Abstract
Rationale
Clarification of alcohol’s effect on stress response during threat is critical to understand motivation for alcohol use and related alcohol-use disorders. Evaluation of stress response dampening (SRD) effects of alcohol has been limited by nonsystematic use of varied experimental methods and measures.
Objectives
This experiment parametrically varied alcohol dose and shock threat intensity among social drinkers to examine their effects on startle potentiation, a physiological measure of the affective component of the stress response.
Methods
96 participants were assigned to one of four beverage groups: placebo and target blood alcohol concentration (BAC) groups of 0.04, 0.075, and 0.11%. Participants viewed colored cues presented in shock and no-shock blocks. Distinct colored cues predicted imminent low, moderate, or high intensity electric shock administration. Startle potentiation during shock threat relative to no-shock cues indexed affective response.
Results
High threat increased startle potentiation relative to moderate/low intensity threat. Startle potentiation decreased as BAC increased. Threat intensity moderated this BAC effect with the strongest BAC effect observed during high threat. Analysis of individual difference moderators revealed reduced effect of BAC among heavier, more problematic drinkers.
Conclusions
Clear alcohol SRD effects were observed. These SRD effects were greatest at higher BACs and during more potent threat. Failure to account for these factors may partially explain inconsistent findings in past laboratory SRD research. Furthermore, they suggest greater reinforcement from alcohol at higher doses and among individuals with greater stress. Moderation of SRD effects by alcohol consumption and problems point to possible important risk factors.
Keywords: Alcohol Dose Response, Blood Alcohol Concentration, Startle Potentiation, Stress Response Dampening, Fear, Anxiety, Threat of Electric Shock, Threat Intensity
The alcohol use-alcohol dependence-stress nexus has been a long-standing focus of research. Empirical work in this area indicates that individuals expect alcohol use to reduce their stress and that this serves as a motive for use among many drinkers (Christiansen et al. 1982; Cooper et al. 1995; Goldman et al. 1987). Drinkers who report stress reduction as a dominant motive for their use are at increased risk for the development of alcohol use disorders (Cooper et al. 1995; Schroder and Perrine 2007). Furthermore, high rates of alcohol use, abuse, and dependence are observed in patients with anxiety disorders (Grant et al. 2004; Kessler et al. 1995). Stress is a potent instigator of relapse among abstinent alcoholics (Brown et al. 1995; Brown et al. 1990) and stress-induced reinstatement of alcohol use has been confirmed in animal models (Lê et al. 1998; Overstreet et al. 2007). Evidence is also rapidly accruing to implicate adaptations in stress neurocircuitry in response to chronic exposure to the acute effects of alcohol and other drugs in the etiology of addiction (Breese et al. 2005; Koob and Volkow 2010; Weiss et al. 2001). Clearly, research on alcohol-stress connections is needed to identify pre-morbid risk factors, understand etiologic mechanisms, and aid development of behavioral and pharmacologic treatments for alcoholism.
Despite its importance to risk and etiological factors in key theories of drinking and alcoholism, the impact of alcohol on affective response to stressors, and the processes and biological mechanisms that mediate this impact, are not well understood (see Curtin and Lang 2007 for a review). Decades of research have established that multiple mechanisms may exist and important moderating individual difference and contextual factors cannot be ignored (e.g. Curtin et al. 1998, 2001; Donohue et al. 2007; Moberg and Curtin 2009; Sayette 1993; Steele and Josephs 1990; Sher 1987). Substantial evidence exists that alcohol may influence stress response indirectly through its detrimental impact on attention and appraisal processes (Curtin et al. 1998, 2001; Sayette 1993; Sher et al. 2007; Steele and Josephs 1990). However, recent evidence suggests that the Stress Response Dampening model thesis (SRD; Levenson et al. 1980; Sher 1987) regarding direct effects of alcohol on stress system neurocircuitry remains viable (Donohue et al. 2007; Moberg and Curtin, 2009; Hefner et al. 2010; Hachiya et al. 2010).
Donohue and colleagues (2007) demonstrated selective reduction in affective response to unpleasant but not pleasant photographic images among intoxicated participants. However, this SRD effect of alcohol was most apparent at higher blood alcohol concentrations, suggesting that alcohol dose may be critical (see also Sher and Walitzer 1986; Stewart et al. 1992). Stressor characteristics may also be important. In a series of experiments, our laboratory has demonstrated direct, selective reduction of affective response to uncertain (e.g., unpredictable, low probability, temporally ill-defined) but not certain (high probable, imminent) threats during intoxication (Moberg and Curtin 2009; Hefner et al. 2010; Hachiya et al. 2010). These findings suggest that alcohol may diminish anxiety that results from activation of Corticotropin Releasing Factor (CRF) and Norepinephrine (NE) sensitive pathways in the extended amygdala in response to unpredictable or otherwise uncertain threats (see Davis et al. 2010, for a review on phasic vs. sustained fear and the extended amygdala). In fact, in an early formulation of the SRD model, Sher (1987) speculated that factors such as alcohol dose, characteristics of the stressor, the nature of the affective response to the stressor (e.g., fear, anxiety, disgust), and individual differences would all prove to be important to understand SRD effects.
