Abstract
Background
Neurotrauma centers have developed management protocols on the basis of evidence obtained from literature analysis and institutional experience. This article reviews our institutional experience in the management of severe traumatic brain injury (TBI) at Simòn Bolivar Hospital, the district trauma center for Bogotá's north zone.
Methods
This is a case control study comparing a group of patients (n: 16) operated for severe TBI between January 2002 and July 2004 according to an institutional management protocol characterized by an early decompressive craniectomy (DC) approach versus a historical control group (n: 20) managed before the implementation of such protocol. Mortality and Glasgow Outcome Score (GOS) at 6 months were used as the main outcome variables.
Results
An early DC protocol implemented within 12 hours from injury in 16 patients with severe isolated TBI and a Marshall score between III or IV was associated with a lesser mortality than the conventional approach with ventriculostomy and Intensive Care Unit (ICU) management alone. The GOS was significantly better in the DC group (p=0.0002) than in the control group.
Conclusion
The use of an early DC protocol for severe TBI patients (Glasgow Coma Scale <9) had a significantly improved outcome compared with the conventional approach with ventriculostomy and ICU management in Simòn Bolivar Hospital in Bogotá, Colombia.
Keywords: Decompressive craniectomy, neurotrauma, severe head trauma, traumatic brain injury
Severe traumatic brain injury (TBI) is associated with a high mortality and morbidity. Increased understanding of the pathophysiology of TBI and the concept of primary versus secondary injury has provided new insight in the early management of TBI. Key factors related to the intrinsic pathology and their clinical implications, especially in patients with severe TBI (defined as a Glasgow Coma Scale [GCS] <9) have allowed the establishment of new and more aggressive protocols across the spectrum of neurosurgical care.
It has become widely accepted that the magnitude of the secondary injury is a function of the quality of the care from the prehospital scene, continuing with the appropriate neurocritical care, until the definitive surgical management is undertaken. These concepts, originated in specialized neurotrauma centers from North America and Europe, have begun to be applied in Latin America after their diffusion facilitated by work groups such as the Brain Trauma Foundation (BTF), the Acute Brain Injury Consortium, the European Brain Injury Consortium, and the International Neurotrauma Society.[1-6]
Colombia is a country with a population of 44 million and a very high incidence of traumatic injury, with a violence-related mortality rate between 50 to 60 per 100,000 habitants in the last 20 years. The annual income per capita is under $1,924 United States dollars (USD) and 21% of the population has a daily income under $1 USD.[7] Therefore, the implementation of these guidelines and recommendations should have a significant effect on public health. Simòn Bolivar Hospital (SBH) is a designated level one trauma center for the north side of Bogotá. The SBH actively participated in the development of a severe TBI management quality program, instituted in Colombia by FUNDCOMA (a member institution of the BTF of New York since 2001). However, since 1999, the SBH was informally implementing the recommendations of the American Association of Neurological Surgeons (AANS) guidelines. Aware of the multiple factors published in several studies.[8-11] describing poor adherence to the TBI guidelines, the staff at SBH begin an aggressive campaign to ensure maximal adherence to these guidelines after 2001.
In 2004, we performed a general overview of the TBI patients who were brought to the operating room (OR) by neurological surgery service with a GCS <9,[12] and we identified 16 patients who were managed according to the SBH early decompressive craniectomy (DC) protocol. We then identified a historical control group of 20 cases, matched according to Marshall score, computed tomography (CT) findings and the GCS, who were managed with ventriculostomy and medical/critical care therapy for intracranial pressure (ICP) control without the DC. Here, we present a comparison of these two groups of patients, which showed a significant difference between them in the incidence of mortality and long-term outcome as determined by Glasgow Outcome Score (GOS).
Materials and Methods
Historical course, development and description of early DC protocol
The 1995 AANS guidelines document was helpful for us to begin changing our approach to the management of TBI at SBH. In 1999, we standardized the use of external ventriculostomy for cerebrospinal fluid (CSF) drainage and monitored severe TBI patients according to the proposed criteria (Table 1).
Table 1.
Indications for ICP monitoring used in Simòn Bolivar Hospital, according to the AANS recommendations (Brain Trauma Foundation, AANS. J Neurotrauma 1996;13:639-734)
| Indications for ICP monitoring according to the AANS guidelines |
|---|
|
Due to the elevated number of patients with severe injuries (many of them with high-velocity penetrating trauma), we instituted early cranial decompression surgery as suggested by earlier reports.[13-16] Early decompression consisted of several techniques such as bi-frontal, temporal windows and bilateral decompression. These interventions were initially performed based on individual criteria of the hospital's attending neurosurgeons. The procedure initially was done as a second-line therapy, after 24 or 48 hours of medical management in patients with a poor response to medical therapy and with an ICP threshold of 25 mmHg. In other cases, we did the procedure in the first 12 hours after trauma. By 2002, early DC was defined as an early surgical intervention usually within 12 hours from injury aimed to diminish the duration of intracranial hypertension in a group of patients who historically had very high mortality according to our experience. In 2002, we decided to standardize the protocol of early DC for the neurotrauma program in part due to the technological limitations for cerebral metabolism monitoring in the SBH intensive care unit (ICU), always striving to obtain better outcomes in this group of patients, including the pediatric population.
Our concept of early DC surgery is based on the following aspects:
1st Phase: Fast and simple decompression technique with external temporary closing.
