Abstract
The satellite RNA of tobacco ringspot virus (STobRV RNA) replicates and becomes encapsidated in association with tobacco ringspot virus. Previous results show that the infected tissue produces multimeric STobRV RNAs of both polarities. RNA that is complementary to encapsidated STobRV RNA, designated as having the (-) polarity, cleaves autolytically at a specific ApG bond. Purified autolysis products spontaneously join in a non-enzymic reaction. We report characteristics of this RNA ligation reaction: the terminal groups that react, the type of bond in the newly formed junction and the nucleotide sequence of the joined RNA. The nucleotide sequence of the ligated RNA shows that joining of the reacting RNAs restored an ApG bond. The junction ApG has a 3'-to-5' phosphodiester bond. Thus the net ligation reaction of STobRV (-)RNA is the precise reversal of autolysis. We discuss this new type of RNA ligation reaction and its implications for the formation of multimeric STobRV RNAs during replication.
Full text
PDF






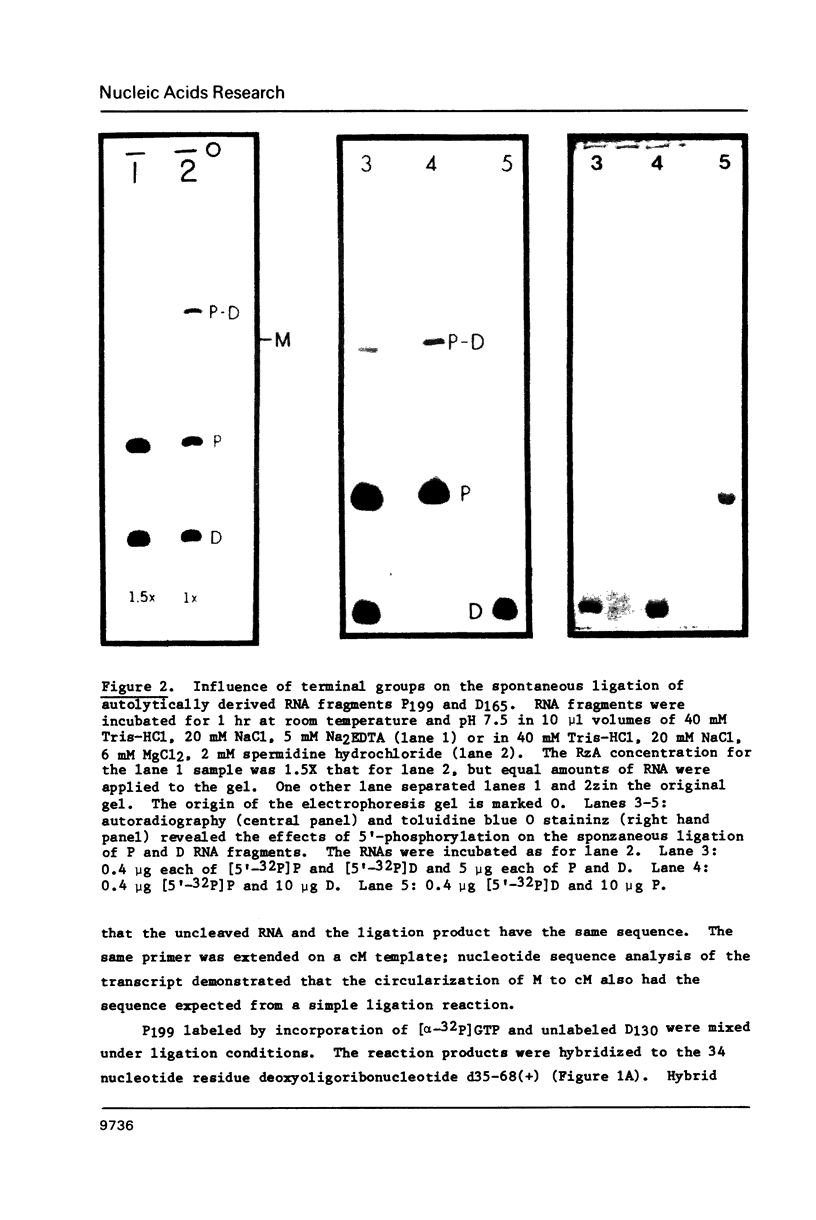

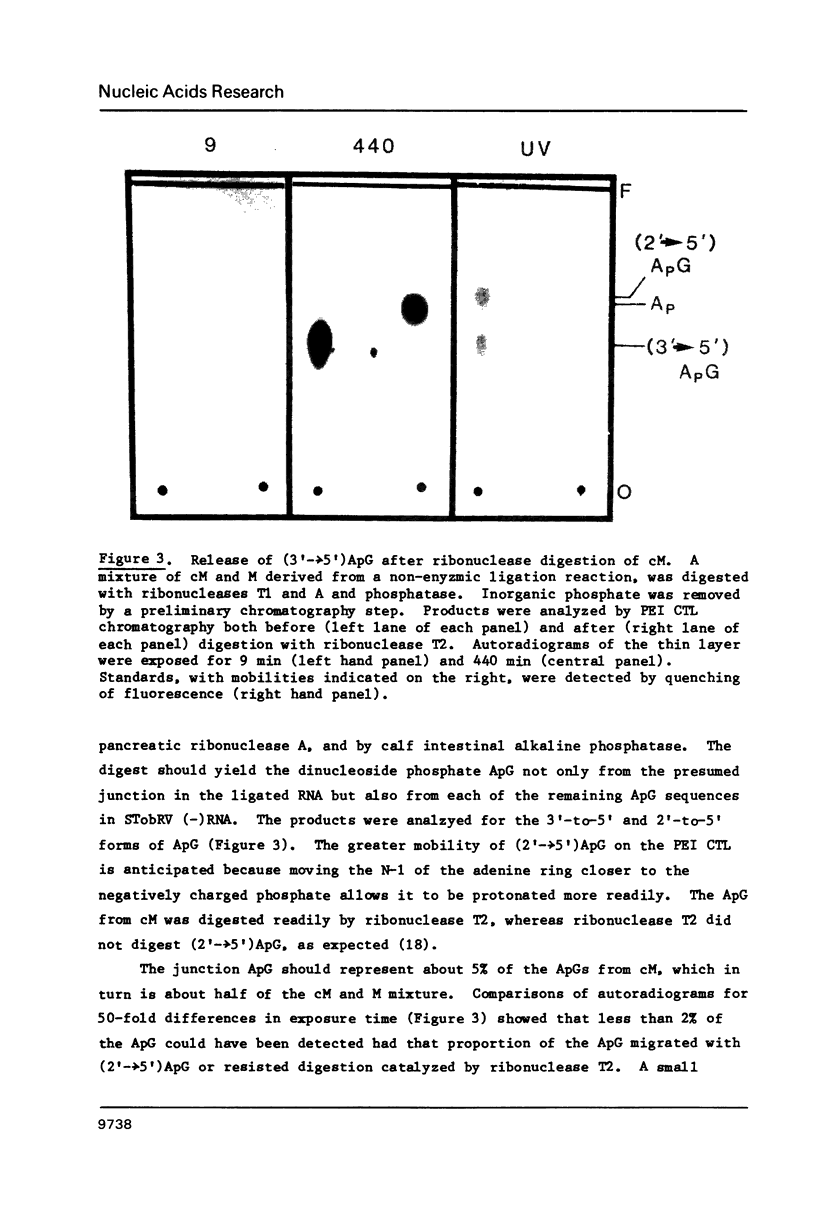





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell. 1986 Jan 31;44(2):207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Shatkin A. J. Origin of splice junction phosphate in tRNAs processed by HeLa cell extract. Cell. 1983 Feb;32(2):547–557. doi: 10.1016/0092-8674(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Greer C. L., Peebles C. L., Gegenheimer P., Abelson J. Mechanism of action of a yeast RNA ligase in tRNA splicing. Cell. 1983 Feb;32(2):537–546. doi: 10.1016/0092-8674(83)90473-7. