Abstract
Purpose
Surgery is curative therapy for pediatric low-grade gliomas (LGGs) in areas of the brain amenable to complete resection. However, LGGs located in areas where complete resection is not possible can threaten both function and life. The purpose of this study was to compare two chemotherapy regimens for LGGs in children younger than age 10 years for whom radiotherapy was felt by the practitioner to pose a high risk of neurodevelopmental injury.
Patients and Methods
Previously untreated children younger than age 10 years with progressive or residual LGGs were eligible. Children were randomly assigned to receive carboplatin and vincristine (CV) or thioguanine, procarbazine, lomustine, and vincristine (TPCV). Children with neurofibromatosis are reported separately.
Results
Of 274 randomly assigned patients who met eligibility requirements, 137 received CV and 137 received TPCV. The 5-year event-free survival (EFS) and overall survival (OS) rates for all eligible patients were 45% ± 3.2% and 86% ± 2.2%, respectively. The 5-year EFS rates were 39% ± 4% for CV and 52% ± 5% for TPCV (stratified log-rank test P = .10; cure model analysis P = .007). On multivariate analysis, factors independently predictive of worse EFS and OS were younger age and tumor size greater than 3 cm2. Tumor location in the thalamus was also associated with poor OS.
Conclusion
The difference in EFS between the regimens did not reach significance on the basis of the stratified log-rank test. The 5-year EFS was higher for TPCV on the basis of the cure model analysis. Differences in toxicity may influence physician choice of regimens.
INTRODUCTION
Low-grade astrocytomas (WHO grade 1 and 2) are the most common brain tumors of childhood.1 Prognosis is excellent for those in whom total excision is possible, with more than 90% survival at 10 years following surgery alone.2,3 Although 10-year event-free survival (EFS) rates as high as 74% have been reported in children with incompletely resected low-grade gliomas (LGGs) treated with conformal radiotherapy, concerns about toxicity of radiotherapy in young children have limited its use in this population.3 Young children are more at risk for late effects of radiotherapy, including decrease in intellectual function, endocrine deficits, second neoplasms, hearing loss, and vasculopathy.4–9 Because of these concerns, several groups have used chemotherapy to delay or replace radiotherapy in young children with tumors in critical locations not amenable to complete resection or when tumors progress after surgery or radiation.10,11
When this trial was planned, the regimens that had previously been studied in LGGs were reviewed for their ability to produce objective responses and preliminary evidence of efficacy in controlling tumor regrowth. The two regimens selected were carboplatin and vincristine (CV)12,13 and thioguanine, procarbazine, dibromodulcitol, lomustine, and vincristine (TPDCV).14,15 Both regimens had a less than 10% tumor progression rate within the first 12 weeks of treatment and both were well tolerated. When the study opened, dibromodulcitol was not available, so the agent was deleted, resulting in the TPCV regimen.
The primary aim of this study was to compare EFS for these two chemotherapy regimens for LGGs in children younger than age 10 years for whom radiotherapy was felt by the practitioner to pose a high risk of neurodevelopmental injury. To the best of our knowledge, this study is the first centrally reviewed and randomized study of chemotherapy for LGGs in young children. Secondary aims were to compare tumor response rates and toxicity of the two regimens and to identify clinical prognostic factors to stratify children on future studies.
PATIENTS AND METHODS
Patients
The Children's Oncology Group Protocol A9952 was opened to Children's Cancer Group (CCG) in April 1997 and to Pediatric Oncology Group (POG) member institutions in August 2000 when the groups merged. It was closed to new patient entry in January 2005. Patients were younger than age 10 years at study entry and had LGGs (WHO grades 1 and 216) with less than 95% resection or residual tumor of more than 1.5 cm2 that were newly diagnosed with residual tumor or were progressive after surgery. Eligible histopathologic diagnoses included low-grade astrocytoma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma, subependymal giant cell astrocytoma, infantile desmoplastic astrocytoma, low-grade oligodendroglioma, oligoastrocytoma, ganglioglioma, and infantile desmoplastic ganglioglioma. In addition, chiasmatic-hypothalamic tumors intrinsic to the optic pathway were eligible without pathologic confirmation. Pathology was centrally reviewed for eligibility by the study neuropathologist (A. Yates). Tumors of all areas of the brain with appropriate histology and residual tumor were eligible, except for intrinsic tumors of the pons and optic nerve tumors without involvement of the optic chiasm. Patients with clinically or radiologically progressive tumors were enrolled within 6 weeks of progression. Patients with newly diagnosed incompletely resected tumors who were symptomatic were enrolled within 6 weeks of surgery or radiologic diagnosis of an optic pathway tumor. Patients must have received no previous treatment for tumor other than surgery. Randomization occurred at study entry, and chemotherapy was started within 3 days of enrollment. The study also included a nonrandomized arm for patients with neurofibromatosis type 1. These results will be reported separately. All patients and/or guardians gave written informed consent according to institutional and National Cancer Institute guidelines, and the protocol was approved by the institutional review boards at all participating centers.