2nd Phase: Transfer to surgical critical care unit for medical management of intracranial hypertension.
3rd Phase: Elective surgery for definitive closing.
Since 2002, this protocol has been applied in patients who fulfill the following admission criteria (Table 2):
Table 2.
Inclusion criteria for the early decompressive craniectomy procedure
(SBH. Neurological Surgery Service Protocol)
| No. Description of inclusion criteria |
|---|
|
Age younger than 50 years.
GCS <9 after emergency room resuscitation (SaO2 >90% and systolic blood pressure [SBP] >90 mm) and after pharmacologic sedation or muscle relaxants have been metabolized if they were used (short action agents in rapid sequence intubation institutional protocols).
Isolated, non-penetrating head injury, without other associated traumas (i.e. abdominal, thoracic or extremity injuries).
CT findings compatible with diffuse injury III or IV of the Marshall classification (The volume and width of the lesions and the midline shift were measured with the CT scan software and correlated with the ABC method for width and the [A/2] – B method for the midline shift).[17,18]
Time from injury <12 hours.
Absence of brain death.
Surgical procedure
The procedure performed in the 16 early DC patients of the study was a decompressive fronto-temporo-parietal craniectomy, uni - or bilaterally according to the CT findings (diffuse edema uni- or bilateral), with dural incision in “H” form (5 cm × 10 cm), auto graft dural patch. In bilateral interventions, an osseous bar was left over the transverse sinus 3 - 4 cm in width with osteotomies at the frontal and occipital level. The osseous graft was saved in the bone bank.
Clinical and radiological definition of severe TBI
Severe TBI (GCS <9) with cerebral edema was defined according to the CT findings, following Marshall's classification III and IV (Fig. 1) (Table 3).[19] Neurological deterioration was characterized as progressive increment in ICP, which was confirmed in some cases with an early ventriculostomy, with a fall in the GCS of more than 2 points, as well as with abnormal motor response, pupillary asymmetry or fixed and dilated pupils.
Fig. 1.
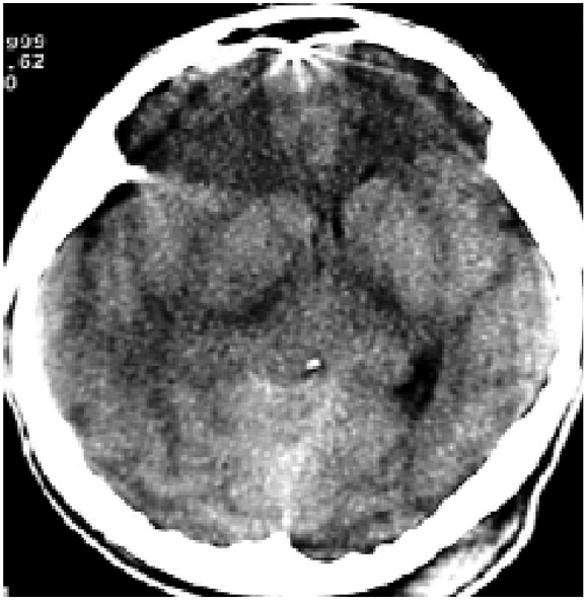
Patient with compressed or absent cisterns with midline shift 0-5 mm; no high or mixed density lesion >25 cc (Marshall III). Diffuse swelling. (Photo: Author).
Table 3.
Marshall's classification of TBI based on initial computed tomography findings[19]
| Category | Definition |
|---|---|
| Diffuse Injury I | No visible intracranial pathology. |
| Diffuse Injury II | Cisterns present with midline shift 0-5 mm and/or: lesion densities present, no high or mixed density lesion >25 cc. May include bone fragments and foreign bodies. |
| Diffuse Injury III (Swelling) | Cisterns compressed or absent with midline shift 0-5 mm; no high or mixed density lesion >25 cc. |
| Diffuse Injury IV | Midline shift >5 mm. No high or mixed density lesion >25 cc. |
| Evacuated mass | Any lesion surgically evacuated. |
| Non-evacuated mass lesion | High or mixed density lesion >25 cc, not surgically evacuated. |
Matching control group
The control group was identified and matched according to the following preoperative criteria:
Age, gender, post-resuscitation pupillary response (unilateral, bilateral or non-pupillary dilatation; dilatation was considered if the size was more than 4 mm and was not reactive), GCS, SBP and heart rate (HR) after initial trauma room resuscitation (SaO2 >90%, SBP >90 mmHg).
Outcome variables
The early DC group and control group were evaluated, and comparisons were made for ICU length of stay, total hospital length of stay, discharge status and GOS.
Statistical analyses
In order to account for the possible influence of GCS, pupils, SBP, Marshall score, age and gender, analysis of covariance models were used with GOS as the dependent variable and treatment effect (pre-versus post-2002) and the remaining variables as covariates. Due to the discreteness of the GOS response, the analysis was repeated with nonparametric rank regression technique to validate robustness of results (Table 4). All analyses were done with SAS Proc REG (SAS Institute Inc. 100 SAS Campus Dr; Cary, NC, USA).
Table 4.
Analysis of variance (ANOVA).◆
| Source | DF | Sum of squares | Mean square | F value | Pr > F |
|---|---|---|---|---|---|
| Model | 7 | 47.39873 | 6.77125 | 6.93 | <.0001 |
| Error | 28 | 27.35127 | 0.97683 | ||
| Corrected Total | 35 | 74.75000 |
The SAS System. REG Procedure. Model 1: GOS=β0 + β1*treatment + β2*GCS + β3*pupil + β4*HR + β5*SBP + β6*CT + β7*age + ε [dependent variable: GOS (Glasgow Outcome Score)].
