Abstract
We report the application of our newly described crystallization technique, which employs silver island films (SIFs) and microwave heating, to rapid crystallization of L-arginine acetate (LAA). Using our technique, LAA crystals (~ 1.2 mm in length) were grown from a 20 μl solution in 1 min on surface functionalized SIFs. In control experiments (glass slides and at room temperature) the growth of LAA crystals (0.1–0.3 mm) took ~ 55 min.
L-Arginine acetate is a promising biological material for nonlinear optics due to its ability to show optical second harmonic generation (SHG).1,2 SHG ability of LAA is due to the presence of chiral carbon atoms and non-centrosymmetric space groups found in LAA crystals. In addition, LAA was shown to have low dielectric constants, which makes them attractive for the microelectronics industry.3 Recently, Renuka et al. studied the synthesis of LAA and growth of the single LAA crystal by the slow evaporation technique.4 In publications to date,1,2,4 the growth of LAA crystals was carried out using slow cooling/evaporation of the solvent. The cooling rate of 0.5 °C per day over 30 days yielded LAA crystals up to 21 × 15 × 3 mm3 in size. Subsequently, there is still a need for a new method that affords rapid growth of LAA crystals that can be used for nonlinear optics.
In this regard, the Aslan Research Group has recently developed a new crystallization technique, called Metal-Assisted and Microwave-Accelerated Evaporative Crystallization (MA-MAEC), for the rapid and selective crystallization of amino acids5,6 The MA-MAEC technique is based on the combined use of metal nanostructures and low power microwave heating. In MA-MAEC,5 metal (i.e., silver island films, SIFs) nanostructures serve as (i) selective nucleation sites for crystal growth and (ii) as a microwave-transparent medium for the creation of thermal gradients between the warmer solution and the silver nanostructures that remain at room temperature after microwave heating. The microwave heating affords significant reduction in the time of the evaporative crystallization process. The MA-MAEC technique also utilizes the well-known affinity of silver nanostructures towards amine groups.7 In this regard, it is thought that the amine groups of amino acids assemble onto silver nanostructures that serve as nucleation sites for the growth of crystals.
In addition, the Aslan Research Group has also demonstrated the use of surface functional groups that can be introduced to metal nanostructures for rapid crystallization of amino acids using the MA-MAEC technique.6 In this regard, the surface modification of metal nanostructures was carried out using self-assembled monolayers of bi-functional molecules (a thiol group that is chemisorbed on to metal nanostructures and a terminal group with –COOH, –NH2 and –CH3 functionality). These added functionalities were proven to improve the selective nucleation and growth of amino acids due to the interactions of the functional groups of the amino acids with the functional groups on the metal nanostructures. For example, primary –NH2 of amino acids can assemble onto –COOH modified metal nanostructures through – NH3+/–COO− group interactions (i.e., electrostatic). The orientation of amino acid assembly onto the engineered surfaces is thought to be dependent on the type of functional group present on SIFs.6
In this communication, the application of the MA-MAEC technique to rapid crystallization of LAA is presented. Using the MA-MAEC technique, significant increase in the size of LAA crystals was observed. The total time for complete evaporation of solvent varied between 55 min for blank glass slides and 1 min for surface modified SIFs at room temperature and using MA-MAEC, respectively. In addition, the growth of LAA crystals at time intervals, starting with the initial deposition of LAA solution on to surface modified SIFs until the complete evaporation of solvent occurs, was monitored using optical microscopy. LAA crystals were characterized by Raman spectroscopy and powder XRD measurements, which showed that LAA crystals grown on surface modified SIFs using the MA-MAEC technique had identical characteristic peaks of LAA crystals grown using traditional evaporative crystallization.
SIFs were deposited onto blank glass microscope slides by allowing them to soak in a heated silver nitrate/D-glucose solution as previously described.8 The surface modification of freshly prepared SIFs with functional molecules: hexamethylenediamine (HMA), 1-undecanethiol (UDET), 11-mercaptoundecanoic acid (MUDA), was carried out as previously described.6 The contact of angles of a water drop on these surfaces was measured to confirm the presence of these functional molecules on the surface.