Study Design
Patients were randomly assigned to CV or TPCV (Figs 1 and 2). The objective response to chemotherapy was determined at 6 months and end of therapy by the institution and by central review by the study neuroradiologists (G.V. and T.N.B.), who also retrospectively reviewed the baseline magnetic resonance imaging (MRI) scans for eligibility and the MRI demonstrating progression. MRI evaluations were performed at least every 3 months while the patient was on therapy, every 3 months the first year off therapy, then at 6-month intervals until 5 years off therapy. The protocol did not include further surgery or radiation.
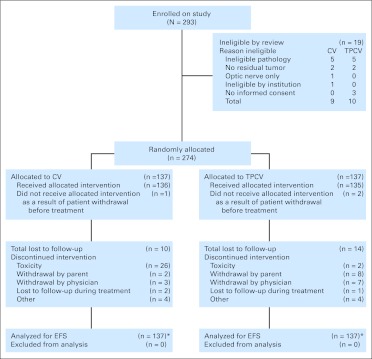
Fig 1.
CONSORT diagram. CV, carboplatin and vincristine; EFS, event-free survival; TPCV, thioguanine, procarbazine, lomustine, and vincristine. (*) Patients lost to follow-up or off study were censored at date last seen.
Fig 2.
Treatment schema for induction and maintenance therapy for two regimens. Regimen A: carboplatin (CP) and vincristine (VCR). Regimen B: TPCV, thioguanine (TG), procarbazine (PCB), CCNU (lomustine), and vincristine (VCR). ANC, absolute neutrophil count.
Evaluation of Tumor Response
Tumor size was estimated from maximal bidimensional measurements by using the product of longest diameter and its longest perpendicular diameter for solid components of each lesion, excluding cysts. Because enhancement can vary depending on technique and timing, the fluid attenuated inversion recovery (FLAIR) and T2-weighted images were primarily used by the central reviewers to determine response. Complete response was defined as complete disappearance of all known disease, partial response as at least 50% reduction in maximal tumor area with no new lesions or progression of any lesion, minor response as a 25% to 50% reduction, stable disease as a change less than ± 25% in tumor area, and progressive disease as an increase of ≥ 25% in tumor area in any site of residual tumor or reappearance of tumor at any site. The date of progression for this analysis was based on the institutional report since that was the date the patient ended protocol therapy.
Statistical Analysis
Patients who did not have neurofibromatosis were randomly assigned to one of the experimental regimens on study enrollment, stratified by site of disease (hypothalamic/optic v other), status of entry (progressive low-grade astrocytoma v newly diagnosed, incomplete resection), and pathology (pilocytic v fibrillary v other; Fig 1). The primary end points for analysis of treatment efficacy were EFS and overall survival (OS). EFS was defined as the time to first disease progression, disease recurrence, death from any cause, or occurrence of a second malignant neoplasm (SMN). OS was defined as the time to death from any cause. The accrual target was 280 to 340 eligible, correctly randomly assigned patients enrolled over a 4-year period. Assuming EFS no greater than 50% at 5 years, with the majority of progressions occurring within the first 4 years, 5% yearly loss to follow-up rate, and final analysis occurring after a minimum of 1 year follow-up, and based on the log-rank test, a sample size of at least 280 randomly assigned patients ensured that a decision to carry the treatment forward with the higher observed 5-year EFS would be correct with at least 80% probability if the true difference in 5-year EFS were 5% and would be correct with at least 95% chance if there were a 10% difference. This sample size provided at least 80% power to detect a 1.95-fold difference in failure rate on the basis of a two-sided log-rank test with 5% type I error.17,18 Interim monitoring was based on the method of Lan-Demets.19 The primary planned randomized treatment comparison was based on the log-rank test stratified for the risk group. Because it appeared that the proportional hazards assumption on which the validity of the log-rank test depends may not hold (ie, the survival curves crossed), a secondary, unplanned analysis of the difference in long-term EFS in the two randomly assigned groups was also performed. This was based on a log normal nonmixture parametric cure model analysis, adjusting for the stratification factor.20 Details of this analysis, which does not depend on the proportional hazard assumption, are provided in the Appendix (online only). Patients were analyzed according to their original assigned treatment without regard to which treatment they actually received and without regard to any deviations from that treatment that might have occurred or any nondisease events that may have occurred after the patients were enrolled. The only patients excluded from the analysis were the ineligible patients as shown in Figure 1.