To examine whether or not treatment groups were different from each other, we used the following model: GOS = (β0 + β1*treatment + β2*GCS + β3*pupil + β4*HR + β5*SBP + β6*CT + β7*age + β8*sex + ε) where, β0 ∼ β8 were unknown parameters and ε ∼ N (0,σ2). Treatment = 0 (if ventriculostomy) or 1 (if early DC), pupil = 1 (if bilateral), 2 (if unilateral), or 3 (if no dilatation) and sex = 1 (if male) or 2 (if female). According to the results of the model, we concluded that the sex variable was not significant, so we reduced the model to: GOS= (β0 + β1*treatment + β2*GCS + β3*pupil + β4*HR + β5*SBP + β6*CT + β7*age + ε).
Results
Demographics
In the SBH, from the informal implementation of the guidelines in March 1999 to July 2004 (cut-off for this review), 524 patients were taken to the OR because of head trauma (Table 5). The most prevalent surgical indication was acute epidural hematoma evacuation in 139 patients (26.5%), followed by acute subdural hematoma evacuation in 104 (20%), correction of depressed skull fracture in 73 (14%), multiple lesion treatment in 70 (13.4%), chronic subdural hematoma evacuation in 60 (11.4%), treatment of gunshot wounds in 38 (7.3%), intracerebral hematoma evacuation in 20 (3.8%), treatment of skull thermal injury in 12 (2.3%), and treatment of newborn obstetrical trauma in 8 (1.3%) patients.
Table 5.
Distribution of 524 TBI patients brought to the operating room between March 1999 and July 2004 according to the surgical procedure
| Surgical procedure | Patients | % of total patients |
|---|---|---|
| Epidural hematoma evacuation | 139 | 26.5 |
| Acute subdural hematoma evacuation | 104 | 20 |
| Treatment of depressed skull fracture | 73 | 14 |
| Treatment of multiple lesions* | 70 | 13.4 |
| Chronic subdural hematoma evacuation | 60 | 11.4 |
| Treatment of gunshot wound | 38 | 7.3 |
| Intracerebral hematoma evacuation | 20 | 3.8 |
| Treatment of thermal skull injury | 12 | 2.3 |
| Treatment of obstetrical trauma | 8 | 1.3 |
Multiple lesions are related to the finding of more than one injury type in the same patient
(e.g. epidural + intracerebral hematoma, etc.) (Simon Bolivar Hospital, Neurosurgical Service. Patient database, 2004)
Of the total patient group, 204 (38.9% of the total operated) had GCS <9 (severe TBI). The most common intervention in the severe TBI group was ventriculostomy in 179 patients (88%). Of this subgroup of patients, 26 (14.5% of 179 patients) underwent some kind of cranial decompression surgery, but only 16 of them (9%) were operated according to the early DC protocol; timing of surgery was 3-10 hours (mean: 6.4 hours) (Table 6) (Fig. 2).
Table 6.
Early DC group
| No | Age (yrs) |
Sex | PRP | PR SBP mmHg |
PR HR bpm |
CT Findings (Marshall) |
PR GCS | ICU Days |
In-Hospital Days |
DS | GOS 6 M |
DEC Type/Time(hrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | M | U | 110 | 75 | 3 | 8 | 5 | 10 | L | 5 | U/3 |
| 2 | 40 | F | B | 140 | 56 | 4 | 4 | 17 | 35 | L | 2 | B/6 |
| 3 | 45 | M | U | 125 | 60 | 4 | 4 | 5 | 5 | D | 1 | U/5 |
| 4 | 20 | F | B | 100 | 58 | 4 | 4 | 6 | 6 | D | 1 | B/10 |
| 5 | 1 | M | U | 105 | 126 | 4 | 8 | 7 | 19 | L | 5 | U/6 |
| 6 | 15 | F | N | 129 | 53 | 4 | 4 | 9 | 21 | L | 4 | U/9 |
| 7 | 1 | M | B | 120 | 99 | 4 | 4 | 7 | 7 | D | 1 | U/9 |
| 8 | 2 | F | U | 115 | 121 | 4 | 4 | 9 | 25 | L | 5 | U/5 |
| 9 | 9 | M | U | 130 | 64 | 3 | 4 | 7 | 21 | L | 4 | U/4 |
| 10 | 20 | F | B | 143 | 54 | 4 | 4 | 6 | 6 | D | 1 | B/6 |
| 11 | 25 | F | U | 150 | 50 | 3 | 4 | 15 | 57 | L | 3 | B/6 |
| 12 | 24 | F | B | 140 | 60 | 4 | 4 | 20 | 43 | L | 3 | B/9 |
| 13 | 5 | M | U | 122 | 78 | 4 | 4 | 7 | 29 | L | 5 | U/7 |
| 14 | 21 | F | B | 156 | 63 | 4 | 4 | 12 | 39 | L | 2 | U/6 |
| 15 | 8 | M | U | 138 | 68 | 4 | 4 | 7 | 21 | L | 5 | U/6 |
| 16 | 34 | F | B | 140 | 64 | 4 | 4 | 12 | 31 | L | 3 | B/6 |
PRP: Post-resuscitation pupils (U: Unilateral dilatation; B: Bilateral dilatation; N: No dilatation); PR SBP: Post-resuscitation systolic blood pressure (mmHg); PR HR: Post-resuscitation heart rate (beats per minute); CT: Computed tomography; PR GCS: Post-resuscitation Glasgow Coma Scale; ICU: Intensive care unit; DS: Discharge status; GOS: Glasgow Outcome Score; DEC: Decompression surgery (data from Simon Bolivar Hospital Medical Records Unit).