We believe that progress clarifying alcohol-stress relations has been slowed by the use of complicated tasks that do not provide the necessary precision and control to isolate specific mechanisms (e.g., staged social interactions with confederates, self-disclosing speeches; see Sayette, 1993 for review of the diversity of stressors in SRD research). In contrast, research in affective neuroscience has relied extensively on cued threat of electric shock tasks to explicate psychological and neurobiological mechanisms involved in the affective response to stressors in animals and humans (Davis et al. 2010; Delgado et al. 2006; LeDoux 1998; Phelps 2006). In these tasks, visual or auditory cues are repeatedly paired with electric shock administration. Mechanisms involved in affective learning can be investigated via “fear conditioning” procedures in these tasks (LeDoux 1995). In humans, the cue-shock relationship can be established via instruction to examine differences in the expression of affective response among psychiatric groups or during drug intoxication or deprivation (Moberg and Curtin 2009; Hogle et al. 2006, 2010; Baas et al. 2002). These flexible cued threat tasks allow for careful, parametric manipulation of cue characteristics, cue-shock contingencies, and participant response requirements to examine important influences on affective response such as attention to threats (e.g., Curtin et al. 2001; Newman et al. 2010), the role of threat uncertainty (e.g., Moberg and Curtin 2009; Grillon et al. 2006; Davis et al. 2010) and threat intensity (e.g., the experiment in this report). Comparable tasks can be used with animals and humans to facilitate identification of neurobiological mechanisms and encourage translation of findings from animal models to humans. As such, cued threat of shock tasks are an attractive paradigm within which to systematically evaluate the SRD properties of alcohol.
Much of the affective neuroscience research with cued threat of shock tasks has relied on startle potentiation as the primary measure of defensive system activation in response to threat (Grillon 2008; Davis et al. 2010). The use of startle potentiation to index affective response to threat among rodents, non-human primates, and humans has provided an important animal-human translational bridge in this research (Davis 2006; Davis et al. 2008). The use of startle potentiation to examine SRD effects of alcohol offers the promise of similar benefit. However, unfortunately, much SRD research to date has relied primarily on measures of affective response that are too indirect to implicate neurobiological mechanisms (e.g., self-report) and/or are influenced by numerous non-affective processes that complicate interpretation (e.g., heart rate) (but see Curtin et al. 1998; Moberg and Curtin 2009; Sripada et al. 2011).
This report describes an experiment designed to examine putative SRD effects of alcohol on startle potentiation to cued threat of shock. This experiment is situated within a larger program of research in our laboratory that is systematically examining drug administration and deprivation effects on startle potentiation in this cued threat of shock paradigm (alcohol: Curtin et al. 2001; Moberg and Curtin 2009; Hefner et al. 2010; Hachiya et al. 2010; tobacco: Hogle and Curtin 2006; Hogle et al. 2010; marijuana: Gloria et al. 2009). In this report, we explicitly examine alcohol dose response and expect to observe (linear) decreases in startle potentiation with increasing blood alcohol concentration produced by higher alcohol doses. We introduce a novel manipulation of threat intensity to vary affective response magnitude systematically. Affective response magnitude has been identified as an important parameter of affective style that may be governed by distinct neurocircuitry and display important individual differences (Davidson et al. 2000). Croissant et al. (2006) have provided recent indirect evidence to implicate affective response magnitude in alcohol SRD effects as well. Finally, we test the possible moderating role of SRD relevant individual differences in trait affectivity (positive emotionality, negative emotionality and constraint/disinhibition), alcohol use, and alcohol problems.
Method
Participants
Ninety-six participants were recruited from the university community via campus flyers and online advertisements. Two participants were removed due to excessive artifact on the primary dependent measure. Two participants were removed as GLM outliers for the primary dependent measure (i.e., Studentized residual with Bonferroni corrected p< .05). Thus, all analyses are reported for N=92 (46 women). Preliminary study eligibility was assessed during a phone screening session. Participants were required to be at least 21 years of age and to report recent experience (within the last year) with the dose of alcohol to be administered in the study (i.e., 4 drinks in one episode for males, 3 for females). Potential participants were excluded if they reported a history of alcohol-related problems (i.e. score of 5 or higher on the SMAST (Selzer, Vinokour, & van Rooijen, 1975)), use of a psychiatric medication in the past year, or a medical condition for which alcohol use was contraindicated. Participants who met these criteria were scheduled for an experimental session and told to abstain from alcohol and other drug use for 24 hours, and all food and beverages other than water for four hours, prior to their experimental session. Participants were compensated $10/hour, or, for those in an introductory psychology course, 2 extra credit points/hour.
General Procedure
Consent and Screening
On arrival at the laboratory, participants provided proof of age and signed a consent form approved by the University of Wisconsin Institutional Review Board. All participants completed a medical screening questionnaire to verify their health status. Female participants were administered an in-stream urine pregnancy test (Northwest Andrology & Cryobank, Inc., Spokane, WA), with a negative result required for participation. A pre-experiment blood alcohol concentration (BAC) of 0.00% was verified via breathalyzer (Alcosensor IV; Intoximeters Inc., St. Louis, MO). Participants were first informed about the electric shock administration during the consent procedure and were offered an opportunity to ask questions about it at this time. They were also informed that they could discontinue participation at any time. One participant discontinued participation due to discomfort with the electric shock.
Baseline Startle Response Assessment
Prior to beverage group assignment, participants completed a brief procedure to assess their startle response magnitude during a neutral baseline procedure. Participants viewed a series of 12 colored squares presented on a 21 inch CRT monitor. Each square was presented for 5 s with a variable duration inter-trial interval (ITI, range = 10 - 20 s). Mean baseline startle response was calculated to 8 startle-eliciting acoustic probes presented during these cues in this baseline procedure (see Startle Potentiation Measurement section below). As per standard analytic procedures in our laboratory (Donohue et al. 2007; Moberg and Curtin 2009; Hogle et al. 2010; see also Miller and Chapman 2001), baseline startle response is used as a covariate to control for individual differences in resting startle response to increase power for analyses of startle potentiation (described below).
