Abstract
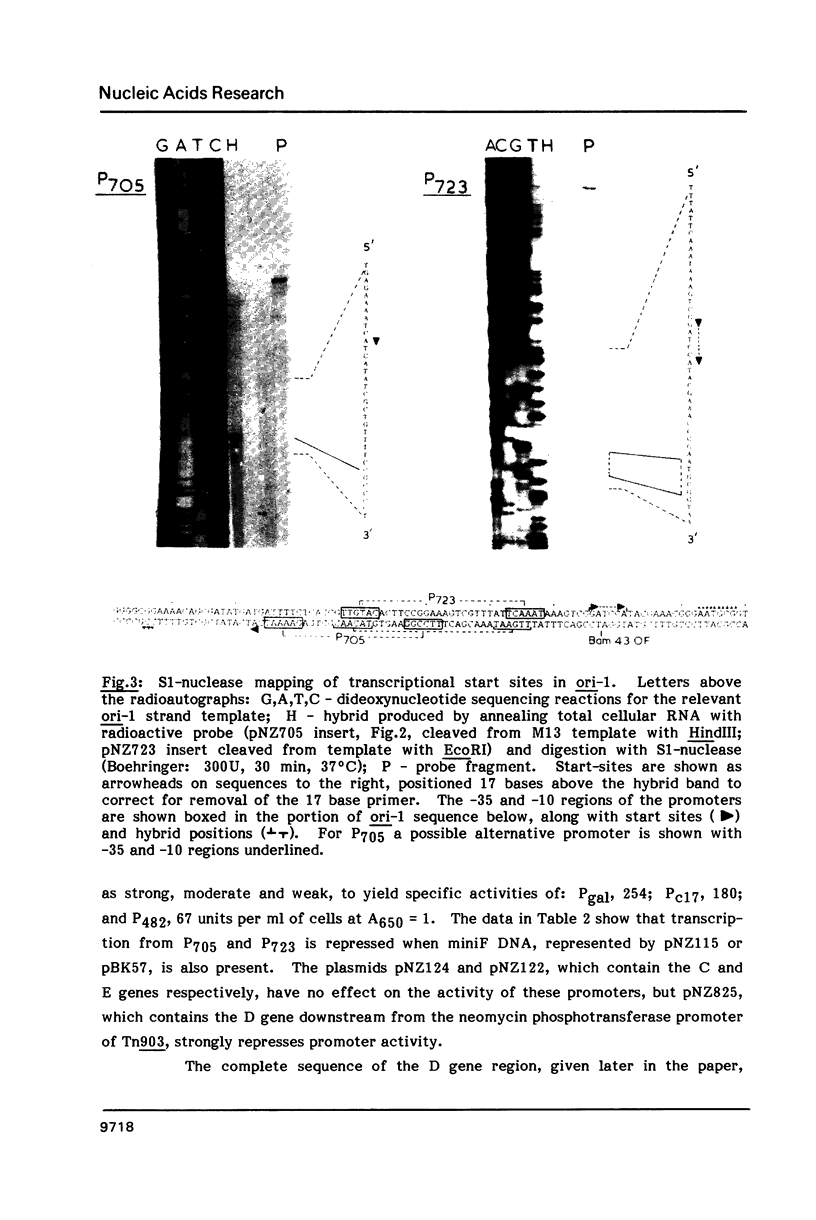
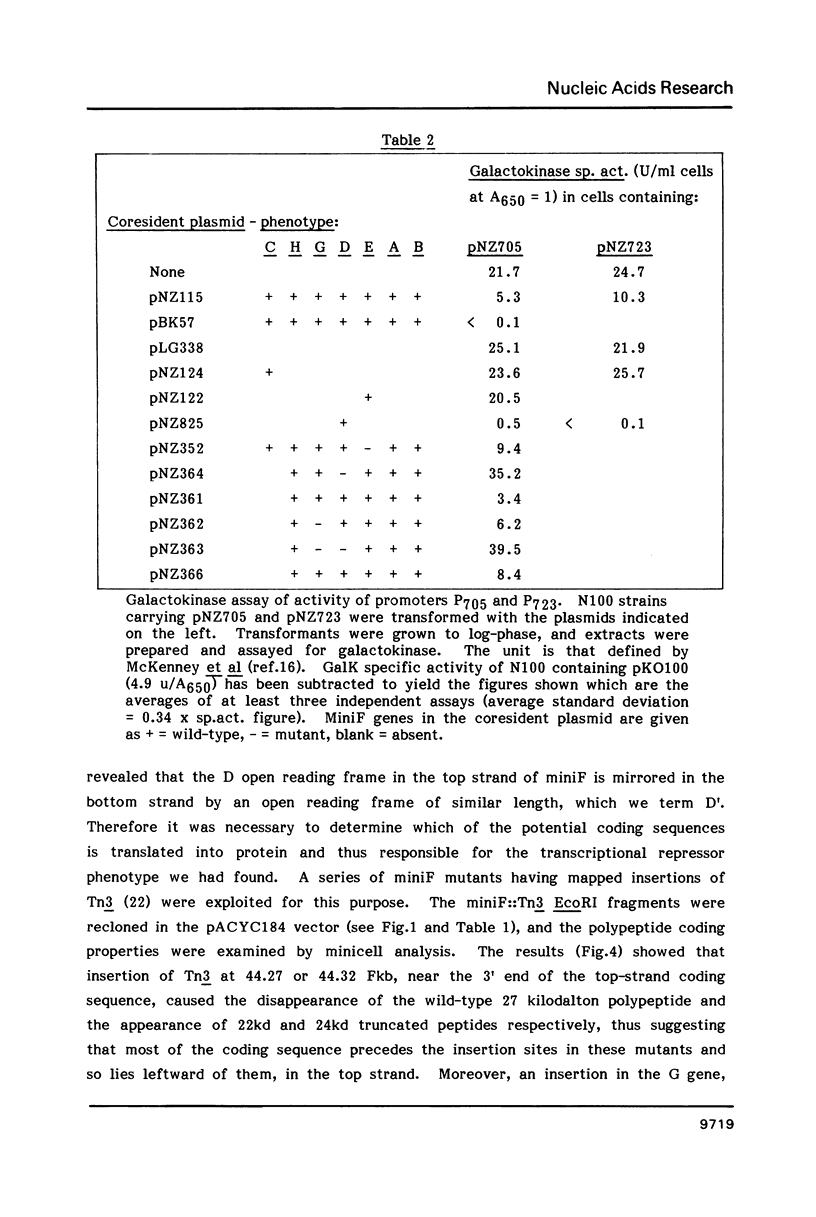
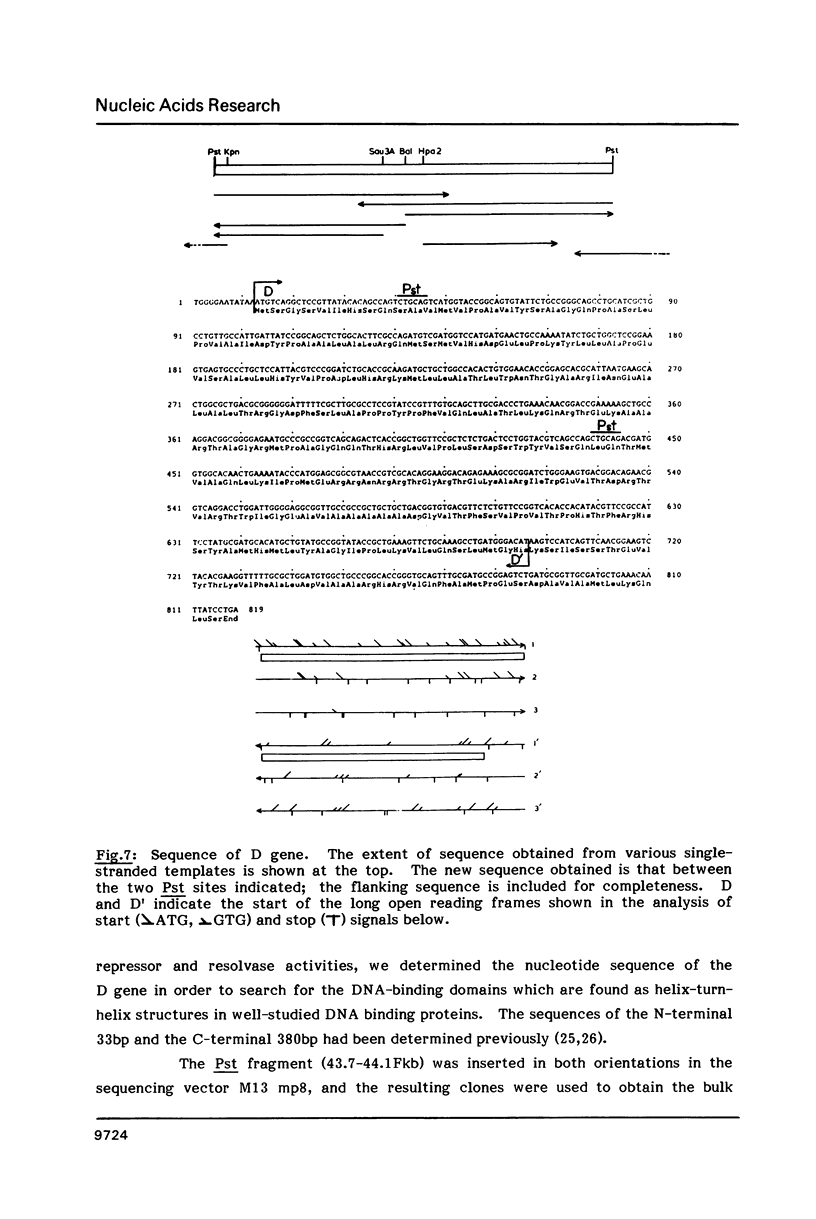
Two activities of the D protein of the miniF plasmid have been found. Divergent promoters in ori-1 ("primary" replicative origin) of miniF are both repressed in cells which produce D protein. The mobilization of plasmids containing the ori-1 region by the F conjugation system is also repressed by D protein. In the former case D appears to act as a transcriptional repressor, whereas in the latter case D protein acts by resolving cointegrates of F and the mobilized plasmid. D protein resolves dimers whose monomer units contain the rfsF sequence needed for recA-independent, site-specific recombination of F. The nucleotide sequence of the D gene was determined. The D gene region contains two oppositely-oriented open reading frames which have the same reading phase and substantially overlap. Transposon insertion mutants were used to show that the gene for D protein occupies the top-strand (left-to-right) open reading frame.