Fig. 2.
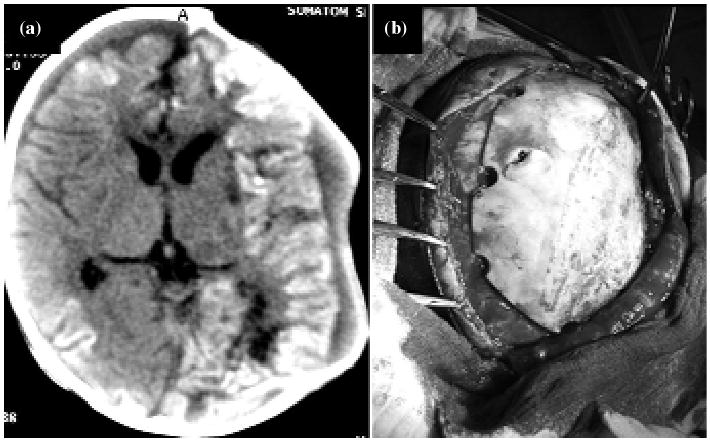
Patient under early DC procedure. (a) Satisfactory evolution. MRI shows the skull defect and post-traumatic parenchyma changes. (b) Third phase of reconstruction and definitive close with the patient's osseous graft from bone bank. (Photo: Author).
The baseline variables in each group were similar (Table 7). The mean age for the early DC group was 18.3 years compared with 24.3 years for the control group. The Revised Trauma Score (RTS) mean for both groups was 5.1. The mean of the post-resuscitation GCS was 4.5 for the early DC group and 4.4 for the control group. In the early DC group, 13 patients (81.2%) had a Marshall score of IV and 3 patients (18.8%) had a Marshall score of III in the CT findings. The Marshall score in the control group was IV in 17 patients (85%) and III in 3 patients (15%). Twelve patients (75%) in the early DC group were discharged alive and 4 patients (25%) died in the hospital. The mortality in the control group was 13 patients (65%); 7 patients (35%) were discharged alive.
Table 7.
Ventriculostomy control group
| No | Age (yrs) |
Sex | PRP Pupils |
PR SBP mmHg |
PR HR bpm |
CT Findings (Marshall) |
PR GCS | ICU Days |
In-Hospital Days |
DS | GOS 6 M |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | M | U | 140 | 67 | 4 | 4 | 3 | 3 | D | 1 |
| 2 | 41 | M | U | 153 | 80 | 3 | 4 | 6 | 6 | D | 1 |
| 3 | 21 | F | B | 127 | 64 | 4 | 5 | 2 | 2 | D | 1 |
| 4 | 16 | M | N | 120 | 87 | 4 | 4 | 6 | 6 | D | 1 |
| 5 | 15 | F | U | 135 | 90 | 4 | 4 | 13 | 27 | L | 2 |
| 6 | 34 | M | B | 130 | 54 | 4 | 4 | 3 | 3 | D | 1 |
| 7 | 7 | M | B | 110 | 64 | 4 | 4 | 2 | 2 | D | 1 |
| 8 | 35 | F | U | 127 | 87 | 3 | 8 | 7 | 14 | L | 3 |
| 9 | 9 | M | N | 100 | 89 | 4 | 4 | 6 | 12 | L | 3 |
| 10 | 15 | F | B | 140 | 62 | 4 | 4 | 4 | 4 | D | 1 |
| 11 | 34 | F | B | 130 | 76 | 4 | 4 | 2 | 2 | D | 1 |
| 12 | 43 | M | N | 143 | 77 | 4 | 4 | 10 | 23 | L | 2 |
| 13 | 19 | M | U | 120 | 87 | 4 | 4 | 9 | 9 | D | 1 |
| 14 | 4 | M | U | 99 | 100 | 4 | 4 | 8 | 17 | L | 3 |
| 15 | 21 | M | B | 137 | 70 | 4 | 4 | 4 | 4 | D | 1 |
| 16 | 30 | M | U | 140 | 66 | 4 | 4 | 2 | 2 | D | 1 |
| 17 | 7 | M | N | 100 | 90 | 4 | 4 | 11 | 31 | L | 3 |
| 18 | 24 | F | U | 120 | 58 | 4 | 4 | 12 | 26 | L | 2 |
| 19 | 31 | M | N | 141 | 80 | 3 | 7 | 7 | 7 | D | 1 |
| 20 | 44 | M | B | 129 | 75 | 4 | 4 | 2 | 2 | D | 1 |
PRP: Post-resuscitation pupils (U: Unilateral dilatation; B: Bilateral dilatation; N: No dilatation); PR SBP: Post-resuscitation systolic blood pressure (mmHg); PR HR: Post-resuscitation heart rate (beats per minute); CT: Computed tomography; PR GCS: Post-resuscitation Glasgow Coma Scale; ICU: Intensive care unit; DS: Discharge status; GOS: Glasgow Outcome Score (data from Simon Bolivar Hospital Medical Records Unit).
