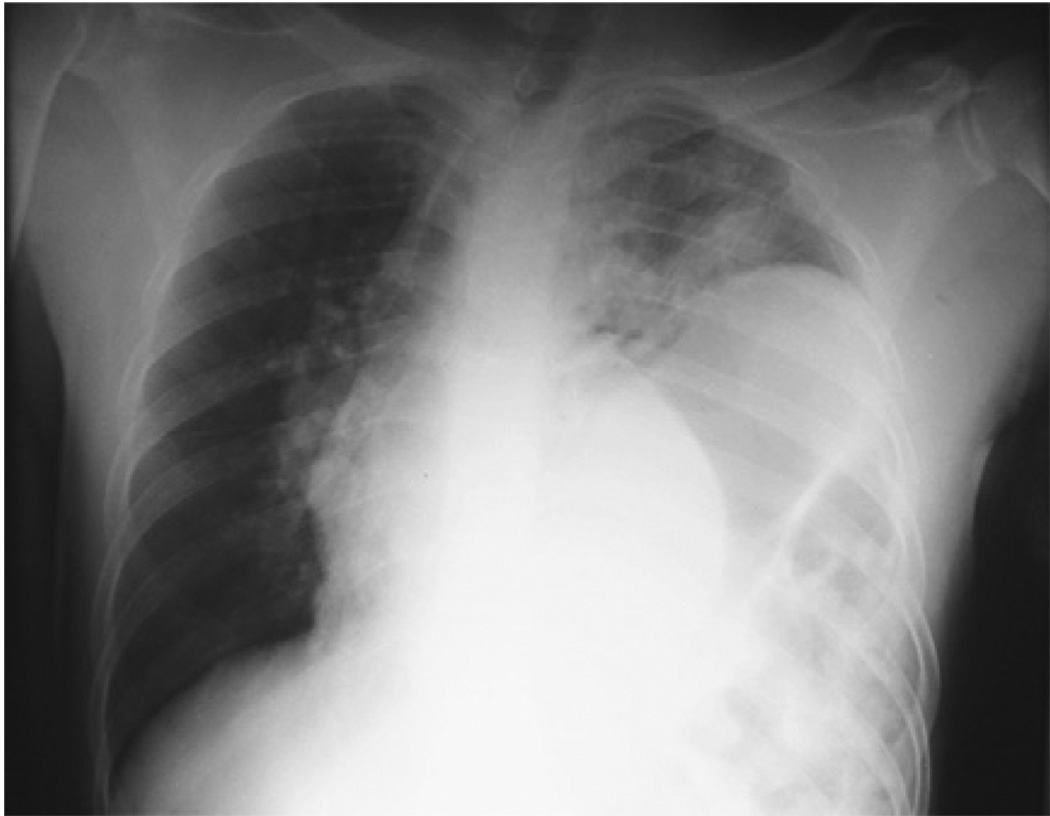
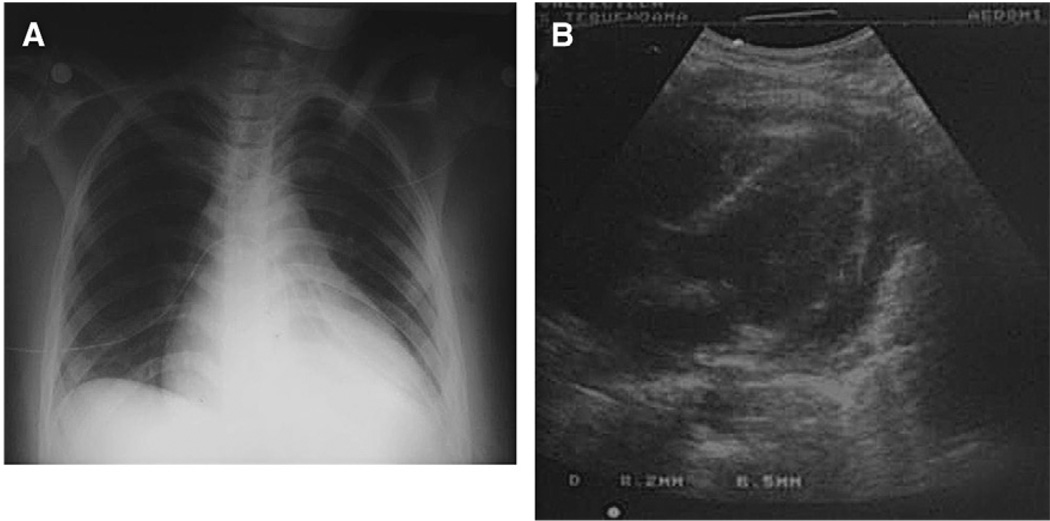
The elevation of the plateau pressure discards airway obstruction, and some clinical clues permit us to diagnose or to suspect specific entities: thorax asymmetry with an increase of chest volume on the compromised side, tracheal deviation to the opposite side, resonance, and diminished breath sounds of the compromised side in tension pneumothorax; tracheal deviation to the compromised side, decreased chest volume, dullness, and decreased breath sounds in massive atelectasis (Fig. 3), and increase of the chest volume of the compromise side, tracheal deviation to the opposite side, dullness, and diminished breath sounds of the compromised side in massive diaphragmatic hernia (Fig. 4).
Fig. 3.
Monobronchial intubation in a BTI patient. He was intubated in the emergency room and transported to the ICU. In the first evaluation there were desaturation, tracheal deviation to the left, and diminished breath sounds in the left hemythorax. Chest radiographs shows the tip of the tube in the main right bronchi, and signs of loss of volume in the left lung.
Fig. 4.
Massive left diaphragmatic hernia. The patient was dyspneic, tachycardic, and had tracheal deviation to the right and diminished breath sounds of the left hemythorax. (Courtesy of Diego Rivera, MD, Hospital Universitario del Valle, Cali, Columbia.)
Tension pneumothorax is confirmed by the insertion of a 12 Fr intravenous (IV) catheter in the second intercostal space. A chest radiograph gives clues to differentiate the other entities. Compartment abdominal syndrome diagnosis is confirmed by measurement of bladder pressure. Definitive treatment is made by surgery.
Evaluation and treatment of the circulatory state
The objectives of the circulatory assessment during the initial evaluation of the trauma patient (recognizing the state of circulatory shock, identifying the bleeding source, controlling the hemorrhage, and replacing IV fluids and eventually blood components), must be kept during critical care reanimation. Some of the most common errors reported during this phase of the treatment are related to the failure in the accomplishment of these principles [7,9,10].
Recognizing a state of shock
Relying exclusively on the vital signs to diagnose hypovolemic shock may be misleading. Supine tachycardia or hypotension are specific but not sensitive to detecting moderate bleedings. Sensitivity of supine hypotension (blood pressure <95 mm Hg) was only 33%, while specificity was 96%, to detect a blood loss of 630 to 1150 mL, in healthly volunteers. In other words, the presence of these signs confirm hypovolemia, but their absence do not discard it [38]. Celoria and colleagues [39] failed to predict a low wedge pressure or a low cardiac output, based on clinical parameters in one third of a group of surgical critical patients; additionally, most of the patients in whom they predicted low wedge pressure or cardiac output, had normal values. Shoemaker and colleagues [40] showed that vital signs were not able to differentiate survivors from nonsurvivors in a group of shock patients. Abou-Khalil and colleagues [41] found evidence of persistent hypoperfusion in 80% of critically traumatized patients under hemodynamic reanimation, despite the normalization of their vital signs.
Hypoperfusion, frequently resulting from a low cardiac output (compared with the required for that current physiological status) [42], causes cellular damage, activation of inflammatory response, and increases multiple organ dysfunction syndrome and death risks, if not corrected in a few hours [43–48].
The aforementioned arguments constitute the rational basis for perfusion monitoring and perfusion-based resuscitation in trauma patients, and for invasive monitoring when the hypovolemia or hypoperfusion do not correct themselves despite adequate volume substitution.
Volume reposition
There has been a lot of controversy about the fluid that must be employed in the resuscitation of hemorrhagic shock patients.
When compared with crystalloids, colloid solutions expand faster in the intravascular space, and remain there longer [49,50], which in turn, produces a quicker restoration of the cardiac output and oxygen transport variables, with less infused volume and less edema formation. When endpoints such as mortality and morbidity are examined, colloids and crystalloids are equally effective. Roberts and colleagues [51] found in a meta-analysis, a pooled relative risk from 1.02 (95% confidence interval, 0.93 to 1.11), in 42 analyzed controlled clinical trials, with a total of 7576 patients included. The same results were achieved when the analysis was performed for each different colloid. In a large randomized controlled clinical trial published recently, albumin 4% or normal saline were administered during the first 4 days of the treatment of critical care patients. The study included 6997 individuals. Mortality was the same in both groups, as well as the complications possibly attributable to the fluid regimes. The differences in the amount of fluid administered were small [52].
In view of the proven absence of benefits of colloids, the absence of harm of crystalloids, and the considerable expense difference favoring crystalloids, these solutions must be considered the first choice for the resuscitation of trauma patients [53].
The required volume to resuscitate a trauma patient exceeds the basal needs for several reasons: at least two thirds of the given crystalloids are re-distributed to the interstitium, making it necessary to administer three times the lost volume; the shift of fluids to the interstitium will continue from a few hours to several days, in proportion to the depth and duration of the shock [54,55]. Additionally, the patient may still have some degree of active hemorrhage. In consequence, the needed volume cannot be predicted, and must be carefully titrated to reach specific goals to avoid persistent hypovolemia or excessive fluid supply.
Endpoints for the resuscitation
Correcting overt signs of hypovolemia (hypotension, tachycardia, altered mental status, and oliguria) constitutes the first step in the resuscitation of a trauma patient. However, achieving these goals cannot be considered satisfactory, because, as already mentioned, they fail to identify hidden hypoperfusion in the state of compensated shock. In consequence, resuscitation directed to the early identification and correction of perfusion deficit has been proposed.
Early monitoring with a pulmonary artery catheter has been advocated, and resuscitation toward supraphysiologic values of cardiac index, oxygen delivery, and oxygen consumption has been proposed, and several controlled clinical trials have been performed. Their results were consolidated in three meta-analyses. The first one found methodologic limitations of the primary studies, which made it difficult to reach solid conclusions [56]. The authors found a nonstatistically significant trend toward a reduced mortality, a shorter ICU stay, and a significant mortality reduction in the experimental group, when the analysis was limited to the studies in which the hemodynamic optimization started before the surgical procedure. Ivanov and coworkers [57] found in their meta-analysis a significant reduction in morbidity when the hemodynamic resuscitation of critically ill patients was guided by the pulmonary artery catheter. Kern and Shoemaker [58] re-viewed the impact of hemodynamic optimization, directed to supranormal values, in high-risk patients. They concluded that the mortality was reduced in the studies in which the treatment was instituted before organ failure was established, and when the mortality of the control group exceeded 20%.