LAA was synthesized according to the modified version of the previously published procedure.2 In this regard, 5 mL of 3 M L-arginine solution (Sigma-Aldrich, USA) was mixed with 5 mL acetic acid and the resultant solution was heated to 30 °C until L-arginine completely dissolved. The crystallization of LAA was carried out according to the following procedure: a fixed volume (20 μl) of freshly prepared LAA solution at 30 °C was pipetted onto a blank glass slide (control experiment) or blank SIFs or surface modified SIFs and were either heated in a conventional microwave oven (Emerson Model No: MW87845B, 0.7 cu. ft, 700 W output power, 100% power level) or incubated at room temperature (22 ± 1 °C, Control experiment). The time taken for the solution to completely evaporate was recorded. LAA crystals were characterized by optical microscopy, Raman spectroscopy and powder X-Ray Diffraction (XRD).
Table 1 summarizes the results for the crystallization of LAA using the MA-MAEC technique and control experiments. In this regard, the water contact angle for water on all surfaces, the range of crystal size and the total time to completely evaporate LAA solutions are listed. In order to confirm that SIFs were successfully modified with –COOH (using MUDA), –NH2 (using HMA) and –CH3 (using UDET) functional groups, the contact angles for the blank glass slides, blank SIFs and surface modified SIFs were measured. It is important to note that the introduction of –COOH, –NH2 and –CH3 functional groups to the SIFs surfaces results in SIFs to be hydrophilic, moderately hydrophobic and very hydrophobic, respectively. The contact angle for blank glass slides was measured to be ~ 12° (hydrophilic), which was increased to ~ 28° after the introduction of SIFs to the glass surface. When –COOH functional groups are introduced to blank SIFs, the contact angle for water decreased to ~ 26° (from 28° for blank SIFs), which implies the presence of –COOH functional groups. The introduction of –NH2 and –CH3 groups to blank SIFs, resulted in the increase of the contact angle for water to ~ 30° and ~ 45°, respectively. It is also important to note that the optical density (i.e. surface plasmon resonance peak at 420 nm = 0.3) of SIFs were the same in all these experiments.
Table 1.
Comparison of the time taken by L-arginine acetate in deionized water to completely crystallize on glass and Silver Island Films (SIFs) at room temperature and microwave heating (duty cycle = 1). Sample size is 20 microliters.
| Blank glass (no functional groups) | Blank SIFs (no functional groups) | MUDA_SIFs (–COOH groups) | HMA_SIFs (–NH2 groups) | UDET_SIFs (–CH3 groups) | ||
|---|---|---|---|---|---|---|
| Water contact angle | 12 ± 0.1° | 28 ± 1° | 26 ± 3° | 30 ± 5° | 45 ± 3° | |
| RT | Time | 55 ± 5 min | 11 ± 5 min | 8 ± 1 min | 6 ± 1 min | 7 ± 1 min |
| Size range | 104–295 mm | 285–505 mm | 250–450 mm | 850–1170 mm | 125–383 mm | |
| MW | Time | 15 ± 3 min | 8 ± 0.1 min | 1 ± 0.1 min | 2 ± 0.5 min | 7 ± 1 min |
| Size range | ND | 380–550 mm | 110–510 mm | 344–530 mm | ND | |
ND - Not Determined
For a 20 μL of LAA solution, the complete evaporation time on blank glass slides at room temperature (a control sample) was recorded to be 55 ± 5 min. (We note that LAA crystals appeared within 10 min (Fig. S2, see ESI†) and the complete evaporation of the solvent was carried out to investigate the effect of solvent evaporation on the crystallization of LAA). The size range for LAA crystals grown on blank glass slides at room temperature was 104–295 μm. At room temperature, the complete evaporation of solvent on blank SIFs took only 11 min. This can be attributed to the faster heat transfer from the solution to the silver nanostructures due the large difference between the thermal conductivity of silver (429 W m−1 K), water (0.61 W m−1 K) and glass (1.1 W m−1 K). The crystallization of LAA on SIFs and at room temperature resulted in the size range for LAA crystals to be 285–505 μm.