Outcomes
The GOS[20] was better in the early DC group than in the control group. In the early DC group, 7 of the 12 patients (43.7%) who were discharged alive had a GOS between 4 and 5 (minor deficits or disabled but independent), while none of the patients were in this range in the control group. Of the 7 living patients (35%) in the control group, all had a GOS between 2 and 3 (disabled, not independent or with minimal responsiveness). Baseline variables other than pupillary response and treatment were not significant in this model (Table 8). The mean GOS in the early DC group was significantly higher than in the control group (p=0.0002, ANOVA analysis). The robustness of the result was verified using a rank regression analysis and the p value for the regression was 0.0008. The difference between mean GOS score for the early DC group and for the control group was estimated as 1.53 with a 95% confidence interval for the difference being (0.81-2.32) (Table 9).
Table 8.
Characteristics and variable averages of damage control group and control group
| Variable | Early DC group | Control group |
|---|---|---|
| Mean age | 18.3 y | 24.3 y |
| Sex (M/F) | (7/11) | (14/6) |
| Post-Resuscitation GCS | 4.5 | 4.4 |
| Post-Resuscitation HR | 71.8 bpm | 76.1 bpm |
| Post-Resuscitation SBP | 128.9 mmHg | 127 mmHg |
| Marshall score (IV/III) | (13 / 3) | (17 / 3) |
| Mean ICU days | 9.4 d | 5.9 d |
| Mean In-Hospital days | 23.4 d | 10.1 d |
| Discharge status (Alive/Dead) | (12 / 4) | (7 / 13) |
| 6-Month GOS (1/2/3/4/5) | (4/2/3/2/5) | (13/3/4/0/0) |
M: Male; F: Female; GCS: Glasgow Coma Scale; HR: Heart rate; SBP: Systolic blood pressure; ICU: Intensive care unit; GOS: Glasgow Outcome Score.
Table 9.
Results of the ANOVA analysis for each independent variable
| Variable | DF | Parameter estimate | Standard error | t value | Pr > |t| |
|---|---|---|---|---|---|
| Intercept | 1 | -0.07576 | 3.95726 | -0.02 | 0.9849 |
| Treatment | 1 | 1.57130 | 0.36905 | 4.26 | 0.0002 |
| GCS | 1 | 0.23750 | 0.19224 | 1.24 | 0.2270 |
| Pupil | 1 | 0.64781 | 0.26390 | 2.45 | 0.0206 |
| SBP | 1 | 0.00004773 | 0.01453 | 0.00 | 0.9974 |
| CT findings | 1 | -0.12298 | 0.59525 | -0.21 | 0.8378 |
| HR | 1 | 0.00751 | 0.01269 | 0.59 | 0.5586 |
| Age | 1 | -0.03106 | 0.01739 | -1.79 | 0.0848 |
Pr > ItI = P value.
Discussion
According to the evidence, three key factors in neurosurgery have been identified as the causes of mortality, especially in the first 24 to 48 hours of the primary injury: hypoxia, hypotension and intracranial hypertension. The combination of these three factors has been recognized as a lethal combination.[21-27] The first and second factors are susceptible to prehospital management and stabilization, based on an organized emergency system, trained personnel and appropriate equipment adopted in the ambulances. However, the management of intracranial hypertension has been the critical factor, especially when considering trauma response racing against time.
Cerebral edema as a result of global and focal hypoperfusion processes and favored by ionic changes resulting from anaerobic cellular dysfunction has an important role in the increase of ICP, especially in the first 48 hours after primary injury.[28-31] This process has been appropriately determined in specialized TBI centers in North America and Europe with techniques like Xenon CT, PtiO2, microdialysis, etc,[32-34] In Colombia, the access to this kind of technology is not feasible, especially in public health care institutions such as the SBH, which paradoxically, are the busiest trauma centers with the highest trauma patient volume, including low resource and indigent population groups.