It seems that the hemodynamic optimization only can be beneficial if started early after the trauma and is directed to the correction of the underlying perfusion deficit. So, markers of anaerobic metabolism, such as lactate or base excess, must be evaluated and followed to trace the recovery of shock at the cellular level.
Lactic acidosis has been associated with alterations in oxygen supply for the past 50 years [59,60]. Its prognostic value in hemorrhagic shock was established in terms of identifying the risk of death as well as in predicting infectious complications and multiorgan failure [61,62]. Several authors have shown that the early clearance of the lactic acidosis correlates with a better chance of survival. All patients who corrected the acidosis in the first 24 hours survived. Mortality increased as the time elapsed to normalization was longer [63,64].
Other measurements of metabolic acidosis have shown a correlation with mortality [65,66]. The most extensively studied is base deficit. It has been shown in animal and clinical studies of hemorrhagic shock that the severity of metabolic acidosis determined by this method correlates with a higher probability of death [67–69], as well as with a higher risk of multiple organ dysfunction syndrome [43,48]. The velocity of normalization of the base deficit has not been related with the prognosis. Lactate and base deficit have been compared. They showed a good correlation in the first evaluation [65], but lactate showed a better performance after the resuscitation with IV fluids, and in the identification of compensated shock [64].
Regional analysis of perfusion could give the most sensitive approximation [70]. Preliminary reports regarding regional CO2 measurement in sensible microvascular beds, as manifestation of hypoperfusion, are promising [71–73]. Measurement of oxygen tension or saturation in certain tissues has permitted the identification of occult hypoperfusion in animal studies and in some limited clinical experiences published [71,74–78]. Unfortunately, these methods have not been fully evaluated, and none of them has reached widespread use.
Transfusion threshold and coagulopathy correction
The transfusion of red blood cells is a common practice in trauma patients. It was found in a multicenter trial that 55% of the trauma patients who were admitted to the ICU received transfusions during their stay [79].
The decision of transfusing red blood cells (RBCs) is, in part, motivated by the aim of improving the oxygen-carrying capacity of the blood and reducing the oxygen debt: “the higher hemoglobin level is the best.” This concept has been challenged by the understanding of the tolerance of low levels of hemoglobin by the critically ill patient, and by the knowledge of the detrimental effects of transfusion in terms of immunosuppresion [80,81], increased risk of infection [82], increased risk of blood-borne infection acquisition [79], increased risk of multiorgan failure [43,48], and death [83], when compared with matched critically ill patients.
The threshold for RBC transfusion was examined in a randomized clinical trial, in which 838 euvolemic critically ill patients with hemoglobin levels below 9.0 g/dL within 72 hours after admission were assigned to receive RBC transfusions to maintain the Hb concentration between 10.0 and 12.0 g/dL (Liberal group), or to receive RBC transfusions only when Hb concentration fell below 7.0 to maintain the Hb concentration between 7.0 and 9.0 g/dL (Restrictive group). Overall 30-day mortality, length of stay, and morbidity were not different for either group [84]. Hill and co-workers [85], who undertook a meta-analysis comparing liberal and restrictive transfusion strategies, confirmed the conclusions reached by these researchers. They found an additionally 40% reduction in exposure to transfusions in the patients allocated in the restrictive groups.
The current recommendation for critical care patients younger than 55 years and without significant heart disease is to administer transfusions to maintain hemoglobin concentrations between 7.0 and 9.0 g/dL.
Coagulopathy is a common event in trauma patients. Its origin is multicausal, and the main etiologic factors are hypothermia, fibrinolysis activated by tissular trauma and by endothelial damage, and dilution of coagulation factors and platelets [86–91]. Occasionally, a traumatized patient has a pre-existing condition such as liver or hematologic diseases or anticoagulant treatment that can predispose to bleeding [92], and must be identified in the anamnesis.
The entity is recognized by bleeding from different places such as intravenous sites, the nasogastric tube, the vesical catheter or the surgical drains, and nonmechanical bleeding in the surgical field. This complication as so many other things is better to prevent than to have to treat later. Prevention is accomplished by hypothermia prevention and treatment [93–97] (Box 1) and shock limitation, which in turn, is guaranteed by rapid interruption of the hemorrhage and aggressive hemodynamic resuscitation. Prophylactic administration of platelets or plasma usually do not prevent coagulopathy [98,99].
Box 1. Hypothermia prevention and treatment
Rationalize ICU temperature
Keep the patient’s skin dry
Avoid unnecessary body exposition
Limit cavities exposition
Administer warmed fluids and blood
Administer humidified gases
Correct shock as early as possible
-
Actively warm the patient.
Surface warming (forced warmed air and resistive warmers, better than circulating water mattresses)
Continuous arteriovenous rewarming (very effective)
Cardiopulmonary bypass (the most effective)
Cavities rewarming (not recommended)
The characterization of coagulopathy is made in most of the cases by simple laboratory tests: platelet count, activated partial thromboplastin time, prothrombin time-international normalized ratio, and fibrinogen concentration, which guide the administration of fresh frozen plasma, platelets, and cryoprecipitate [87–91]. Damage control surgery is a powerful ancillary tool in the coagulopathic patient who is being operated on. It permits the expeditious control of the nonmechanical bleeding, limits the extension of the surgery, and stops the vicious circle of bleeding–hypothermia–coagulopathy.
The hypotense or hypoperfused trauma patient in the ICU
Frequently, a critically traumatized patient shows hypotension or persistence of the indicators of hypoperfusion. It often results from a mismatch between the fluids required and the fluids administered. This situation must be distinguished from occult bleeding and from intrathoracic hypertension that may result from a tension pneumothorax [29,30], a massive diaphragmatic hernia [31–34], or intra-abdominal hypertension [36,37]. On rare occasions cardiac tamponade will result from a missed cardiac wound or from liquid accumulation in the pericardial sac after a wound or an incision (Table 2) [100–102].
Table 2.
Differential diagnosis of the hypotense or hypoperfused trauma patient
| Condition | Filling pressures | Airway pressures | Observations |
|---|---|---|---|
| Ongoing hemorrhage | Low | Low or normal | Hb drops Source of bleeding is found if looked for |
| Fluids shifts, or vasodilation |
Low | Low or normal | Hb does not drop Source of bleeding is not found |
| Myocardial depression or contusion |
High | Low or normal | Echocardiogram shows contractility alterations |
| Cardiac tamponade | High | Low or normal | Subxyphoid echo or subxyphoid pericardial window identify pericardial effusion |
| Tension pneumothorax or massive diaphragmatic hernia |
High | High | Clinical diagnosis Radiologic confirmation if clinical signs are nor clear |
| Intraabdominal Hypertension |
High | High | Oliguria Elevated intravesical pressure |
Persistent hemorrhage must be identified and treated immediately. It is easily recognized when there is external bleeding coming from the drains or the surgical wound (Fig. 5). It is more difficult to recognize when the patient has not been operated on, or when the drains or the chest tube become occluded by clots. In these cases the reexamination of the patient, aided by a chest radiograph, an ultrasound directed to detect liquid in the cavities [103–105], and a high suspicion index will help to diagnose the complication. The treatment should not be delayed, and will consist of surgical exploration with the strong consideration of performing damage control procedures. Angiographic embolization may be employed in selected cases; which depends on the experience of the team in these cases and the resources available (Fig. 6) [106–112].
Fig. 5.
Patient with massive bleeding due to a gunshot wound to the liver. It was controlled by a catheter with a balloon. The photography shows blood coming from the wound. The patient was reexplored and a wound of a diaphragmatic vein was found and treated.
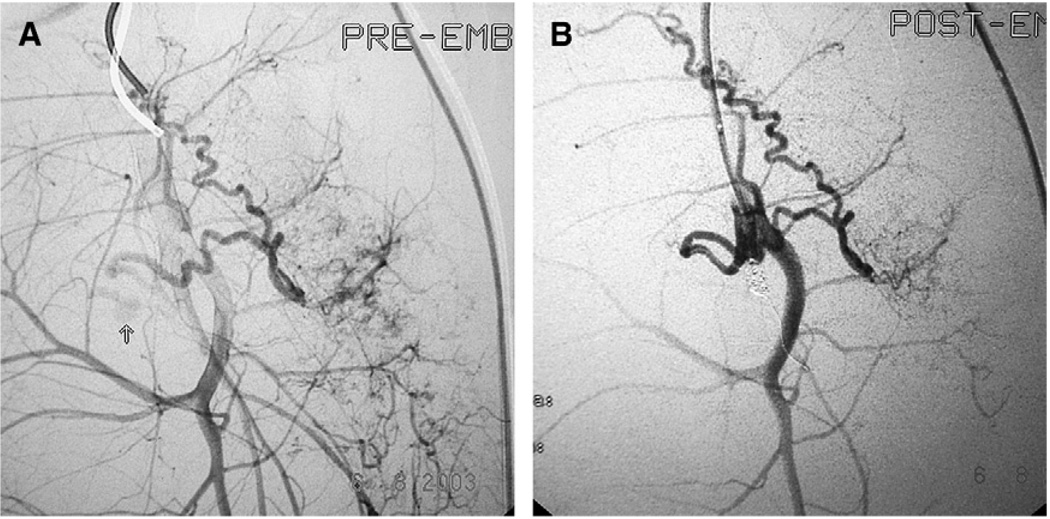
Fig. 6.
Bleeding control in a patient with pelvic fracture. (Courtesy of Diego Rivera, MD, Hospital Universitario del Valle, Calle, Columbia.) (A) The arteriography identifies a torn artery bleeding. (B) The arteriography after coils embolization shows that the bleeding has stopped.
Conditions that course with elevation of the intrathoracic pressure decrease the venous return and the cardiac output, with potential disastrous consequences that will not subside unless the cause be removed. The hemodynamic profile includes low cardiac output, high filling pressures, and high peripheral vascular resistances. The elevated intrathoracic pressure hinders lung expansion and oxygenation. The mechanically ventilated patient shows elevated airway pressures, hypoxemia, and sometimes hypercarbia [31,36,37]. The physical examination can identify asymmetry in tension pneumothorax and diaphragmatic hernia. As the presence of a chest tube does not preclude the development of a pneumothorax (Fig. 7), the presence of a silo in the abdominal wall does not prevent abdominal hypertension (Fig. 8). The diagnosis is based on clinical grounds in some instances, but in the cases of diaphragmatic hernia and abdominal hypertension a chest radiograph and the urinary bladder pressure must be obtained. The correct treatment must be performed immediately, and sometimes, due to the enormous instability, the surgical procedure must be performed in the ICU [113,114].