Beverage Group Manipulation
An equal number of male and female participants were randomly assigned to each of four beverage groups (placebo and target BACs of 0.04%, 0.075%, and 0.11%). All participants, regardless of beverage group assignment, were informed that they had been assigned to the “alcohol group” and would receive a moderately impairing dose of alcohol, equivalent to 3 drinks in a 160 lb. man in an hour, which should produce a BAC of approximately 0.08%. The beverage was comprised of 100 proof vodka (Smirnoff Blue Label) and a juice mixer in a 3:1 ratio of mixer to alcohol. The alcohol dose was calculated based on each participant’s height, weight, age, and gender to produce the specified target BAC approximately 30 minutes after completion of beverage consumption (see Curtin and Fairchild 2003 for details regarding the dosing formula). Participants assigned to the placebo group received beverages consisting of fruit juice mixed with water poured from a vodka bottle in their presence. The total volume of these placebo beverages was matched to the .075% BAC group beverage with water replacing the equivalent volume of alcohol. Outside of their view, the drinks were misted with alcohol and 2 mls of alcohol were floated on top of the beverages to provide sensory stimuli to support the placebo manipulation. The total beverage was divided into 4 drinks, each consumed in 10 minutes, for a total drinking period of 40 minutes. The experimental session began 15 minutes after the end of the drinking period. Participants’ BACs were measured at two points during the experiment: just prior to the start of the main task and immediately following the completion of the main task. Each participant’s mean BAC across these two measurement times was included in the general linear model analyses reported below to assess the effects of alcohol on startle potentiation.
Shock Tolerance Assessment
Following the drinking period and immediately prior to the start of the cued threat task, participants reported their subjective response to a series of increasing intensity, 200 ms duration, electric shocks to assess their maximum tolerance threshold as per standardized procedures in our laboratory (e.g., Curtin et al. 2001; Hogle and Curtin 2006; Hogle et al. 2010, Moberg and Curtin 2009). Shocks were administered across the distal phalanges of the index and ring fingers of left hand. The three shock intensities used in the main experiment (described below) were set to 33%, 66% and 100% of each participant’s maximum reported shock tolerance. Calibration of shock intensities to each participant’s subjective maximum tolerance threshold was designed to minimize individual differences in sensitivity and any possible analgesic effects associated with alcohol. The cued threat task began immediately after assessment of shock tolerance.
Cued Threat Task
Participants were instructed that they would complete five blocks of trials and that the duration of the task was approximately 13 minutes. In each block, participants viewed a series of 8 colored square cues presented on a 21 inch CRT monitor for 5 s each and separated by a variable inter-trial interval (10 – 20 s, mean=15 s). There were two block types: shock and no-shock. Participants completed three shock threat blocks separated by two no-shock threat blocks. A message was presented on the monitor to indicate the onset of each block type. During the shock threat blocks, participants viewed a series of pseudo-randomly intermixed colored square cues with the text “low”, “moderate” or “high” in the center of each square, to indicate the intensity of the shock that they were to receive on each trial. Square color also served as an indicator of shock intensity (i.e., distinct colors were used for the three different shock intensity cues and the no-shock cue). Participants were instructed that electric shocks would be administered only during the shock threat cues and that no shocks would ever be administered during the inter-trial interval. Shocks were administered at 4.5s post-cue onset during all shock cues (i.e., 24 shocks total). During no-shock blocks, participants were instructed that no shocks would be administered either during the cues or the inter-trial intervals.
Measures
Startle Potentiation
Electromyographic activity in the orbicularis oculi muscle was sampled at 2000Hz with a bandpass filter (30-500Hz) from electrodes placed under the right eye according to published guidelines (Blumenthal et al. 2005;Van Boxtel et al. 1998). Eyeblink startle response was measured in response to startle-eliciting noise probes (50ms of 102 dB white noise with near instantaneous rise time). Twenty-four noise probes were presented during a subset of low, moderate and high shock threat and no-threat cues at 4 seconds post cue onset (6 noise probes per cue type). Eight additional noise probes were presented during the inter-trial interval to decrease predictability of the noise probes. Serial position of the probes was counterbalanced within-subjects and a minimum of 13 s separated each probe from any previous startle eliciting event (e.g. another probe, electric shock). Data reduction and processing methods followed published guidelines (Blumenthal et al. 2005). Specifically, offline processing included signal epoching (-50 – 250 ms period surrounding noise probe), rectification, and smoothing (30 Hz low-pass). Trials with greater than 40 μV deflections in the 50ms pre-probe baseline were rejected as artifact (i.e. unstable baseline). As described earlier, two participants were removed from the sample because more than 20% of their probe trials contained excessive artifact. Peak eyeblink response between 20-120 ms post-probe onset was scored relative to mean 50 ms pre-probe baseline. Startle potentiation (i.e., increase in startle response during threat cue relative to no-threat cue) was scored separated for each shock threat intensity and served as the primary dependent measure of fear response.
Post Experimental Questionnaire
Immediately following completion of the cued threat task, participants responded to three questions to assess the integrity of the placebo manipulation. Specifically, participants 1) evaluated the alcohol content of their experimental beverages in terms of standard (12-oz beer, 5 oz glass of wine, or 1.5 oz shot of liquor) alcoholic drinks, 2) rated their peak intoxication on a 0 – 4 point scale with “Not at all intoxicated” and “Extremely intoxicated” as anchors, and, 3) estimated their peak blood alcohol concentration (they were prompted to consider that 0.08% was the legal driving limit and 0.05% is considered mildly intoxicated by most drinkers).