Full text
PDF


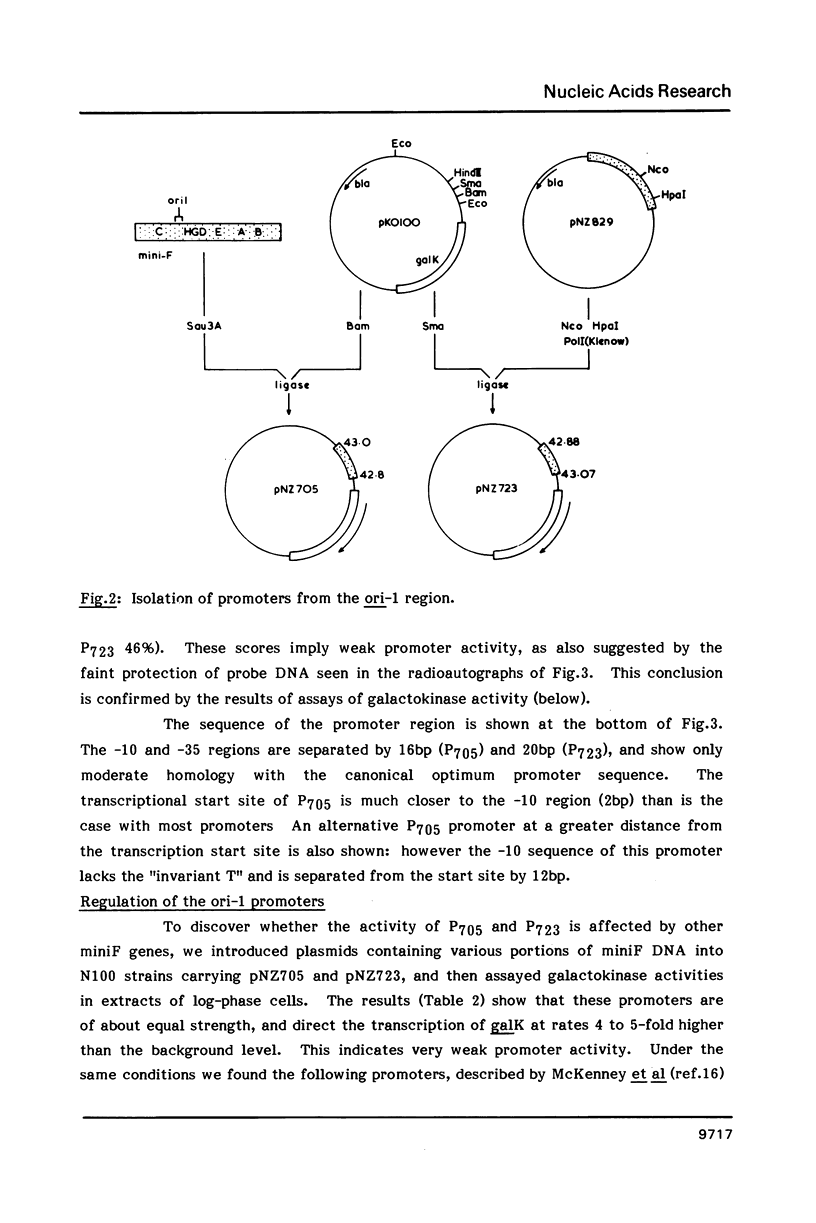








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Austin S., Ziese M., Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981 Sep;25(3):729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Bergquist P. L., Downard R. A., Caughey P. A., Gardner R. C., Lane H. E. Analysis of mini-F plasmid replication by transposition mutagenesis. J Bacteriol. 1981 Sep;147(3):888–899. doi: 10.1128/jb.147.3.888-899.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L., Jamieson A. F., Gardner R. C., Lane D. Replication mutants of the F-plasmid of Escherichia coli K-12. I. Isolation and characterization of temperature-sensitive replication mutants of F'-gal+. Plasmid. 1980 Mar;3(2):165–178. doi: 10.1016/0147-619x(80)90107-9. [DOI] [PubMed] [Google Scholar]
- Bex F., Karoui H., Rokeach L., Drèze P., Garcia L., Couturier M. Mini-F encoded proteins: identification of a new 10.5 kilodalton species. EMBO J. 1983;2(11):1853–1861. doi: 10.1002/j.1460-2075.1983.tb01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Ebbers J., Eichenlaub R. Complementation of replication-deficient deletion derivatives of plasmid mini-F. J Bacteriol. 1981 Sep;147(3):736–743. doi: 10.1128/jb.147.3.736-743.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub R., Figurski D., Helinski D. R. Bidirection replication from a unique origin in a mini-F plasmid. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1138–1141. doi: 10.1073/pnas.74.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Caughey P. A., Lane D., Bergquist P. L. Replication mutants of the F-plasmid of Escherichia coli. II. Cloned replication regions of temperature-sensitive mutants. Plasmid. 1980 Mar;3(2):179–192. doi: 10.1016/0147-619x(80)90108-0. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Malcolm L., Bergquist P. L., Lane H. E. IncD, a genetic locus in F responsible for incompatibility with several plasmids of the IncFI group. Mol Gen Genet. 1982;188(2):345–352. doi: 10.1007/BF00332699. [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Abremski K. Mechanism of strand cleavage and exchange in the Cre-lox site-specific recombination system. J Mol Biol. 1985 Feb 5;181(3):351–362. doi: 10.1016/0022-2836(85)90224-4. [DOI] [PubMed] [Google Scholar]