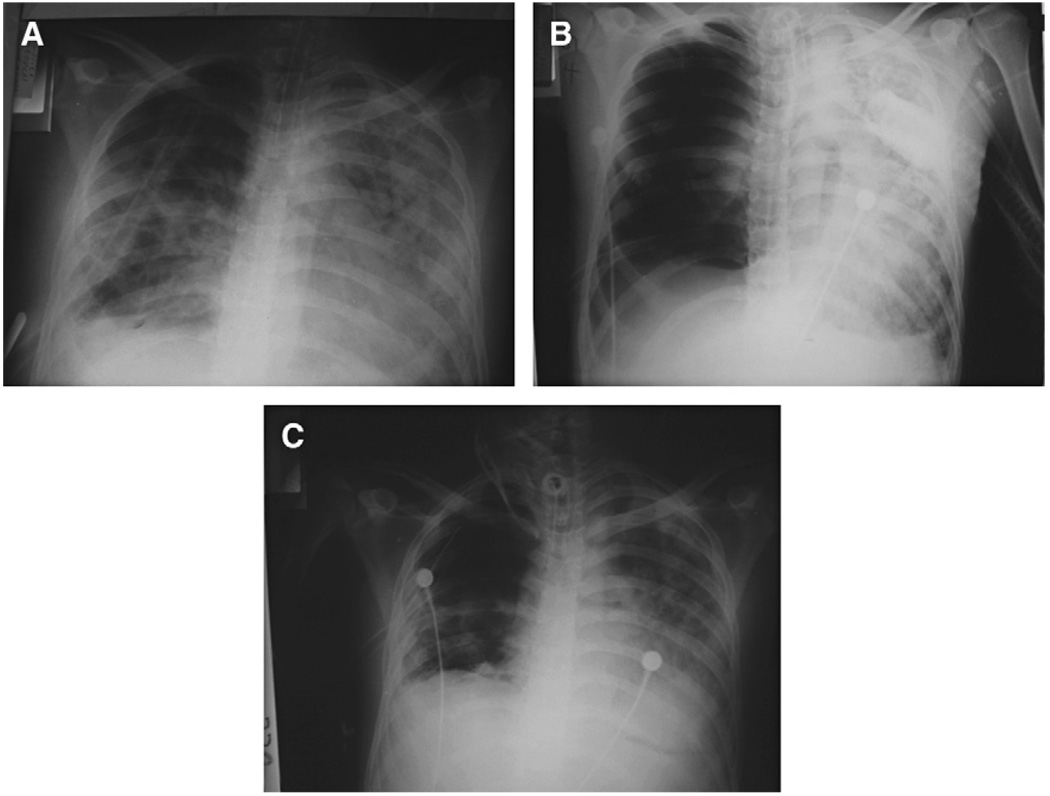
Fig. 7.
Tension pneumothorax in a patient with a chest tube. (A) Chest radiographs 5 days after a right thoracotomy and tractotomy of the three lobes. (B) Chest radiographs 3 days later. A massive right pneumothorax with left deviation of the mediastinal structures, compression of the left lung, and a chest tube are seen. The patient looked restless and dyspneic. He had tachycardia and resonance of the right thorax, with diminished breath sounds. (C) Chest radiography after the insertion of a second chest tube.
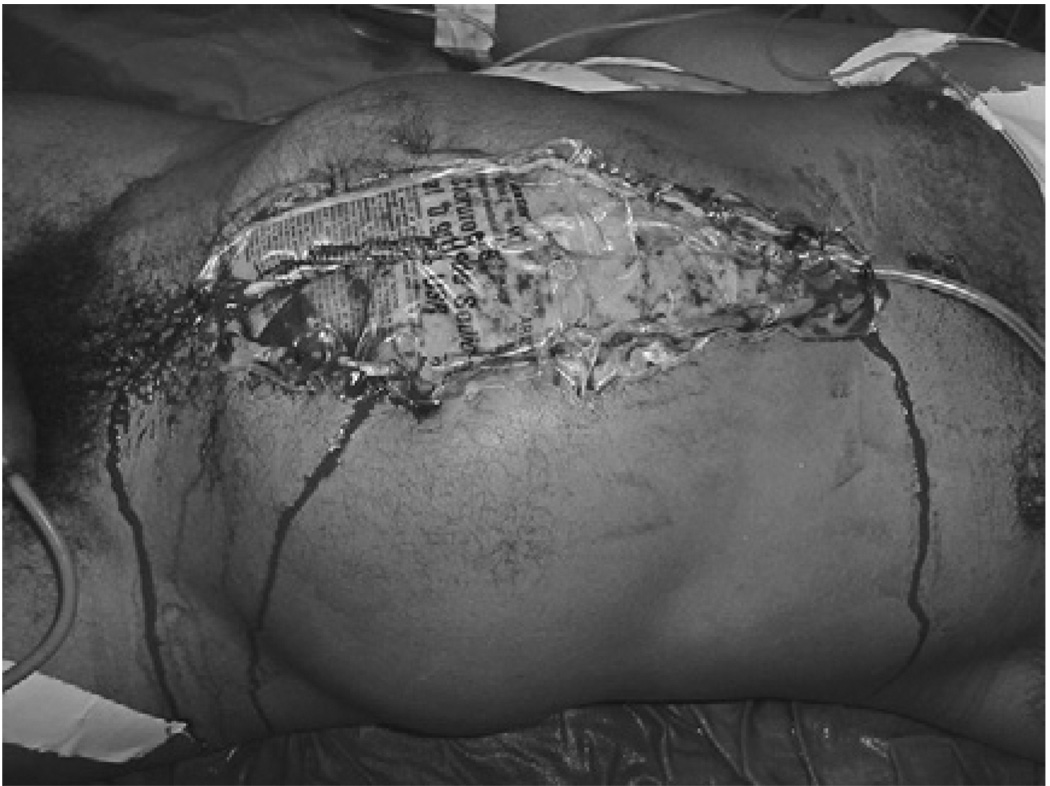
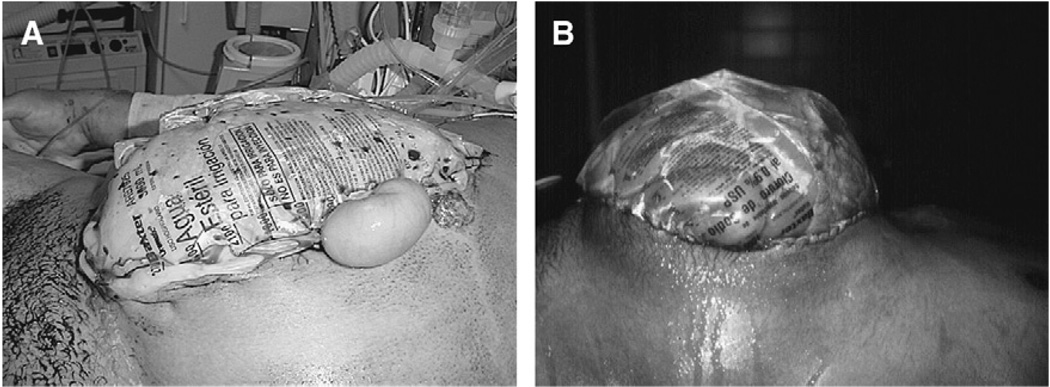
Fig. 8.
A second episode of intraabdominal hypertension in a patient with intraabdominal hypertension after massive fluid resuscitation for an extraabdominal trauma. (A) Eighteen hours after a laparotomy and collocation of a Bogota Bag. The patient developed a scenario of difficult ventilatory support, with progressive elevation of the airway pressures, requirement of high oxygen concentrations, and high PEEP levels; hypotension despite IV fluids and high dose of norepinefrine. The urinary bladder pressure risen to 28 cm H2O. (B) After the decompression the respiratory and hemodynamic changes reverted to normal. The patient requires a bigger silo than before.
Cardiac tamponade must be suspected in the presence of elevated filling pressures. Neck vein distension diminished heart sounds and paradoxal pulse are absent in most cases. Subxyphoid echo will confirm the diagnosis [115], and subxyphoid pericardial window will be diagnostic in traumatic hemopericardium and therapeutic in inflammatory pericardial effusion (Fig. 9) [100,101,116,117].
Fig. 9.
Cardiac tamponade of late presentation. The patient had received a precordial stab-wound. A left chest tube was inserted because of hemothorax. A subxyphoid window did not show pericardial effusion. (A) The patient developed dyspnea 5 days later. The chest radiograph shown enlargement of the cardiac silhouette. (B) A new subxyphoid echo showed a pericardial effusion. The patient was treated with a subxyphoid pericardial window; 400 cc of sterile liquid were obtained.
Independently of the cause of the hypotension or the hypoperfusion signs, the monitoring must be increased and include at least a radial artery catheter and a central venous catheter. The infused volume must be titrated to reach specific goals: the mean arterial blood pressure must be of 65 mmHg or more during the first 30 minutes, and the lactate and base deficit must show a clear trend to normalize in the first 6 hours, with complete normalization at the end of the first day (Table 3).
Table 3.
Endpoints of the resuscitation
| Parameter | Goal | Observations |
|---|---|---|
| MAP | >65 mm Hg in the first 30 minutes |
TBI patients need MAP >70 mm Hg |
| Lactic Acidosis | <2.1 mmol/L | Trend to reduction in the first 6 hours Complete clearance in 24 hours |
| Base deficit | >−5.0mEq/L | Trend to reduction in the first 6 hours Complete clearance in 24 hours |
| Hemoglobin | 7.0–9.0 g/dL | Patients with significant heart disease or older than 55 years may need higher levels |
| SatP | >94% | With the lowest PEEP that permits keeping Fio2<0.6 |
Abbreviations: MAP, mean arterial pressure; PEEP, positive end expiratory pressure; TBI, traumatic brain injury.
To avoid infusing excessive amounts of IV solutions and its complications, the condition of responsiveness to volume must be identified [50,118–120]. The filling heart pressures have been used traditionally to accomplish this task, being that the pulmonary artery wedge pressure is considered as the standard. Other methods such as the right ventricular end-diastolic volume, measured with a specially designed pulmonary artery catheter or the aortic blood velocity, and left ventricle end-diastolic area determined by echocardiography have been tested [121]. The accuracy of all these static variables to determine the status of responsiveness is low, motivating the search of dynamic, more reliable measurements, of which the variations of the arterial pressure with the respiratory cycle have proven to be accurate and easy to obtain [122,123]. Other objectives to be accomplished in the initial hours is a hemoglobin concentration > 7.0 g/dL and a pulse oximetry (SatP) >94%.
A pulmonary artery catheter must be considered in the first few hours if there is not a clear response. This is to facilitate the decisions about the amount of fluids to be given and the necessity of administering inotropes if the cardiac output is judged not to be enough despite an adequate status of intravascular volume or vasopressors if the cardiac output is good but hypotension persists, due to a very low peripheral vascular resistance.