The complete evaporation of solvent on SIFs after the introduction of –COOH (using MUDA), –NH2 (using HMA) and –CH3 (using UDET) functional groups was comparable to that of blank SIFs. However, the size of LAA crystals grown on SIFs with –NH2 functional groups was up to 12-fold and 5-fold larger as compared to blank glass slides and blank SIFs (and SIFs with –COOH and –CH3 functional groups), respectively. This is thought to be due to the selective assembly of LAA on to SIFs with –NH2 functional groups through –COO− groups of LAA with the –NH2 functional groups of HMA on SIFs. Since LAA is acidic and the –NH2 group on LAA is also converted to acetate, the assembly of LAA on to SIFs with –COOH and –CH3 functional groups was random, similar to the assembly of LAA on to blank glass slides. On the other hand, the time of complete evaporation was significantly shorter due the presence of SIFs.
As mentioned above, in addition to SIFs, the MA-MAEC technique also employs low power microwave heating. Subsequently, the identical experiments summarized above were repeated with microwave heating (instead of evaporation at room temperature). Microwave heating of LAA solution on blank glass slides resulted in a reduction of the total evaporation time to 15 min, an ~ 4-fold reduction in time. However, LAA crystals grown on blank glass slides with microwave heating did not have definite shapes and the size range was indiscernible. The introduction of microwave heating resulted in significant reduction in the total evaporation time for LAA solution on surface modified SIFs. For example, the crystallization of LAA on SIFs with –NH2 functional groups was completed in 2 min using the MA-MAEC technique. This corresponds to up to ~ 30-fold reduction in crystallization time as compared to crystallization on blank glass slides at room temperature. The size range for LAA crystals grown on SIFs with –NH2 functional groups and microwave heating was 344–530 μm. On the other hand, the use of SIFs with –CH3 functional groups and microwave heating did not result in any discernible LAA crystals, which was assessed using an optical microscope, Raman spectroscopy or XRD measurements. After complete evaporation of the solvent on SIFs with –CH3 functional groups using microwave heating, only amorphous matter was obtained. This can be attributed to the rapid and random assembly of LAA molecules on to SIFs with –CH3 functional groups that did not yield the crystalline structure of LAA.
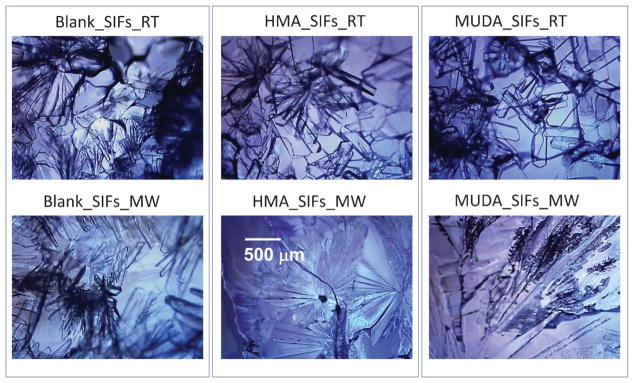
Fig. 1 and Fig. S1 (see ESI†) show the optical images of LAA crystals grown (after complete evaporation of the solvent) on blank SIFs and SIFs with –COOH and –NH2 functional groups at room temperature and by the MA-MAEC technique. One can see that LAA crystals were separated from one another on SIFs with –COOH functional groups at room temperature, as compared to polycrystals of LAA grown on other surface modified and blank SIFs. These observations can be explained by the selective assembly of LAA molecules as described in the previous paragraphs. It is thought that the hydrophilic/hydrophobic nature of the surface modified SIFs play a role in the nucleation of LAA crystals. The functional groups on SIFs are thought to lower the interfacial energy between SIFs and LAA crystals, which promotes the heterogeneous nucleation of LAA crystals. For example, since SIFs with –COOH functional groups are hydrophilic in nature, initial LAA solution is spread over a wider surface area, which affords crystal nucleation at a larger number of sites on the SIFs surface. This resulted in the spread of LAA crystals over a wider surface area, and hence the separation of LAA crystals is observed. In comparison, a larger number of polycrystals of LAA is grown on a smaller surface area on SIFs with –CH3 functional groups (Fig. S1, see ESI†).