Traditionally, patients with severe head injuries, without obvious surgical lesions and with significant cerebral edema (associated with midline shift and diminished basal cisterns), were managed with external ventriculostomy and transferred to the ICU for standard non-surgical management of intracranial hypertension (CSF drainage, sedation and paralysis, hyper osmolar solutions, barbiturates and hyperventilation or hypothermia). The mortality of this specific group of patients in our institution was high compared with the standard mortality of the Traumatic Coma Data Bank for the same Marshall group of patients (between 40% and 50%), even with all therapeutic interventions including brain monitoring measurements like Jv02, transcranial Doppler and the cerebral perfusion pressure (CPP) measurement.[35-37]
An alternative therapy emerged within management protocols based on scientific communications of specialized groups: decompressive craniectomy. Most studies made with DC before the 1980's showed poor results, having great methodological faults in their elaboration.[38-41] Between 1980 and 1990, studies that were published showed a new possibility for therapeutic intervention.[42-44] In the 1990's, classics studies like Polin's in 1997[16] and Welch Guerra's in 1999[15] allowed the creation of a methodological structure for the selection of patients who would probably benefit from the procedure. Since 2000, decompression has gained in importance. Subsequent studies by Munch in 2000[45] and Coplin in 2001[46] provided specifics on the safety and feasibility of craniectomy and duraplasty for elevated ICP management. Literature reviews by Berger, Ruf, Figaji, Hutchinson, Albanise, Jaeger, Kontopoulos, Ziai, Spagnolo, and Meier, etc, from 2002 to 2003, reported the possible benefits of the procedure and were demonstrated in specific patient populations and at specific times. Such reports also generated new questions, especially on the ethical issues, because of the important number of patient outcomes of permanent vegetative state after being submitted to emergency decompressions.[47-56] Between 2005 and 2006, there were several series showing the benefits of the procedure especially in the pediatric population. The early procedure was under consideration looking for a specific timing.[57-61] In the series reported here, we suggest that early decompression diminishes ICP and increases the volumetric capacity of expansion of the cranial vault.[62,63] Despite these encouraging findings, other studies have demonstrated that early craniectomy can increase the cerebral edema (increase the transmural gradient of hydrostatic pressure in the capillary bed) and can induce infarcts with hemorrhagic transformation until cortical necrosis;[64] however, these findings were not present in our early DC population. The follow-up scans of the early DC group were more consistent with the new experimental studies that have shown different results with no edema expansion within the first 24 hours.[65] There is not enough evidence-based data in the literature at the present time to propose these interventions as the standard of care. There are several multicenter studies underway trying to answer these questions, including that of Bullock in the United States,[66] the Multicenter Cooperative Hispano-American study coordinated by the Vall d'Hebron Hospital's neurotrauma group in Spain,[67] and the Rescue ICP group in Europe.[68]
Our experience, however, appears to indicate that in properly selected patients, a systematic approach (designated here as early DC), when instituted within the first hours after the traumatic event, had beneficial effects in our patients.
We have presented our experience with our early DC protocol and with a model that dates to 2002. Some aspects of it are different from what it is available in the literature today, but it has the same basic objective of “minimizing” the secondary brain injury through a methodical and standardized approach that rests on the three phases described above. Obviously, for it to become a reality, a neurosurgical trauma team has to be available 24 hours, 365 days a year, and synchronization between the emergency room, OR and the ICU is vitally important. We hope to continue with the evaluation of this procedure and wait for the results of studies of the scientific international associations.
In conclusion, the systematic approach of DC in neurotrauma patients can be applicable early in patients with severe TBI. Early application of this DC protocol within less than 12 hours from injury in young patients with a GCS <9, a Marshall CT finding between III or IV, and isolated TBI was associated with significantly less mortality than the conventional approach with ventriculostomy and ICU management in the SBH population. Hemispheric cranial decompression in patients with severe head injury, who otherwise may not have been previously considered as surgical candidates, may turn out to be a better alternative management when compared with simple ventriculostomy and medical therapy in the ICU. The basic principle relies on prompt intervention aimed at early control of elevated ICP. The ethical dilemma remains, as there may be a number of patients with poor functional outcomes. Further evaluation of quality of life and long-term results will be necessary to understand the full extent of such interventions.
References
- 1.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Trauma systems. J Neurotrauma. 2000;17:457–62. doi: 10.1089/neu.2000.17.457. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for the management of severe head injury. Introduction. J Neurotrauma. 1996;13:643–5. doi: 10.1089/neu.1996.13.643. [DOI] [PubMed] [Google Scholar]
- 3.Brain Trauma Foundation: Guidelines for surgical management of traumatic brain injury. BTF (New York), Neurosurgery. 2006;58:S2–S120. [Google Scholar]
- 4.Gabriel EJ, Ghajar J, Jagoda A, Pons PT, Scalea T, Walters BC, Brain Trauma Foundation Guidelines for prehospital management of traumatic brain injury. J Neurotrauma. 2002;19:111–74. doi: 10.1089/089771502753460286. [DOI] [PubMed] [Google Scholar]