Initial neurologic evaluation of the trauma patient
Errors in diagnosis, monitoring, and treatment of brain trauma patients are among the most frequently reported in the intensive care phase treatment of the trauma patient. Of the errors reported by Duke and colleagues [9], 54% contributed to death. In the publication by Davis and coworkers [10], 60% of the monitoring errors were classified as neurologic, while 12% of the management errors corresponded to this category. One fourth of the neurologic errors of this study contributed to death.
Usually the condition of severe brain trauma (Glasgow coma scale of 8 or less) has been diagnosed in the prehospital phase or in the emergency room. In these cases the tracheal intubation has been achieved previously and the patient is transferred to the ICU directly from the computed axial tomography (CT) suite if surgery is not indicated, or from the surgical theater when a surgical operation was required. Occasionally, the cause of the emergency intervention has been extracranial, and in such cases it is possible that the CT had not yet been done. Usually the possibility of an intracranial lesion has been ruled out with measurement of the intracranial pressure (ICP), completed by an air ventriculography [124]. If an intracranial lesion has not been ruled out, the arrangements to perform the CT must be made.
The optimal resuscitation constitutes the first step in the optimal treatment of the brain trauma patient, in virtue of the acknowledged deleterious role of secondary insults such as hypotension and hypoxia [125,126]. Maintaining SatP at a level >94%, the systolic blood pressure above 90 mm Hg, the mean arterial blood pressure above 70 mm Hg, and the PaCO2 around 35 mm Hg seem reasonable goals [124].
The oxygenation goal must be rapidly obtained by manipulating FiO2, while PEEP is titrated. Concerns about the worsening of ICP with PEEP have not been confirmed [127,128]. In any case, it must be titrated at the lowest possible level. Sedation must be used to permit ventilatory support, and contribute to lower ICP. Care must be taken to avoid hemodynamic instability due to an excessive dose of sedatives. Neuromuscular blockade should be used only if indispensable, as continuous protracted relaxation, used to facilitate the ICP management, does not improve the results, pro-longs ICU stay, and increases complication risks [129].
Isotonic crystalloids (preferable normal saline), should be used to reach the blood pressure goal. Vasopressors should be used briefly to sustain blood pressure, while the volume resuscitation is performed. If continuous administration is necessary due to hypotension despite the absence of hypovolemia, then phenyleprine or noradrenaline are preferred [130,131].
The patient must be maintained normothermic, as deliberate hypothermia has failed in improve the prognosis of patients with traumatic brain injury [132,133]. The head must be elevated, after the normovolemia has been restituted, because under these circumstances it improves ICP and cerebral perfusion pressure (CPP). The torso must not be flexed until the spine has been cleared, so the head will be elevated by reversed Trendelenberg position [130].
The ICP measurement must be started as early as can be permitted by the patient’s stabilization process. The ICP is managed with the goal of main-taining CPP above 50–60 mm Hg. The ICP threshold above which interventions are warranted is 20 mm Hg [124], and they are applied in a sequential fashion, with the addition of a new treatment when the present one is inadequate (Box 2).
Box 2. Management of the traumatic brain injury patient
Basic management
SatP >94%
Paco2 around 35 mm Hg
MAP >70 mm Hg
Normothermia
Head elevated to 30° (if normovolemic)
ICP management goals
ICP <20 mm Hg
CPP >50 mm Hg
First line measures to control ICP
Intermittent ventricular drainagea
Hyperventilation to Paco2 30–35 mm Hg
Mannitol bolus (0.25 g/Kg)a
Second tier therapies for intracranial hypertension
Barbiturates
Hypothermia
Optimized hyperventilation (Paco2 to 25–30 mm Hg)
Decompresive craniotomy
CPP, cerebral pressure of perfusion; ICP, intracranial pressure; MAP, mean artery pressure; SatP. pulse-oximetry.
a Consider hypertonic saline
When intracranial hypertension is diagnosed, ventricular drainage is the first intervention to be employed if available [124]. When other ICP measurement methods are employed, an intraventricular catheter must be inserted. Its safety and effectiveness have been proven: the risk of hemorrhage associated with its insertion is low, and the risk of infection less than 2% [134]. The drainage must be intermittent, with a maximum of five times per hour, with a duration of 2 minutes each. Continuous drainage makes the ICP measurement inaccurate. The ICP is continuously measured.
If intracranial hypertension persists despite the maximum of five drainages per hour, hyperventilation is indicated. The ventilator is set to drive the PaCO2 to 30 mmHg. Monitoring the CO2 expired makes this task easy, provided that the monitor is calibrated against the PaCO2.
If hyperventilation does not control the ICP, mannitol must be administered in a bolus at a dose of 0.25 g/kg. Administering it in infusion or at higher doses do not improve the result [135]. Osmolarity and intravascular volume status must be monitored, because hyperosmolarity or hypovolemia may occur and negatively affect the prognosis.
Along the process, care must be taken to maintain adequate oxygenation and blood pressure. The persistence on intracranial hypertension despite the above-mentioned therapies must reach the suspicion of an intracranial mass. In this situation, a new CT must be obtained [130]. Additionally, a second tier therapy must be considered [124].
The first of the second tier therapies is optimized hyperventilation. There is a small group of patients who could benefit from lower the PaCO2 below 30 mm Hg. It is undertaken if a method such as jugular venous saturation, which permits monitoring global brain ischemia, is being used [136]. The parameters of the ventilator are modified to increase slowly minute volume, until ICP controls or jugular venous saturation reaches its lower threshold (60–70%).
Barbirurates have long been used in the treatment of intracranial hypertension. Although their effect may be deleterious in the initial management compared with mannitol, barbiturates improve survival probability when used in patients with intracranial hypertension, refractory to other therapies [137]. The most commonly employed is pentobarbital sodium, at an IV load doses of 10 mg/kg over 30 minutes, followed by an infusion of 5 mg/kg/h, for 3 hours and then maintained at 1 to 3 mg/kg/h. The infusion is administered until ICP control for 24 hours; then is reduced by 50% per day. The aim is to induce profound coma, with burst suppression on electroencephalogram. Barbiturates can produce severe hypotension. Patients must be monitored carefully, to avoid potential disastrous consequences.
In recent years, decompressive craniectomy has emerged as a potential second tier therapy. It allows the brain to swell, without further ICP increasing. It has been used lately in intracranial hypertension refractory to other treatments [138]. The technique carries with it a high mortality that, in part, can be attributed to several secondary injuries previously suffered by the patients. It is strongly recommended in patients with complications from the other therapies. The results could be better if applied earlier in the course of intracranial hypertension [139].
Hypertonic saline has been used in the treatment of intracranial hypertension instead of mannitol [140–142]. In spite of the impressive support given by animal studies, the clinical evidence fails to show the expected benefits [143].
Prophylactic anticonvulsivants do not provide any protection against posttraumatic epilepsy, and should not be given prophylactically [124]. They are indicated when the patient has had seizures or when its detection will be impossible, such as when neuromuscular blockade is used.
Corticosteroids have been used for many years in the treatment of the traumatic brain injury. Literature evidence does not support its use. A mega randomized controlled clinical trial showed that corticosteroids group had a higher mortality than the control group. In consequence, this group of drugs must not be used in patients with traumatic brain injury [144,145].
Missed injuries and tertiary survey
Delayed diagnosis of lesions has been reported to occur between 0.5% to 38%, in different trauma populations [146–152]. When the analysis concentrates on high energy trauma, the incidence exceeds 10%.The most common undiagnosed injuries in the primary and secondary surveys are fractures located on long bones, ribs, and clavicles [150,153]. Less frequent but not less important are fractures of the spine, face, and pelvis [154]; with a much lower reported frequency are intrathoracic and intraabdominal lesions. Visceral and vascular missed wounds are more frequent in series with penetrating trauma mechanisms [146,148,155].
The impact of delayed diagnosis has been determined: they cause a change in the treatment in one third to two thirds of the affected patients, with requirement of a surgical intervention in 20% of the cases [150,156–158]. Sharma and coworkers [159] found missed injuries in 58% of the analyzed autopsies, with negative impact on survival in 3% of them. Hollow viscus perforation is infrequent in blunt trauma [160], but delays in diagnosis and treatment result in a significant increase in morbidity and mortality [161]. Hemorrhage has been reported between 18% and 25% of all preventable deaths, some of them corresponding to an intracavital bleeding not timely recognized [8,155,162].
The reasons associated with delayed diagnosis have been investigated, and include trauma severity, conditions that alter the process of attention, conditions that complicate the clinical evaluation, and errors in the process (Box 3) [147,152,154,163,164].
Box 3. Causes of missed injuries
Trauma severity
Multiple systems
Severe brain injury
Conditions that complicate the complete clinical evaluation
Altered consciousness
Brain trauma
Early sedation-intubation
Intoxication
Early surgical intervention
Altered process of attention
Referral
Workload excess
Error
Inadequate physical examination
Inaccurate interpretation of diagnostic investigations
Inadequate surgical sequence
To limit the number and the impact of the lesions diagnosed lately, a “tertiary survey” has been proposed [147,151,165]. It consists of a systematic re-view of the patient at the completion of the first day. The patient must be reexamined, and all the diagnostic investigations must be reevaluated. All the detected lesions must be cataloged. The participation of the trauma surgeons and the radiology team increases the probability of detecting undiagnosed lesions, and may reduce preventable deaths [166]. Missed lesions were reduced between 39% and 57% in prospective trials in which a tertiary survey was performed [147,150,167].