- Jaffé A., Ogura T., Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985 Sep;163(3):841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbane J. J., Malamy M. H. F factor mobilization of non-conjugative chimeric plasmids in Escherichia coli: general mechanisms and a role for site-specific recA-independent recombination at orV1. J Mol Biol. 1980 Oct 15;143(1):73–93. doi: 10.1016/0022-2836(80)90125-4. [DOI] [PubMed] [Google Scholar]
- Kline B. C. A review of mini-F plasmid maintenance. Plasmid. 1985 Jul;14(1):1–16. doi: 10.1016/0147-619x(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Komai N., Nishizawa T., Hayakawa Y., Murotsu T., Matsubara K. Detection and mapping of six miniF-encoded proteins by cloning analysis of dissected miniF segments. Mol Gen Genet. 1982;186(2):193–203. doi: 10.1007/BF00331850. [DOI] [PubMed] [Google Scholar]
- Lane D., Hill D., Caughey P., Gunn P. The mini-F primary origin. Sequence analysis and multiple activities. J Mol Biol. 1984 Dec 5;180(2):267–282. doi: 10.1016/s0022-2836(84)80004-2. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol Gen Genet. 1977 Apr 29;152(3):175–182. doi: 10.1007/BF00268815. [DOI] [PubMed] [Google Scholar]
- Manis J., Kline B. Recombination between an Flac and a mini-FKmr plasmid deleted for an origin of replication. Plasmid. 1978 Sep;1(4):480–491. doi: 10.1016/0147-619x(78)90006-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Miki T., Yoshioka K., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. I. The 42.84-43.6 F segment couples cell division of the host bacteria with replication of plasmid DNA. J Mol Biol. 1984 Apr 25;174(4):605–625. doi: 10.1016/0022-2836(84)90086-x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- O'Connor M. B., Malamy M. H. Role of the F factor oriV1 region in recA-independent illegitimate recombination. Stable replicon fusions of the F derivative pOX38 and pBR322-related plasmids. J Mol Biol. 1984 May 25;175(3):263–284. doi: 10.1016/0022-2836(84)90348-6. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Phua S. H., Bergquist P. L., Lane H. E. Effects of Tn5 insertion in the incD region on mini-F maintenance and polypeptide synthesis. Mol Gen Genet. 1982;188(2):353–355. doi: 10.1007/BF00332700. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Tanimoto K., Iino T. Additional genes essential for replication of the mini-F plasmid from origin I. Mol Gen Genet. 1985;198(2):358–359. doi: 10.1007/BF00383020. [DOI] [PubMed] [Google Scholar]
- Tanimoto K., Iino T. An essential gene for replication of the mini-F plasmid from origin I. Mol Gen Genet. 1984;196(1):59–63. doi: 10.1007/BF00334092. [DOI] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]