Nonparametric EFS and OS curves were computed by using the product-limit (Kaplan-Meier) estimates, with SEs via the Greenwood formula. Multivariate Cox regression analysis was used to analyze possible prognostic factors for the risk of recurrence. Cumulative incidences for toxicity were obtained by using life table methods, with an event defined as the first occurrence of a primary toxicity. The time scale used is the time in days to first occurrence of a key acute or subacute toxicity since the start of therapy. Patients who had progression or recurrence of disease were censored in these analyses. The test of differences in toxicity rates between treatments was based on the log-rank test. Our analysis is based on the data cutoff of June 2010.
RESULTS
Patient Characteristics
Between April 1997 and January 2005, the study enrolled and randomly assigned 293 patients. After central review, there were 19 ineligible patients, nine for CV and 10 for TPCV. Reasons for exclusion for CV and TPCV are shown in Figure 1. Characteristics of patients at the time of enrollment are provided in Table 1. In all, 137 patients were randomly assigned to each regimen.
Table 1.
Patient Characteristics
| Characteristic | CV (n = 137) |
TPCV (n = 137) |
All (N = 274) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 63 | 46 | 66 | 48 | 129 | 47 |
| Female | 74 | 54 | 71 | 52 | 145 | 53 |
| Age, years | ||||||
| < 1 | 19 | 14 | 15 | 11 | 34 | 12 |
| 1-5 | 69 | 50 | 67 | 49 | 136 | 50 |
| 5-10 | 49 | 36 | 55 | 40 | 104 | 38 |
| Race | ||||||
| White | 104 | 76 | 84 | 61 | 188 | 69 |
| Hispanic | 15 | 11 | 23 | 17 | 38 | 14 |
| African American | 9 | 7 | 17 | 12 | 26 | 9 |
| Other/unknown | 9 | 7 | 13 | 9 | 22 | 8 |
| Status at entry | ||||||
| Newly diagnosed | 98 | 72 | 99 | 72 | 197 | 72 |
| Progressive low-grade astrocytoma | 38 | 28 | 37 | 27 | 75 | 27 |
| Unknown | 1 | 1 | 1 | 1 | 2 | 1 |
| Amount of residual tumor, cm2 | ||||||
| ≤ 1.5 | 15 | 11 | 4 | 3 | 19 | 7 |
| 1.5-3.0 | 28 | 20 | 38 | 28 | 66 | 24 |
| > 3 | 79 | 58 | 73 | 53 | 152 | 55 |
| Unknown/not measurable | 15 | 11 | 22 | 16 | 37 | 14 |
| Extent of resection | ||||||
| No surgery | 24 | 18 | 20 | 15 | 44 | 16 |
| Biopsy only (< 10%) | 48 | 35 | 49 | 36 | 97 | 35 |
| Partial/subtotal (10%–95%) | 57 | 42 | 62 | 45 | 119 | 43 |
| Radical subtotal (> 95%) | 7 | 5 | 4 | 3 | 11 | 4 |
| Unknown | 1 | 1 | 2 | 1 | 3 | 1 |
| Institutional pathology | ||||||
| Juvenile pilocytic astrocytoma | 60 | 44 | 64 | 47 | 124 | 45 |
| Low-grade fibrillary astrocytoma | 12 | 9 | 16 | 12 | 28 | 10 |
| Low-grade astrocytoma, NOS | 15 | 11 | 12 | 9 | 27 | 10 |
| Other eligible diagnosis | 10 | 7 | 11 | 8 | 21 | 8 |
| No biopsy/insufficient tumor tissue | 40 | 29 | 34 | 25 | 74 | 27 |
| Tumor site | ||||||
| Optic chiasm/hypothalamus | 71 | 52 | 67 | 49 | 138 | 50 |
| Thalamus | 11 | 8 | 14 | 10 | 25 | 9 |
| Other supratentorial | 20 | 15 | 23 | 17 | 43 | 16 |
| Posterior fossa/brainstem | 25 | 18 | 25 | 18 | 50 | 18 |
| Spinal cord | 6 | 4 | 3 | 2 | 9 | 3 |
| Unknown/missing | 4 | 3 | 5 | 4 | 9 | 3 |
Abbreviations: CV, carboplatin and vincristine; NOS, not otherwise specified; TPCV, thioguanine, procarbazine, lomustine, and vincristine.