Self-Report Individual Difference Measures
Participants responded to the self report measures after completion of the cued threat task prior to dismissal. The measures were administered via computer. Broadband personality scales (Positive Emotionality, Negative Emotionality, and Constraint) were derived from the MPQ-Brief Form (Patrick, Curtin, & Tellegen, 2002). Participants’ alcohol consumption was measured as Drinks/Week, calculated by multiplying their reported frequency of drinking occasions per week by their average number of drinks per occasion. Number of alcohol problems in past year was based on participants’ responses to the Young Adult Alcohol Problems Screening Test (YAAPST; Hurlbut & Sher, 1992).
Results
Beverage Group and Placebo Manipulation Checks
To verify the success of our placebo manipulation, we compared the placebo and three alcohol beverage groups on the three placebo manipulation check questions that were completed at the conclusion of the experiment. Participants’ evaluation of their beverage content in terms of standard alcoholic drinks (e.g., a 12 oz beer) varied significantly by Beverage Group, F(3,88)= 3.36, p= .022, such that placebo participants estimated their beverages to contain 2.7 standard alcoholic drinks, low dose participants reported 3.5 drinks, moderate dose participants reported 3.9 drinks and high dose participants reported 4.0 drinks. Pairwise contrasts with placebo were significant for moderate (p= .008) and high (p= .006) but not low alcohol groups (p= .094). Participants’ perceived level of intoxication (measured on 0 – 4 Likert scale) varied significantly by Beverage Group, F(3,88)= 12.08, p< .001, with mean intoxication levels of 1.0, 1.8, 1.9, and 2.2 for placebo, low, moderate and high dose groups, respectively. Pairwise contrasts with placebo were significant for all alcohol groups (ps <. .001). Participants’ estimated BACs were not significantly different across Beverage Groups, F(3,88)= 0.37, p= .775, with mean estimated BAC levels of .077%, .073%, .094%, and .090% for placebo, low, moderate and high dose groups, respectively. Thus, we were successful in establishing an expectation of alcohol consumption and intoxication among participants in all beverage groups. However, as is typical with these manipulations, we were not entirely successful in matching level of expectations about consumption and intoxication across groups.
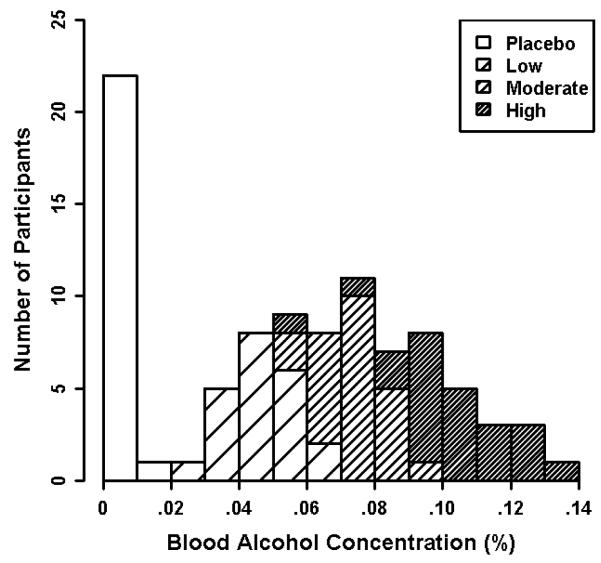
Analysis of mean achieved BACs confirmed that our dosing procedure was successful at producing a wide range of BACs (.000% - .139%). Mean BAC (mean of assessments at start and end of task) differed significantly by Beverage Group, F(2,66)= 103.7, p< .001. As expected, there was also considerable variability in BACs within each beverage group as displayed in Figure 1. Specifically, the low dose group had a mean BAC of .050% (SD= .010; range = .024 - .066%). The moderate dose group had a mean BAC of .074% (SD= .009; range = .051 - .093%). The high dose group had a mean BAC of .103% (SD= .018; range = .058 - .139%). Given this substantial variability in BACs within beverage groups, all subsequent analyses use each individual’s quantitatively measured Mean BAC rather than categorical Beverage Group as the focal independent variable to increase power and precision1.
Fig 1. Histogram of participants’ mean achieved blood alcohol concentration (BAC) by Beverage Group.
BAC was averaged over measurements obtained immediately pre-task and post-task.
Main Analyses
Startle potentiation during threat cues was analyzed in a General Linear Model (GLM) with repeated measures for Threat Intensity (low vs. moderate vs. high). Additive between-subjects regressors for Mean BAC and Baseline Startle Response were included in the GLM2,3. Huynh-Feldt corrected p-values are reported for all multi-df effects involving Threat Intensity. Significant Threat Intensity effects were decomposed with two planned orthogonal contrasts (1. Moderate vs. Low intensity and 2. High vs. the average of Moderate and Low intensity). Raw GLM coefficients (Bs) are reported to document effect sizes as appropriate.
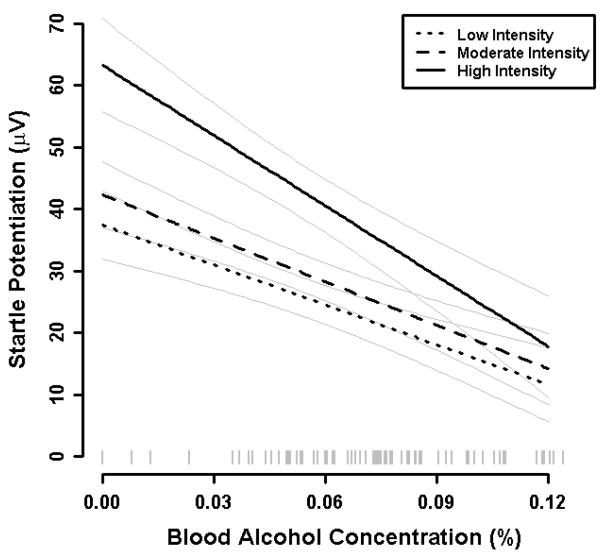
Significant (non-zero) startle potentiation was observed across threat intensity levels at BAC= .00%, B= 47.7 μV, t(89)= 8.44, p< .001. However, a significant effect of Threat Intensity was also observed, which confirms our manipulation of shock intensity altered the magnitude of startle potentiation as intended, F(2, 178)= 19.27, p< .001. Follow-up contrasts indicated that startle potentiation was non-significantly increased during moderate (M= 42.3 μV, SE= 5.3) vs. low intensity shock threat (M= 37.4 μV, SE= 5.5), B= 4.9 μV, t(89)= 1.35, p= .181 . Startle potentiation was significantly increased during high (M= 63.3 μV, SE= 7.5) vs. moderate/low intensity shock threat, B= 23.4 μV, t(89)= 5.29 , p< .001. Despite this Threat Intensity effect, significant (non-zero) startle potentiation was observed at all three threat intensities (ps< .001).