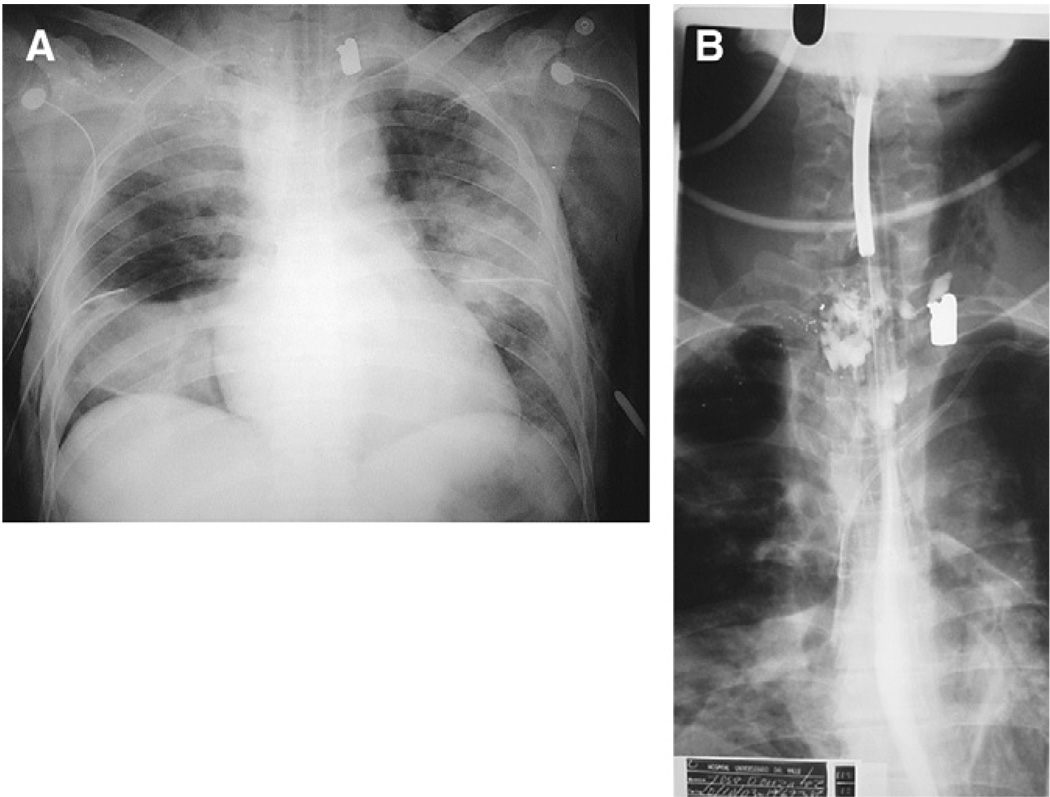
Of paramount importance is the diagnosis of occult bleeding and hollow viscera perforation. The first situation was discussed earlier. The second one requires experience to detect that the evolution moves away from the expected pattern: the fluids requirements are higher than the usual, there is no tolerance to the enteral feeding, there is no tolerance of the weaning from the ventilator, and there are new and unexpected organ dysfunctions. In such cases a perforation of a hollow viscera must be considered. Endoscopy and esophagogram will permit the diagnosis of an esophageal wound (Fig. 10), and in some cases abdominal CT will help to diagnose an abdominal hollow viscus perforation. In these cases, diagnostic peritoneal lavage, a laparoscopy, or an exploratory laparotomy will identify an intestinal perforation missed by image methods. Surgical treatment must be performed without hesitation.
Fig. 10.
Missed esophageal perforation. (A) The patient received a gunshot wound in the right periclavicular region. He was intubated and mechanically ventilated after massive subcutaneous emphysema and right pneumothorax. Bronchoscope, esophagoscope, and arteriography were performed. All negative. Three days later he continued on mechanical ventilation with right inferior lobe atelectasis and progressive lung infiltrates. (B) A missed esophageal perforation was suspected and a contrast esophagogram performed, which identifies the lesion.
Other considerations during the first day
Critically traumatized patients pose significant infection risk. Antibiotic administration is indicated in abdominal penetrating trauma, and in open fractures. The indication is less clear previous to the insertion of a chest tube [168–173]. In any case, protracted administration is not indicated, provided that it does not confer additional protection and increases antibiotic-related complications, and in some instances the risk of nosocomial infections [174–176]. Randomized clinical trials and comparative nonrandomized studies have proven it [177–181]. Comprehensive guides have been developed, regarding short antibiotic courses [173,182]. Operative site infection is best prevented by early surgical treatment, when indicated: early control of bleeding, early measures to control spillage from bowel perforations, gentle manipulation of the tissues, avoidance of unnecessary maneuvers, and complete debridement of dead or severely contaminated tissues. Irrigating the cavities with warm normal saline, to remove all the contaminants and blood remnants is a final complementary step. Nosocomial infections are pre-vented by avoiding unnecessary use of invasive dispositives. Adequate insertion technique, appropriated care, and removal of them as early as possible are recommended when they are indispensable [183–186].
An enteral access for nutrition must be gained from the first day, as early enteral feeding reduces infection risk [187–191].
Severely traumatized patients have increased risk of thromboembolic complications [192,193]. Prophylactic measures must be instituted from the first day. Pharmacologic prophylaxis with a low molecular weight heparin is the choice for the nonbleeding patient. When bleeding risk is considered to be increased, intermittent pneumatic compression is indicated [194–198].
Summary
Increasingly in an ever more violent society, trauma surgeons are going to be placed in stressful situations, calling for crucial split-second decisions. Then only their skill and that of their support staff can significantly reduce ICU mortality.
ICU trauma patients must be resuscitated toward specific goals. Ventilation must be directed to keep blood oxygenation at safe levels, hemodynamic support to the early correction of perfusion deficit, and neurologic support to avoid secondary insults and to maintain a cerebral perfusion pressure. All these with less intense support must be possible, to avoid complications attributable to treatment.
Respiratory, hemodynamic or neurologic complications may arise, with catastrophic consequences if not treated in a timely and appropriate manner. A systematic and ordered approach by priorities will permit identifying the cause of the crisis. The solution will consist of adjustments in the treatment in some cases, but frequently a surgical intervention will be crucial in cases such as tension pneumothorax, massive diaphragmatic hernia, intra-abdominal hypertension, and occult bleeding. Identifying its indication is a key determining factor and prompt and precise execution definitive.
Acknowledgments
Funded in part by Fogerty International Center NIH Grant No. 1 D43 TW007560-01.
References
- 1.Krug EG, Dahlberg LL, Mercy JA, et al. Geneva: World Health Organization; World report on violence and health. 2002
- 2.Peden M, Scurfield R, Sleet D, et al. Geneva: World Health Organization; World report on road traffic injury prevention. 2004
- 3.Wayatt J, Beard D, Gray A, et al. The time of death after trauma. BMJ. 1995;310:1502. doi: 10.1136/bmj.310.6993.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jatt A, Khan MR, Zafar H, et al. Peer review audit of trauma deaths in a developing country. Asian J Surg. 2004;27:54–58. doi: 10.1016/s1015-9584(09)60247-5. [DOI] [PubMed] [Google Scholar]
- 5.Stewart RM, Myers JG, Dent DL, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevent. J Trauma. 2003;54:66–71. doi: 10.1097/00005373-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Durham R, Shapiro D, Flint L. In-house trauma attendings: is there a difference? Am J Surg. 2005;190:960–966. doi: 10.1016/j.amjsurg.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Esposito TJ, Sandddal TL, Reynolds SA, et al. Effect of a voluntary trauma system on pre-ventable death and inappropriate care in a rural state. J Trauma. 2003;54:633–670. doi: 10.1097/01.TA.0000058124.78958.6B. [DOI] [PubMed] [Google Scholar]
- 8.Maio MR, Burney RE, Gregor MA, et al. A study of preventable trauma mortality in rural Michigan. J Trauma. 1996;41:83–90. doi: 10.1097/00005373-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Duke GJ, Morley PT, Cooper DJ, et al. Management of severe trauma in intensive care units and surgical wards. Med J Aust. 1999;170:416–419. doi: 10.5694/j.1326-5377.1999.tb127815.x. [DOI] [PubMed] [Google Scholar]
- 10.Davies JW, Hoyt DB, McArdle MS, et al. The significance of critical care errors in causing preventable death in trauma patients in a trauma system. J Trauma. 1991;31:813–818. doi: 10.1097/00005373-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Quiroz F, Garcia A, Perez M. Análisis de mortalidad prevenible en 150 casos de trauma carotídeo. Panam J Trauma. 1995;5:116. [Google Scholar]
- 12.Nunn JF. Applied respiratory physiology. 4th edition. Cambridge: Butterworth-Heinemann; 1993. [Google Scholar]
- 13.Chang DW. AARC clinical practice guideline in-hospital transport of the mechanically ventilated patient—2002 revision & update. Respir Care. 2002;47:721–723. [PubMed] [Google Scholar]
- 14.Mort T. Emergency tracheal intubation: complications associated with repeated laryngo-scopic attempts. Anest Analg. 2004;99:607–613. doi: 10.1213/01.ANE.0000122825.04923.15. [DOI] [PubMed] [Google Scholar]
- 15.Wong DT, Lai K, Chung FF, et al. Cannot Intubate-cannot ventilate and difficult intubation strategies: results of a Canadian National Survey. Anesth Analg. 2005;100:1439–1446. doi: 10.1213/01.ANE.0000148695.37190.34. [DOI] [PubMed] [Google Scholar]
- 16.Mort T. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA guidelines in the remote location. J Clin Anesth. 2004;16:508–516. doi: 10.1016/j.jclinane.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Krinsley SJ, Barone JE. The drive to survive. Unplanned extubation in the ICU. Chest. 2005;128:560–566. doi: 10.1378/chest.128.2.560. [DOI] [PubMed] [Google Scholar]
- 18.Epstein SK, Nevins ML, Chung J. Effect of unplanned extubation on outcome of mechanical ventilation. Am J Respir Crit Care Med. 2001;163:1755–1756. doi: 10.1164/ajrccm.163.7.16372b. [DOI] [PubMed] [Google Scholar]
- 19.DeLassence A, Alberti C, Assoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit.A prospective multicenter study. Anesthesiology. 2002;97:148–156. doi: 10.1097/00000542-200207000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia FN, Bajan K, Raje KV. Airway accidents in intubated intensive care unit patients: an epidemiological study. Crit Care Med. 2000;28:659–664. doi: 10.1097/00003246-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Carrion MI, Ayuso D, Marcos M, et al. Accidental removal of endotracheal and nasogastric tubes and intravascular catheters. Crit Care Med. 2000;28:63–66. doi: 10.1097/00003246-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Needham DM, Thompson DA, Holzmueller CG, et al. A system factors analysis of airway events from the Intensive Care Unit Safety Reporting System (ICUSRS) Crit Care Med. 32:2227–2233. doi: 10.1097/01.ccm.0000145230.52725.6c. 204. [DOI] [PubMed] [Google Scholar]
- 23.Mort T. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg. 1998;86:1171–1176. doi: 10.1097/00000539-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Pesiri AJ. Two-year study of the prevention of unintentional extubation. Crit Care Nurs Q. 1994;17:35–39. doi: 10.1097/00002727-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Arney KL, Judson MA, Sahn SA. Airway obstruction arising from blood clot three reports and a review of the literature. Chest. 1999;115:293–300. doi: 10.1378/chest.115.1.293. [DOI] [PubMed] [Google Scholar]
- 26.Collins KA, Presnell SE. Asphyxia by tracheobroncial trombus. Am J Forensic Med Pathol. 2005;26:327–329. doi: 10.1097/01.paf.0000188078.43884.77. [DOI] [PubMed] [Google Scholar]
- 27.Shalmovitz GZ, Halpern P. Delated obstruction of endotracheal tubes by aspirated foreign bodies: report of two cases. Ann Emerg Med. 2004;43:630–633. doi: 10.1016/j.annemergmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Sprung J, Bourke DL, Harrison G, et al. Endotracheal tube and tracheobronchial obstruction as causes of hypoventilation with high inspiratory pressures. Chest. 1994;105:550–552. doi: 10.1378/chest.105.2.550. [DOI] [PubMed] [Google Scholar]
- 29.DeLassence A, Timsit JF, Taflet M, et al. Pneumothorax in the intensive care unit incidence, risk factors, and outcome. Anesthesiology. 2006;104:5–13. doi: 10.1097/00000542-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Chen KY, Jern JS, Liao WY, et al. Pneumothorax in the ICU. Patient outcomes and prognostic factors. Chest. 2002;122:678–683. doi: 10.1378/chest.122.2.678. [DOI] [PubMed] [Google Scholar]