Treatment Failures
Of 274 eligible patients, 151 experienced a treatment failure event, as defined by their treating institution, and 38 have died. Patients who did not experience EFS were followed for a median of 5.7 years as of the data cutoff date. Two patients treated with TPCV had SMNs, but there were none in the CV treatment group. The SMN was the first event in one patient. The SMNs were myelodysplastic syndrome (not otherwise specified) at 5.5 years after diagnosis in a patient who also had recurrence of primary tumor and subsequent treatment with temozolomide and a papillary thyroid carcinoma 6.5 years after primary diagnosis.
Overall Outcome and Treatment Effect
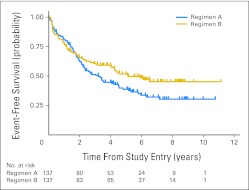
Five-year EFS and OS rates for 274 randomly assigned patients were 45% ± 3.2% and 86% ± 2.2%, respectively. Five-year EFS was 39% ± 4% for the CV regimen and 52% ± 5% for the TPCV regimen (stratified log-rank test P = .1; Fig 3). On the basis of the secondary unplanned cure model, there was evidence that the EFS outcome in these two treatment arms differed in general (P = .025; likelihood ratio χ2, 2 df). This general difference was attributable mostly to the difference in the long-term EFS (P = .007; likelihood ratio χ2, 1 df), and in part to nonproportionality (ie, the survival curves were similar in early follow-up but differed only later; P = .027; likelihood ratio χ2, 1 df).
Fig 3.
Event-free survival for patients randomly assigned to regimen A (CV: carboplatin and vincristine) or regimen B (TPCV: thioguanine, procarbazine, CCNU [lomustine], and vincristine).
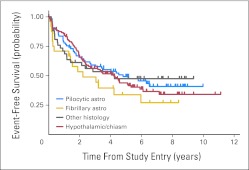
Five-year EFS by stratum was 49% ± 6% for patients with pilocytic astrocytoma, 44% ± 5% for those with hypothalamic/optic chiasmal tumors, and 34% ± 10% for those with fibrillary astrocytoma (Appendix Fig A1, online only). Five-year OS was 86% ± 3% for CV and 87% ± 7% for TPCV (log-rank P = .52). Five-year OS was 88% ± 4% for patients with pilocytic astrocytoma, 87% ± 3% for those with hypothalamic/optic chiasmal tumors, and 79% ± 8% for those with fibrillary astrocytoma.
Prognostic Factors
Multivariate analysis revealed two factors that were independently predictive of EFS: age and the amount of residual tumor (Table 2). The relative risk for progression/relapse was 3.4 times higher (95% CI, 1.99 to 5.66) in patients who were younger than age 1 year than in those who were older than age 5 years. Five-year EFS was 19% ± 7% for patients younger than age 1 year, 51% ± 4% for those age 1 to 5 years, and 64% ± 4% for those older than 5 years. The relative risk for progression/relapse was 0.65 times lower (95% CI, 0.44 to 0.97) in patients with residual tumor less than 3.0 cm2 than in patients with residual tumor ≥ 3.0 cm2. None of the following variables had an association with EFS that reached the nominal significance level P < .05: sex, race, histology, extent of resection, tumor enhancement, treatment at diagnosis versus at progression, or objective tumor response to chemotherapy. Three factors were independently associated with OS: age, tumor site, and amount of residual tumor (Table 2).
Table 2.