- 5.Brain Trauma Foundation, Society of Critical Care Medicine, American Association of Neurological Surgeons, Join Sections of Pediatrics and Neurotrauma, American Academy of Pediatrics, World Federation of Pediatric Intensive and Critical Care Societies, American College of Emergency Physicians, Congress of Neurological Surgeons. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4:1–72. [Google Scholar]
- 6.European Brain Injury Consortium: Guidelines for Management of Severe Head Injury in Adults. Acta Neurochir (Wien) 1997;139:286–94. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- 7.Paredes Zapata GD. Terrorism in Colombia. Prehosp Disaster Med. 2003;18:80–7. doi: 10.1017/s1049023x00000807. [DOI] [PubMed] [Google Scholar]
- 8.Hesdorffer DC, Ghajar J, Iacono L. Predictors of compliance with the evidence-based guidelines for traumatic brain injury care: a survey of United States trauma centers. J Trauma. 2002;52:1202–9. doi: 10.1097/00005373-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Murray GD, Teasdale GM, Braakman R, Cohadon F, Dearden M, Iannotti F, et al. The European Brain Injury Consortium survey of head injuries. Acta Neurochir (Wien) 1999;141:223–36. doi: 10.1007/s007010050292. [DOI] [PubMed] [Google Scholar]
- 10.Palmer S, Bader MK, Qureshi A, Palmer J, Shaver T, Borzatta M, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. Americans Associations for Neurologic Surgeons. J Trauma. 2001;50:657–64. doi: 10.1097/00005373-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Vukic M, Negovetic L, Kovac D, Ghajar J, Glavic Z, Gopcevic A. The effect of implementation of guidelines for the management of severe head injury on patient treatment and outcome. Acta Neurochir (Wien) 1999;141:1203–8. doi: 10.1007/s007010050419. [DOI] [PubMed] [Google Scholar]
- 12.Rubiano A. Neurological Surgery Program Reviews. El Bosque University; Bogotá: 2004. Surgical Management of Head Trauma in Simon Bolivar Hospital; pp. 1–80. [Google Scholar]
- 13.Alexander E, Ball MR, Laster DW. Subtemporal decompression: radiological observations and current surgical experience. Br J Neurosurg. 1987;1:427–33. doi: 10.3109/02688698708999632. [DOI] [PubMed] [Google Scholar]
- 14.Gaab MR, Rittierodt M, Lorenz M, Heissler HE. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien) 1990;51:326–8. doi: 10.1007/978-3-7091-9115-6_110. [DOI] [PubMed] [Google Scholar]
- 15.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187–96. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 16.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–94. doi: 10.1097/00006123-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Chesnut RM, Ghajar J, Maas AIR, Marion DW, Servadei F, et al. Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17:614–9. [Google Scholar]
- 18.Stocchetti N, Croci M, Spagnoli D, Gilardoni F, Resta F, Colombo A. Mass volume measurement in severe head injury: accuracy and feasibility of two pragmatic methods. J Neurol Neurosurg Psychiatry. 2000;68:14–7. doi: 10.1136/jnnp.68.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall L, Gautille T, Klauver M, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75:s28–s35. [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.Andrews PJ, Piper IR, Dearden NM, Miller JD. Secondary insults during intrahospital transport of head-injured patients. Lancet. 1990;335(8685):327–30. doi: 10.1016/0140-6736(90)90614-b. [DOI] [PubMed] [Google Scholar]
- 22.Chesnut RM. Avoidance of hypotension: conditio sine qua non of successful severe head-injury management. J Trauma. 1997;42(5 Suppl):S4–9. doi: 10.1097/00005373-199705001-00002. [DOI] [PubMed] [Google Scholar]
- 23.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–5. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 25.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54:312–9. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 26.Marmarou A, Anderson R, Ward J, et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:s59–s66. [Google Scholar]
- 27.Wald SL, Shackford SR, Fenwick J. The effect of secondary insults on mortality and long-term disability after severe head injury in a rural region without a trauma system. J Trauma. 1993;34:377–81. doi: 10.1097/00005373-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Hlatky R, Valadka AB, Robertson CS. Intracranial hypertension and cerebral ischemia after severe traumatic brain injury. Neurosurg Focus. 2003;14(4):e2. doi: 10.3171/foc.2003.14.4.2. [DOI] [PubMed] [Google Scholar]
- 29.Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991;74:407–14. doi: 10.3171/jns.1991.74.3.0407. [DOI] [PubMed] [Google Scholar]
- 30.Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17:1137–52. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- 31.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–83. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 32.Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360–8. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- 33.Haitsma IK, Maas AI. Advanced monitoring in the intensive care unit: brain tissue oxygen tension. Curr Opin Crit Care. 2002;8:115–20. doi: 10.1097/00075198-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Johnston AJ, Gupta AK. Advanced monitoring in the neurology intensive care unit: microdialysis. Curr Opin Crit Care. 2002;8:121–7. doi: 10.1097/00075198-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Cormio M, Valadka AB, Robertson CS. Elevated jugular venous oxygen saturation after severe head injury. J Neurosurg. 1999;90:9–15. doi: 10.3171/jns.1999.90.1.0009. [DOI] [PubMed] [Google Scholar]
- 36.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–62. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 37.van Santbrink H, Schouten JW, Steyerberg EW, Avezaat CJ, Maas AI. Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir (Wien) 2002;144:1141–9. doi: 10.1007/s00701-002-1012-8. [DOI] [PubMed] [Google Scholar]
- 38.Clark K, Nash TM, Hutchison GC. The failure of circumferential craniotomy in acute traumatic cerebral swelling. J Neurosurg. 1968;29:367–71. doi: 10.3171/jns.1968.29.4.0367. [DOI] [PubMed] [Google Scholar]