- 31.Burshell SA, Takiguchi SA, Myers SA, et al. Unilateral lung hyperinflation and auto-positive end-expiratory pressure due to a ruptured right hemidiaphragm. Crit Care Med. 1996;24:1418–1421. doi: 10.1097/00003246-199608000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Guth AA, Pachler HL, Kim V. Pitfalls in the diagnosis of blunt diaphragmatic injury. Am J Surg. 1995;170:5–9. doi: 10.1016/s0002-9610(99)80242-6. [DOI] [PubMed] [Google Scholar]
- 33.Voeller GL, Reisser JR, Fabian TC, et al. Blunt diaphragmatic injuries: a five- year experience. Am Surg. 1990;56:28–31. [PubMed] [Google Scholar]
- 34.Lee WC, Chen RJ, Fang JF, et al. Rupture of the diaphragm after blunt trauma. Eur J Surg. 1990;160:479–483. [PubMed] [Google Scholar]
- 35.Balogh Z, McKinley B, Cocanour CS, et al. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–544. doi: 10.1016/s0002-9610(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 36.Balogh Z, McKinley B, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–861. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- 37.Ertel W, Oberholzer A, Platz A, et al. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747–1753. doi: 10.1097/00003246-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 38.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 39.Celoria G, Steingrub JS, Vickers-Lati M, et al. Clinical assessment of hemodynamic values in two surgical intensive care units. Effects on therapy. Arch Surg. 1990;125:1036–1039. doi: 10.1001/archsurg.1990.01410200100016. [DOI] [PubMed] [Google Scholar]
- 40.Shoemaker WC, Appel PL, Kran H. Physiologic patterns in surviving and nonsurviving shock patients. Arch Surg. 1973;106:630–636. doi: 10.1001/archsurg.1973.01350170004003. [DOI] [PubMed] [Google Scholar]
- 41.Abou-Khalil B, Scalea TM, Trooskin SZ. Hemodynamic responses to shock in young trauma patients: need for invasive monitoring. Crit Care Med. 1994;22:633–639. doi: 10.1097/00003246-199404000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Dutton RP. Shock and trauma anesthesia. Anest Clin North Am. 1999;17:83–95. [Google Scholar]
- 43.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:1036–1039. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 44.Moore FA, Haenel JB, Moore EE, et al. Incommensurate oxygen consumption in response to maximal oxygen availability predicts postinjury multiple organ failure. J Trauma. 1992;33:58–65. doi: 10.1097/00005373-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Shoemaker WC, Appel PL, Kran H. Hemodynamic and oxygen transport responses insur-vivors and nonsurvivors of high risk surgery. Crit Care Med. 1993;21:977–990. doi: 10.1097/00003246-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Kirton O, Windsor J, Wedderburn R, et al. Failure of splanchnic resuscitation in the acutely injured trauma patient correlates with multiple organ system failure and length of stay in the ICU. Chest. 1998;113:1064–1069. doi: 10.1378/chest.113.4.1064. [DOI] [PubMed] [Google Scholar]
- 47.Garcia A. Resuscitación en trauma y cirugía mayor. Rev Col Cirug. 2001;16:26–38. [Google Scholar]
- 48.Losada HF, Garcia A. Riesgo de disfunción orgánica múltiple, después de toracotomía por trauma. Rev Med Sur (Chile) 2001;23:46–53. [Google Scholar]
- 49.Griffel MI, Kauffman B. Pharmacology of colloids and crystalloids. Crit Care Clin. 1992;8:235–253. [PubMed] [Google Scholar]
- 50.Grocott MPW, Mythen MC, Gan TJ. perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–1106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 51.Roberts I, Alderson P, Bunn F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004;(1):CD000567. doi: 10.1002/14651858.CD000567.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Investigators TSAFES. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 53.Mora RRA, Alí A, Borráez O, et al. Reunión de consenso: terapia de fluídos en pacientes críticamente enfermos. Acta Col Cuidado Intensivo. 2005;8:52. [Google Scholar]
- 54.Shires GT, Cohn D, Carrico CT. Fluid therapy in hemorrhagic shock. Arch Surg. 1964;88:688–692. doi: 10.1001/archsurg.1964.01310220178027. [DOI] [PubMed] [Google Scholar]
- 55.Riddez L, Hahn RG, Brismar B, et al. Central and regional hemodynamics during acute hypovolemia and volume substitution in volunteers. Crit Care Med. 1997;25:635–640. doi: 10.1097/00003246-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Heyland DK, Cook DJ, King D, et al. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24:617–624. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 57.Ivanov R, Allen J, Calvin JE. The incidence of major morbidity in critically ill patients managed with pulmonary artery catheters: a meta-analysis. Crit Care Med. 2000;28:615–619. doi: 10.1097/00003246-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–1692. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Huckabee WE. Relationships of pyruvate and lactate during anaerobic metabolism: I. Effect of infusion of pyruvate or glucose and of hyperventilation. J Clin Invest. 1958;37:244–254. doi: 10.1172/JCI103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huckabee WE. Relationships of pyruvate and lactate during anaerobic metabolism: II.Exercise and formation of O-debt. J Clin Invest. 1958;37:255–263. doi: 10.1172/JCI103604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broder G, Weil MH. Excess lactate—an index of reversibility of shock in human patients. Science. 1964;143:1457–1459. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 62.Crowl AC, Young JS, Kahler DM, et al. Occult hypoperfusion is associated with increased morbidity in patients undergoing early femur fracture fixation. J Trauma. 2000;48:260–267. doi: 10.1097/00005373-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Abramson D, Scalea TM, Hitchcock R, et al. Lactate clearance and survival following injury. J Trauma. 1993;35:548–549. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Husain FA, Martin MJ, Mullenix PS, et al. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–491. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32:1120–1124. doi: 10.1097/01.ccm.0000125517.28517.74. [DOI] [PubMed] [Google Scholar]
- 66.FitzSullivan E, Salim A, Demetriades D, et al. Serum bicarbonate may replace the arterial base deficit in the trauma intensive care unit. Am J Surg. 2005;190:941–946. doi: 10.1016/j.amjsurg.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 67.Dunham CM, Siegel JH, Weireter L, et al. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit Care Med. 1991;19:231–243. doi: 10.1097/00003246-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 68.Davis JW, Shackford SR, Holbrook TL, et al. Base deficit as a sensitive indicator of compensated shock and tissue oxygen utilization. Surg Gynecol Obstet. 1991;173:473–476. [PubMed] [Google Scholar]
- 69.Rutherford EJ, Morris JA, Reed GW, et al. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Porter J, Ivatury RR. In search of the optimal end points of resuscitation in trauma patients: a review. J Trauma. 1998;88:908–914. doi: 10.1097/00005373-199805000-00028. [DOI] [PubMed] [Google Scholar]
- 71.Tatevossian RG, Wo CC, Velmahos GC, et al. Transcutaneous oxygen and CO2 as early warning of tissue hypoxia and hemodynamic shock in critically ill emergency patients. Crit Care Med. 2000;28:2248–2253. doi: 10.1097/00003246-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Hameed SM, Cohn SM. Gastric tonometry. The role of mucosal pH measurement in the management of trauma. Chest. 2003;123:475s–481s. doi: 10.1378/chest.123.5_suppl.475s. [DOI] [PubMed] [Google Scholar]
- 73.Marik PE, Bankov A. Sublingual capnometry versus traditional markers of tissue oxygenation in critically ill patients. Crit Care Med. 2003;31:818–822. doi: 10.1097/01.CCM.0000054862.74829.EA. [DOI] [PubMed] [Google Scholar]
- 74.Knudson MM, Bermudez KM, Doyle CA, et al. Use of tissue oxygen tension measurements during resuscitation from hemorrhagic shock. J Trauma. 1997;42:608–614. doi: 10.1097/00005373-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 75.McKinley BA, Butler BD. Comparison of skeletal muscle PO2, PCO2, and pH with gastric tonometric P(CO2) and pH in hemorrhagic shock. Crit Care Med. 1999;27:1869–1877. doi: 10.1097/00003246-199909000-00027. [DOI] [PubMed] [Google Scholar]
- 76.Venkatesh B, Meacher R, Muller MJ, et al. Monitoring tissue oxygenation during resuscitation of major burns. J Trauma. 2001;50:485–494. doi: 10.1097/00005373-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 77.Benaron DA, Parachikov IH, Friedland S, et al. Continuous, noninvasive, and localized microvascular tissue oximetry using visible light spectroscopy. Anesthesiology. 2004;100:1469–1475. doi: 10.1097/00000542-200406000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Crookers BA, Cohn SM, Bloch S, et al. Can near-infrared spectroscopy identify the severity of shock in trauma patients? J Trauma. 2005;58:806–816. doi: 10.1097/01.ta.0000158269.68409.1c. [DOI] [PubMed] [Google Scholar]
- 79.Shapiro MJ, Gettinger A, Corwin HL, et al. Anemia and blood transfusion in trauma patients admitted to the intensive care unit. J Trauma. 2003;55:269–274. doi: 10.1097/01.TA.0000080530.77566.04. [DOI] [PubMed] [Google Scholar]
- 80.van de Watering LM, Hermans J, Houbiers JGA, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery a randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 81.van Hilten JA, van de Watering LM, van Bockel JH, et al. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. BMJ. 2004;328:1281–1284. doi: 10.1136/bmj.38103.735266.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Claridge J, Sawyer RG, Schulman AW, et al. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:556–572. [PubMed] [Google Scholar]
- 83.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 84.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Eng J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 85.Hill SR, Carless PA, Henry DA, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. The Cochrane Database of Systematic Reviews Cochrane Database Syst Rev. 2002;(2):CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 86.Gubler KD, Gentilello LH, Hassantash SA, et al. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994;36:847–851. doi: 10.1097/00005373-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Garcia A. Coagulopatía asociada al trauma. Rev Col Cirug. 1996;11:17–23. [Google Scholar]