Multivariate Risk Factors Affecting Outcome
| Prognostic Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Affecting event-free survival | |||
| Age, years | |||
| ≥ 5 | 1.0 | < .001 | |
| 1-5 | 1.8 | 1.21 to 2.73 | |
| < 1 | 3.4 | 1.99 to 5.66 | |
| Residual tumor, cm2 | |||
| > 3.0 | 1.0 | .03 | |
| < 3.0 | 0.65 | 0.44 to 0.97 | |
| Affecting overall survival | |||
| Age, years | |||
| ≥ 5 | 1.0 | .001 | |
| 1-5 | 0.94 | 0.35 to 2.56 | |
| < 1 | 6.0 | 2.04 to 17.4 | |
| Tumor site | |||
| Thalamus | 1.0 | .001 | |
| Midbrain | 0.20 | 0.049 to 0.83 | |
| Hypothalamus/optic chiasm | 0.1 | 0.037 to 0.35 | |
| Other | 0.12 | 0.03 to 0.43 | |
| Residual tumor, cm2 | |||
| > 3.0 | 1.0 | .03 | |
| < 3.0 | 0.31 | 0.1 to 0.91 |
Tumor Response
Tumor responses to each regimen at the end of chemotherapy (after CV maintenance cycle 8 or TPCV cycle 8), as coded by central review, are listed in Table 3. There were 44 patients (32%) in the CV regimen and 34 patients (25%) in the TPCV regimen who were not evaluable for response because they went off therapy for allergic reactions to carboplatin, parent choice, physician choice, or did not submit scans before the last course of chemotherapy.
Table 3.
Tumor Response at End of Chemotherapy by Central Review
| Review Response | CV |
TPCV |
||||
|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | |
| Complete/partial | 33 | 35 | 27 to 46 | 31 | 30 | 22 to 40 |
| Minor | 14 | 15 | 9 to 24 | 23 | 22 | 15 to 31 |
| Stable disease | 16 | 17 | 11 to 26 | 16 | 16 | 10 to 24 |
| Progressive disease/recurrence/off study because of progressive disease | 30 | 32 | 26 to 42 | 33 | 32 | 24 to 42 |
| Total | 93 | 100 | 103 | 100 | ||
Abbreviations: CV, carboplatin and vincristine; TPCV, thioguanine, procarbazine, lomustine, and vincristine.
Toxicity
Table 4 describes cumulative incidence of toxicity by the end of chemotherapy with comparisons between the CV and TPCV treatment groups. All the allergic reactions reported were attributed to carboplatin. When the study opened, patients were required to be removed from carboplatin therapy for any grade allergic reaction. On August 11, 2000, the protocol was amended to allow patients with grade 1 to 2 allergic reactions to remain on study if they did not progress to grade 3 to 4. A total of 26 patients went off therapy for allergic reactions. The incidence of peripheral nervous system grade 3 to 4 toxicity was 19% for CV and 19% for TPCV because of the vincristine in both regimens. TPCV also had more reported CNS grade 3 to 4 toxicity. For both regimens, neurologic symptoms from tumor were reported as CNS events related to drug treatment in several patients. For CV, six patients (2.2%) were coded as having CNS toxicity when cranial nerve deficits and leg weakness were present and accompanied by other signs of vincristine toxicity. This did not occur with TPCV. The primary difference was that 11 patients had grade 3 or 4 episodes of transient weakness or seizures with TPCV, although only one patient given CV had this problem.
Table 4.
Cumulative Incidence of Toxicity by End of Chemotherapy
| Key Toxicity | Grade 3 or 4 |
Grade 4 Only |
||||
|---|---|---|---|---|---|---|
| CV | TPCV | P | CV | TPCV | P | |
| Absolute neutrophil count | 94 | 77 | < .001 | 73 | 48 | < .001 |
| Platelets | 21 | 48 | < .001 | 8 | 26 | < .001 |
| Hemoglobin | 35 | 40 | .91 | 6.1 | 7.9 | .68 |
| ALT | 3 | 11 | .004 | 1.2 | 2.8 | .09 |
| Total bilirubin | 0.0 | 1.5 | .16 | 0.0 | 0.7 | .32 |
| Creatinine | 1.2 | 0.0 | 1.00 | 1.2 | 0.0 | 1.00 |
| Creatinine clearance | 0.0 | 0.9 | .33 | 0.0 | 0.0 | 1.00 |
| Pulmonary | 2.1 | 3.3 | .44 | 1.0 | 2.6 | .34 |
| Calcium | 4.8 | 2.5 | .69 | 2.9 | 1.7 | .64 |
| Magnesium | 2.8 | 2.3 | .65 | 0.7 | 0.0 | .32 |
| Peripheral nervous system | 19 | 19 | .92 | 0.0 | 0.0 | 1.00 |
| CNS | 12 | 24 | .004 | 0.7 | 3.4 | .19 |
| Allergy | 10 | 0 | < .001 | 2.8 | 0.0 | .08 |
| Infection | 23 | 26 | .39 | 0.0 | 1.7 | .16 |
Abbreviations: CV, carboplatin and vincristine; TPCV, thioguanine, procarbazine, lomustine, and vincristine.