A significant effect of Mean BAC was observed across threat intensity levels such that startle potentiation decreased 2.76 μV on average for every .01% increase in BAC, B= −2.76 μV, t(89)= 3.36, p= .001. However, the effect of Mean BAC was significantly moderated by Threat Intensity, F(2,178)= 3.85, p= .028 (see Figure 2). Follow-up contrasts indicated that the magnitude of the Mean BAC effect was comparable across moderate (-2.35 μV per .01%) vs. low intensity shock threat (-2.15 μV per .01%), B= −0.20 μV, t(89)= 0.37, p= .713. However, the magnitude of the mean BAC effect was significantly increased during high (-3.79 μV per .01%) vs. moderate/low shock intensity, B= −1.54 μV, t(89) = 2.39, p= .019. Despite these differences in the magnitude of the Mean BAC effect across threat intensities, the simple effects of Mean BAC were significant at each threat intensity level (ps< .009).
Fig. 2. Startle potentiation by BAC and threat intensity.
Startle potentiation was calculated as startle magnitude during shock threat cues vs. no-shock cues. Dark lines display the relationship between BAC and startle potentiation separately for each level of threat intensity (low vs. moderate vs. high). Light grey lines represent +1 standard error bands for point estimates of mean startle potentiation at each threat intensity from the general linear model for this analysis. A rug plot of observed BACs for participants in the sample is included along the x-axis.
Individual Difference Moderators
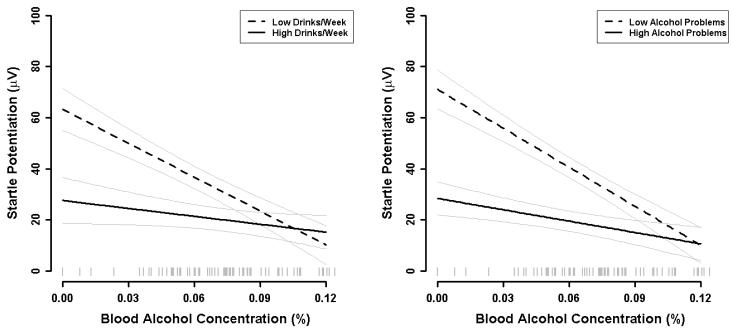
Supplemental analyses were conducted to determine if Mean BAC effects were moderated by relevant individual differences in personality (Positive Emotionality, Negative Emotionality, and Constraint), alcohol use (drinks/week), self-reported recent (past year) alcohol problems4, or shock tolerance levels. Additive and interactive effects of each quantitative individual difference measure were evaluated in separate GLMs. A significant Drinks/Week X Mean BAC effect was observed, B= 18.88 μV, t(85) = 2.20, p= .031, indicating that the magnitude of the BAC effect decreased with increasing alcohol consumption (see Figure 3, left panel). Similarly, a significant Alcohol Problems X Mean BAC effect was observed, B= 56.15 μV, t(85) = 2.92, p= .005, indicating that the magnitude of the BAC effect decreased with increasing self report of alcohol problems in this past year (see Figure 3, right panel). Not surprisingly, these measures of alcohol use and alcohol problems were significantly correlated, r= 0.60, t(88)= 7.05, p< .001. Furthermore, when both alcohol use and alcohol problems are included in the same GLM, neither effect remained significant. Thus, these two effects involved shared variance across alcohol use and problems. No other significant Individual Difference X Mean BAC or Individual Difference X Mean BAC X Threat Intensity effects were observed. Furthermore, shock tolerance levels were unrelated to startle potentiation, which confirms the success of our procedure to titrate shock intensities based on individual’s subjective response to the shock stimulus.
Fig. 3. Individual different moderators of BAC effect.
Left panel: Startle potentiation by BAC for participants with low and high weekly alcohol consumption. Point estimates for low and high alcohol consumption were obtained at + 1 standard deviation relative to the sample mean consumption (i.e., 0.5 vs. 18.4 drinks/week). Light grey lines represent standard error bands for point estimates from the general linear model for this analysis. A rug plot of observed BACs for participants in the sample is included along the x-axis.
Right panel: Startle potentiation by BAC for participants with low and high past year alcohol problems. Alcohol problems were measured with the Young Adult Alcohol Problems Screening Test (Hurlbut and Sher 1992). Point estimates for low and high alcohol problems were obtained at + 1 standard deviation relative to the sample mean problems (i.e., 1.7 vs. 8.2 past year problems). Light grey lines represent standard error bands for point estimates from the general linear model for this analysis. A rug plot of observed BACs for participants in the sample is included along the x-axis.