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiberstis P. A., Haseloff J., Zimmern D. 2' phosphomonoester, 3'-5' phosphodiester bond at a unique site in a circular viral RNA. EMBO J. 1985 Mar;4(3):817–822. doi: 10.1002/j.1460-2075.1985.tb03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor virus. VI. Processive mode of action of avian myeloblastosis virus polymerase. J Virol. 1976 Sep;19(3):932–939. doi: 10.1128/jvi.19.3.932-939.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Schneider I. R. Satellite of tobacco ringspot virus RNA lacks detectable mRNA activity. Virology. 1977 Jul 1;80(1):222–224. doi: 10.1016/0042-6822(77)90396-8. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Uhlenbeck O. C. Joining of RNA molecules with RNA ligase. Methods Enzymol. 1983;100:52–59. doi: 10.1016/0076-6879(83)00045-2. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Tyc K., Kikuchi Y., Konarska M., Filipowicz W., Gross H. J. Ligation of endogenous tRNA 3' half molecules to their corresponding 5' halves via 2'-phosphomonoester,3',5'-phosphodiester bonds in extracts of Chlamydomonas. EMBO J. 1983;2(4):605–610. doi: 10.1002/j.1460-2075.1983.tb01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. A., McHale A. H. Nonenzymic joining of oligoadenylates on a polyuridylic acid template. Science. 1976 Apr 2;192(4234):53–54. doi: 10.1126/science.1257755. [DOI] [PubMed] [Google Scholar]
- Verlander M. S., Lohrmann R., Orgel L. E. Catalysts for the self-polymerization of adenosine cyclic 2', 3'-phosphate. J Mol Evol. 1973 Nov 27;2(4):303–316. doi: 10.1007/BF01654098. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]