There were two patients who went off therapy for toxicity on the TPCV regimen. The percentage of patients who had therapy modified because of toxicity for each course of maintenance (two cycles) for CV and TPCV, respectively, were course 1, 13.04% and 1.24%; course 2, 13.66% and 4.97%; course 3, 10.56% and 8.07%; and course 4, 4.97% and 12.42%. Twenty-one patients receiving TPCV and 11 receiving CV discontinued treatment for reasons other than event, completion of therapy, or toxicity: withdrawal by parent (8 and 2), withdrawal by doctor (7 and 3), lost to follow-up (1 and 2), and other/unknown (5 and 4).
DISCUSSION
The A9952 clinical trial was initiated in 1997 to validate the approach of using chemotherapy for LGG to improve survival and delay radiotherapy in young children and to compare effectiveness and toxicity of the two regimens. The 5-year EFS was 39% ± 4% for CV and 52% ± 5% for TPCV. EFS with the TPCV regimen was similar to that of CV in the first 2 years, but EFS was higher long-term for TPCV, based on the cure model analysis. Although the strength of this evidence of a difference in EFS provided by the planned stratified log-rank test (P = .1), did not reach the traditional P < .05 level, we also performed a secondary, unplanned, and admittedly data driven cure model analysis. Data driven analyses should be viewed with some skepticism, since one is testing for the difference that one sees without having an a priori expectation that it would exist. We think, though, that this analysis suggests that TPCV prevents tumor progression in a higher proportion of patients than does CV.
TPCV had slightly more toxicity when the allergic reactions to carboplatin were excluded. The concern by some physicians has been the potential for toxicity of the TPCV regimen, especially the risk of second neoplasms. The objective toxicity data presented in this article show that this may have been feared out of proportion to the facts. The one patient with secondary leukemia was also treated with temozolomide, an alkylator with potential risk for second neoplasms. The 15-year follow-up of the TPDCV regimen of 33 patients also showed only one second neoplasm, an osteosarcoma in the radiation field.21
Both regimens have efficacy that is comparable to or superior to that of other regimens in recent trials. For example, weekly vinblastine was reported in nine children with LGGs who had allergic reactions to carboplatin and low toxicity. Response was evaluated from diagnosis and included response to both CV and vinblastine. Median follow-up was 10 months, so EFS was not determined.22 St. Jude Children's Research Hospital reported a study of tamoxifen and carboplatin with two of 14 patients having an objective response.23 Massimino et al24,25 in Italy studied cisplatin and etoposide in children younger than 21 years with LGGs who achieved a 70% response rate but at the expense of high-frequency ototoxicity. This same group reported 37 children, median age 6 years, treated with a lower dose cisplatin and etoposide regimen with less ototoxicity and 3-year EFS of 65%. The French Society of Pediatric Oncology studied the combination of procarbazine, carboplatin, vincristine, etoposide, cisplatin, and cyclophosphamide.26 They achieved a 34% progression-free survival rate at 5 years in children younger than age 5 years when enrolled. The Children's Oncology Group (COG) and others have also studied oral temozolomide in small groups of children with recurrent LGGs.27,28 The COG phase II study of temozolomide found one partial response in 21 children with LGGs, but 41% had stable disease through all 12 courses of the chemotherapy. Khaw et al27 treated 13 children ages 3.8 to 15.2 years with progressive LGG with temoxolomide, and the 3-year EFS was 57%. Finally, new regimens that include bevacizumab and irinotecan29 have been evaluated in large groups of previously treated children with recurrent hypothalamic juvenile pilocytic astrocytoma, with seven of 10 tumors having objective responses.28
The prognostic factors identified by this study can form the basis for future clinical trials. The importance of this is illustrated by the difficulty of comparing the recent clinical trials mentioned earlier that included different age groups, no information about tumor size, and different end points. For LGGs, as for other childhood tumors, there should be some risk stratification so that results of phase II trials can be adequately compared, thus avoiding comparisons between patient groups with different prognoses. In addition, treatment can be reduced for groups with better prognosis to permit more tailored treatment approaches in the future. Risk stratification by biologic markers and further individualization of care should be explored. To provide this biologic information, tumor biopsy, when feasible, will be important in future studies since new potentially prognostic genetic abnormalities, such as IDH and BRAF, that were not known at the time of our study, have been identified.30,31 With exciting new studies that show alterations in the BRAF gene in pediatric LGG, biologically targeted treatments may be possible in the future.30,32
Supplementary Material
Acknowledgment
We recognize Allan Yates, MD, who provided the neuropathologic review for this study and who passed away suddenly in August 2010. We are grateful for his years of dedicated support as a review neuropathologist for the Children's Oncology Group.