Fig. 1.
Optical microscope images of L-arginine acetate crystallized on blank SIFs and surface engineered SIFs (HMA and MUDA) at room temperature (RT) and microwave heating (MW). The scale bar shown in the middle figure applies to all images here.
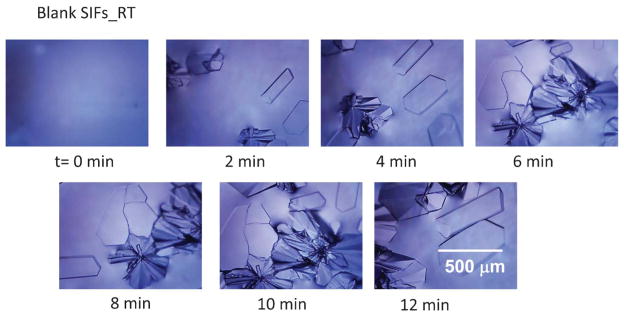
In order to partially elucidate the crystallization process, optical images of the initial LAA solution and the growing crystals on blank glass slides, blank SIFs and SIFs with –COOH functional groups were taken at time intervals as indicated in Fig. 2 and Fig. S2–S3 (see ESI†). It is important to note that, in all these experiments the growth of crystals was observed on an optical microscope stage without interruption. Fig. 2 shows the time progression of growth of LAA crystals on blank SIFs at room temperature. As expected, no crystals were observed in the initial LAA solution at the time the first image (t = 0 min) was taken. After only 2 min, multiple LAA crystals appeared on the SIFs surface and continued to grow in size and number until the solvent was completely evaporated. Fig. 2 also shows that single and polycrystals of LAA are formed on blank SIFs at room temperature. On blank glass slides (Fig. S2, see ESI†) initial polycrystals of LAA appeared after 10 min and continued to grow in number until the solvent completely evaporated after 55 min. It is interesting to note the morphological differences between the single and polycrystals of LAA crystals grown at room temperature. The single LAA crystals are typically larger and wider than LAA polycrystals.
Fig. 2.
Timed images of L-arginine acetate crystallized on blank SIFs at room temperature.
Although not completely elucidated, the observation of single and polycrystals of LAA on the engineered surfaces can also be attributed to the heterogeneous distribution of silver nanoparticles on the surface, which serve as heterogeneous nucleation sites. In this regard, the size of SIFs were previously reported5 as ~ 80 nm and the distance between the silver nanoparticles varies between a few nm up to several hundred nm. It is thought that single LAA crystals were formed on silver nanoparticles further away from each other, whereas single LAA crystals growth can occur on the non-silvered part of the surface after the nucleation process on the silver nanoparticles. On the other hand, LAA polycrystals were thought to form on silver nanoparticles relatively closer together, which can serve as multiple nucleation sites.
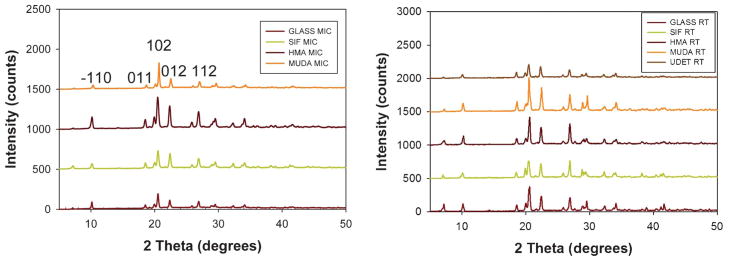
The characterization of LAA crystals grown on blank glass slides and surface modified and blank SIFs at room temperature and using the MA-MAEC technique was carried out using powder XRD and Raman spectroscopy. As shown in Fig. 3, on all surfaces the same polymorph of LAA was obtained. We note that the powder XRD data for LAA crystals grown on SIFs with –CH3 functional groups using microwave heating did not show any characteristic diffraction peaks. In addition, the XRD data for LAA crystals grown on blank glass slides using microwave heating showed weak characteristic diffraction peaks.