Discussion
This report describes an experiment designed to examine SRD effects to threats of varying intensity across a range of blood alcohol concentrations. We confirmed the primary SRD thesis that alcohol dampens stress response as measured by startle potentiation to cued threat of electric shock. Furthermore, this SRD effect was clearly dose dependent such that greater reduction in startle potentiation was observed with increasing BAC. This experiment included a novel manipulation of threat intensity to vary affective response magnitude. This threat characteristic proved to be an important moderator of the BAC effect on stress response such that the most robust alcohol SRD effect was displayed to the most potent shock threat stressor. Finally, we identified two clinically relevant individual difference moderators of the alcohol SRD effect: drinks per week and past year alcohol problems. We address the implications of each of these findings in more detail below, along with their limitations and important future directions.
This study’s demonstration that the magnitude of alcohol’s SRD effects vary with BAC joins a surprisingly small set of SRD experiments that have explicitly examined alcohol dose response in humans (e.g., Donohue et al. 2007, Sher et al. 1986; Stewart et al. 1992). This routine failure to consider alcohol dose response and/or higher alcohol doses, combined with sample sizes that are adequately powered to detect only medium effect sizes at best (Cohen 1992), may account for much of the inconsistency observed in laboratory tests of alcohol SRD effects. Clear empirical support has been provided for mediation of alcohol effects on stress response indirectly via attention and threat appraisal processes (Curtin et al. 1998, 2001; Sayette 1993; Sher et al. 2007). However, this experiment demonstrates that alcohol can reduce stress response to a visually simple, easily appraised, focal threat that was described to participants prior to alcohol administration. We believe this finding provides strong evidence that more direct SRD mechanisms exist and must continue to be explored.
Affective neuroscientists have used manipulations of threat uncertainty in animals and humans to parse anxiety and fear and their putatively distinct underlying neurocircuitry. In particular, these scientists have implicated CRF and NE sensitive pathways in the extended amygdala, including the bed nucleus of the stria terminalis (BNST), in anxiety during uncertain threats (Davis et al. 2010; Grillon 2008). In other recent research (Moberg and Curtin 2009; Hefner et al. 2010; Hachiya et al. 2010), we have documented a selective SRD effect to uncertain (e.g., unpredictable, low probability, temporally ill-defined, or otherwise ambiguous) but not certain (high probability, imminent) threats at moderate alcohol doses. Unfortunately, the current experiment remains silent on this distinction. Shock intensity for each cue was unambiguously indicated by distinct colors and text labels. However, the use of use of random, intermixed, low, moderate, and high intensity cues within blocks of trials may have engaged anxiety rather than fear neurocircuitry due to the trial-by-trial uncertainty regarding the next scheduled threat cue. Future research with this task could present cues in separate blocks of different intensities to remove this uncertainty. In addition, event related neuroimaging methods can be collected in this cued threat of shock paradigm (Wager et al. 2004; LaBar et al. 1998) to more directly examine neural mechanisms underlying these alcohol SRD effects. Of course, alcohol dose effects should continue to be examined in these future experiments.
The confirmation that alcohol SRD increased with increasing BAC in the current experiment has important potential etiological relevance as well. Many drinkers use alcohol to cope with stress at least occasionally (Cooper et al. 1995). Drinkers that more strongly endorse stress-coping motives for their use are at increased risk for alcohol-related problems (Cooper et al. 1995; Schroder and Perrine 2007). The BAC effect observed in this experiment suggests that greater SRD reinforcement will be offered to these drinkers by increasing their quantity of alcohol use on any individual drinking occasion. This increased reinforcement at higher doses is significant because consumption aimed at achieving higher BACs is associated with an array of varied health hazards. Furthermore, stronger or more persistent neuroadapations in alcoholism-relevant etiologic mechanisms also may be expected to occur with larger alcohol doses that result in higher BACs (Robinson and Berridge, 2003; Koob and Volkow 2010).
This experiment included a novel manipulation of threat intensity that produced statistically robust variation in affective response magnitude across within-subject intensity levels. Affective response magnitude has been identified as an important parameter of affective style that represents a principal ingredient to fundamental dimensions of personality and vulnerability factors that govern risk for psychopathology generally Davidson et al. 2000; see also Davidson et al. 1999; Patrick et al. 2002). In this experiment, the magnitude of the BAC effect varied systematically with threat intensity such that the largest alcohol SRD effect was observed to the most potent threat that elicited the strongest affective response. This moderating effect of threat intensity may point to another factor to account for the inconsistent observations of alcohol SRD effects in the laboratory. Many of the stressors that are used in laboratory experiments with humans are relatively weak. For example, startle potentiation during unpleasant pictures is substantially smaller than during threat of shock (e.g. see Donohue et al. 2007, Stritzke et al. 1995). In addition, other common stressors (e.g., social interactions, cognitive dissonance, self-disclosing speeches; Wilson and Abrams 1977; Steele et al. 1981; Sayette et al. 1992) are difficult to quantify with respect to intensity and likely subject to large individual differences. As such these experiments may have artificially reduced or otherwise underestimated the magnitude of alcohol SRD effects observed to more robust and/or well-controlled stressors. More importantly, this threat intensity moderation of the alcohol SRD effect may point to an important mechanism responsible for the escalating feedback loop between alcohol use and stress in individuals with alcohol use disorders. Specifically, as problems associated with alcohol use intensify, the drinker may find that alcohol provides greater SRD reinforcement, particularly at higher BACs. Of course, increasing alcohol use to cope with stress may result in still further intensification of alcohol related problems, resulting in still greater immediate alcohol SRD reinforcement.