Appendix
Analysis Based on Parametric Cure Model
An unplanned analysis of event-free survival (EFS) based on a parametric cure model is presented in our report. This Appendix provides the details of this analysis. A complete description of analysis using parametric cure models (PCMs) is provided by Sposto (Sposto R: Stat Med 21:293-312, 2002).
The nonmixture PCM used in our report takes the form:
where π is the cure rate (or alternatively, the proportion of long-term event-free survivors), and F(t) is any proper continuous cumulative distribution function. In the current analysis, F(t) was the lognormal function:
where Φ(·) is the standard normal distribution function, λ is a scale parameter, and γ is a shape parameter. This function was found by likelihood criteria to provide the best fit to the data compared with a Weibull function or logistic function.
The hazard for the nonmixture PCM is:
where f(t) is the density function corresponding to F(t). Both π and F(t) (or equivalently, f(t)) can depend on covariates, as we describe. One can see from the third equation that when only π depends on covariates but f(t) does not, the model will satisfy the proportional hazards (PH) assumption.
In the current analysis, π and F(t) were allowed to depend on covariates. The cure rate was modeled as complementary log log, that is:
Covariates were introduced in the scale parameter of F(t) through a log-linear function:
where γ was a scalar parameter.
For the particular analyses presented in our report, z1 was an indicator of treatment group, and z2,…,z8 were indicators of the different strata.
Model parameters were estimated via maximum likelihood, and through this, various meaningful likelihood ratio (LR) tests could be formed. In particular, three LR tests are referred to in our report. All of these are with reference to the complete model that includes the parameters.
LR test of general difference in EFS between the two treatment groups.
This is a 2-df test of the null hypothesis a1 = b1 = 0. This is a test of whether treatment has any influence at all, either in long-term EFS (through parameter π) or in the distribution of failure times among patients who experience an event (through the scale parameter λ), controlling for the effect of the stratification variables. This test was χ2df2 = 7.38, p = 0.025.
LR test of the treatment effect on long-term EFS (ie, cure rate).
This is a 1-df test of the null hypothesis b1 = 0, and a test of the difference in cure rate resulting from treatment after controlling for stratification variables and the influence of treatment on the time to failure in those for whom treatment fails. This test was χ1df2 = 7.27, p = 0.007.
LR test of the treatment effect on the distribution of failure times among those for whom treatment fails.
This is a 1-df test of the null hypothesis b1 = 0, and is a test of whether the treatment effect on EFS has a non-PH component to it. This test was χ1df2= 4.88, p =0.027.
Fig A1.
Event-free survival by stratum at study entry. Astro, astrocytoma.
Footnotes
Supported by Grant No. U10 CA98543 from the National Institutes of Health to the Chairman of the Children's Oncology Group.