- 88.DeLougery T. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin. 2004;20:13–24. doi: 10.1016/s0749-0704(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 89.Wolberg AS, Meng SH, Monroe DM, III, et al. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221–1228. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- 90.Hardy J-F, de Moerloose P, Samama M. Massive transfusion and coagulopathy: patho-physiology and implications for clinical management. Can J Anesth. 2004;51:293–310. doi: 10.1007/BF03018233. [DOI] [PubMed] [Google Scholar]
- 91.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. BJA. 2005;95:130–139. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 92.McKenna R. Postoperative medical complications.Abnormal coagulation in the postoperative priod contributing to excessive bleeding. Med Clin North Am. 2001;85:1277–1310. doi: 10.1016/s0025-7125(05)70378-3. [DOI] [PubMed] [Google Scholar]
- 93.Gentilello L, Jurkovich G, Stark M, et al. Is hypothermia in the victim of major trauma protective or harmful?: A randomized, prospective study. Ann Surg. 1997;226:439–449. doi: 10.1097/00000658-199710000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531–543. doi: 10.1097/00000542-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 95.Kober A, Scheck T, Fulesdi B, et al. Effectiveness of resistive heating compared with passive warming in treating hypothermia associated with minor trauma: a randomized trial. Mayo Clin Proc. 2001;76:369–375. doi: 10.4065/76.4.369. [DOI] [PubMed] [Google Scholar]
- 96.Petrone P, Kuncir EJ, Asensio J. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165–1178. doi: 10.1016/s0733-8627(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 97.Negishi C, Hasegawa K, Mukai S, et al. Resistive-heating and forced-air warming are comparably effective. Anest Analg. 2003;96:1683–1687. doi: 10.1213/01.ANE.0000062770.73862.B7. [DOI] [PubMed] [Google Scholar]
- 98.Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190:91–99. doi: 10.1097/00000658-197907000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reed RL, Ciavarella D, Heimbach DM, et al. Prophylactic platelet administration during massive transfusion. A prospective, randomized, double-blind clinical study. Ann Surg. 1986;203:48–58. doi: 10.1097/00000658-198601000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yugueros P, Sarmiento J, Ferrada R. Síndrome postpericardiotomía. Rev Col Cirug. 1993;8:90–100. [Google Scholar]
- 101.Campbell NC, Thompson SR, Muckard DJJ, et al. Review of 1198 cases of penetrating cardiac trauma. Br J Surg. 1997;84:1737–1740. [PubMed] [Google Scholar]
- 102.Orliaguet G, Ferjani M, Riuo B. The heart in blunt trauma. Anesthesiology. 2001;95:544–548. doi: 10.1097/00000542-200108000-00041. [DOI] [PubMed] [Google Scholar]
- 103.Tso P, Rodriguez A, Cooper C, et al. Sonography in blunt abdominal trauma: a preliminary progress report. J Trauma. 1992;33:39–44. doi: 10.1097/00005373-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Rozicki GS, Ocshner MG, Jaffin JH, et al. Prospective evaluation of surgeons’ use of ultrasound in the evaluation of trauma patients. J Trauma. 1993;34:516–527. doi: 10.1097/00005373-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 105.McKenney KL, McKenney MG, Cohn SM, et al. Hemoperitoneum score helps determine need for therapeutic laparotomy. J Trauma. 2001;50:650–656. doi: 10.1097/00005373-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Sclafani SSJA, Shaftan GW, Scalea TM, et al. Nonoperative salvage of computed tomography—diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma. 1995;39:818–827. doi: 10.1097/00005373-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Velmahos G, Demetriades D, Chahwan S, et al. Angiographic embolization for arrest of bleeding after penetrating trauma to the abdomen. Am J Surg. 1999;178:367–373. doi: 10.1016/s0002-9610(99)00212-3. [DOI] [PubMed] [Google Scholar]
- 108.Velmahos G, Chahwan S, Falabella A, et al. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24:539–545. doi: 10.1007/s002689910087. [DOI] [PubMed] [Google Scholar]
- 109.Velmahos GC, Toutoouzas K, Vassiliu P, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002;52:3003–3008. doi: 10.1097/00005373-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 110.Kushimoto S, Arai M, Aiboshi J, et al. The role of interventional radiology in patients requiring damage control laparotomy. J Trauma. 2003;54:171–176. doi: 10.1097/00005373-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 111.Mohr A, Lavery RF, Barone A, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2005;55:1077–1082. doi: 10.1097/01.TA.0000100219.02085.AB. [DOI] [PubMed] [Google Scholar]
- 112.Yeh MW, Hom JK, Schecter WP, et al. Endovascular repair of an actively hemorrhaging gunshot injury to the abdominal aorta. J Vasc Surg. 2005;42:1007–1009. doi: 10.1016/j.jvs.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 113.Barba CA. The intensive care unit as an operating room. Surg Clin North Am. 2000;80:957–973. doi: 10.1016/s0039-6109(05)70108-0. [DOI] [PubMed] [Google Scholar]
- 114.Mayberry JC. Bedside open abdominal surgery. Utility and wound management. Crit Care Clin. 2000;16:151–172. doi: 10.1016/s0749-0704(05)70102-0. [DOI] [PubMed] [Google Scholar]
- 115.Rozicki GS, Feliciano DV, Ocshner MG, et al. The role of ultrasound in patients with possible penetrating cardiac wounds: a prospective multicenter study. J Trauma. 1999;46:543–552. doi: 10.1097/00005373-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 116.Bar-Nathan M, Richardson D, Garcia A. Indicaciones de toracotomia en trauma. In: Rodriguez A, Ferrada R, editors. Texto de trauma. Sociedad Panamericana de Trauma. Cali: Feriva; 1997. [Google Scholar]
- 117.Ferrada A, Rodriguez A. Trauma cardiaco. Tratamiento quirurgico. Rev Col Cirug. 2001;16:5–15. [Google Scholar]
- 118.Lowell JA, Schifferdecker C, Driscoll DF, et al. Postoperative fluid overload: not a benign problem. Crit Care Med. 1990;18:728–733. doi: 10.1097/00003246-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 119.Venn R, Steela A, Richardson P, et al. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth. 2002;88:65–71. doi: 10.1093/bja/88.1.65. [DOI] [PubMed] [Google Scholar]
- 120.Balogh Z, McKinley B, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of intraabdominal compartment. Arch Surg. 2003;138:637–643. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 121.Michard F, Teboul JF. Predicting fluid responsiveness in ICU patients.A critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 122.Weiss YG, Oppenheim-Eden A, Gilon D, et al. Systolic pressure variation in hemodynamic monitoring after severe blast injury. J Clin Anesth. 1999;11:132–135. doi: 10.1016/s0952-8180(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 123.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 124.Bullock R, Chesnut RM, Clifton G, et al. Guidelines for the management of severe head injury—revision. J Neurotrauma. 2000;17:457–627. [Google Scholar]
- 125.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1994;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 126.Jeremitsky E, Omert L, Dunham M, et al. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;55:388–389. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 127.Huyhn T, Messer M, Sing RF, et al. Positive end-expiratory pressure alters intracranial and cerebral perfusion pressure in severe traumatic brain injury. J Trauma. 2002;53:488–492. doi: 10.1097/00005373-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 128.Caricato A, Conti G, Della Corte F, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58:571–576. doi: 10.1097/01.ta.0000152806.19198.db. [DOI] [PubMed] [Google Scholar]
- 129.Hsiang JK, Chesnut RM, Crisp CB, et al. Early, routine paralysis for intracranial pressure control in severe head. Crit Care Med. 1994;22:1471–1476. doi: 10.1097/00003246-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 130.Chesnut RM. Management of brain and spine injuries. Crit Care Clin. 2004;20:25–55. doi: 10.1016/s0749-0704(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 131.Feinstein AJ, Patel MB, Sanui M, et al. Resuscitation with pressors after traumatic brain injury. J Am Coll Surg. 2005;201:536–545. doi: 10.1016/j.jamcollsurg.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 132.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Eng J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 133.Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev. 2004;(4):CD001048. doi: 10.1002/14651858.CD001048.pub2. [DOI] [PubMed] [Google Scholar]
- 134.Ghajar R. Intracranial pressure monitoring techniques. New Horiz. 1995;3:395–399. [PubMed] [Google Scholar]
- 135.Marshall LF, Smith RW, Rausher LA, et al. Mannitol dose requirements in brain injured patients. J Neurosurg. 1978;48:169–172. doi: 10.3171/jns.1978.48.2.0169. [DOI] [PubMed] [Google Scholar]
- 136.Cruz J. The first decade of continuous monitoring of jugular bulb oxyhemoglobinsaturation: management strategies and clinical outcome. Crit Care Med. 1998;26:344–351. doi: 10.1097/00003246-199802000-00039. [DOI] [PubMed] [Google Scholar]
- 137.Eisenberg H, Frankiwski R, Contant C, et al. The comprehensive central nervous system trauma centers.High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15–23. doi: 10.3171/jns.1988.69.1.0015. [DOI] [PubMed] [Google Scholar]
- 138.Gaab MR, Rittierodt M, Lorenz M, et al. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir. 1990;51:s326–s328. doi: 10.1007/978-3-7091-9115-6_110. [DOI] [PubMed] [Google Scholar]
- 139.Bullock MR, Chesnut R, Ghajar R, et al. Surgical management of traumatic brain lesions. Neurosurgery. 2006;58:s225–s246. doi: 10.1227/01.NEU.0000210365.36914.E3. [DOI] [PubMed] [Google Scholar]
- 140.Berger S, Schurer L, Hartl R, et al. Reduction of post-traumatic intracranial hypertension by hypertonic/hyperoncotic saline/dextran and hypertonic mannitol. Neurosurgery. 1995;37:87–107. doi: 10.1227/00006123-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 141.Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 142.Peterson B, Khanna S, Fisher B, et al. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 143.Cooper J, Myles P, McDermott F, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury. JAMA. 2004;291:1350–1377. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- 144.Roberts I, Yates D, Sandercock P, et al. Effect of Intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial) Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 145.Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 146.Scalea TM, Phillips TF, Goldstein AS, et al. Injuries missed at operation: nemesis of the trauma surgeon. J Trauma. 1988;28:962–966. [PubMed] [Google Scholar]