- 39.Cooper PR, Hagler H, Clark WK, Barnett P. Enhancement of experimental cerebral edema after decompressive craniectomy: implications for the management of severe head injuries. Neurosurgery. 1979;4:296–300. doi: 10.1227/00006123-197904000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Kerr FW. Radical decompression and dural grafting in severe cerebral edema. Mayo Clin Proc. 1968;43:852–64. [PubMed] [Google Scholar]
- 41.Venes JL, Collins WF. Bifrontal decompressive craniectomy in the management of head trauma. J Neurosurg. 1975;42:429–33. doi: 10.3171/jns.1975.42.4.0429. [DOI] [PubMed] [Google Scholar]
- 42.Crone KR, Kelly DL., Jr Subtemporal decompression in the management of refractory intracranial hypertension in severe closed head injury. Neurosurgery. 1985;16:726–8. [Google Scholar]
- 43.Gower DJ, Lee KS, McWhorter JM. Role of subtemporal decompression in severe closed head injury. Neurosurgery. 1988;23:417–22. doi: 10.1227/00006123-198810000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Karlen J, Stula D. Decompressive craniotomy in severe craniocerebral trauma following unsuccessful treatment with barbiturates. Neurochirurgia (Stuttg) 1987;30:35–9. doi: 10.1055/s-2008-1053653. Article in German. [DOI] [PubMed] [Google Scholar]
- 45.Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315–23. doi: 10.1097/00006123-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Coplin WM, Cullen NK, Policherla PN, Vinas FC, Wilseck JM, Zafonte RD, et al. Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma. 2001;50:1050–9. doi: 10.1097/00005373-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Albanèse J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury: Evaluation of the effects at one year. Crit Care Med. 2003;31:2535–8. doi: 10.1097/01.CCM.0000089927.67396.F3. [DOI] [PubMed] [Google Scholar]
- 48.Berger S, Schwarz M, Huth R. Hypertonic saline solution and decompressive craniectomy for treatment of intracranial hypertension in pediatric severe traumatic brain injury. J Trauma. 2002;53:558–63. doi: 10.1097/00005373-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666–73. doi: 10.1007/s00381-003-0804-3. [DOI] [PubMed] [Google Scholar]
- 50.Hutchinson PJ, Kirkpatrick PJ. Decompressive craniectomy in head injury. Curr Opin Crit Care. 2004;10:101–4. doi: 10.1097/00075198-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003;74:513–5. doi: 10.1136/jnnp.74.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kontopoulos V, Foroglou N, Patsalas J, Magras J, Foroglou G, Yiannakou-Pephtoulidou M, et al. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered? Acta Neurochir (Wien) 2002;144:791–6. doi: 10.1007/s00701-002-0948-z. [DOI] [PubMed] [Google Scholar]
- 53.Meier U, Gräwe A, König A. The importance of major extracranial injuries by the decompressive craniectomy in severe head injuries. Acta Neurochir Suppl. 2005;95:55–7. doi: 10.1007/3-211-32318-x_12. [DOI] [PubMed] [Google Scholar]
- 54.Ruf B, Heckmann M, Schroth I, Hügens-Penzel M, Reiss I, Borkhardt A, et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Crit Care. 2003;7:R133–8. doi: 10.1186/cc2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spagnuolo E, Costa G, Calvo A, Johnston E, Tarigo A. Descompresive craniectomy in head injury. Intractable I.C.P. Neurocirugia (Astur) 2004;15:36–42. Article in Spanish. [PubMed] [Google Scholar]
- 56.Ziai WC, Port JD, Cowan JA, Garonzik IM, Bhardwaj A, Rigamonti D. Decompressive craniectomy for intractable cerebral edema: experience of a single center. J Neurosurg Anesthesiol. 2003;15:25–32. doi: 10.1097/00008506-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Josan VA, Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. hilds Nerv Syst. 2006;22:1268–74. doi: 10.1007/s00381-006-0064-0. [DOI] [PubMed] [Google Scholar]
- 58.Reithmeier T, Speder B, Pakos P, Brinker G, Löhr M, Klug N, Ernestus RI. Delayed bilateral craniectomy for treatment of traumatic brain swelling in children: case report and review of the literature. Childs Nerv Syst. 2005;21:249–53. doi: 10.1007/s00381-004-1044-x. [DOI] [PubMed] [Google Scholar]
- 59.Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106(4 Suppl):268–75. doi: 10.3171/ped.2007.106.4.268. [DOI] [PubMed] [Google Scholar]
- 60.Figaji AA, Fieggen AG, Argent A, Peter JC. Surgical treatment for “brain compartment syndrome” in children with severe head injury. S Afr Med J. 2006;96(9 Pt 2):969–75. [PubMed] [Google Scholar]
- 61.Kan P, Amini A, Hansen K, White GL, Jr, Brockmeyer DL, Walker ML, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105(5 Suppl):337–42. doi: 10.3171/ped.2006.105.5.337. [DOI] [PubMed] [Google Scholar]
- 62.Burkert W, Plaumann H. The value of large pressure-relieving trepanation in treatment of refractory brain edema. Animal experiment studies, initial clinical results. Zentralbl Neurochir. 1989;50:106–8. Article in German. [PubMed] [Google Scholar]
- 63.Hatashita S, Hoff JT. The effect of craniectomy on the biomechanics of normal brain. J Neurosurg. 1987;67:573–8. doi: 10.3171/jns.1987.67.4.0573. [DOI] [PubMed] [Google Scholar]
- 64.Moody RA, Ruamsuke S, Mullan SF. An evaluation of decompression in experimental head injury. J Neurosurg. 1968;29:586–90. [Google Scholar]
- 65.Zweckberger K, Erös C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23:1083–93. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]
- 66.Bullock R. Management of brain injury by decompressive craniectomy (Comment) Neurosurgery. 2000;47:322–3. doi: 10.1097/00006123-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Ibanez J. Tienen alguna indicacion las craniectomias descompresivas en el tratamiento de la hipertension endocraneana refractaria? Presentacion de un estudio multicentrico multinacional sobre la eficacia de las craniectomias descompresivas en las lesiones difusas tipo III y IV del Traumatic Coma Data Bank. PIC 2000. 2000;1:179–93. [Google Scholar]
- 68.Compagnone C, Murray GD, Teasdale GM, Maas AI, Esposito D, Princi P, et al. The management of patients with intradural post-traumatic mass lesions: a multicenter survey of current approaches to surgical management in 729 patients coordinated by the European Brain Injury Consortium. Neurosurgery. 2005;57:1183–92. doi: 10.1227/01.neu.0000279218.53504.fe. [DOI] [PubMed] [Google Scholar]