Presented in part at the 12th International Symposium on Neuro-Oncology, Nara, Japan, June 6-9, 2006; 13th International Symposium on Neuro-Oncology, Chicago, IL, June 29-July2, 2008; and 40th Congress of the International Society of Pediatric Oncology, Berlin, Germany, September 21-23, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joann L. Ater, Claire M. Mazewski, Roger J. Packer, Michael Prados, Richard Sposto, Gilbert Vezina, Jeffrey H. Wisoff, Ian F. Pollack
Provision of study materials or patients: Joann L. Ater, Roger J. Packer, Michael Prados, Ian F. Pollack
Collection and assembly of data: Joann L. Ater, Tianni Zhou, Emiko Holmes, Timothy N. Booth, Ken H. Lazarus, Roger J. Packer, Richard Sposto, Gilbert Vezina
Data analysis and interpretation: Joann L. Ater, Tianni Zhou, Emiko Holmes, Claire M. Mazewski, David R. Freyer, Roger J. Packer, Richard Sposto, Gilbert Vezina
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pollack IF. Brain tumors in children. N Engl J Med. 1994;331:1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: A decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 5.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 6.Mulder RL, Kremer LC, van Santen HM, et al. Prevalence and risk factors of radiation-induced growth hormone deficiency in childhood cancer survivors: A systematic review. Cancer Treat Rev. 2009;35:616–632. doi: 10.1016/j.ctrv.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83:583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 8.Collet-Solberg PF, Sernyak H, Satin-Smith M, et al. Endocrine outcome in long-term survivors of low-grade hypothalamic/chiasmatic glioma. Clin Endocrinol (Oxf) 1997;47:79–85. doi: 10.1046/j.1365-2265.1997.2211032.x. [DOI] [PubMed] [Google Scholar]
- 9.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy AT, Packer RJ. Chemotherapy for low-grade gliomas. Childs Nerv Syst. 1999;15:506–513. doi: 10.1007/s003810050539. [DOI] [PubMed] [Google Scholar]
- 11.Moore BD, 3rd, Ater JL, Copeland DR. Improved neuropsychological outcome in children with brain tumors diagnosed during infancy and treated without cranial irradiation. J Child Neurol. 1992;7:281–290. doi: 10.1177/088307389200700308. [DOI] [PubMed] [Google Scholar]
- 12.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 13.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 14.Petronio J, Edwards MS, Prados M, et al. Management of chiasmal and hypothalamic gliomas of infancy and childhood with chemotherapy. J Neurosurg. 1991;74:701–708. doi: 10.3171/jns.1991.74.5.0701. [DOI] [PubMed] [Google Scholar]
- 15.Prados MD, Edwards MS, Rabbitt J, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein LV, Gail MH, Santner TJ. Planning the duration of a comparative clinical trial with loss to follow-up and a period of continued observation. J Chronic Dis. 1981;34:469–479. doi: 10.1016/0021-9681(81)90007-2. [DOI] [PubMed] [Google Scholar]
- 18.Sposto R, Sather HN. Determining the duration of comparative clinical trials while allowing for cure. J Chronic Dis. 1985;38:683–690. doi: 10.1016/0021-9681(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 19.Demets DL. Group sequential procedures: Calendar versus information time. Stat Med. 1989;8:1191–1198. doi: 10.1002/sim.4780081003. [DOI] [PubMed] [Google Scholar]
- 20.Sposto R. Cure model analysis in cancer: An application to data from the Children's Cancer Group. Stat Med. 2002;21:293–312. doi: 10.1002/sim.987. [DOI] [PubMed] [Google Scholar]
- 21.Mishra KK, Squire S, Lamborn K, et al. Phase II TPDCV protocol for pediatric low-grade hypothalamic/chiasmatic gliomas: 15-year update. J Neurooncol. 2010;100:121–127. doi: 10.1007/s11060-010-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636–2642. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 23.Walter AW, Gajjar A, Reardon DA, et al. Tamoxifen and carboplatin for children with low-grade gliomas: A pilot study at St. Jude Children's Research Hospital. J Pediatr Hematol Oncol. 2000;22:247–251. doi: 10.1097/00043426-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 25.Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100:65–71. doi: 10.1007/s11060-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 26.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: Dependence on age and the quality of the response to chemotherapy—Results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Khaw SL, Coleman LT, Downie PA, et al. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49:808–811. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: A report from the Children's Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 29.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Deshmukh H, Gutmann RJ, et al. Alterations of BRAF and HIPK2 loci predominate in sporadic pilocytic astrocytoma. Neurology. 2009;73:1526–1531. doi: 10.1212/WNL.0b013e3181c0664a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.