- 147.Enderson BL, Reath DB, Meadors J, et al. The tertiary trauma survey: a prospective study of missed injury. J Trauma. 1990;30:666–669. [PubMed] [Google Scholar]
- 148.Hirshberg A, Wall MJ, Mattox KL, et al. Causes and patterns of missed injuries in trauma. Am J Surg. 1994;168:299–303. doi: 10.1016/s0002-9610(05)80152-7. [DOI] [PubMed] [Google Scholar]
- 149.Buduhan G, McRitchie DI. Missed injuries in patients with multiple trauma. J Trauma. 2000;49:600–605. doi: 10.1097/00005373-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 150.Vles WJ, Veen EJ, Roukema JD, et al. Consequences of delayed diagnoses in trauma patients: a prospective study. J Am Coll Surg. 2003;197:596–602. doi: 10.1016/S1072-7515(03)00601-X. [DOI] [PubMed] [Google Scholar]
- 151.Brooks A, Holroyd B, Riley B. Missed injury in major trauma patients. Injury. 2004;35:407–410. doi: 10.1016/S0020-1383(03)00219-5. [DOI] [PubMed] [Google Scholar]
- 152.Jimenez-Gomez LM, Amunategui I, Sanchez JM, et al. Missed injuries in patients with multiple trauma: analysis of a trauma registry. Cir Esp. 2005;78:303–307. doi: 10.1016/s0009-739x(05)70939-8. [DOI] [PubMed] [Google Scholar]
- 153.Sharma OP, Scala-Barnett DM, Oswanski MF, et al. Clinical and autopsy analysis of delayed diagnosis and missed injuries in trauma patients. Am Surg. 2006;72:174–179. [PubMed] [Google Scholar]
- 154.Born CT, Ross SE, Iannacone WM, et al. Delayed identification of skeletal injury in multisystem trauma: the “missed” fracture. J Trauma. 1989;29:1643–1646. doi: 10.1097/00005373-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 155.Garcia A, Lalsie R, Paredes J, et al. Mortalidad prevenible por trauma en Cali. Colombia,1998. Acta Col Cuidado Intensivo. 2001;4:106–107. [Google Scholar]
- 156.Rizoli SB, Boulanger BR, McLellan BA, et al. Injuries missed during initial assessment of blunt trauma patients. Accid Anal Prev. 1994;26:681–686. doi: 10.1016/0001-4575(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 157.Furnival RA, Woodward GA, Schunk JE. Delayed diagnosis of injury in pediatric trauma. Pediatrics. 1996;98:56–62. [PubMed] [Google Scholar]
- 158.Meijer JMR, Janssens M, Hammacher ER. Injuries missed in dealing with severely wounded accident victims in the emergency room. Ned Tijdschr Geneeskd. 1999;40:1742–1745. [PubMed] [Google Scholar]
- 159.Sharma BR, Gupta M, Harish D, et al. Missed diagnoses in trauma patients vis-a-vis significance of autopsy. Injury. 2005;36:976–983. doi: 10.1016/j.injury.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 160.Watts DD, Fahkhry S the EAST Multi-institutional HVI Research Group. Incidence of hollow viscus injury in blunt trauma: an analysis from 275,557 trauma admissions from the EAST Multi-Institutional Trial. J Trauma. 2003;54:289–294. doi: 10.1097/01.TA.0000046261.06976.6A. [DOI] [PubMed] [Google Scholar]
- 161.Fakhry SM, Brownstein M, Watts DD, et al. Relatively short diagnostic delays (<8 hours) produce morbidity and mortality in blunt small bowel injury: an analysis of time to operative intervention in 198 patients from a multicenter experience. J Trauma. 2000;48:408–414. doi: 10.1097/00005373-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 162.Sirlin CB, Brown MA, Andrade-Barreto O, et al. Abdominal trauma: clinical value of negative screening US scans. Radiology. 2004;230:661–668. doi: 10.1148/radiol.2303021707. [DOI] [PubMed] [Google Scholar]
- 163.Ball CG, Kirkpatrick AW, Laupland KB, et al. Factors related to the failure of radio-graphic recognition of occult posttraumatic pneumothoraces. Am J Surg. 2005;189:541–546. doi: 10.1016/j.amjsurg.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 164.Ball CG. Are occult pneumothoraces truly occult or simply missed? J Trauma. 2006;60:294–298. doi: 10.1097/01.ta.0000202462.96207.18. [DOI] [PubMed] [Google Scholar]
- 165.Grossman MD, Born C. Tertiary survey of the trauma patient in the intensive care unit. Surg Clin North Am. 2000;80:805–824. doi: 10.1016/s0039-6109(05)70097-9. [DOI] [PubMed] [Google Scholar]
- 166.Hoff WS, Sicoutris CP, Lee SY, et al. Formalized radiology rounds: the final component of the tertiary survey. J Trauma. 2004;56:291–295. doi: 10.1097/01.TA.0000105924.37441.31. [DOI] [PubMed] [Google Scholar]
- 167.Bifl WL, Harrington DT, Cioffi WC, et al. Implementation of a tertiary trauma survey decreases missed injuries. J Trauma. 2003;54:38–44. doi: 10.1097/00005373-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 168.Maxwell RA, Campbell DJ, Fabian TC, et al. Use of presumptive antibiotics following tube thoracostomy for traumatic hemopneumothorax in the prevention of empyema and pneumonia—a multi-center trial. J Trauma. 2004;57:742–749. doi: 10.1097/01.ta.0000147481.42186.42. [DOI] [PubMed] [Google Scholar]
- 169.Grover FL, Richardson JD, Fewel JG, et al. Prophylactic antibiotics in the treatment of penetrating chest wounds. A prospective double-blind study. J Thorac Cardiovasc Surg. 1977;74:528–536. [PubMed] [Google Scholar]
- 170.LeBlanc KA, Tucker WY. Prophylactic antibiotics and closed tube thoracostomy. Surg Gynecol Obstet. 1985;160:259–263. [PubMed] [Google Scholar]
- 171.Nichols RL, Smith JW, Muzic AC, et al. Preventive antibiotic usage in traumatic thoracic injuries requiring closed tube thoracostomy. Chest. 1994;106:1493–1498. doi: 10.1378/chest.106.5.1493. [DOI] [PubMed] [Google Scholar]
- 172.Gonzalez RP, Holevar MR. Role of prophylactic antibiotics for tube thoracostomy in chest trauma. Am Surg. 1998;64:617–620. [PubMed] [Google Scholar]
- 173.Luchette FA, Barrie PS, Oswanski MF, et al. Practice management guidelines for prophylactic antibiotic use in tube thoracostomy for traumatic hemopneumothorax: the EAST practice management guidelines work group. J Trauma. 2000;48:753–757. doi: 10.1097/00005373-200004000-00027. [DOI] [PubMed] [Google Scholar]
- 174.Namias N, Harvill S, Ball S, et al. Cost and morbidity associate with antibiotic prophylaxis in the ICU. J Am Coll Surg. 2000;190:503–504. doi: 10.1016/s1072-7515(98)00287-7. [DOI] [PubMed] [Google Scholar]
- 175.Velmahos G, Toutzas KG, Sarkisyan G, et al. Severe trauma is not an excuse for prolonged antibiotic prophylaxis. Arch Surg. 2002;137:537–542. doi: 10.1001/archsurg.137.5.537. [DOI] [PubMed] [Google Scholar]
- 176.Hoth JJ, Franklin GA, Stassen NA, et al. Prophylactic antibiotics adversely affect nosocomial pneumonia i trauma patients. J Trauma. 2000;55:549–554. doi: 10.1097/01.TA.0000083334.93868.65. [DOI] [PubMed] [Google Scholar]
- 177.Dellinger EP, Wertz HJ, Lennard ES, et al. Efficacy of short-course antibiotic prophylaxis after penetrating intestinal injury. Arch Surg. 1986;121:23–30. doi: 10.1001/archsurg.1986.01400010029002. [DOI] [PubMed] [Google Scholar]
- 178.Demetriades D, Lakhoo M, Pezikis A, et al. Short-course antibiotic prophylaxis in penetrating abdominal injuries: ceftriaxone versus cefoxitin. Injury. 1991;22:20–24. doi: 10.1016/0020-1383(91)90154-7. [DOI] [PubMed] [Google Scholar]
- 179.Fabian TC, Croce MA, Payne LW, et al. Duration of antibiotic therapy for penetrating abdominal trauma: a prospective trial. Surgery. 1992;112:788–795. [PubMed] [Google Scholar]
- 180.Sarmiento JM, Aristizabal G, Rubiano J, et al. Prophylactic antibiotics in abdominal trauma. J Trauma. 1994;37:803–806. doi: 10.1097/00005373-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 181.Kirton O, O’Neill PA, Ketsner M, et al. Perioperative antibiotic use in high-risk penetrating hollow viscus injury: a prospective randomized, double-blind, placebo-control trial of 24 hours versus 5 days. J Trauma. 2000;49:822–832. doi: 10.1097/00005373-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 182.Luchette FA, Borzotta AP, Croce MA, et al. Practice management guidelines for prophylactic antibiotic use in penetrating abdominal trauma: the EAST practice management guidelines work group. J Trauma. 2000;48:508–518. doi: 10.1097/00005373-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 183.Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059–2093. doi: 10.1378/chest.120.6.2059. [DOI] [PubMed] [Google Scholar]
- 184.Mollitt DL. Infection control: avoiding the inavitable. Surg Clin North Am. 2002;82:365–378. doi: 10.1016/s0039-6109(02)00011-7. [DOI] [PubMed] [Google Scholar]
- 185.Coffin SE, Zaoutis KE. Infection control, hospital epidemiology, and patient safety. Infect Dis Clin North Am. 2005;19:647–665. doi: 10.1016/j.idc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 186.Garcia A, Duque P, Urrutia L, et al. Factores de Riesgo de la UTI Asociada a la sonda vesical. Rev Col Cirug. 2005;20:135–143. [Google Scholar]
- 187.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma. 1986;26:874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 188.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma-reduced septic morbidity. J Trauma. 1989;29:916–922. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 189.Kudsk KA, Croce MA, Fabian TC, et al. enteral versus parenteral feeding effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 191.Jacobs DG, Jacobs DO, Kudsk K, et al. Practice management guidelines for nutritional support of the trauma patient. J Trauma. 2004;57:660–679. doi: 10.1097/01.ta.0000135348.48525.a0. [DOI] [PubMed] [Google Scholar]
- 192.Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Eng J Med. 1994;331:1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 193.Knudson MM, Ikossi DG, Khaw L, et al. Thromboembolism after trauma an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–498. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: The EAST Practice Management Guidelines Work Group. J Trauma. 2002;53:142–164. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 195.Kirn J, Gearheart MM, Zurick A, et al. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J Trauma. 2002;53:38–433. doi: 10.1097/00005373-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 196.Garcia A. Enfermedad tromboembólica en trauma. Panam J Trauma. 2003;10:25–39. [Google Scholar]
- 197.Alejandro KV, Acosta JA, Rodriguez PA. Bleeding manifestations after early use of low-molecular-weight heparins in blunt splenic injuries. Am Surg. 2003;69:1006–1009. [PubMed] [Google Scholar]
- 198.Geerts WH, Pinneo GF, Heith JA, et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338s–400s. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]