Fig. 3.
Powder XRD data for L-arginine acetate crystallized (left) at room temperature (right) using microwave heating on all surfaces glass slides. We were not able to obtain a presentable XRD data for LAA crystals grown on UDET-modified SIFs using microwave heating.
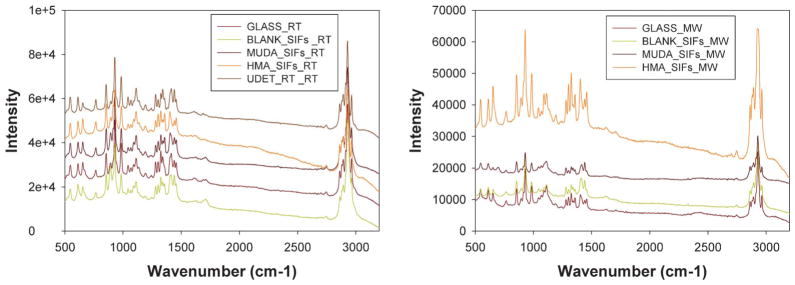
Fig. 4 shows the Raman spectrum of LAA crystallized on blank glass slides, blank SIFs and surface modified SIFs at room temperature and using MA-MAEC technique. Since all peaks on the Raman spectra for all samples studied here have identical wavenumbers for LAA crystals grown at room temperature on glass surfaces, we conclude that the use of surface modified SIFs and microwave heating did not alter the molecular structure of LAA using MA-MAEC technique.
Fig. 4.
Raman spectra of L-arginine acetate crystallized (left) at room temperature (right) using microwave heating on all surfaces glass slides.
Conclusions
The successful use of surface engineered SIFs (–COOH (using MUDA), –NH2 (using HMA) and –CH3 (using UDET) functional groups) in rapid crystallization of L-arginine acetate is demonstrated. Significant improvements in LAA crystal size was observed on SIFs with –NH2 functional groups at room temperature as compared to those crystals grown on blank glass slides and blank SIFs. In addition, using the MA-MAEC technique, the crystallization time of LAA on surface engineered SIFs was reduced to 1–7 min as compared to 55 min on blank glass slides at room temperature. Raman spectroscopy and powder XRD measurements showed that LAA crystals grown on surface engineered SIFs using the MA-MAEC technique had identical characteristic peaks of LAA crystals grown on blank glass slides using traditional evaporative crystallization. The observations made in this study imply that the MA-MAEC technique has the potential to develop into a method in which large size and high-quality LAA crystals can be produced in a much faster time than traditional crystallization techniques.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Additional microscope images and Raman spectra of LAA crystals grown on all surfaces. See DOI: 10.1039/c2ce25380a
References
- 1.Monaco SB, Davis LE, Velsko SP, Wang FT, Eimerl D, Zalkin A. J Cryst Growth. 1987;85:252–255. [Google Scholar]
- 2.Pal T, Kar T, Bocelli G, Rigi L. Cryst Growth Des. 2003;3:13–16. [Google Scholar]
- 3.Meena M, Mahadevan CK. Mater Lett. 2008;62:3742–3744. [Google Scholar]
- 4.Renuka N, Vijayan N, Rathi B, Babu RR, Nagarajan K, Haranath D, Bhagavannarayana G. Optik - International Journal for Light and Electron Optics. 2012;123:189–192. [Google Scholar]
- 5.Pinard MA, Aslan K. Cryst Growth Des. 2010;10:4706–4709. doi: 10.1021/cg101059c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alabanza AM, Pozharski E, Aslan K. Cryst Growth Des. 2012;12:346–353. doi: 10.1021/cg2011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagastine RR, Grieser F. Langmuir. 2004;20:6742–6747. doi: 10.1021/la049307s. [DOI] [PubMed] [Google Scholar]
- 8.Abel B, Akinsule A, Andrews C, Aslan K. Nano Biomed Eng. 2011;3:184–191. doi: 10.5101/nbe.v3i3.p184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.