Two significant individual difference moderators of the alcohol SRD effect were identified in this experiment: drinks per week and past year alcohol problems. As expected, these two individual differences were significantly correlated and their moderating effect was produced by their shared variance. Heavy alcohol users who reported higher numbers of recent alcohol related problems generally displayed reduced affective response to shock threat, regardless of their level of intoxication. However, these same individuals also experienced substantially diminished alcohol SRD effects. This diminished SRD effect could be interpreted as a risk factor for alcoholism within Schuckit’s Low Response (LR) to Alcohol model (Schuckit et al. 2009; Schuckit and Smith 2006). These relationships between reduced alcohol SRD, high levels of alcohol use, and increased alcohol problems are intriguingly consistent with this LR model. Drinkers who experience reduced alcohol SRD may consume more alcohol to obtain the desired SRD effects, resulting in concomitant problems associated with the heavy hazardous drinking referenced earlier. Of course, speculation about causal ordering among these correlated variables must be advanced very cautiously until replicated in designs that can rule out alternative explanations. For example, reduced alcohol SRD among heavy drinkers may simply reflect acquired tolerance, with a spurious connection to alcohol problems via increased alcohol use. Alternatively, reduced alcohol SRD among heavy, problem users may reflect a floor effect that would not be observed in the real world with more potent stressors. Regardless, these connections warrant further investigation. In particular, initial alcohol SRD effects prior to substantial alcohol use should be tested as a prospective marker for subsequent alcohol problems.
The temporal ordering of alcohol consumption and stressor appraisal remains an important, understudied issue with potential implications for both mechanism and clinical significance of observed SRD effects. Sayette (1993) reported that larger SRD effects are typically observed in the laboratory when stressors are appraised following alcohol consumption. In contrast, many real world examples of stress motivated drinking involve alcohol use for ‘relief’ subsequent to stressor exposure. The temporal ordering of alcohol consumption and stressor appraisal is difficult to define definitively in the current experiment. Participants received detailed description of the shock threat during the consent procedure immediately after arriving at the laboratory. However, all shocks were administered following beverage consumption. Future research should examine this issue more carefully.
Substantial advances in ecological momentary assessment of real world stressors, affective response and alcohol and other drug use have occurred in the past decade (Bopp et al. 2010; McCarthy et al. 2006; Piper et al. 2008). Preliminary coarse assessments have documented that stress-drinking covariation is greater among individuals who develop alcohol problems (Schroder and Perrine 2007). These advances set the stage for an important next step for laboratory alcohol SRD research. The cued threat of shock paradigm has proved sensitive to detect alcohol SRD effects in controlled laboratory settings. We must now demonstrate that individual differences in these SRD effects measured in the laboratory are motivationally relevant for drinkers in their day-to-day lives. Questions about whether laboratory SRD individual differences predict alcohol use, development of alcohol problems, risk for relapse among alcoholics, and/or stress-alcohol covariation in the real world remain. Furthermore, it remains to be demonstrated whether chronic heavy alcohol use alters the nature of the drinker’s stress response itself as emerging models in animals and humans suggest (Sinha 2008; Breese et al. 2010; Koob and Volkow, 2010; Weiss et al. 2001).
Table 1.
| BAC | YAP | DrkWk | Nem | Pem | Con | Age | |
|---|---|---|---|---|---|---|---|
| BAC | -- | ||||||
| YAP | −.044 | -- | |||||
| DrkWk | .135 | .601 | -- | ||||
| Nem | −.012 | .118 | −.085 | -- | |||
| Pem | .080 | .158 | .118 | −.180 | -- | ||
| Con | .094 | .067 | −.082 | −.165 | −.052 | -- | |
| Age | −.041 | −.07 | −.023 | −.131 | −.031 | .045 | -- |
| Mean | .056 | 5.0 | 9.4 | 33.8 | 77.7 | 71.9 | 22 |
| SD | .039 | 3.2 | 9.0 | 13.7 | 14.5 | 14.9 | 1.5 |
| Min | 0 | 0 | 0.25 | 10 | 29 | 34 | 21 |
| Max | 0.139 | 16 | 60.5 | 94 | 101 | 103 | 29 |
Acknowledgements
This research was support by a grant to John J. Curtin from NIAAA (R01AA15384).
Footnotes
Prior research in our laboratory has attempted similar control via defining beverage groups based on actual observed rather than target BACs (Donohue et al. 2007). However, that strategy does not fully account for variation in BACs within each beverage group. Use of quantitative BAC in General Linear Model analyses is superior for this reason. Regardless, results from analysis of categorical Beverage Group (significant Beverage Group and Beverage Group X Threat Intensity effects) are comparable to results reported below for quantitative Mean BAC.
Between-subject regressors were modeled additively because significant interactions involving Mean BAC and Baseline Startle Response were not observed in preliminary models. No significant additive or interactive effects of Sex were observed in preliminary models. Therefore Sex was removed from the final reported GLM. BAC was linearly transformed (BAC X 100) for descriptive purposes such that a 1 unit change in our BAC regressor was equivalent to .01% change in Mean BAC. Of course, linear transformations do not affect model fit or significant tests.
To examine possible additive and/or interactive effects of BAC limb (i.e., whether participants BAC was ascending vs. descending during the task), we quantified BAC change as post-task BAC minus pre-task BAC. As such, positive scores code for participants on the ascending limb and negative scores code for participants on the descending limb. We added this additional regressor to our GLM in a preliminary analysis. No significant overall effect of BAC change on startle potentiation was observed, F(1,87) = 0.001, p = .972. BAC change did not significantly moderate either the overall mean BAC effect or the BAC X Threat intensity effect, F (1,87) = 0.02, p=.887 and F(2,174) = 1.42, p = .245, respectively, indicating that the BAC effects reported in the main analysis were consistent regardless of whether participants were ascending or descending. Finally, when partial effects of BAC and BAC X Threat intensity are examined in this model that controls for BAC change, their effects remain significant, F(1, 87) = 10.30, p = .002 and F(2, 174) = 3.21, p = .049, respectively.
Two participants were missing data on all self-reported individual difference measures and are therefore excluded from these analyses (N=90).
References
- Baas JMP, Grillon C, Böcker KBE, Brack AA, Morgan CA, III, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bopp JM, Miklowitz DJ, Goodwin GM, Stevens W, Rendell JM, Geddes JR. The longitudinal course of of bipolar disorder as revealed through weekly text messaging: a feasibility study. Bipolar Disord. 2010;12:327–334. doi: 10.1111/j.1399-5618.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2010;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: Inhibition by CRF1 and benzodiazepine-receptor antagonists and a 5-HT1a – receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability, and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol. 1990;99(4):344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Goldman MS, Inn A. Development of alcohol-related expectancies in adolescents: Separating pharmacological from social-learning influences. J Consult Clin Psychol. 1982;50:336–344. doi: 10.1037//0022-006x.50.3.336. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Croissant B, Rist F, Demmel R, Olbrich R. Alcohol-induced heart rate response dampening during aversive and rewarding stress paradigms in subjects at risk for alcohol. Intl J Psychophysiology. 2006;61:253–261. doi: 10.1016/j.ijpsycho.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112(3):424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR. Alcohol and emotion: Insights and directives from affective science. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. American Psychological Association; Washington, DC: 2007. pp. 191–213. [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychol Sci. 2001;12:527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WGK. Alcohol and fear-potentiated startle: The role of competing cognitive demands in the stress-reducing effects of intoxication. J Abnorm Psychol. 1998;107:547–565. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9(2):228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Ann N Y Acad Sci. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT. Acoustic startle reflex in rhesus monkeys: A review. Rev Neurosci. 2008;19:171–185. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology Reviews. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, Lang AR. Intoxication level and emotional response. Emotion. 2007;7:103–112. doi: 10.1037/1528-3542.7.1.103. [DOI] [PubMed] [Google Scholar]
- Gloria R, Jaber JN, Baker TB, Curtin JJ. The effect of temporal precision and probability on the response to threat of shock: a fear-potentiated startle study. Psychophysiology. 2009;46(s1):s80. [Google Scholar]
- Goldman MS, Brown SA, Christiansen BA. Expectancy theory: Thinking about drinking. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. Guilford Press; New York: 1987. pp. 181–226. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou P, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. 2nd edn Guilford Press; New York: 1999. pp. 14–53. [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Avenevoli S, Daurignac E, Merikangas KR. Fear-potentiated startle to threat, and prepulse inhibition among young adult nonsmokers, abstinent smokers, and nonabstinent smokers. Biol Psychiatry. 2007;62:1155–1161. doi: 10.1016/j.biopsych.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Hachiya LY, Moberg CA, Curtin JJ. Alcohol effects on affective response during variable and fixed duration threat. Alcohol Clin Exp Res. 2010;34(s2):117A. [Google Scholar]
- Hefner KR, Jaber JN, Grant AM, Curtin JJ. Alcohol intoxication: Selective reduction of anxiety in the face of uncertain threat. Psychophysiology. 2009;46(s1):s64. [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biol Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. J Am Coll Health. 1992;41:49–58. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Addiction and the brain antireward system. Ann Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;36:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Ann Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Alcohol and stress response dampening: Pharmacological effects, expectancy, and tension reduction. J Abnorm Psychol. 1980;89(4):528–538. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. J Abnorm Psychol. 2006;115:454–66. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable vs. predictable threat. J Abnorm Psychol. 2009;118(2):335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry. 2010;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res. 2007;31(9):1473–1481. doi: 10.1111/j.1530-0277.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assmt. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Ann Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Ann Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol’s effects on stress responses in social drinkers. Psychol Bull. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Smith DW, Beiner MJ, Wilson GT. The effect of alcohol on emotional response to a social stressor. J Stud Alcohol. 1992;53:541–545. doi: 10.15288/jsa.1992.53.541. [DOI] [PubMed] [Google Scholar]
- Schroder KEE, Perrine MW. Covariations of emotional states and alcohol consumption: evidence from 2 years of daily data collection. Soc Sci & Med. 2007;65(12):2588–602. doi: 10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. J Stud Alcohol. 2006;67:215–227. doi: 10.15288/jsa.2006.67.215. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Trim R, Bucholz KK, Edenberg HJ, Hesselbrock V, Kramer JJ, Dick DM. An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol. 2009;70:436–445. doi: 10.15288/jsad.2009.70.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Stress response dampening. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. Guilford Press; New York: 1987. pp. 227–271. [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, Wood MD. Stress-response-dampening effects of alcohol: Attention as a mediator and moderator. J Abnorm Psychol. 2007;116(2):362–377. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS. Individual differences in the stress-response dampening effect of alcohol: A dose-response study. J Abnorm Psychol. 1986;95:159–167. doi: 10.1037//0021-843x.95.2.159. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage. 2011;55:371–380. doi: 10.1016/j.neuroimage.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C, Josephs R. Alcohol myopia: Its prized and dangerous effects. Am Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Steele CM, Southwick LL, Critchlow B. Dissonance and alcohol: Drinking your troubles away. J Pers Soc Psychol. 1981;41:831–846. doi: 10.1037//0022-3514.41.5.831. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, Pihl RO. The effects of alcohol on the cardiovascular stress response in men at high risk for alcoholism: A dose response study. J Stud Alcohol. 1992;53:499–506. doi: 10.15288/jsa.1992.53.499. [DOI] [PubMed] [Google Scholar]
- Van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35:690–697. [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Ann NY Acad Sci: 2001:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Abrams D. Effects of alcohol on social anxiety and physiological arousal: Cognitive versus pharmacological processes. Cog Ther and Res. 1977;1:195–210. [Google Scholar]